Abstract
Purpose
Dynamic contrast-enhanced-MRI (DCE-MRI) can provide information regarding tumor perfusion and permeability and has shown prognostic value in certain tumors types. The goal of the present study was to assess the prognostic value of pretreatment DCE-MRI in head and neck squamous cell carcinoma (HNSCC) patients with nodal disease undergoing chemoradiation therapy or surgery.
Methods and Materials
Seventy-four patients with histologically proven squamous cell carcinoma and neck nodal metastases were eligible for the study. Pretreatment DCE-MRI was performed on a 1.5T MRI. Clinical follow-up was a minimum of 12 months. DCE-MRI data were analyzed using Tofts model. DCE-MRI parameters were related to treatment outcome (progression free survival [PFS] and overall survival [OS]). Patients were grouped as no evidence of disease (NED), alive with disease (AWD), dead with disease (DOD) or dead of other causes (DOC). Prognostic significance was assessed using the log rank test for single variables and Cox proportional hazards regression for combinations of variables.
Results
At last clinical follow-up, for stage III, all 12 pts were NED, for stage IV, 43 patients were NED, 4 were AWD, 11 were DOD, and 4 were DOC. Ktrans is volume transfer constant. In a stepwise Cox regression skewness of Ktrans was the strongest predictor for stage IV patients (PFS and OS: p<0.001).
Conclusion
Our study shows that skewness of Ktrans was the strongest predictor of PFS and OS in stage IV HNSCC patients with nodal disease. This study suggests an important role for pretreatment DCE-MRI parameter Ktrans as a predictor of outcome in these patients.
Keywords: Dynamic Contrast Enhanced-MRI (DCE-MRI), head and neck squamous cell carcinoma (HNSCC), volume transfer constant (Ktrans)
Introduction
The American Cancer Society estimated that in 2010 approximately 49,260 new cases of oral cavity, pharyngeal and laryngeal cancers would be diagnosed, and that 11,480 deaths would occur from these cancers in the United States (1). Treatment for advanced (stage III or IV) head and neck squamous cell carcinoma (HNSCC) usually consists of combined chemoradiation therapy, or complete surgical resection followed by adjuvant chemo- and/or radiation therapy (2–5). Despite advances in the treatment options available, the overall survival rate of HNSCC patients with advanced disease has not improved substantially over the past decade (6). Thus, an a priori predictor of outcome could prove extremely valuable by allowing oncologists to intervene with alternative therapies if necessary.
Reported tumor-based prognostic factors for locoregional control of HNSCC include the presence and extent of nodal metastases, T-stage, tumor site, tumor size, human papillomavirus (HPV) tumor positivity, and other biological markers (7–10). In advanced HNSCC, the T stage and nodal disease at initial presentation are the most important predictors of outcome (8). The use of individual and combined markers to predict outcome in HNSCC has shown conflicting results. For example, different investigators have recorded different degrees of correlation between tumor suppressor gene p53 status and outcome (11–13). Epidermal growth factor receptor (EGFR) over-expression has been shown to correlate strongly with advanced tumor stage, shorter disease-free survival and overall survival in HNSCC (14). Recently, HPV-positive HNSCC has been shown to respond to treatment better than non-HPV-positive HNSCC (15). Preliminary evidence supports the potential role of such biomarkers in disease management, but their value needs to be tested in prospective validation studies (7).
Non-invasive measurement of tumor perfusion and permeability using gadopentetate dimeglumine (Gd-DTPA)-based dynamic contrast-enhanced MRI (DCE-MRI) has shown promise in predicting treatment response and outcome in selected tumors (16–21). DCE-MRI involves assessing changes in signal intensity over time. With proper quantitative analysis, the data may provide parameters reflecting tumor-vessel permeability, tumor perfusion, and extracellular-extravascular volume fraction (22–25). Studies have suggested that DCE-MRI parameters such as Ktrans (volume transfer constant) and primary tumor blood volume (BV) may predict early response in HNSCC patients treated with chemoradiation (20, 26). The present study aims to assess the prognostic value of pretreatment DCE-MRI parameters in HNSCC patients with nodal disease undergoing chemoradiation therapy or surgery.
Materials and Methods
Patient Selection
The institutional review board (IRB) granted a waiver of informed consent for this retrospective study that included 74 patients with histologically proven squamous cell carcinoma (SCC) and neck nodal metastases. Their clinical characteristics are listed in Table 1. Out of 74 patients, 61 patients had primary treatment with chemoradiation and 13 underwent surgery (Table 1). Patients received treatment as per standard guidelines (4, 5, 27, 28) (see Table 2 for details).
Table 1.
Pretreatment characteristics of the HNSCC patients
| Characteristic | Chemoradiation therapy (N=61) | Surgery (N=13) |
|---|---|---|
| Age | ||
| 18–69 yr -no. (%) | 55 (90) | 11 (85) |
| ≥70 yr -no. (%) | 6 (10) | 2 (15) |
| Median | 57 | 53 |
| Range | 38–83 | 41–79 |
|
| ||
| Sex -no. (%) | ||
| Male | 53 (87) | 10 (77) |
| Female | 8 (13) | 3 (23) |
|
| ||
| KPS (%) | ||
| Median | 90 | 90 |
| Range | 80–100 | 90–100 |
|
| ||
| Alcohol consumption -no. (%) | ||
| Yes | 54 (89) | 11 (85) |
| No | 7 (11) | 2 (15) |
|
| ||
| Tobacco history -no. (%) | ||
| Yes | 42 (69) | 9 (69) |
| No | 19 (31) | 4 (31) |
|
| ||
| Clinical Stage -no. (%) | ||
| III | 10 (16) | 2 (15) |
| IV | 51 (84) | 11(85) |
|
| ||
| Primary tumor location -no. (%) | ||
| Oropharynx | 61 (100) | 3 (23) |
| Oral Cavity | 0 | 4 (31) |
| Unknown primary | 0 | 6 (46) |
|
| ||
| HPV status -no. (%) | ||
| Positive | 16 (26) | 3 (23) |
| Negative | 21 (34) | 8 (62) |
| Unknown | 24 (40) | 2 (15) |
Table 2.
Treatment details of chemoradiation for the 61 HNSCC patients
| Treatment | Number (%) of patients |
|---|---|
| Definitive IMRT | 61 (100) |
| Radiation dose (Gy) | 70 |
|
| |
| Chemotherapy | 61 (100) |
| Cisplatin | 40 (66) |
| Cetuximab | 12 (20) |
| Carboplatin/5-FU | 9(14) |
Abbreviations: IMRT= intensity-modulated radiotherapy; and FU = fluorouracil
DCE-MRI Methodology
All patients had a baseline DCE-MRI performed before surgery or chemoradiation therapy (mean 16 ± 11 days). MR Images were acquired on a 1.5-Tesla Excite™ scanner (General Electric, Milwaukee, WI) with a 4-channel neurovascular phased-array coil for signal reception and a body coil for transmission. The MR imaging protocol for the neck survey included rapid scout images, multiplanar (axial, coronal and sagittal) T2-weighted, fat-suppressed, fast-spin-echo images, and multi-planar T1-weighted images (29). A neuroradiologist identified the largest lymph node in the neck region for DCE-MRI study. DCE-MRI data acquisition and analysis have been described previously (29, 30). DCE-MR images were acquired using a fast multi-phase spoiled gradient echo sequence. Antecubital vein catheters delivered a bolus of 0.1 mmol/kg Gd-DTPA (Magnevist; Berlex Laboratories, Wayne, NJ, USA) at 2 cc/s, followed by saline flush using an MR-compatible programmable power injector (Spectris; Medrad, Indianola, PA, USA). The entire node was covered contiguously with 5–7 mm thick slices, zero gap, yielding 3–6 slices with 3.75–7.5 sec temporal resolution. Acquisition parameters were as follows: TR = 9 ms, TE = 2 ms, 30° flip angle (α), 2 excitations, 15.63-kHz receive bandwidth, field of view (FOV) = 18–20cm, slice thickness = 5–6 mm, zero gap, 256×128 matrix that was zero filled to 256 × 256 during image reconstruction, and 40–80 time course data points collected.
All DCE-MRI analyses were conducted by an investigator blinded to patient identity, treatment group, scan date, and patient outcome. DCE-MRI data were analyzed with IDL 5.4 (Research Systems Inc., Boulder Co). For the tumor tissue time course data, regions of interest (ROIs) were manually drawn by the same neuroradiologist who outlined the contrast-enhanced lymph nodes for signal intensity measurements. All the slices containing the tumor were outlined and analyzed. Quantitative DCE-MRI analyses of the tumor tissue time course data were performed using the Tofts model (24). For functional analysis of tissue microcirculation, Tofts et al. (24) proposed in a consensus paper kinetic parameters to describe tumor and tissue permeability using two compartment (ie, blood plasma and extravascular extracellular space (EES)) model. A population-based arterial input function was used (29). The data were fit to the Tofts model to determine the kinetic parameters Ktrans (the volume transfer constant between the plasma and the EES in min−1), ve (the volume fraction of the EES, which is dimensionless) and kep (the rate constant describing the contrast transfer between the EES and plasma in min−1, which equals the ratio Ktrans/ve). DCE-MRI analyses of each ROI were done on a pixel-by-pixel basis. Histogram analysis was done for all pixels within the ROI, which yielded median, standard deviation, and skewness. Histograms were normalized to the total number of tumor voxels to allow direct comparison between patients. The standard deviation describes the width of the distribution and is indicative of the tumor heterogeneity (31). The skewness characterizes the asymmetry of the distribution.
Patient Assessment
A complete medical history was obtained and clinical tumor assessment was performed before treatment. Clinical follow-up to detect distant metastases or local failure included clinical evaluation and imaging studies. Planned clinical disease evaluation was performed approximately 1–3 months during the first year and included imaging as clinically indicated but was not routine. Local-regional control was assessed at approximately 3 months after completion of treatment. All patients alive had a minimum of one year of clinical follow-up (range: 13–64 months, median 40 months). Data was censored at the time of last follow-up. The primary end points calculated were progression-free survival (PFS) and overall survival (OS), consistent with published literature (5). Each patient’s status on last follow-up was classified as no evidence of disease (NED), alive with disease (AWD), dead with disease (DOD) or dead of other causes (DOC) (32).
HPV (human papilloma virus) in situ hybridization
Assays were performed on 4μm whole sections of a paraffin embedded tissue specimens. Tissue for 48 of 74 patients was available for HPV staining. Staining was performed on an automated stainer using INFORM HPV III family 16 probe (Ventana Medical system, Tucson AZ) according to manufacturer instructions. The probe has affinities to HPV genotypes 16, 18, 31, 33, 39, 45, 51, 52, 56, 58, and 66. Adequate positive and negative control slides were included in each batch. A HPV-positive tumor was defined as a tumor for which there was specific staining of tumor-cell nuclei for HPV in the analysis.
Statistical Analysis
There were three data types per patient: outcomes (PFS and OS), potential prognostic variables (demographic and clinical variables that may influence outcome), and DCE-MRI parameters (Ktrans, ve and kep). The distributions of the DCE-MRI parameters for the ROIs were summarized as median, mean, standard deviation and skewness. Relationships between DCE-MRI parameters and prognostic variables were assessed by Spearman correlation coefficients for numeric variables and t-test or analysis of variance for categorical variables. T-stage was treated as a numeric variable (1, 2, 3, 4, assigning 2.5 for unknown T-stage). N-stage 3 was combined with N-stage 2 since there were only two cases of Stage 3. Survival analyses were done for PFS and OS using: the Kaplan-Meier method to compute survival curves, the log-rank test to assess univariate statistical significance of prognostic variables, and Cox proportional hazards regression to assess statistical significance of combinations of prognostic variables. Significant prognostic factors were determined, and the prognostic significance of DCE-MRI parameters was assessed using Cox regression to adjust for significant prognostic factors. Subset analysis was done for stage IV patients and patients who received chemo-radiation as primary treatment. Results were considered to be statistically significant with a p-value < 0.05. Statistical computations were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
All 12 clinical stage III patients were NED at last clinical follow-up. Of the clinical stage IV patients, 43 were NED, 4 were AWD, 11 were DOD, and 4 were DOC at last clinical follow-up. The average pretreatment values for the median, standard deviation and skewness of Ktrans, ve and kep for stage III and stage IV patients are provided in Table 3. Figure 1 shows representative pretreatment DCE-MRI data. Figure 2 shows representative case of HPV positive and negative staining. Patterns of failure were reviewed. Among 61 patients who received chemoradiation as primary treatment, the initial site of failure was distant for 8 patients and simultaneous distant and locoregional failure for 2 patients. No patient in the chemoradiation cohort had locoregional disease only as the first site of failure. Five patients underwent neck surgery within 4 months of completion of chemoradiation, but this is not considered locoregional failure, as neck dissection is part of multimodality locoregional therapy. All 13 patients who underwent surgery as primary treatment were further treated with chemoradiation or radiation alone. Seven patients received radiation while 6 received chemoradiation post surgery. Patterns of failures showed that one patient had regional failure and two patients had distant failure.
Table 3.
Pretreatment values (mean±SEM) of DCE-MRI parameters in neck nodal metastases of stage III patients (n=12) and stage IV patients (n=58)* who have NED, AWD, or DOD.
| Patients | median Ktrans | median ve | median kep | skewness of Ktrans | skewness of ve | skewness of kep | std of Ktrans | std of ve | std of kep |
|---|---|---|---|---|---|---|---|---|---|
| Stage III: NED (n=12) | 0.31 ±0.04 | 0.56 ±0.05 | 0.63 ±0.07 | 3.45 ±1.26 | 0.17 ±0.19 | 3.05 ±0.60 | 0.17 ±0.02 | 0.23 ±0.02 | 0.59 ±0.11 |
| Stage IV:NED (n=43) | 0.26 ±0.02 | 0.50 ±0.02 | 0.60 ±0.04 | 0.73 ±0.13 | 0.39 ±0.10 | 2.53 ±0.31 | 0.13 ±0.006 | 0.24 ±0.009 | 0.44 ±0.03 |
| Stage IV:AWD (n=4) | 0.20 ±0.06 | 0.39 ±0.06 | 0.49 ±0.06 | 1.96 ±1.16 | 0.77 ±0.09 | 4.30 ±1.39 | 0.12 ±0.01 | 0.23 ±0.04 | 0.57 ±0.13 |
| Stage IV:DOD (n=11) | 0.19 ±0.03 | 0.52 ±0.08 | 0.47 ±0.05 | 1.63 ±0.51 | 0.11 ±0.22 | 3.19 ±0.67 | 0.18 ±0.04 | 0.30 ±0.01 | 0.54 ±0.11 |
The mean±SEM for patients in DOC is not included as there were four patients in this group.
Figure 1.
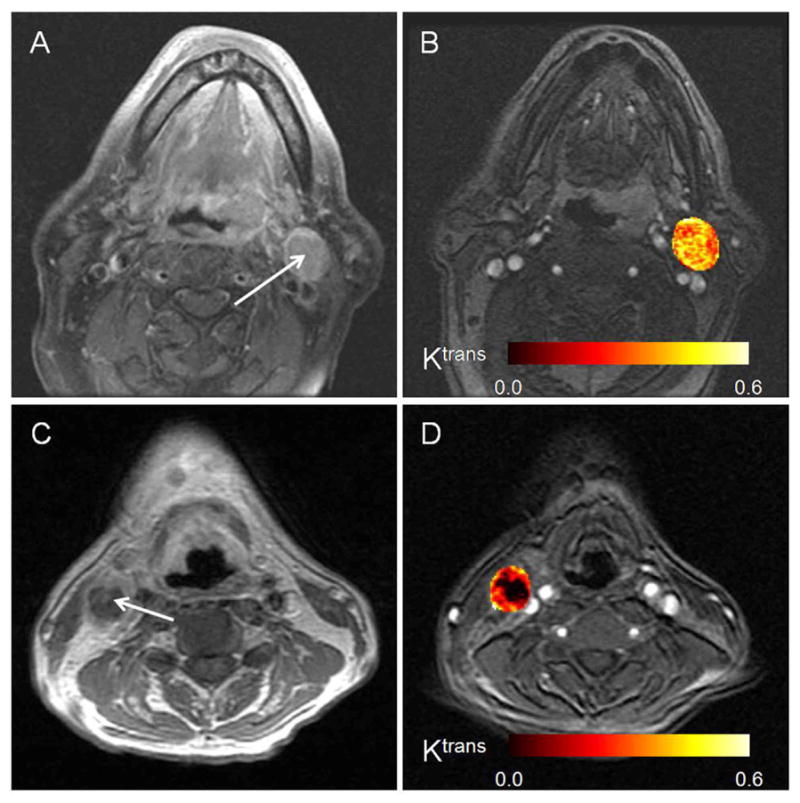
(a) MR images illustrating left neck lymph node (with arrow) of patient who continued to be NED for 57 months after concurrent chemoradiation therapy (male, 56 years old, primary tonsil cancer). (b) A corresponding post-contrast axial image extracted from the DCE-MRI scan, on which the color, calculated parametric Ktrans map of the node is overlaid. (c) MR images illustrating right neck lymph node (with arrow) of patient who died five months after surgery due to distant recurrence (male, 56 years old, primary base of tongue cancer). (d) A corresponding post-contrast axial MR image extracted from the DCE-MRI scan, on which the color, calculated parametric Ktrans map of the node is overlaid. The heterogeneity of the necrotic node can be appreciated in the Ktrans map
Figure 2.
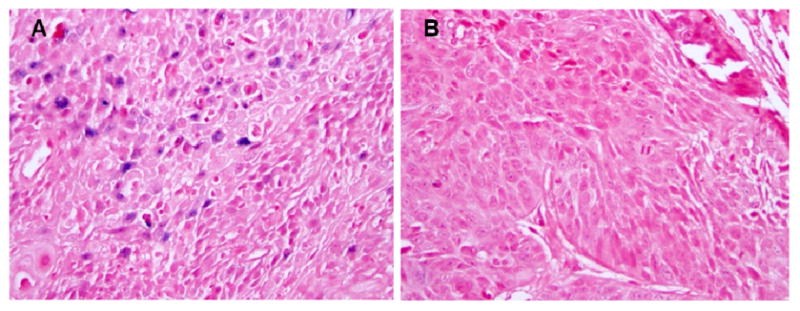
(a) Positive HPV in-situ hybridization on a tumor from a patient who received chemoradiation and continued to be NED at 30 months (female, 79 years old, primary oropharynx cancer). (b) Negative HPV tumor from a patient who received chemoradiation and died of disease after 27 months of treatment (male, 52 years old, primary oropharynx cancer).
The pretreatment DCE-MRI parameters appeared to give information independent of demographic and clinical as they were not, in general, significantly related to prognostic factors. Correlation coefficients with T-stage, age, and nodal size were in most cases very small (−0.1 < r < 0.1), and the few in the ranges −0.3 < r < − 0.2 or 0.2 < r < 0.3 were not statistically significant after adjusting for multiple statistical significance testing. Similarly, DCE-MRI parameters did not differ significantly according to categorical variables such as sex, type of treatment, or risk factors such as smoking history. These results were not shown since they were ancillary and not significant. Age was partially confounded with sex, since women were on average significantly older than men in this study sample. Since both could not be entered in the same Cox regression analysis; while age was given preference in regression analyses, replacing with sex would yield similar results.
Prognostic factors for survival
Univariate survival analyses of PFS and OS showed significant prognostic value for T stage (numeric); p=0.004 (PFS), p=0.010 (OS); N stage (1vs 2/3): p=0.028 (PFS) and p=0.050 (OS); clinical stage (III vs IV): p=0.034 (PFS) and p=0.056 (OS); age: p=0.008 (PFS) and p=0.013 (OS), sex: p=0.008 (PFS) and p=0.009 (OS) and treatment (chemoradiation vs surgery): p=0.210 (PFS) and p=0.039 (OS). Disease site, tobacco history and alcohol addiction were non-significant. There was no difference in survival between HPV-positive and HPV-negative patients; the 26 patients (35%) without HPV data had slightly better survival than patients with HPV data, suggesting that poorer risk patients had tissue available for assay. Loco-regional control was achieved in all but 2 patients, so this variable was not considered further. In Cox regression analysis, only T-stage and either age or sex had a jointly significant relationship to PFS or OS.
DCE-MRI parameters and survival
Cox regression analyses of the additive value of DCE-MRI parameters for predicting survival, adjusting for the two primary prognostic factors T-stage and age, showed the following. Significant for PFS were skewness of Ktrans (p=0.011), std ve (p=0.043) and marginally, median Ktrans (p=0.060). The latter two did not add significant prognostic value to skewness of Ktrans. Significant predictors of OS were mean ve (p=0.007), skewness of Ktrans (p=0.012) and marginally, median Ktrans (p=0.067) or std Ktrans (p=0.068). The latter three did not add significant prognostic value to mean ve.
Subset analyses
Survival analyses based on 61/74 patients who received chemoradiation as primary treatment yielded similar results to analyses based on the full sample. Analysis was also performed on the subset comprising 62 Stage IV patients, since the 12 Stage III patients experienced 100% PFS during the follow-up period. Cox regression results showed similar results for both PFS and OS: more skewness of Ktrans (p<0.0001 PFS, p<0.001 OS), higher T-stage (p=0.004 PFS, p=0.009 OS) and older age (p=0.028 PFS, p=0.042 OS) were jointly associated with shortened OS. Figure 3A-B shows Kaplan-Meier survival curves for skewness of Ktrans.
Figure 3.
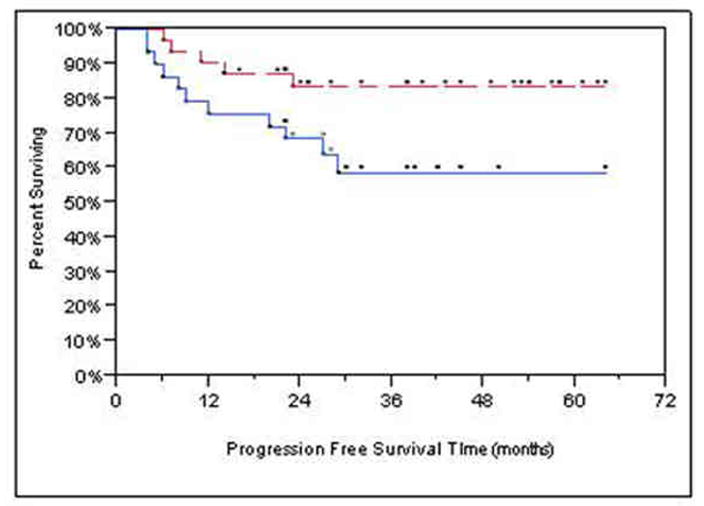
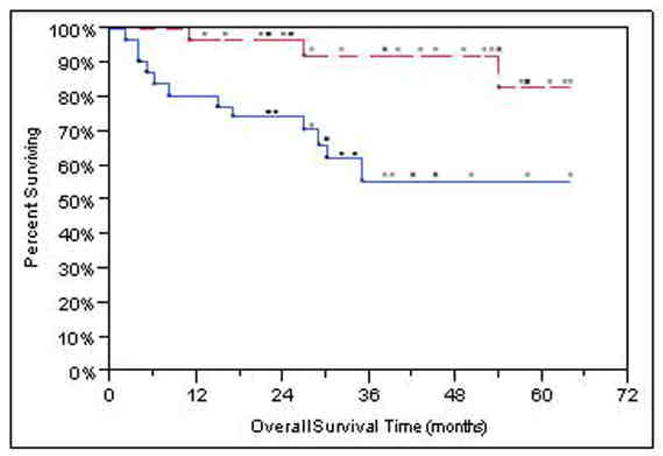
Kaplan-Meier graphs with the upper, red, dashed lines representing skewness of Ktrans < median (better prognosis) and the lower, blue, solid lines are for > median (worse prognosis). The dots above each line represent censored observations. (a) Kaplan-Meier progression-free survival plot. Patients stratified at median Ktrans skewness. (b) Kaplan-Meier overall survival plot. Patients stratified at median Ktrans skewness.
Discussion
The data in the present study indicate that lower skewness (i.e., less asymmetry in the distribution) of Ktrans may be predictive of better outcome in HNSCC patients with stage IV nodal disease. There is overwhelming evidence that tumors are heterogeneously perfused (33). Imaging vascular heterogeneity by DCE-MRI has been shown to be useful for understanding tumor biology and predicting outcome (34). Jackson et al. reported that tumor heterogeneity is better reflected by the distribution of the DCE-MRI parameter values than by their mean or median (34). Studies in gliomas, breast and rectal cancers have shown that tumor heterogeneity measures, such as the upper part of the distribution (95th percentile) or the skewness of the distribution, of DCE-MRI parameter values correlate with overall survival, tumor grade or radiation treatment outcome (35–38). Tumor heterogeneity in HNSCC has been attributed in part to regions of hypoxia and necrosis within the tumor (39). In a study of 28 HNSCC patients with stage IV disease, Brizel et al. found that tumor hypoxia (measured by pO2) adversely affected prognosis (40). Recently, in a study of 13 patients, our group showed that hypoxic metastatic neck lymph nodes were poorly perfused (i.e., had significantly lower kep and Ktrans values) compared with nonhypoxic nodes (30). Additionally, hypoxic nodes had a more asymmetric distribution of kep values than did non-hypoxic nodes (30). These thirteen patients were also included in the present study.
In advanced HNSCC, tumor stage and neck node involvement are widely recognized as negative prognostic factors (8, 41, 42). Our results have shown that in Cox regression analysis, only T-stage and either age or sex had a jointly significant relationship to PFS or OS. The present study examined imaging measurements from neck nodal metastases only. The primary endpoints of the analysis in the present study were PFS (including assessment of all tumor sites) and OS, rather than just disease progression at the primary site. Our results show that information about tumor vascularity from a priori DCE-MRI may help in stratifying HNSCC patients with stage IV nodal disease into for risk-adjusted treatment selection.
DCE-MRI parameters have shown promise as early markers of response in HN cancers (20, 26). Cao, et al. performed quantification of blood volume (BV) and blood flow (BF) from DCE-MRI data before therapy and 2 weeks after initiation of chemoradiation in 14 HNSCC patients; they found that an increase in available primary tumor BV during RT was associated with local-regional control (26). The median follow-up for the ten surviving patients in their study was 9.7 months (range, 5.3–27 months) (26). In a cohort of 33 HNSCC patients who were treated with chemoradiation, Kim, et al. found that the average pretreatment Ktrans value of the complete response group was significantly higher (P = 0.001) than that of the partial response group at 6-month follow-up (20). Our study has a larger number of patients (n=74 patients) and longer clinical follow up (minimum one year).
The use of functional imaging (with CT, PET or MRI) is gaining acceptance in the management of patients with HN cancers (43). DCE-MRI measures changes in signal intensity while perfusion CT measures changes in tissue attenuation during a dynamic contrast infusion (43). In a study of 105 HNSCC patients who underwent perfusion CT followed by radiotherapy, Hermans, et al. showed that patients with lower median perfusion values had a significantly higher local failure rate (p<0.05) (44). The results confirmed the hypothesis that less-perfused tumors respond poorly to radiotherapy (44). Consistent with this hypothesis, we found that the skewness of the perfusion parameter Ktrans was higher in stage IV patients who had heterogeneous, poorly perfused tumors and died of disease thereafter.
A few studies have shown that 18F-fluorodeoxyglucose (18F-FDG) uptake intensity in HNSCC has some prognostic value (45–47). These studies have used the maximum standardized uptake value (SUVmax) as a predictor of outcome (45–47). Perfusion CT and PET, unlike DCE-MRI, use ionizing radiation, which has known risks. Efforts have been initiated by the National Cancer Institute Cancer Imaging Program to develop DCE-MRI as a mainstay of diagnostic imaging that can provide quantitative data for use in multicenter clinical trials (48). DCE-MRI is rapidly gaining acceptance as a tool for early assessment of therapeutic response in clinical trials (18). In a Phase II study of HNSCC patients treated with sunitinib, a significant decrease in Ktrans was seen in three of the four patients who received DCE-MRI monitoring (21).
Our study has a few limitations. First, the study design was retrospective, and the patients were not consecutive. Second, patients received different treatments; however, this reflects daily practice at the Center. Thirdly, we had HPV status on 48 (65%) patients; it would have been better if we had the data for the whole population, particularly since the oropharynx was the predominant primary site. Finally, our study included no direct pathological validation of heterogeneity (necrosis). Future prospective validation studies are needed to confirm our findings in a larger patient population and known predictors that were not significant in the present study should be pursued.
Conclusion
Skewness of Ktrans was a strong predictor of progression-free survival and overall survival in HNSCC patients with stage IV nodal disease. This finding suggests an important role for this pretreatment DCE-MRI parameter as a predictor of outcome in HNSCC patients with advanced disease.
Acknowledgments
Study Funding: National Cancer Institute/National Institutes of Health (grant number 1 R01 CA115895). We thank Ada Muellner for editing the manuscript.
Footnotes
All authors in this manuscript have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. pp. 1–68. [Google Scholar]
- 2.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Trotti A, Pfister DG, et al. Head and neck cancer: recent advances and new standards of care. J Clin Oncol. 2006;24:2603–2605. doi: 10.1200/JCO.2006.07.1464. [DOI] [PubMed] [Google Scholar]
- 5.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Di B, Shang Y, et al. Clinicopathologic risk factors for distant metastases from head and neck squamous cell carcinomas. Eur J Surg Oncol. 2009;35:1348–1353. doi: 10.1016/j.ejso.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Awada A, de Castro G., Jr Head and neck cancer emerging strategies: advances and new challenges. Curr Opin Oncol. 2009;21:191–193. doi: 10.1097/CCO.0b013e32832a6e05. [DOI] [PubMed] [Google Scholar]
- 8.Baatenburg de Jong RJ, Hermans J, Molenaar J, et al. Prediction of survival in patients with head and neck cancer. Head Neck. 2001;23:718–724. doi: 10.1002/hed.1102. [DOI] [PubMed] [Google Scholar]
- 9.Mukherji SK, O’Brien SM, Gerstle RJ, et al. Tumor volume: an independent predictor of outcome for laryngeal cancer. J Comput Assist Tomogr. 1999;23:50–54. doi: 10.1097/00004728-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Rudat V, Dietz A, Schramm O, et al. Prognostic impact of total tumor volume and hemoglobin concentration on the outcome of patients with advanced head and neck cancer after concomitant boost radiochemotherapy. Radiother Oncol. 1999;53:119–125. doi: 10.1016/s0167-8140(99)00119-x. [DOI] [PubMed] [Google Scholar]
- 11.Casado S, Forteza J, Dominguez S, et al. Predictive value of P53, BCL-2, and BAX in advanced head and neck carcinoma. Am J Clin Oncol. 2002;25:588–590. doi: 10.1097/00000421-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Osman I, Sherman E, Singh B, et al. Alteration of p53 pathway in squamous cell carcinoma of the head and neck: impact on treatment outcome in patients treated with larynx preservation intent. J Clin Oncol. 2002;20:2980–2987. doi: 10.1200/JCO.2002.06.161. [DOI] [PubMed] [Google Scholar]
- 13.Quon H, Liu FF, Cummings BJ. Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck. 2001;23:147–159. doi: 10.1002/1097-0347(200102)23:2<147::aid-hed1010>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Almadori G, Cadoni G, Galli J, et al. Epidermal growth factor receptor expression in primary laryngeal cancer: an independent prognostic factor of neck node relapse. Int J Cancer. 1999;84:188–191. doi: 10.1002/(sici)1097-0215(19990420)84:2<188::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 16.Devries AF, Griebel J, Kremser C, et al. Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res. 2001;61:2513–2516. [PubMed] [Google Scholar]
- 17.Hawighorst H, Knapstein PG, Knopp MV, et al. Uterine cervical carcinoma: comparison of standard and pharmacokinetic analysis of time-intensity curves for assessment of tumor angiogenesis and patient survival. Cancer Res. 1998;58:3598–3602. [PubMed] [Google Scholar]
- 18.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 19.Johansen R, Jensen LR, Rydland J, et al. Predicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRI. J Magn Reson Imaging. 2009;29:1300–1307. doi: 10.1002/jmri.21778. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Loevner LA, Quon H, et al. Prediction of Response to Chemoradiation Therapy in Squamous Cell Carcinomas of the Head and Neck Using Dynamic Contrast-Enhanced MR Imaging. AJNR Am J Neuroradiol. 2010;31:262–268. doi: 10.3174/ajnr.A1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machiels JP, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006–01. J Clin Oncol. 2010;28:21–28. doi: 10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann U, Brix G, Knopp MV, et al. Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med. 1995;33:506–514. doi: 10.1002/mrm.1910330408. [DOI] [PubMed] [Google Scholar]
- 23.Port RE, Knopp MV, Hoffmann U, et al. Multicompartment analysis of gadolinium chelate kinetics: blood-tissue exchange in mammary tumors as monitored by dynamic MR imaging. J Magn Reson Imaging. 1999;10:233–241. doi: 10.1002/(sici)1522-2586(199909)10:3<233::aid-jmri3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 25.Yankeelov TE, Gore JC. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology:Theory, Data Acquisition, Analysis, and Examples. Current Medical Imaging Reviews. 2007;3:91–107. doi: 10.2174/157340507780619179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y, Popovtzer A, Li D, et al. Early prediction of outcome in advanced head-and-neck cancer based on tumor blood volume alterations during therapy: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72:1287–1290. doi: 10.1016/j.ijrobp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee NY, O’Meara W, Chan K, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2007;69:459–468. doi: 10.1016/j.ijrobp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Klem ML, Mechalakos JG, Wolden SL, et al. Intensity-modulated radiotherapy for head and neck cancer of unknown primary: toxicity and preliminary efficacy. Int J Radiat Oncol Biol Phys. 2008;70:1100–1107. doi: 10.1016/j.ijrobp.2007.07.2351. [DOI] [PubMed] [Google Scholar]
- 29.Shukla-Dave A, Lee N, Stambuk H, et al. Average arterial input function for quantitative dynamic contrast enhanced magnetic resonance imaging of neck nodal metastases. BMC Med Phys. 2009;9:4. doi: 10.1186/1756-6649-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen JF, Schoder H, Lee NY, et al. Noninvasive Assessment of Tumor Microenvironment Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging and (18)F-Fluoromisonidazole Positron Emission Tomography Imaging in Neck Nodal Metastases. Int J Radiat Oncol Biol Phys. 2010;77:1403–1410. doi: 10.1016/j.ijrobp.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CH, Choi JW, Kim KA, et al. Usefulness of standard deviation on the histogram of ultrasound as a quantitative value for hepatic parenchymal echo texture; preliminary study. Ultrasound Med Biol. 2006;32:1817–1826. doi: 10.1016/j.ultrasmedbio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Bockmuhl U, Ishwad CS, Ferrell RE, et al. Association of 8p23 deletions with poor survival in head and neck cancer. Otolaryngol Head Neck Surg. 2001;124:451–455. doi: 10.1067/mhn.2001.114794. [DOI] [PubMed] [Google Scholar]
- 33.Gillies RJ, Schornack PA, Secomb TW, et al. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1:197–207. doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson A, O’Connor JP, Parker GJ, et al. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13:3449–3459. doi: 10.1158/1078-0432.CCR-07-0238. [DOI] [PubMed] [Google Scholar]
- 35.de Lussanet QG, Backes WH, Griffioen AW, et al. Dynamic contrast-enhanced magnetic resonance imaging of radiation therapy-induced microcirculation changes in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1309–1315. doi: 10.1016/j.ijrobp.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 36.Issa B, Buckley DL, Turnbull LW. Heterogeneity analysis of Gd-DTPA uptake: improvement in breast lesion differentiation. J Comput Assist Tomogr. 1999;23:615–621. doi: 10.1097/00004728-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Jackson A, Kassner A, Annesley-Williams D, et al. Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tumor grade. AJNR Am J Neuroradiol. 2002;23:7–14. [PMC free article] [PubMed] [Google Scholar]
- 38.Mills SJ, Patankar TA, Haroon HA, et al. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;27:853–858. [PMC free article] [PubMed] [Google Scholar]
- 39.Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist. 2008;13 (Suppl 3):21–26. doi: 10.1634/theoncologist.13-S3-21. [DOI] [PubMed] [Google Scholar]
- 40.Brizel DM, Sibley GS, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 41.Koch WM, Ridge JA, Forastiere A, et al. Comparison of clinical and pathological staging in head and neck squamous cell carcinoma: results from Intergroup Study ECOG 4393/RTOG 9614. Arch Otolaryngol Head Neck Surg. 2009;135:851–858. doi: 10.1001/archoto.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendt TG, Bank P. Prognostic factors in squamous cell carcinoma of the head and neck. Onkologie. 2002;25:208–212. doi: 10.1159/000064313. [DOI] [PubMed] [Google Scholar]
- 43.Emonts P, Bourgeois P, Lemort M, et al. Functional imaging of head and neck cancers. Curr Opin Oncol. 2009;21:212–217. doi: 10.1097/cco.0b013e32832a2322. [DOI] [PubMed] [Google Scholar]
- 44.Hermans R, Meijerink M, Van den Bogaert W, et al. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1351–1356. doi: 10.1016/s0360-3016(03)00764-8. [DOI] [PubMed] [Google Scholar]
- 45.Liao CT, Chang JT, Wang HM, et al. Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys. 2009;73:764–771. doi: 10.1016/j.ijrobp.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Machtay M, Natwa M, Andrel J, et al. Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck. 2009;31:195–201. doi: 10.1002/hed.20942. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki H, Hasegawa Y, Terada A, et al. FDG-PET predicts survival and distant metastasis in oral squamous cell carcinoma. Oral Oncol. 2009;45:569–573. doi: 10.1016/j.oraloncology.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Evelhoch J, Garwood M, Vigneron D, et al. Expanding the use of magnetic resonance in the assessment of tumor response to therapy: workshop report. Cancer Res. 2005;65:7041–7044. doi: 10.1158/0008-5472.CAN-05-0674. [DOI] [PubMed] [Google Scholar]


