Abstract
Background
Cachexia, also known as muscle wasting, is a complex metabolic condition characterized by loss of skeletal muscle and a decline in physical function. Muscle wasting is associated with cancer, sarcopenia, chronic obstructive pulmonary disease, end-stage renal disease, and other chronic conditions and results in significant morbidity and mortality. GTx-024 (enobosarm) is a nonsteroidal selective androgen receptor modulator (SARM) that has tissue-selective anabolic effects in muscle and bone, while sparing other androgenic tissue related to hair growth in women and prostate effects in men. GTx-024 has demonstrated promising pharmacologic effects in preclinical studies and favorable safety and pharmacokinetic profiles in phase I investigation.
Methods
A 12-week double-blind, placebo-controlled phase II clinical trial was conducted to evaluate GTx-024 in 120 healthy elderly men (>60 years of age) and postmenopausal women. The primary endpoint was total lean body mass assessed by dual energy X-ray absorptiometry, and secondary endpoints included physical function, body weight, insulin resistance, and safety.
Results
GTx-024 treatment resulted in dose-dependent increases in total lean body mass that were statistically significant (P < 0.001, 3 mg vs. placebo) and clinically meaningful. There were also significant improvements in physical function (P = 0.013, 3 mg vs. placebo) and insulin resistance (P = 0.013, 3 mg vs. placebo). The incidence of adverse events was similar between treatment groups.
Conclusion
GTx-024 showed a dose-dependent improvement in total lean body mass and physical function and was well tolerated. GTx-024 may be useful in the prevention and/or treatment of muscle wasting associated with cancer and other chronic diseases.
Keywords: Muscle wasting, Selective androgen receptor modulator, Cachexia, Physical function, Lean body mass
Introduction
Skeletal muscle accounts for nearly half of whole body protein mass and plays a critical role in overall health by providing strength for daily physical function [1]. In healthy individuals, skeletal muscle homeostasis requires a balance of anabolic and catabolic processes, resulting in continuous renewal of muscle proteins without a net change in overall muscle mass. As the primary reservoir of amino acids, muscle is the mediator of whole body protein and is a source of precursors for hepatic gluconeogenesis.
Muscle also plays an important role in blood glucose regulation and metabolic health [2]. Muscle loss is a strong predictor of physical disability, frailty, and loss of independence and has been linked to poor balance, decreased gait speed, falls, and fractures [3–6]. Healthy adults lose approximately 1–2% of their skeletal muscle mass per year after the age of 50 [7]. Additionally, Baumgartner et al. estimates that 13–24% of persons under the age of 70 and up to 50% of individuals over the age of 80 suffer from significant muscle loss [8].
Accelerated muscle loss occurs in many acute and chronic conditions including cancer, chronic kidney disease, diabetes, acquired immune deficiency syndrome, chronic obstructive pulmonary disease, congestive heart failure, rheumatoid arthritis and hypogonadism, sepsis, and burn injury [1, 9–12]. Patients have profound muscle loss because of increased protein demand, a heightened inflammatory state, increased resting energy expenditure, and atrophy from disuse. Muscle wasting is an independent risk factor for poor health outcomes in patients with chronic diseases [11]. The prevalence of muscle wasting in cancer patients increases to greater than 80% prior to death from malignancy and represents the cause of death in over 20% of cancer patients [13–16]. To date, no therapy is approved for the prevention and treatment of muscle wasting.
Although anabolic steroids and testosterone have been used to treat muscle wasting, their risks often outweigh the potential benefits for many patients [17]. The use of these agents is limited by their inability to separate the desired anabolic effects of steroids in the muscle and bone from unwanted side effects of testosterone, partly due its conversion to estrogen in adipose tissue and dihydrotestosterone (DHT) in the skin and prostate [18]. Concerns related to increased risk or severity of benign prostatic hyperplasia or prostate cancer in men, virilization in women, and cardiovascular side effects in certain populations limit widespread use of testosterone and other anabolic steroids [19]. Additionally, due to limitations in oral bioavailability, testosterone replacement therapy is only available in transdermal and intramuscular formulations which may also limit its usefulness due to adverse skin reactions and wide fluctuations in the serum concentrations of testosterone that are observed [20].
There is an unmet medical need for an anabolic agent with an improved benefit/risk profile. The ideal agent should demonstrate anabolic selectivity in muscle and bone without suppressing luteinizing hormone (LH), lack cross-reactivity with other steroid receptors, allow oral dosing, and avoid conversion to DHT and estradiol [21]. The need for such an agent has stimulated the development of selective androgen receptor modulators (SARMs).
GTx-024 (enobosarm; United States Adopted Names Council official generic name) is an orally bioavailable nonsteroidal SARM with tissue-selective anabolic and androgenic pharmacologic activity [22–26]. In animal studies, GTx-024 increased muscle mass and bone density while having limited effects on other androgen-responsive tissues including the prostate and seminal vesicles. In fact, prostate size was reduced at doses that increased muscle mass in intact, male rats. In a phase I study in 48 healthy 18- to 45-year-old males and 23 elderly males with truncal obesity, GTx-024 administered for 14 days increased lean body mass and had a favorable tolerability profile without clinically apparent adverse effects involving the skin or prostate (data on file). A phase II, randomized, double-blind, placebo-controlled study was conducted to evaluate the effects of GTx-024 on total lean body mass and physical function in elderly men and postmenopausal women. Secondary endpoints included safety of GTx-024, effects on fat mass, bone mineral density, blood glucose, and insulin. This study is the first to demonstrate the ability of a nonsteroidal, orally bioavailable SARM to increase lean muscle mass and improve physical function.
Patients and methods
This was a randomized, double-blind, placebo-controlled, and multicenter study conducted under the World Medical Association Declaration of Helsinki, International Conference on Harmonization Guidance for Good Clinical Practices. The study documents were reviewed and approved by an Independent Ethics Committee and Medicines and Healthcare products Regulatory Agency, UK. The study was conducted from 11 July 2006 to 20 October 2006. The primary endpoint was to assess the effect of GTx-024 on total lean body mass measured by dual energy X-ray absorptiometry (DXA). Secondary endpoints included assessments of the effects of GTx-024 on physical function measured by the Stair Climb Test [27], bone mineral density (BMD), body weight, fat mass and insulin resistance, sebum production, lipids, serum hormones, hair growth in females (hirsutism), and safety and tolerability.
The subjects included in this study were healthy volunteers without evidence of disease recruited from the general population around the phase I study units in London (Richmond Pharmacology, Ltd.), Plymouth (Veeda Clinical Research), and Dundee (Drug Development Solutions, Limited) in the UK, Belfast in Northern Ireland (MDS Pharma Services), and Hamburg, Germany (MDS Pharma Services). The subjects were recruited through advertisement and database searches. The subjects were non-obese (body mass index (BMI) <30) and were not required to have mobility disability. The subjects were required to sign an informed consent, agree to return to the study center at least ten times during the outpatient portion of this study, and have a white blood cell count ≥3,000/mm3, platelet count ≥100,000/mm3, serum creatinine ≤1.5 mg/dL, alanine aminotransferase and aspartate aminotransferase <1.25 times the upper limit of normal, and total bilirubin <1.5 mg/dL. Females required clinical confirmation of menopausal status, and males were required to be over the age of 60 and have a serum prostate specific antigen of ≤4.0 ng/mL or a negative prostate biopsy (no prostate cancer) within 6 months of evaluation. The subjects were not permitted to initiate diet modification or initiate or discontinue any exercise program during the study. No dietary supplementation was provided.
Subjects were randomly assigned to one of five different dose groups (doses of 0.1, 0.3, 1 and 3 mg of GTx-024 and placebo) and were instructed to take the study medication daily for 86 days at approximately the same time each day, which included a period of approximately 70 days as an outpatient. The doses for this study were chosen from the pharmacokinetic, safety and tolerability data from the previous trials with GTx-024, and the clinical trial material was provided by GTx, Inc. Each dose group contained 24 subjects and was randomized in a 1:1:1:1:1 fashion. Randomization was stratified such that each dose group contained 12 males and 12 females.
A baseline pre-study assessment of the subjects was completed within 3 days of the first administered dose, and the final assessment of efficacy was conducted at day 86. Body composition, including BMD, was assessed at baseline, day 30, and day 86 by DXA. Physical function was evaluated using the Stair Climb Test at baseline, day 30, and day 86 [27]. The Stair Climb Test requires that step switch pads be placed on steps 1, 4, 8, and 12. The switch pads record the time for each subject to proceed up the stairs. Subjects were asked to climb the stairs, one step at a time as quickly as possible without assistance, and were asked to only use the handrail if they felt like they were losing their balance. Study personnel were instructed not to provide encouragement during the test. The height of the stairs, the subject's weight, and the time interval between each step pad were recorded. The power exerted was calculated as follows:
Hair growth in females was assessed by a modified Ferriman–Gallwey Scale [28]. In the modification of this scale, hair growth was assessed at each of six anatomical sites: upper lip, chin, upper arms, chest, upper abdomen, and upper back in the females participating in the protocol. Hair growth was scored based on a 4-point scale. Scoring was 0 for no terminal hair growth to 4 for maximal hair growth.
Sebum was assessed at baseline and days 30 and 86 using a DermaLab® handheld dual tape sebum reader module, Cortex Technology (Denmark). Two sebum measurements were taken on each test day. The measurements were taken on the forehead in two distinct areas on each of the mornings.
Dosing was without regard to food intake except on days 1 and 15 when blood samples for pharmacokinetic sampling were collected after the subjects had fasted from approximately 10 h prior to dose until 4 h after dosing and on day 86 when fasting glucose and insulin were collected for measurement of insulin resistance, calculated as the product of fasting glucose and fasting insulin divided by a constant based on the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) [29].
Statistical analyses
Subjects included in the analyses were the evaluable population defined as subjects in the intent-to-treat population that completed the study. PROC MIXED of SAS® Version 8.2 [30] was used to estimate and test treatment effects for changes from baseline to day 86 for lean body mass, stair climb power, serum hormones, lipids, glucose and insulin levels, and other lab values, including changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Though some endpoints were measured at day 30, the analyses that are reported reflect changes from baseline to day 86. Overall treatment effect estimates and their associated standard errors are the differences in the least square means between the placebo arm and the treatment arm reported from a model with treatment as the only factor. References to estimates, standard errors, and associated P values within gender are from models that include treatment arm, gender, and the treatment by gender interaction terms. The statistical tests are T tests comparing the cell means of interest. P values reported are two sided and are unadjusted for multiple comparisons. P values <0.05 are considered significant.
Results
The demographics and anthropometric characteristics of the subjects are summarized in Table 1. Mean age of the study population was 63.3 ± 5.6 years, with no statistically significant differences between treatment groups. There were no differences in body weight, height, or BMI between treatment groups. One hundred and 15 subjects completed the study. Five subjects were discontinued from the study prior to completion. Three subjects withdrew consent due to personal reasons (one subject receiving the 0.3-mg dose and two subjects receiving the 1-mg dose of GTx-024). One subject was discontinued due to an adverse event of urinary tract infection after a single dose of GTx-024 (1-mg dose). One subject was discontinued by the investigator due to ALT elevation (3-mg dose). However, the ALT elevation was not considered a serious adverse event.
Table 1.
Patient demographics
| Placebo | 0.1 mg | 0.3 mg | 1 mg | 3 mg | |
|---|---|---|---|---|---|
| n = 24 | n = 24 | n = 24 | n = 24 | n = 24 | |
| Gender | |||||
| Female | 12 (50.0%) | 12 (50.0%) | 12 (50.0%) | 12 (50.0%) | 12 (50.0%) |
| Male | 12 (50.0%) | 12 (50.0%) | 12 (50.0%) | 12 (50.0%) | 12 (50.0%) |
| Race | |||||
| Black/African American | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Other | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| White | 23 (95.8%) | 23 (95.8%) | 24 (100%) | 24 (100%) | 24 (100%) |
| Age (years) | |||||
| Median (min, max) | 62.5 (48,70) | 62.5 (52,79) | 63.5 (52,76) | 64.0 (48,79) | 64.5 (55,74) |
| Weight (kg) | |||||
| Median (min, max) | 68.95 (56.1, 94.0) | 74.9 (52.2, 93.8) | 69.95 (50.7, 106.3) | 76.4 (49.0, 100.7) | 70.55 (50.1, 97.3) |
| Height (cm) | |||||
| Median (min, max) | 166.5 (153, 186) | 164.8 (149, 187) | 167.0 (153, 184) | 173.5 (152, 191) | 168.0 (151, 187) |
| BMI (kg/m2) | |||||
| Median (min, max) | 25.15 (20.8, 31.0) | 26.85 (21.5, 31.6) | 24.80 (19.6, 31.4) | 26.05 (19.1, 31.4) | 21.35 (20.1, 30.7) |
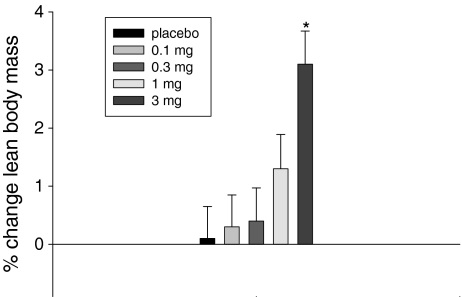
As shown in Fig. 1, GTx-024 elicited a dose-dependent increase in total lean body mass, with subjects in the 3-mg-dose group gaining a statistically significant (P < 0.001) average of 1.3 kg ± 0.3 (±1 SE) of lean mass compared to placebo (Table 2). The change in lean mass at the 1-mg dose averaged 0.7 kg ± 0.3 greater than placebo but was not statistically significant (P = 0.055). A statistically significant (P = 0.049) decrease in total fat mass was also observed at the 3-mg dose, with the 1-mg dose approaching statistical significance (P = 0.085). The differences in changes in fat mass from baseline in the 3-mg group compared to placebo were similar in males and females with approximately a loss of 0.6 kg relative to placebo. No differences in total body weight were observed, indicating that the shift to more lean body composition in the 3-mg-dose group was achieved, at least partially, at the expense of body fat.
Fig. 1.
Percentage change from baseline to day 86/EOS in total lean body mass: evaluable population. EOS end of study, *P < 0.001 3 mg vs. placebo (T test)
Table 2.
Changes in primary and secondary endpoints
| Baseline | SD | Absolute change | SD | P value treatment versus placebo | |
|---|---|---|---|---|---|
| Lean body mass (g) | |||||
| Placebo | 44,614.8 | 9,674.22 | −73.2 | 1,126.77 | |
| 0.1 mg | 46,399.5 | 9,350.43 | 164 | 868.19 | 0.474 |
| 0.3 mg | 45,257.8 | 10,102.75 | 78 | 1,150.33 | 0.651 |
| 1 mg | 48,145.3 | 10,589.96 | 588.7 | 1,257.47 | 0.055 |
| 3 mg | 45,030.7 | 10,255.4 | 1,246.3 | 1,287.92 | <.001* |
| Fat mass (g) | |||||
| Placebo | 20,806.6 | 8,688.95 | 304.7 | 1,105.24 | |
| 0.1 mg | 23,354.5 | 6,019.07 | 222.7 | 958.04 | 0.793 |
| 0.3 mg | 21,554.8 | 6,693.72 | −65.4 | 1,054.9 | 0.242 |
| 1 mg | 22,561.4 | 5,659.38 | −255.1 | 947.95 | 0.085 |
| 3 mg | 20,492.9 | 6,932.34 | −321.9 | 1,281.95 | 0.049* |
| Total body weight (kg) | |||||
| Placebo | 68.0 | 12.01 | −0.1 | 2.32 | |
| 0.1 mg | 72.5 | 10.61 | 0.4 | 1.28 | 0.51 |
| 0.3 mg | 68.6 | 15.87 | 0.9 | 4.88 | 0.196 |
| 1 mg | 72.9 | 13.65 | 0.3 | 1.67 | 0.55 |
| 3 mg | 67.5 | 13.48 | 0.9 | 1.74 | 0.178 |
| Stair climb speed (s) | |||||
| Placebo | 5.0003 | 1.24036 | −0.2 | 0.84768 | |
| 0.1 mg | 4.3008 | 1.09389 | 0.6 | 1.788 | 0.010* |
| 0.3 mg | 4.3789 | 1.17799 | 0 | 0.79584 | 0.507 |
| 1 mg | 4.7289 | 1.04263 | −0.2 | 0.78467 | 0.953 |
| 3 mg | 4.9847 | 1.59781 | −0.8 | 0.86947 | 0.08 |
| Power (W) | |||||
| Placebo | 280.72 | 87.807 | 20.35 | 38.835 | |
| 0.1 mg | 331.77 | 91.95 | −16.68 | 60.608 | 0.012* |
| 0.3 mg | 307.47 | 96.69 | 14.45 | 49.106 | 0.69 |
| 1 mg | 324.7 | 100.826 | 10.68 | 50.955 | 0.522 |
| 3 mg | 298.69 | 97.232 | 49.67 | 50.317 | 0.049* |
*P < 0.05
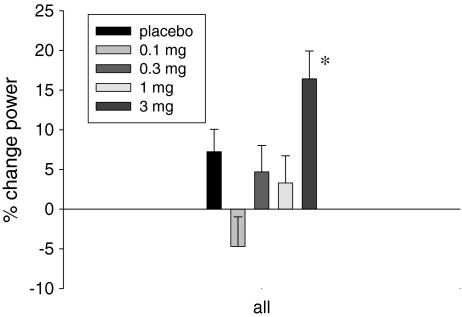
GTx-024 demonstrated a dose-dependent decrease in the time required for elderly men and postmenopausal women to climb 12 steps. The reduction in time was observed at every segment of the stair climb (i.e., 1–4, 4–8, 8–12, and 1–12 stairs; data not shown) within the GTx-024 3-mg-dose group. A statistically significant (P = 0.013) improvement in stair climb power was observed in the GTx-024 3-mg-dose group (Fig. 2).
Fig. 2.
Percentage change from baseline to day 86/EOS in stair climb power: evaluable population. EOS end of study, *P = 0.013 3 mg vs. placebo (T test)
GTx-024 3 mg significantly (P = 0.006) decreased blood glucose an average of 6.9 ± 2.5 (milligrams per deciliter), while a trend toward a significant (P = 0.052) reduction of 2.2 ± 1.1 μIU/mL in blood insulin was also observed in this group compared to placebo. Additionally, insulin resistance (calculated based on the HOMA-IR [29]) was reduced in the GTx-024 1-mg and 3-mg treatment groups (placebo = 2.6% ± 8.6, 1 mg = −9.3% ± 5.5, 3 mg = −27.5% ± 7.6: P = 0.013 3 mg vs. placebo).
The incidence of adverse events was similar among groups and between GTx-024- and placebo-treated subjects. The most common adverse events were headache and back pain (Table 3). No SAEs were reported during the study.
Table 3.
Summary of adverse events
| Adverse event frequency by treatment | |||||
|---|---|---|---|---|---|
| Number of subjects reporting event (%) | |||||
| Placebo | 0.1 mg | 0.3 mg | 1 mg | 3 mg | |
| Nausea | 2 (8.3%) | 2 (8.3%) | 5 (20.8%) | 3 (12.5%) | 1 (4.2%) |
| Diarrhea | 3 (12.5%) | 2 (8.3%) | 5 (20.8%) | 6 (25.0%) | 2 (8.3%) |
| Fatigue | 2 (8.3%) | 1 (4.2%) | 3 (12.5%) | 0 (0.0%) | 2 (8.3%) |
| Influenza-like illness | 2 (8.3%) | 3 (12.5%) | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) |
| Nasopharyngitis | 3 (12.5%) | 2 (8.3%) | 3 (12.5%) | 4 (16.7%) | 0 (0.0%) |
| Alanine aminotransferase increase | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) | 1 (4.2%) | 5 (20.8%) |
| Back pain | 4 (16.7%) | 4 (16.7%) | 1 (4.2%) | 9 (37.5%) | 3 (12.5%) |
| Pain in extremity | 6 (25.0%) | 2 (8.3%) | 2 (8.3%) | 3 (12.5%) | 1 (4.2%) |
| Headache | 4 (16.7%) | 7 (29.2%) | 7 (29.2%) | 9 (37.5%) | 5 (20.8%) |
| Cough | 1 (4.16%) | 1 (4.2%) | 3 (12.5%) | 3 (12.5%) | 1 (4.2%) |
| Pharyngolaryngeal pain | 3 (12.5%) | 2 (8.3%) | 2 (8.3%) | 2 (8.3%) | 3 (12.5%) |
Decreases in serum triglycerides were noted in the GTx-024 1- and 3-mg-dose groups compared to placebo, with the 3-mg-dose group approaching statistical significance (Table 4). A statistically significant reduction in total cholesterol was observed in the 0.3-, 1-, and 3-mg GTx-024 dose groups compared to placebo. No significant effect on low-density lipoprotein (LDL) was observed among treatment groups. High-density lipoprotein (HDL) was reduced in a dose-dependent manner in GTx-024-treated subjects compared to placebo, with 17% and 27% decreases noted at the 1- and 3-mg doses, respectively. Importantly, the average total cholesterol/HDL ratio in the 1-mg- and 3-mg-dose groups was between 3.5 and 5 at baseline and end of the study.
Table 4.
Summary of lipid parameters
| Baseline | SD | Absolute change | SD | P value | |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | |||||
| Placebo | 195.9 | 35.83 | 4.8 | 17.46 | |
| 0.1 mg | 197.8 | 27.31 | −6.3 | 20.03 | 0.088 |
| 0.3 mg | 204.4 | 29.84 | −14.3 | 19.88 | 0.004* |
| 1 mg | 197.1 | 29.87 | −19 | 26.34 | <.001* |
| 3 mg | 203.1 | 35.1 | −15.3 | 26.95 | 0.003* |
| HDL (mg/dL) | |||||
| Placebo | 49.9 | 10.2 | 0 | 4.88 | |
| 0.1 mg | 50.9 | 9.49 | −4.3 | 4.72 | 0.027* |
| 0.3 mg | 55.3 | 13.99 | −6.3 | 4.86 | 0.001* |
| 1 mg | 52.1 | 10.44 | −8.9 | 6.18 | <.001* |
| 3 mg | 52.8 | 10.99 | −14.7 | 10.58 | <.001* |
| LDL (mg/dL) | |||||
| Placebo | 130 | 34.02 | 7.5 | 13.95 | |
| 0.1 mg | 128 | 22.91 | 5.5 | 16.48 | 0.734 |
| 0.3 mg | 130.7 | 31.57 | −0.2 | 15.67 | 0.206 |
| 1 mg | 125.2 | 23.83 | 3.9 | 27.16 | 0.564 |
| 3 mg | 130.6 | 29.68 | 4.6 | 27.44 | 0.629 |
| Triglycerides (mg/dL) | |||||
| Placebo | 114.8 | 39.66 | 7.2 | 34.43 | |
| 0.1 mg | 137.4 | 76.17 | 5.8 | 46.96 | 0.952 |
| 0.3 mg | 126 | 80.69 | 2.4 | 50.18 | 0.838 |
| 1 mg | 112.9 | 49.14 | −12.8 | 31.14 | 0.4 |
| 3 mg | 153.5 | 182.89 | −36.6 | 155.64 | 0.06 |
In men, no statistically significant differences from placebo in change from baseline values for free testosterone, DHT, estradiol, follicle-stimulating hormone (FSH), or LH were observed at any GTx-024 dose. However, sex hormone-binding globulin (SHBG) was significantly (P = 0.048) reduced with GTx-024 3 mg versus placebo, −15.8 ± 7.9 nmol/L. The decrease in SHBG was accompanied by a reduction of serum total testosterone in subjects treated with 1 mg (P < 0.001) or 3 mg (P < 0.001) of GTx-024 compared to placebo, −6.4 ± 1.1 nmol/L and −7.4 ± 1.0 (Table 5).
Table 5.
Summary of changes in serum hormones
| Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | SD | Absolute change | SD | P value | Baseline | SD | Absolute change | SD | P value | |
| Free testosterone (pmol/L) | ||||||||||
| Placebo | 43.57 | 20.5 | −11.21 | 17.97 | 7.16 | 4.75 | −0.27 | 0.87 | ||
| 0.1 mg | 25.55 | 9.78 | 0.54 | 7.89 | 0.003* | 7.25 | 5.05 | −2 | 3.06 | 0.718 |
| 0.3 mg | 22.13 | 10.37 | 2.06 | 8.33 | <.001* | 6.63 | 3.6 | −0.4 | 1.97 | 0.887 |
| 1 mg | 39.63 | 10.84 | −11.03 | 6.71 | 0.966 | 5.41 | 4.38 | −0.24 | 0.61 | 0.922 |
| 3 mg | 36.5 | 16.56 | −8.24 | 7.64 | 0.413 | 5.64 | 2.94 | −1.4 | 0.995 | |
| Total testosterone (nmol/L) | ||||||||||
| Placebo | 18.98 | 5.05 | 0.5 | 3.39 | 1.05 | 0.45 | −0.03 | 0.35 | ||
| 0.1 mg | 12.76 | 2.33 | −0.49 | 2.05 | 0.348 | 0.96 | 0.34 | −0.02 | 0.25 | 0.995 |
| 0.3 mg | 16.18 | 4.99 | −1.8 | 4.2 | 0.053 | 1.07 | 0.5 | −0.04 | 0.32 | 0.981 |
| 1 mg | 19.65 | 6.58 | −6.03 | 3.9 | <.001* | 0.95 | 0.41 | −0.09 | 0.31 | 0.909 |
| 3 mg | 16.02 | 5.59 | −6.86 | 3.51 | <.001* | 0.93 | 0.58 | −0.17 | 0.21 | 0.918 |
| SHBG (nmol/L) | ||||||||||
| Placebo | 51.17 | 20.61 | −10.03 | 8.62 | 68.6 | 40.39 | −16.5 | 28.42 | ||
| 0.1 mg | 36.25 | 14.34 | −6.09 | 7.02 | 0.627 | 47.67 | 15.26 | −1.09 | 16.98 | 0.064 |
| 0.3 mg | 44.58 | 11.2 | −11.36 | 10.88 | 0.775 | 62.92 | 17.53 | −21.17 | 16.73 | 0.564 |
| 1 mg | 48.3 | 20.73 | −18.81 | 15.76 | 0.265 | 83 | 37.63 | −51.84 | 35.95 | <.001* |
| 3 mg | 42.28 | 13.9 | −25.82 | 9.32 | 0.048* | 64.61 | 25.55 | −51.95 | 24.3 | <.001* |
| FSH (IU/L) | ||||||||||
| Placebo | 6.02 | 2.1 | −0.14 | 0.8 | 66.73 | 35.55 | −1.54 | 5.86 | ||
| 0.1 mg | 6.63 | 5.9 | −0.24 | 1.43 | 0.978 | 74.58 | 17.99 | 2.78 | 10.32 | 0.216 |
| 0.3 mg | 7.59 | 5.22 | −0.23 | 0.57 | 0.968 | 82.28 | 32.98 | −4.45 | 20.11 | 0.393 |
| 1 mg | 5.96 | 2.55 | −0.97 | 2 | 0.929 | 91.72 | 25.61 | −5.11 | 7.19 | 0.25 |
| 3 mg | 7.58 | 5.39 | −1.03 | 0.74 | 0.791 | 82.31 | 33.02 | −9.82 | 5.5 | 0.014* |
| LH | ||||||||||
| Placebo | 3.58 | 1.3 | 0.4 | 1.51 | 25.04 | 12.45 | −3.32 | 4.13 | ||
| 0.1 mg | 4.19 | 2.57 | −0.75 | 0.77 | 0.466 | 23.01 | 5.42 | 1.15 | 4.69 | 0.010* |
| 0.3 mg | 5.24 | 2.57 | −0.01 | 1.68 | 0.834 | 29.23 | 9.54 | −1.75 | 5.27 | 0.403 |
| 1 mg | 4.34 | 1.11 | −1 | 1.69 | 0.476 | 37.46 | 9.96 | −1.63 | 6.66 | 0.78 |
| 3 mg | 4.83 | 1.74 | −0.54 | 1.59 | 0.543 | 31.74 | 12.39 | −5.5 | 6.23 | 0.039* |
| Estradiol (pmol/L) | ||||||||||
| Placebo | 111.26 | 29.27 | −14.96 | 21.95 | 190.21 | 294.96 | −141.26 | 309.5 | ||
| 0.1 mg | 115.83 | 47.84 | −21 | 28.75 | 0.775 | 91.18 | 32.89 | −5.63 | 12.51 | 0.021* |
| 0.3 mg | 114.2 | 28.15 | −14.38 | 23.85 | 0.988 | 77 | 6.23 | −3.25 | 7.89 | 0.013* |
| 1 mg | 125.04 | 30.99 | −32.28 | 28.65 | 0.679 | 107.12 | 104.86 | −28.22 | 31.03 | 0.045* |
| 3 mg | 120.84 | 26.3 | −44.21 | 27.67 | 0.472 | 139.57 | 170.14 | −47.29 | 98.64 | 0.014* |
In women, changes from baseline in free testosterone, total testosterone, DHT, and estradiol levels did not differ between GTx-024 and placebo. Observed decreases in LH and FSH in women with GTx-024 3 mg versus placebo were statistically significant, but the reduction in LH and FSH in postmenopausal women had no effect on other serum hormones. SHBG was significantly reduced with GTx-024 1 mg (P < 0.001) and 3 mg (P < 0.001) compared to placebo, −39.3 ± 8.4 and −36.4 ± 8.2 nmol/L, respectively (Table 5).
GTx-024 did not have effects on sebum production or hair growth in women. Results of clinical laboratory tests were generally unremarkable. No significant increases in total bilirubin, GGT, or alkaline phosphatase were observed at any GTx-024 dose. Small, statistically significant increases in hemoglobin and hematocrit were observed with GTx-024 3 mg compared to placebo. Although transient increases in ALT to above the upper limit of normal were observed in eight subjects in this study, the ALT observations in seven of eight subjects had resolved while on drug such that no subject had clinically significantly, abnormal levels of ALT or AST at the end of study. One subject was discontinued due to an elevation in ALT 4.2 times the upper limit of normal. The ALT level in that subject returned to normal levels after discontinuation of the study drug.
Discussion
Muscle wasting is associated with significant morbidity and mortality [11, 13, 14]. The potential risk profile of testosterone replacement therapy and anabolic steroids limits their clinical use for a variety of indications that may otherwise benefit from increasing lean mass, improved physical function, and improved insulin resistance. SARMs in various stages of preclinical and clinical drug development offer an important option to meet these unmet medical needs [25, 26].
GTx-024 is the first SARM to reach advanced clinical trials and demonstrate a strong efficacy and safety profile [21]. In this randomized, double-blind, placebo-controlled study, GTx-024 increased total lean body mass and improved physical function with no evidence of androgenic side effects in elderly men and postmenopausal women. The increase in total lean body mass with GTx-024 was accompanied by an improvement in physical function as demonstrated by a clinically meaningful improvement in the Stair Climb Test.
GTx-024 3 mg also significantly reduced fasting glucose levels, and a trend toward reduction in blood insulin was observed. These effects resulted in a decrease in insulin resistance from baseline in the 1-mg and 3-mg treatment groups calculated based on the HOMA-IR. Of note, such decrease in insulin resistance was similar to that observed with metformin and glipizide, drugs used in the treatment of diabetes [31, 32]. Low levels of testosterone in men are associated with insulin resistance and type II diabetes, while testosterone therapy is known to improve metabolic parameters [33–35]. This observation may have potential implications for the use of SARMs in prediabetic or diabetic individuals, as commonly seen in patients with chronic kidney disease, chronic obstructive pulmonary disease, or metabolic syndrome [36–38].
GTx-024 was well tolerated with an adverse event profile similar to placebo during 3 months of daily treatment. Compared to placebo, GTx-024 decreased total cholesterol, HDL, and triglycerides. These findings are consistent with previous reports that oral anabolic therapy influences serum lipid profiles [39, 40]; however, it is important to note that the average LDL/HDL ratio for all GTx-024 doses remained in the low cardiovascular risk category. Furthermore, the beneficial effects of GTx-024 on insulin sensitivity and triglycerides, known associations of hypogonadism with metabolic syndrome and cardiovascular risk [33–35], as well as the low cardiovascular risk observed in randomized clinical trials of testosterone supplementation [18], suggest that the overall cardiovascular risk/benefit ratio for GTx-024 is low.
The pharmacologic effects of GTx-024 on serum hormone levels were unremarkable. Notable but expected reductions in SHBG and total testosterone in men were observed. The reductions in SHBG in men and women (−61% and −80%, respectively, at the 3-mg dose) exceed those observed in men treated with a 600-mg intramuscular testosterone enanthate (−31%) [41]. Decreases in total testosterone accompanied the observed decreases in SHBG; however, no significant decreases in free testosterone were observed in men or women at any dose of GTx-024. GTx-024-associated reductions in FSH and LH that were observed in postmenopausal women were not clinically significant.
In this 3-month study, GTx-024 showed no difference in BMD compared to placebo (data not shown). Changes in BMD were not necessarily expected as the treatment period was likely too short to detect a benefit. In preclinical studies, GTx-024 demonstrated both anabolic and antiresorptive activity in bone. Future research is warranted as the potential dual beneficial effects of GTx-024 and other SARMs on muscle and bone may provide a unique advantage to currently available agents for osteoporosis that solely modify bone strength.
Conclusion
There are currently no approved therapies available for the prevention or treatment of muscle wasting. GTx-024 is a novel SARM that was well tolerated in elderly men and postmenopausal women and resulted in significant increases in total lean body mass and improvements in physical function. Importantly, this study provides evidence that GTx-024 provides beneficial anabolic effects on total lean body mass and physical function without the adverse consequences often seen with testosterone and other anabolic steroids. These data support the development of GTx-024 for treatment and prevention of muscle wasting in patients with chronic diseases.
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [42].
Conflicts of interest
James T. Dalton, K. Gary Barnette, Casey E. Bohl, Michael L. Hancock, Domingo Rodriguez, Shontelle T. Dodson, Ronald A. Morton, and Mitchell S. Steiner are employees of and have stock and stock options with GTx, Inc.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia. Int J Biochem Cell Biol. 2005;37(5):1084–1104. doi: 10.1016/j.biocel.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Baracos VE. Management of muscle wasting in cancer-associated cachexia. Cancer. 2001;92(6 Suppl):1669–1677. doi: 10.1002/1097-0142(20010915)92:6+<1669::AID-CNCR1495>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–S47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 4.Tan BHL, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11(4):400–407. doi: 10.1097/MCO.0b013e328300ecc1. [DOI] [PubMed] [Google Scholar]
- 5.Guralnick JM. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56:2118–2123. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76(2):473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 9.Mitch WE, Price SR. Mechanisms activated by kidney disease and the loss of muscle mass. Am J Kidney Dis. 2001;38(6):1337–1342. doi: 10.1053/ajkd.2001.29249. [DOI] [PubMed] [Google Scholar]
- 10.Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61(5 Pt 1):157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- 11.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachex Sarcopenia Muscle. 2010;1(1):1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1S–5S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 13.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69(4):491–497. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 14.Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 15.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315(7117):1219–1222. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Nutrition in cancer care (PDQ). National Cancer Institute 2010 contract no.: 04/06/2011.
- 17.Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004;33(6):548–555. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2(3):146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivas-Shankar U, Wu FC. Drug insight: testosterone preparations. Nat Clin Pract Urol. 2006;3(12):653–665. doi: 10.1038/ncpuro0650. [DOI] [PubMed] [Google Scholar]
- 21.Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;84(10):3459–3462. doi: 10.1210/jc.84.10.3459. [DOI] [PubMed] [Google Scholar]
- 22.Bohl CE, Chang C, Mohler ML, Chen J, Miller DD, Swaan PW, et al. A ligand-based approach to identify quantitative structure-activity relationships for the androgen receptor. J Med Chem. 2004;47(15):3765–3776. doi: 10.1021/jm0499007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Hwang DJ, Chung K, Bohl CE, Fisher SJ, Miller DD, et al. In vitro and in vivo structure-activity relationships of novel androgen receptor ligands with multiple substituents in the B-ring. Endocrinology. 2005;146(12):5444–5454. doi: 10.1210/en.2005-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Wu D, Hwang DJ, Miller DD, Dalton JT. The para substituent of S-3-(phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-prop ionamides is a major structural determinant of in vivo disposition and activity of selective androgen receptor modulators. J Pharmacol Exp Ther. 2005;315(1):230–239. doi: 10.1124/jpet.105.088344. [DOI] [PubMed] [Google Scholar]
- 25.Gao W, Kim J, Dalton JT. Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands. Pharm Res. 2006;23(8):1641–1658. doi: 10.1007/s11095-006-9024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, et al. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem. 2009;52(12):3597–3617. doi: 10.1021/jm900280m. [DOI] [PubMed] [Google Scholar]
- 27.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88(5):604–609. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute Inc . SAS/STAT user's guide, version 8. Cary: SAS Institute Inc.; 1999. [Google Scholar]
- 31.Bulcao C, Ribeiro-Filho FF, Sanudo A, Roberta Ferreira SG. Effects of simvastatin and metformin on inflammation and insulin resistance in individuals with mild metabolic syndrome. Am J Cardiovasc Drugs. 2007;7(3):219–224. doi: 10.2165/00129784-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson JG, Lehtovirta M, Ehrnstrom B, Salmela S, Groop L. Long-term beneficial effects of glipizide treatment on glucose tolerance in subjects with impaired glucose tolerance. J Intern Med. 2006;259(6):553–560. doi: 10.1111/j.1365-2796.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi BG, McLaughlin MA. Why men's hearts break: cardiovascular effects of sex steroids. Endocrinol Metab Clin North Am. 2007;36(2):365–377. doi: 10.1016/j.ecl.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Guay A, Jacobson J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med. 2007;4(4 Pt 1):1046–1055. doi: 10.1111/j.1743-6109.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 35.Lunenfeld B. Testosterone deficiency and the metabolic syndrome. Aging Male. 2007;10(2):53–56. doi: 10.1080/13685530701390800. [DOI] [PubMed] [Google Scholar]
- 36.Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167(6):551–561. doi: 10.1001/archinte.167.6.551. [DOI] [PubMed] [Google Scholar]
- 37.Jamerson KA. Preventing chronic kidney disease in special populations. Am J Hypertens. 2005;18(4 Pt 2):106S–111S. doi: 10.1016/j.amjhyper.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Peralta CA, Kurella M, Lo JC, Chertow GM. The metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens. 2006;15(4):361–365. doi: 10.1097/01.mnh.0000232875.27846.7e. [DOI] [PubMed] [Google Scholar]
- 39.Cantrill JA, Dewis P, Large DM, Newman M, Anderson DC. Which testosterone replacement therapy? Clin Endocrinol (Oxf) 1984;21(2):97–107. doi: 10.1111/j.1365-2265.1984.tb03448.x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson PD, Cullinane EM, Sady SP, Chenevert C, Saritelli AL, Sady MA, et al. Contrasting effects of testosterone and stanozolol on serum lipoprotein levels. JAMA. 1989;261(8):1165–1168. doi: 10.1001/jama.261.8.1165. [DOI] [PubMed] [Google Scholar]
- 41.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 42.von Haehling S, Morley JE, Coats AJS, Anker SD. (2010) Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]