Abstract
Background
Under physiological conditions, the melanocortin system is a crucial part of the complex network regulating food intake and energy expenditure. In pathological states, like cachexia, these two parameters are deregulated, i.e., food intake is decreased and energy expenditure is increased—a vicious combination leading to catabolism. Agouti-related protein (AgRP), the endogenous antagonist at the melanocortin-4 receptor (MC-4R), was found to increase food intake and to reduce energy expenditure. This qualifies MC-4R blockade as an attractive mode of action for the treatment of cachexia. Based on this rationale, a novel series of small-molecule MC-4R antagonists was designed, from which the orally active compound BL-6020/979 (formerly known as SNT207979) emerged as the first promising development candidate showing encouraging pre-clinical efficacy and safety properties which are presented here.
Methods and results
BL-6020/979 is an orally available, selective and potent MC-4R antagonist with a drug-like profile. It increased food intake and decreased energy expenditure in healthy wild-type but not in MC-4R deficient mice. More importantly, it ameliorated cachexia-like symptoms in the murine C26 adenocarcinoma model; with an effect on body mass and body composition and on the expression of catabolic genes. Moreover, BL-6020/979 showed antidepressant-like properties in the chronic mild stress model in rats and exhibits a favorable safety profile.
Conclusion
The properties of BL-6020/979 demonstrated in animal models and presented here make it a promising candidate suitable for further development towards a first-in-class treatment option for cachexia that potentially opens up the opportunity to treat two hallmarks of the disease, i.e., decreased food intake and increased energy expenditure, with one drug.
Keywords: Cancer cachexia, C26 adenocarcinoma, Melanocortin-4 receptor antagonist, Mouse, Food intake, Metabolic rate
Introduction
Cachexia is a complex wasting syndrome in chronically ill patients that involuntarily lose more than 5% body mass within 12 months or have a body mass index below 20 kg/m2, and in addition exhibit at least three out of the following five symptoms: anorexia, decreased muscle strength, fatigue, low fat-free mass index or increased markers for inflammation [1]. It is a highly prevalent co-morbidity that is associated with a progressively decreasing quality of life and a predictor of poor survival. It is extremely common in cancer patients. Up to 80% of these patients develop cachexia and in more than 20% of patients it is causal for their death [2, 3].
The pathological changes leading to cachexia are reduced energy intake and at the same time an increase in resting energy expenditure [4]. This vicious combination is responsible for an accelerated catabolism [5]. So far, the mechanisms underlying these changes are not well understood. Nevertheless, it has been proposed that cytokines might be key factors for its genesis [6]. They are linked to increased energy expenditure [7], decreased food intake [8], and the depletion of skeletal muscle [9]. Mechanistically, it has been established that cytokines can alter energy balance via the regulation of pro-opiomelanocortin (POMC) expression [2]. Amongst others, α-melanocyte stimulating hormone (α-MSH), a 13 amino acid peptide with appetite-inhibiting effects [10, 11] is a cleavage product of POMC. The most prominent target receptor for α-MSH to exert these anorexic effects is the melanocortin-4 receptor (MC-4R).
Through blockade of the same receptor, however, the exact opposite effects can be elicited. Agouti-related protein (AgRP) is an endogenous antagonist at the MC-4R and has been shown to increase food intake and reduce energy expenditure [12–15]. This led to the hypothesis that the MC-4R is a promising target to tackle these disastrous maladaptations of cachexia [16]. Indeed, it has been demonstrated in a number of rodent studies that interruption of the MC-4R signaling pathway has the potential to ameliorate cancer cachexia [17–19], and it has been suggested that blockade of this pathway could eventually be the key to treating the progression of cachexia [20, 21]. Besides cancer cachexia, MC-4R antagonism might be a mechanism that could be exploited as treatment of additional cancer symptoms such as anxiety and depression [22].
Recently, we have introduced two novel non-peptidic, orally active MC-4 receptor antagonists that increase food intake in healthy animals and ameliorate cancer cachexia in a rodent model [23]. Unfortunately, these early compounds do not yet exhibit an overall favorable and safe profile that would allow for further development into the clinic. Moreover, it has not been shown if they decrease energy expenditure, which would be predicted by the anticipated mechanism of action. In fact, for our earlier compounds, no in vivo evidence for the postulated MC-4R dependent mechanism of action is available [23]. Unpredicted off-target effects of these compounds could potentially be responsible for the beneficial effects of these compounds in the C26 cancer cachexia model.
Here we describe the discovery and characterization of BL-6020/979 (formerly known as SNT207979) as the results of a major effort to identify selective, potent and orally active MC-4 receptor antagonists. Importantly, its general pharmacological properties, tolerability and safety were acceptable, and BL-6020/979 increased food intake in healthy fed animals and was effective in the C26 cancer cachexia model. In addition, BL-6020/979 did indeed lower energy expenditure as previously hypothesized [23]. This effect was absent in MC-4R deficient animals, thus strongly suggesting a mechanism of action as predicted from the in vitro data. Taken together, these results show the high potential of BL-6020/979 for further development towards a first-in-class treatment of cachexia.
Animals, materials, and methods
BL-6020/979 was designed and synthesized as SNT207979 in the Medicinal Chemistry Department at Santhera Pharmaceuticals (Switzerland) Ltd. All animal experiments were approved by the local authorities (Animal Care and Use Committees of Oregon Health and Science University, Portland, USA; Kantonales Verterinäramt Baselland, Liestal, Switzerland (BL246, BL356); Bioethical Committee of the Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland) and were in accordance with international guidelines [24]. If not otherwise stated all animals were held under standard laboratory conditions (12 h light per day, 22 ± 2°C, 40–60% humidity) with food and water available ad libitum.
Pharmacology, safety and toxicology profile of BL-6020/979
The specificity of BL-6020/979 was tested by studying in vitro binding to human MC-4 receptors as well as closely related human MC-3 and MC-5 receptors. An assay based on fluorescence polarization in membrane suspensions prepared from stably transfected HEK293 cells was used. Furthermore, binding to human MC-1R and a standard panel of 71 receptors, ion channels and transporters was measured at a concentration of 10 μM using a radio-ligand binding assay. BL-6020/979 was tested in an in vitro whole cell patch-clamp assay to determine inhibition of human ether-a-go-go related gene (hERG) tail currents in stably transfected HEK293 cells. Inhibition curves were constructed based on the results from three test concentrations (0.1, 1.0, and 10 μM) and the IC50 was determined and averaged. Assays were in accordance with international safety guidelines published by the FDA as S7B of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. The potential of BL-6020/979 to interact with a standard in vitro panel of 41 enzymes, comprising proteases, esterases, kinases, phosphatases, oxidases, and transferases was tested at a concentration of 10 μM.
Another safety parameter that has to be considered in drug development is the potential for undesired drug–drug interaction. In particular, induction or inhibition of the cytochrome P450 (CYP) isoforms is important in this context [25]. Thus, the inhibition potential of BL-6020/979 for the major human CYP isoforms CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 was tested in incubations of human liver microsomes in the presence of isoform-specific probe substrates as previously described [26]. The potential of BL-6020/979 to induce CYP3A4 was assessed in human hepatocytes from three individual donors using midazolam as a probe substrate as described [27].
The cytotoxic potential of BL-6020/979 was investigated in HepG2 hepatoblastoma cells [28] measuring metabolic activity of living cells using the WST-1 cell proliferation reagent (Roche Diagnostics, CH). For a standard assay, HepG2 cells were seeded into a 96-well micro-plate and maintained in culture for 24 h. Then, they were exposed to the test compound over a range of eight concentrations for another 24 h until they were finally incubated with WST-1 for 30 min before measuring the absorbance of the formazan dye formed. The TC50 (Toxic Concentration 50%) is defined as the concentration of tested compound required to reduce the absorbance by 50% relative to the control culture.
Genotoxicity was studied in vitro using automated Ames tests in two bacterial strains (TA98 and TA100), both with and without S9 fraction as described elsewhere [29].
The clastogenic and/or aneugenic potential of BL-6020/979 was tested in vivo in 4-week-old male (n = 18) and 6-week-old female (n = 18) CD-1 mice (Charles River, UK). The dose of BL-6020/979 was determined as maximal tolerated dose in a pre-study with a separate set of animals as 750 mg/kg and 625 mg/kg BL-6020/979 p.o. in males and females. Study animals were dosed daily for two subsequent days with BL-6020/979 or vehicle alone (0.5% carboxymethylcellulose). A third group of animals received a single injection of 4 mg/kg Mitomycin C (a compound with known clastogenic properties) i.p. on the second day which served as a positive control for the assay. On the third day, bone marrow was harvested, smears prepared and microscopically scored for developing (polychromatic) and mature (normochromatic) erythrocytes.
Seven day repeated dose toxicity studies were carried out in mice and rats. Forty-eight mice of the Crl:CD-1 strain were divided into four groups (6 males and 6 females per group), and 50 Crl:WI (HAN) rats were divided into five groups (5 males and 5 females per group). Mice and rats were dosed by oral gavage for up to 7 days at dose levels of 200, 300, or 500 mg/kg/day, and at 100, 200, 300, or 500 mg/kg/day, respectively. Control animals received the vehicle (0.5% carboxymethlycellulose) only. Standard toxicity parameters were recorded during the study. Ten selected organs were weighed and histopathology was carried out.
Lastly, pharmacokinetic studies were performed. Plasma protein binding is of relevance for the interpretation of these studies. Plasma protein binding was determined by equilibrium dialysis in 10% mouse, rat, dog and human plasma at a concentration of 5 μM BL-6020/979 as described elsewhere [30]. Brain penetration and oral bioavailability of BL-6020/979 was evaluated in male CD-1 mice and Sprague–Dawley rats after an oral dose of 50 mg/kg (in 5 ml/kg 10% hydroxypropyl-β-cyclodextrin in 100 mM NaCl solution, n = 3 per time point) as previously described [23]. In addition, fasted male Beagle dogs were dosed orally at 10 mg/kg (n = 3). Brain and plasma samples were analyzed by HPLC followed by mass spectrometry (MS/MS). Brain/plasma ratios were then calculated based on the area under the curve, and in rodents integrity of the blood–brain barrier was determined by co-dosing with Atenolol (absence of Atenolol indicated that the blood–brain barrier was intact).
All in vitro assays except those for MC-3, MC-4, and MC-5 receptors, which were established at Santhera, hERG, which was performed by bSys (Witterswil, Switzerland) and CYP inhibition and plasma protein binding, which was performed by Cyprotex (UK), were performed by MDS (Taiwan) or Cerep (France).
Food intake studies in healthy wild-type mice
Light-phase food intake experiments were conducted as previously described [23]. In short, efficacy was demonstrated following a single subcutaneous administration of 20 mg/kg BL-6020/979 using 6-week-old female NMRI mice. Thereafter, an oral dose–response curve was generated (vehicle, 10, 50, or 100 mg/kg) in NMRI mice housed in triplets dosed 3 h after lights on. Just before and 4 h after administration, the food hopper weight of each triplet, as well as the animals’ individual body mass, was recorded. The measured values from each cage were normalized to 100 g body mass.
Oxygen and food consumption studies in MC-4R deficient mice
Six- to eight-week-old male C57BL/6J (wild-type) and littermate MC4-R−/− mice from MC-4R+/− breeding pairs at Oregon Health and Science University (Portland, USA) [31] were used for food intake and energy expenditure experiments. The strain had previously been backcrossed onto the C57BL/6J background for more than ten generations. For oxygen consumption experiments, mice were maintained in a 12/12 h light/dark cycle with ad libitum access to food (Powdered Laboratory Rodent Diet, Purina 5001; PMI Nutrition International, St. Louis, USA) and water. The administration of BL-6020/979 or vehicle occurred either at onset of light phase (daytime) or on a different day at onset of dark phase (nighttime) and food and gas exchange parameters were recorded for the following 4 and 8 h, respectively (n = 10 per group). Oxygen consumption (VO2) and carbon dioxide production (VCO2) were simultaneously determined by indirect calorimetry (Oxymax, Columbus Instruments, Columbus, USA), while animals were housed in separate chambers at 24 ± 1°C. Mice were first acclimatized to the chambers for 4 days. Measurements were recorded for 4–8 h at the start of the light cycle (08.00–16.00 h) or at the start of the dark cycle (20.00–04.00 h). Parameters were recorded approximately every 3 min with the room air reference taken every 30 min. Basal oxygen consumption was determined for individual mice as the average of the lowest plateau regions corresponding to inactive periods.
For food intake experiments, animals were individually housed for a minimum of 1 week prior to starting each experiment. Animals were habituated for a minimum of 4 days to eating powdered chow (Purina 5001) from containers designed to minimize spill and contamination of the remaining food. On each day before dosing, the mean body weight of each treatment group was determined. To calculate the concentration needed for treatment groups, the dose, the mean body weight, and the solution volume of gavage were considered. Vehicle (10% hydroxypropyl-β-cyclodextrin in 100 mM saline) and working solutions of compound were prepared daily before dosing. Each mouse received the gavage volume calculated as 1% of body mass. After weighing mice and food, gavage was performed at the start of the study interval using animal feeding needles (Popper and Sons, Inc., New Hyde Park, Japan).
C26 adenocarcinoma-induced cachexia model
The C26 colon adenocarcinoma model was used as previously described [23]. In summary, 6-week-old male BALB/c received unilateral subcutaneous injections of approximately 1 × 106 C26 cells in phosphate-buffered saline (PBS) or PBS only (n = 9 per group). Starting on the next day (day 1), animals were treated with 30 mg/kg BL-6020/979 p.o. or vehicle once daily. Wellbeing of the animal, tumor growth, food intake and body mass were recorded daily. Lean and fat mass of each animal were determined by MRI relaxometry (EchoMRI-500, Echo Medical Systems, Houston, USA) on the day of inoculation (day 0) and at the end of the experiment after removal of the tumor (day 15). Furthermore, tissue samples of the gastrocnemius were frozen on dry ice and stored at −80°C for RNA extraction and subsequent gene expression analysis. Total RNA was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hombrechtikon, Switzerland). The initial homogenization step was carried out using the gentleMACS™ M Tubes (Miltenyi Biotec, Bergisch Gladbach, Germany). One microgram of total RNA was reverse transcribed into cDNA using the QuantiTect Rev. Transcription Kit (Qiagen, Hombrechtikon, Switzerland). Quantitative real-time PCR was carried out on the LightCycler® 480 System (Roche, Rotkreuz, Switzerland) using the LightCycler® 480 SYBR Green I Master (Roche, Rotkreuz, Switzerland). The following primer pairs have been used for the target genes: Atrogin-1 fwd AAGCGTTTGATCTTGTCTGA and rev TGCTCTCTTCTTGGGTAACA; Pgc1beta fwd CCTACCCACAAGGACAGCAT and rev ACCTTCCAGAGCAGTCTCCA. And gapdh was used for standardization (Primers: fwd ATTGTCAGCAATGCATCCTG and rev ATGGACTGTGGTCATGAGCC).
Chronic mild stress model in rats
The chronic mild stress model was used as described elsewhere [32]. In short, male Wistar rats (Charles River, Germany; 90–100 g at delivery) were first trained to consume a 1% sucrose solution, and sucrose consumption was monitored at weekly intervals throughout the whole experiment. On the basis of their sucrose intakes in the final baseline test, animals were divided into two matched groups. One group of animals was subjected to the chronic mild stress procedure (two periods of food or water deprivation, two periods of 45° cage tilt, two periods of intermittent illumination, two periods of soiled cage, one period of paired housing, two periods of low intensity stroboscopic illumination, and three periods of no stress; with a duration of 10–14 h for each period) for seven consecutive weeks. The other group of animals (controls) was held in a separate room with no contact to the stressed rats and no exposure to stress conditions. On the basis of their sucrose intakes in the initial 2 weeks of stress, both stressed and control groups were each divided further into matched subgroups (n = 8), and for subsequent 5 weeks they received once daily administration of vehicle (10% hydroxypropy-β-cyclodextrin in 100 mM saline, p.o., 1 ml/kg), BL-6020/979 (p.o., 10 mg/kg) or imipramine (i.p., 10 mg/kg) as the reference treatment.
Statistics
Multiple (repeated measures) analysis of variance (ANOVA) with Dunnett’s or Bonferroni post hoc test where applicable was used in all group comparisons; with the exception of gene expression analysis where the non-parametric H-test was used, and the survival analysis to compare numbers of cachectic animals where the log-rank Mantel–Cox test was used. All tests were carried out two-tailed with α = 0.05 using GraphPad Prism (GraphPad Software, CA, USA). All results are given as means ± SEM except gene expression data, which are presented as median and interquartile range.
Results
Pharmacology and safety profile of BL-6020/979
In vitro binding of BL-6020/979 to the MC-4 receptor followed a sigmoidal dose–response. The calculated average IC50 was 19 nM. Binding of BL-6020/979 to the MC-3 and MC-5 receptors resulted in IC50 values of 2.7 μM and 0.89 μM, respectively. No significant binding was observed at the MC-1R at a test concentration of 10 μM. The resulting selectivity for the MC-4R over the MC-3R was 140-fold and over MC-5R 50-fold. Selectivity over the MC-1R was estimated to be >10,000-fold, based on the binding result at 10 μM.
An MC-4R membrane agonist assay of BL-6020/979 measuring stimulation of cAMP production revealed that the compound has no significant agonistic activity up to the maximal test concentration of 100 μM. Schild plot analysis of BL-6020/979 showed a regression slope significantly less than 1, which might indicate that the antagonism is not strictly competitive. The calculated K B of BL-6020/979 was 39 nM, corresponding to a pA2 value of 7.4.
Among all 71 receptor, transporter and ion channel off-targets in the tested panel, no significant interaction was found. In a patch-clamp assay, BL-6020/979 exhibited an average IC50 of 4.6 μM at the I Kr/hERG channel, indicating that the compound was a weak inhibitor of this channel in vitro. Moreover, BL-6020/979, at a concentration of 10 μM, did not significantly inhibit a range of 41 enzymes, indicating the very low propensity of the compound towards enzyme inhibition.
In vitro toxicology showed a favorable profile for BL-6020/979. It exhibited a TC50 value of 93 μM, indicating low cytotoxic potential. The same is true for the lack of mutagenic potential as shown in a negative AMES test. Furthermore, there was no change in frequency in micro-nucleated cells in vivo in male mice at doses up to 750 mg/kg and females up to 625 mg/kg compared to negative control animals and no evidence of bone marrow toxicity following oral treatment with BL-6020/979 thereby confirming that there is no evidence for clastogenic or aneugenic effects of the compound.
In exploratory tolerability studies that were aimed at determining suitable doses for longer term toxicity studies in mice and rats, BL-6020/979 was administered once daily by oral gavage to both species for 7 days. In mice, doses of up to 500 mg/kg BL-6020/979 were well tolerated in females and males and no adverse effects were found in the post mortem analysis. In rats at necropsy, distension of the gastro-intestinal tract of was observed at 500 mg/kg/day. Consequently, in rats treated for 7 days with BL-6020/979, the NOAEL was considered to be 300 mg/kg/day.
The potential for drug–drug interaction was explored in CYP inhibition and induction assays. BL-6020/979 did not inhibit CYP1A up to the highest test concentration of 25 μM. For CYP2C9, CYP2C19, and CYP2D6, interference of the compound with the detection method of the assay was observed at 25 μM and precluded assessment at this concentration. However, no inhibition of these CYPs was measured at a test concentration of 5 μM. Accordingly, the IC50 for these isoforms were set >5 μM. In the case of CYP3A4, the same interference occurred at a compound concentration of 25 μM, but here 31% inhibition was measured at a test concentration of 5 μM yielding an estimated IC50 for CYP3A4 of 5–10 μM.
In human hepatocytes, no significant induction was apparent at 0.1 μM. The largest extent of induction was measured at a test concentration of 1 μM and amounted to 5-fold or 18% of the positive control response (which gave a 25-fold induction of CYP3A4 activity). However, at the highest test concentration of 10 μM the observed induction was merely 2-fold, corresponding to 7% of the positive control.
Lastly, the pharmacokinetic properties were assayed. BL-6020/979 reaches the brain in mice and rats after oral administration of 50 mg/kg. The brain-to-plasma ratios were about 10% in both species. In mice, plasma levels after 20 mg/kg s.c. were comparable to 100 mg/kg p.o. Peak plasma and brain levels are reached 1 h after dosing. Brain concentration levels for mice, rats and dogs are given in Table 1. Plasma protein binding amounted to approximately 90% across species and amounted to 89% in human plasma.
Table 1.
BL-6020/979 is orally bioavailable in mice, rats and dogs and penetrates the brain
| Mouse | Rat | Dog | |
|---|---|---|---|
| Dose in mg/kg p.o. | 50 | 50 | 10 |
| Cplasma in nM | 1269 ± 404 | 2277 ± 340 | 2426 ± 1036 |
| Cbrain in nM | 110 ± 26 | 151 ± 27 | n.d. |
| b/p ratio in % | 12 | 8 | n.d. |
| PPB in % | 92 | 87 | 93 |
BL-6020/979 was given orally at indicated dose. N = 3 per group, mean ± SD are given
b/p brain-to-plasma-ratio, n.d. not determined, PPB plasma protein binding
BL-6020/979 dose-dependently increases food intake in healthy NMRI mice
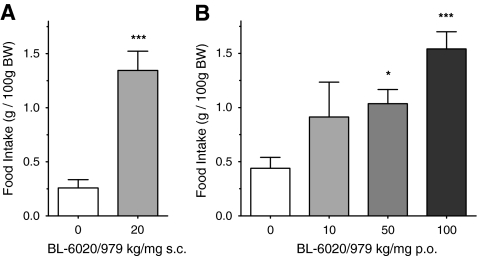
Daytime food consumption was measured for the first 4 h after compound administration at various doses via subcutaneous and oral routes. The results showed an increase in daytime food intake at doses of and above 50 mg/kg via the oral route and 20 mg/kg via subcutaneous injection (Fig. 1). Oral administration of BL-6020/979 increased daytime food intake in a dose-dependent manner, resulting in a 3- to 5-fold increase in the amount of food consumed over a 4 h period. Noteworthy, both the magnitude of effect on food intake as well as the peak plasma levels attained after 20 mg/kg s.c. and 100 mg/kg p.o. administration were comparable. Importantly, the drug-induced feeding occurred in non-fasted animals during the light phase, where, under normal (non-stressed) conditions, food intake is low as seen in the vehicle group of the present experiments.
Fig. 1.
The effect of BL-6020/979 on 4 h light-phase food intake in female mice. Food intake of female NMRI mice after a subcutaneous or b oral application of BL-6020/979 at indicated doses. Animals were housed in groups of three. The food intake (per cage) was recorded 4 h post administration of compound whereby food intake was normalized to 100 g body weight. Bars represent the mean ± SEM cumulative food intake per cage. All BL-6020/979 groups n = 6 cages, and Vehicle n = 12 cages. Significance vs. vehicle in Dunnett’s post hoc test:*p < 0.05 ***p < 0.001
The effects of BL-6020/979 on food intake and energy expenditure are MC-4R mediated
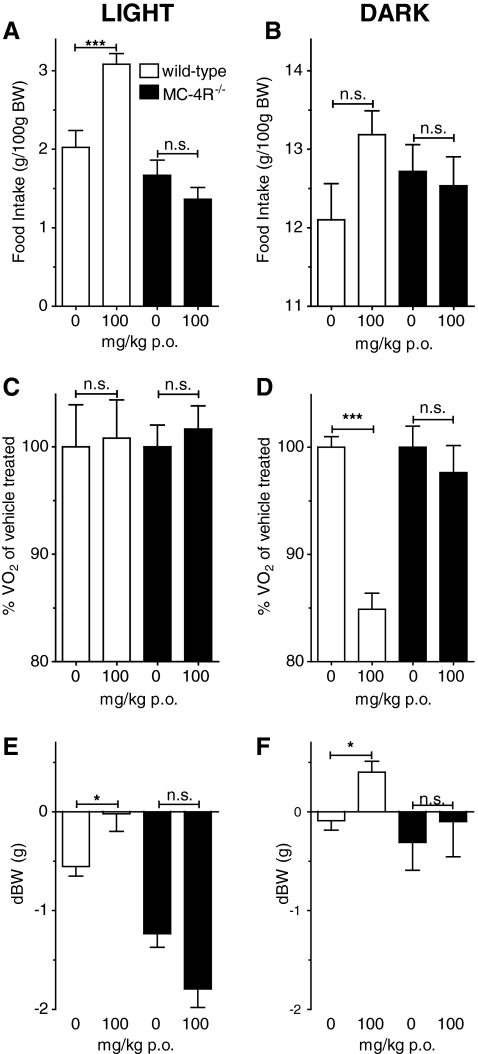
Feeding and energy expenditure data was normalized to body mass because MC-4R−/− mice are known to differ from wild-type control mice in body mass and in baseline feeding characteristics [31]. After daytime application, there was a significant increase in food intake in wild-type mice treated with 100 mg/kg BL-6020/979 p.o. compared with vehicle-treated controls 4 h after application. This effect was not observed in the MC-4R−/− mice. Nighttime dosing led to similar results. Although the BL-6020/979 treated groups of both genotypes did not differ from vehicle controls of each genotype (ANOVA, p = 0.24), BL-6020/979 treated mice showed a tendency to increase food intake in wild-type but not in MC-4R−/− mice (Fig. 2A and B)
Fig. 2.
The effects of BL-6020/979 on food intake and energy expenditure are mediated by MC-4R. a and b Food intake and c and d relative oxygen consumption in wild-type (open bars) and MC-4R−/− (black bars) mice in the first 4 h after application of 100 mg/kg BL-6020/979 p.o. or vehicle. e and f Body mass development in wild-type and MC-4R−/− mice in the first 4 h after application of 100 mg/kg BL-6020/979 p.o. or vehicle. Note that data plotted for the resting (light; a, c, e) and active (dark; b, d, f) period were not measured during one 24-h interval and therefore not directly comparable. See text for more details
The expected overall lower VO2 value was observed in MC-4R−/− animals relative to wild-type controls, therefore the data were normalized to the values for vehicle-treated mice of each genotype [33]. During the first 4 h post dosing at nighttime, the 100 mg/kg BL-6020/979 p.o. treated wild-type mice had a basal VO2 that was significantly lower than the vehicle-treated wild-type group, whilst no such difference was observed in treated and untreated MC-4R−/− mice. In contrast, application of the compound during daytime did not yield any significant differences in wild-type or MC-4R−/−mice (Fig. 2C and D).
In line with the results on the energy intake and expenditure, body mass changed during the course of the experiment. BL-6020/979 treated wild-type mice lost less and gained more body mass after daytime and nighttime application, respectively. In contrast, however, MC-4R−/− mice treated with BL-6020/979 lost more body mass than controls after daytime application, but after nighttime application there was no difference between these groups (Fig. 2E and F).
BL-6020/979 ameliorates cachexia in the murine C26 adenocarcinoma model
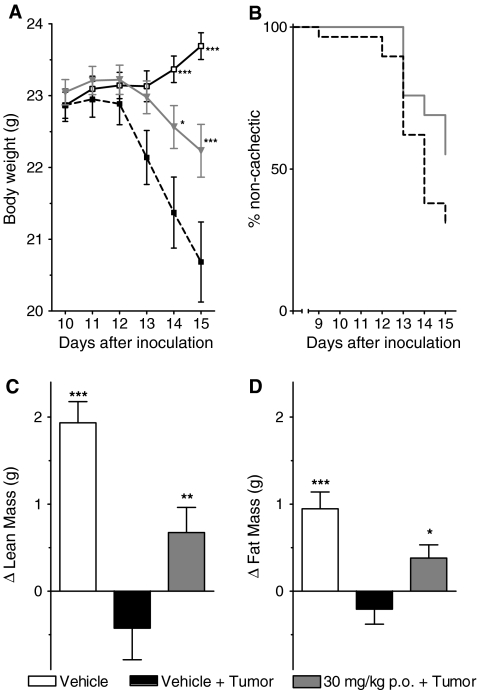
Daily oral administration of BL-6020/979 (p.o., 30 mg/kg) consistently reduced signs of cachexia in three independent experiments (Fig. 3). Data from each of these three separate experiments were similar and, thus, were pooled for further analysis. A tumor developed in all inoculated animals and was palpable around day 4 after inoculation. Importantly, tumor growth was not altered by treatment and final tumor mass was similar in all groups, i.e., 0.99 ± 0.05 g in the 30 mg/kg BL-6020/979 vs. 1.02 ± 0.04 g in the vehicle control group, confirming that no anti-tumor effects of the compound confounded its effect on the cachexia.
Fig. 3.
The effects of BL-6020/979 in C26 tumor-bearing mice. a Mean body weight development of vehicle control (open squares), vehicle + tumor control (closed squares), and BL-6020/979 30 mg/kg (gray triangles) group. b Kaplan–Meyer plot for vehicle control (black line) and BL-6020/979 30 mg/kg (gray line) treated tumor-bearing groups. Occurrence of cachexia was defined as loss of more than 5% of body mass during the course of the experiment. Difference in lean body mass (c) and fat mass (d) between day of tumor inoculation (day 0) and end of experiment (day 15). Note: all graphs are average from three independent experiments, each with n = 9–10 per group. Each value represents mean ± SEM. Statistical difference vs. vehicle + tumor in post hoc testing is signified as *p < 0.05, ***p < 0.001
Vehicle-treated mice started losing body mass around days 10–12 after inoculation. This was ameliorated in the BL-6020/979 treated group, and at the end of the experiment the average body mass of the compound treated mice was significantly higher than that of the vehicle-treated tumor-bearing mice (Fig. 3A).
In addition to the effect on body mass, treatment with 30 mg/kg BL-6020/979 p.o. positively affected body composition whereby both fat and lean mass were significantly increased compared to vehicle-treated tumor-bearing mice, which showed a distinct loss of fat and lean body mass compared to non-tumor-bearing mice (Fig. 3C and D).
Cachexia, defined as loss of more than 5% of body mass in the course of the experiment, did not occur in all tumor-bearing mice. However, cachexia was absent in 16 of 29 BL-6020/979-treated animals but only in 9 of the 29 vehicle-treated animals. Kaplan–Meier analysis of the onset of cachexia (i.e., the day ≥5% body mass was lost) revealed a significant beneficial effect of BL-6020/979 compared to the vehicle-treated controls (p = 0.04, Fig. 3B).
On the gene expression level, and in line with the effects on body composition and body weight, 30 mg/kg BL-6020/979 p.o. prevented deregulation of genes relevant for muscle atrophy, i.e., Atrogin-1 and PGC-1β (Table 2). These genes are typically up-regulated (Atrogin-1) or down- regulated (PGC-1β) during cachexia and other muscle wasting pathologies.
Table 2.
The effects of 30 mg/kg BL-6020/979 p.o. on gene expression in the C26 tumor model
| Group | Atrogin-1 | PGC-1β |
|---|---|---|
| Vehicle | 1.0 (0.9–1.2)*** | 1.1 (0.8–1.2)*** |
| Vehicle + tumor | 20.1 (4.1–28.8) | 0.2 (0.1–0.4) |
| 30 mg/kg BL-6020/979 + tumor | 3.0 (1.4–19.4) | 0.5 (0.2–0.6)*** |
Relative expression levels of Atrogin-1 and PGC-1β in gastrocnemius muscle of mice. Median (interquartile range) are given (n = 27–29/group). Statistical difference in Dunn’s post hoc test vs. Vehicle + tumor with ***p < 0.001
Unexpectedly, there was no clear effect of BL-6020/979 on food intake in the last third (d10–d15) of the experiment, when the cachexia was apparent. The total food consumption of BL-6020/979 and vehicle-treated tumor-bearing mice did not differ significantly in this period although a difference in body mass was observed. However, there was a trend in the expected direction with an increased food intake in BL-6020/979 treated mice (14.5 ± 0.5 g) compared to vehicle-treated mice (13.3 ± 0.7 g); both groups’ food intake was lower (p < 0.001) than that of non-tumor-bearing mice (17.2 ± 0.3 g).
BL-6020/979 has antidepressant-like effects in the rat chronic mild stress model
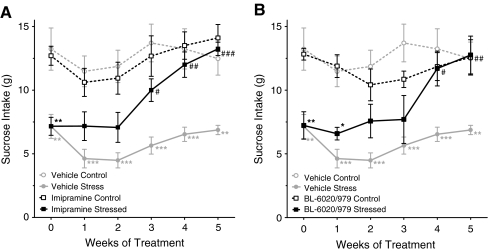
As expected, sucrose consumption gradually decreased in the vehicle-treated stressed animals but not in the non-stressed during the baseline weeks before the rats were treated (data not shown). Imipramine—a known antidepressant—reversed the effect of stress, i.e., the sucrose intake of treated rats was significantly higher than in vehicle-injected stressed rats and was indistinguishable in rats from the control group starting at week 3 of the experiment (Fig. 4A). Similar to imipramine, 10 mg/kg BL-6020/979 p.o. produced significant treatment effects in stressed rats but was inactive in non-stressed controls. This positive effect was apparent only 1 week later than in the imipramine-treated group. Of note, both imipramine-treated groups—the stressed as well as the control group—gained less body mass in the 5 weeks of the treatment period than vehicle-treated controls (control, p < 0.05; stressed, p < 0.001), while body mass gain in BL-6020/979-treated animals was indistinguishable from vehicle-treated stressed and control rats (control and stressed: n.s.; data not shown).
Fig. 4.
Effects of BL-6020/979 in the chronic mild stress model in rats. Consumption of 1% sucrose solution after once daily administration of b 10 mg/kg BL-6020/979 p.o. compared to a 10 mg/kg imipramine i.p. is given for control (open symbols) and stressed (closed symbols) rats. Values for vehicle controls are plotted in gray in (a) and (b). All data given as mean ± SEM (n = 7–8/group). Results of post hoc statistical comparison is of stressed vs. control group of each treatment group is depicted as *p < 0.05, **p < 0.01, ***p < 0.001, and comparison of active compound vs. vehicle alone is signified as # p < 0.05, ## p < 0.01, ### p < 0.001
Discussion
Blockade of the MC-4R by an endogenous antagonist or genetic deletion has previously been proven effective in experimental animal models of cachexia [20]. Likewise peptidic antagonists have been used, and subsequently the development of more drug-like small molecules and even antibodies against this target has been pursued by pharmaceutical companies and academic groups [19, 34–36]. Regarding the prevention of cachexia, the current data strongly suggest that it is indeed the dual effect of increased food intake and decreased energy expenditure which is key to the beneficial features of this mode of action [17]. We have demonstrated, that the novel, orally available, non-peptidic small-molecule MC-4R antagonist BL-6020/979 was not only able to increase food intake and prevent cancer cachexia like previous chemically related compounds [23]. Overall, this promising candidate is clearly superior to previously described earlier lead compounds [23] from the same optimization program. Importantly, its general pharmacological properties, tolerability and safety, were markedly improved. In addition, it decreased energy expenditure in healthy mice. BL-6020/979 displayed high selectivity and low nanomolar affinity towards binding and inhibition of the MC-4R. Consistent with its designated mode of action, its pharmacological activity in vivo is mediated selectively via the MC-4 receptor since BL-6020/979 was without effect in mice lacking this receptor.
The MC-4R antagonist BL-6020/979 significantly increased food intake during the day. A similar trend towards an increase in food intake was detected at the beginning of the night. Inversely, treatment with BL-6020/979 efficiently reduced energy expenditure at night in healthy wild-type animals. When given during the day, this effect was not observed. This is likely the result of the inactive state of the mice during the day which, typically for nocturnal animals [37], reduces their basal level of energy expenditure during the resting phase to a level that cannot be lowered further. Conversely, food intake is highest during the early night, where it might not be possible to stimulate a further increase, and low during the day, when application BL-6020/979 caused a significant increase.
Previously, nonspecific blockade of the MC-4R through intra-cerebroventricular application of the endogenous ligand AgRP or SHU-9119 has been shown to be effective in cancer-induced cachexia models [21, 38]. Similarly, small-molecule MC-4R antagonists have been reported to ameliorate cachexia in rodent models [39, 40] but none of them have been reported to be moved towards clinical development.
The completely non-peptidic small-molecule BL-6020/979 is one of the first MC-4R antagonists that ameliorated cancer cachexia after oral administration. BL-6020/979 showed beneficial effects on body mass and composition in the C26 murine cachexia model as had been shown previously for earlier lead compounds from the same and from a chemically distinct series [23]. Probably as a consequence of its beneficial effect on lean mass, BL-6020/979 also showed effects at the gene expression level in the gastrocnemius. The up-regulation of the ubiquitin ligase Atrogin-1, which has been shown to be essential for muscle degeneration [41], was attenuated. Similarly, the decrease in PGC-1β that is typical for muscle degeneration [42] was prevented (Table 2).
It might be a benefit of the dual effect of MC-4R blockade on food intake and energy expenditure [17] that although no significant effect of BL-6020/979 on food intake could be observed, compound-treated animals did lose significantly less body mass indicating treatment success. This lack of an effect on food intake might be due to the high variability in daily food intake that we observed in our experiments. If averaged over 5 days, there was a trend towards an increased food intake in BL-6020/979 treated mice. In addition, and based on the data from healthy animals (Fig. 2) and the effects of MC-4R blockade described previously [17], we speculate that this positive outcome might have been due to the lowered energy expenditure of the treated animals.
Important for further development, BL-6020/979 is the first MC-4R antagonist with an overall favorable pharmacological profile. In vitro, a low nanomolar affinity at the targeted MC-4R and high selectivity against most closely related melanocortin receptors 3, 5, and 1 was evident. Moreover, at a concentration of 10 μM, the compound exhibited very little off-target activities confirming its excellent selectivity in a standard panel of over 100 receptors, ion channels, transporters, and enzymes. Of note was that BL-6020/979 has a single digit micromolar affinity for the delayed rectifier current-producing K+ channel encoded by hERG. The blockade of this channel is often associated with QT prolongation and torsade des pointes [43]. Retrospective data on marketed drugs on prolonged QT effects have shown that a safety margin of the I Kr/hERG IC50 divided by C max of free drug in plasma concentration of >30 up to ∼45-fold can be considered uncritical for further development [44, 45]. Considering the compound’s plasma protein binding of approximately 90% and an efficacious dose in mice of 50 mg/kg p.o. in the food intake assay in healthy mice, the estimated peak plasma concentration of free drug in vivo amounts to approximately 100 nM. Thus, BL-6020/979 fulfills this consensus industry standard and has a calculated ∼50-fold safety margin for hERG inhibition. Based on this as well as published literature, it is therefore not anticipated that BL-6020/979 will have an effect on QT prolongation in vivo [45].
Moreover, the toxicological profile of BL-6020/979 (in in vitro toxicity and in vivo acute and 7-day repeated dose toxicity studies) in relation to its pharmacological effects supports the further development of BL-6020/979. These data are further endorsed by an in vivo genotoxicity study which did not find any indication of a clastogenic and/or aneugenic potential of BL-6020/979.
In addition to chemotherapy, most cancer patients already receive a cocktail of drugs, e.g., analgesics, antiemetics, and antidepressants [46, 47]. Thus, a potential cachexia drug should exhibit a low potential for induction or inhibition of cytochromes involved in drug metabolism of the co-medications. Indeed, BL-6020/979 did not show any critical inhibitory activity in the human CYP isoforms tested. CYP3A4 induction was assessed in human hepatocytes according to the draft FDA guidance to industry for drug interaction studies. Based on the obtained results (data not shown) BL-6020/979 is not considered to be an inducer of CYP3A4. Taken together, the data indicate a low potential for inhibition—or induction—based drug–drug interactions.
As stated above and like in other chronic diseases in which cachexia develops, cancer is typically accompanied by a whole plethora of co-morbidities treated with a cocktail of various drugs. We were interested to assess whether BL-6020/979 could provide a beneficial co-effect in any of these. For example, depression is a common problem in cancer patients [48]. Interestingly, MC-4R antagonism has been previously investigated as a potential mode of action in the treatment of depression due to anxiolytic and antidepressant-like effects in animal models [22]. We have chosen the chronic mild stress paradigm because of its excellent construct and predictive validity [49] and could demonstrate that BL-6020/979 exhibits antidepressant-like effects comparable to imipramine, a standard treatment in cancer-related depression [50]. Similarly, and also highly relevant for cancer patients, MC-4R antagonism has been shown to be effective in models of neuropathic pain as an analgesic [51]. Equally interesting, a modulating effect on opioid tolerance as has been shown to be mediated by this mechanism [52]. Therefore, BL-6020/979 may open an option to treat not only cachexia itself but also accompanying symptoms, typical of the clinical presentation of the disease. Further studies elucidating these possible additional effects of BL-6020/979 seem warranted.
Cachexia is not only part of the clinical manifestation of many cancer types but also of other diseases like chronic kidney disease [53], liver failure [54], chronic obstructive pulmonary disease (COPD) [55], chronic heart failure [56], and AIDS [57]. Based on its mechanism, MC-4R blockade might be beneficial in the treatment of all of the above mentioned causes of cachexia. It has been suggested that hypothalamic energy homeostasis is disturbed in a similar way in all these diseases by inflammation of key brain regions [6]. It has been shown that MC-4R blockade can ameliorate or even prevent the occurrence of cachexia for most of these underlying pathologies. It remains an interesting question to be answered in future studies whether BL-6020/979 could prove useful in any of these indications.
In summary, melanocortin-4 receptor antagonism in general seems to represent an exciting mode of action not only for the treatment of cancer-induced cachexia but also other forms of wasting disorders [6, 58]. Although several pharmaceutical companies and academic groups worked on the development of small molecules and even antibodies against this target, no treatment is currently available for cachexia patients or even in ongoing clinical testing [34, 35, 59], potentially underscoring the complexity of the MC-4 receptor and the highly challenging medicinal chemistry required, not only to address it, but in particular to identify antagonist compounds with a balanced overall pharmacological and safety profile. In fact, BL-6020/979 is the result of an extensive lead optimization program and fulfills these requirements as the first orally available completely non-peptidic, small-molecule MC-4R antagonist. Its overall profile qualifies this novel chemical entity for development towards treatment of human patients. Consequently, BL-6020/979 is currently in late stage pre-clinical development as a first-in-class treatment option for cancer cachexia. This opens up the long-awaited opportunity to validate the benefits of treating cachexia with MC-4R antagonists in human patients.
Acknowledgments
We thank Dr. Etgar Levy Nissenbaum, BioLineRx Ltd. (Jerusalem, Israel), for helpful discussions and suggestions, Reto Bolliger and Gloria Akyempon for support in the chemical syntheses, and Siglinde Zepter and Tina Pfeiffer-Unckrich for support in chemical analyses and in purification. Part of the data in this manuscript was previously presented at the 5th Cachexia Conference in Barcelona, Spain. All authors certify that they comply with the ethical guidelines of the Journal of Cachexia, Sarcopenia and Muscle [60].
Disclosures
All authors were employees of Santhera Pharmaceuticals at the time of the experiments, except DLM and MP, which were consultants for Santhera Pharmaceuticals (Switzerland) Ltd. Santhera’s MC-4R antagonist program including BL-6020/979 has been licensed to BioLineRx Ltd. for further development.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- MC-4R
Melanocortin-4 receptor
- AgRP
Agouti-related protein
- hERG
Human ether-a-go-go related gene
- PBS
Phosphate-buffered saline
- COPD
Chronic obstructive pulmonary disease
- AIDS
Autoimmune deficiency syndrome
Contributor Information
R. Dallmann, Email: dallmann@pharma.uzh.ch
A. Feurer, Email: achim.feurer@santhera.com
References
- 1.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Scarlett JM, Marks DL. The use of melanocortin antagonists in cachexia of chronic disease. Expert Opin Investig Drugs. 2005;14(10):1233–1239. doi: 10.1517/13543784.14.10.1233. [DOI] [PubMed] [Google Scholar]
- 3.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 4.den Staal-van Brekel AJ, Schols AM, ten Velde GP, Buurman WA, Wouters EF. Analysis of the energy balance in lung cancer patients. Cancer Res. 1994;54(24):6430–6433. [PubMed] [Google Scholar]
- 5.Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome—when all you can eat is yourself. Nat Clin Pract Oncol. 2005;2(3):158–165. doi: 10.1038/ncponc0112. [DOI] [PubMed] [Google Scholar]
- 6.Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100(5):478–489. doi: 10.1016/j.physbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8(5):971–982. doi: 10.1016/S1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 8.Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia–cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7(4):427–434. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- 9.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8(3):255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 10.Martin NM, Smith KL, Bloom SR, Small CJ. Interactions between the melanocortin system and the hypothalamo-pituitary-thyroid axis. Peptides. 2006;27(2):333–339. doi: 10.1016/j.peptides.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Tung YC, Piper SJ, Yeung D, O’Rahilly S, Coll AP. A comparative study of the central effects of specific proopiomelancortin (POMC)-derived melanocortin peptides on food intake and body weight in pomc null mice. Endocrinology. 2006;147(12):5940–5947. doi: 10.1210/en.2006-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kask A, Mutulis F, Muceniece R, Pahkla R, Mutule I, Wikberg JE, et al. Discovery of a novel superpotent and selective melanocortin-4 receptor antagonist (HS024): evaluation in vitro and in vivo. Endocrinology. 1998;139(12):5006–5014. doi: 10.1210/en.139.12.5006. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Meal patterns and foraging in melanocortin receptor knockout mice. Physiol Behav. 2005;84(1):129–133. doi: 10.1016/j.physbeh.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, et al. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes. 2001;50(2):248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- 15.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, et al. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. doi: 10.1210/en.139.10.4428. [DOI] [PubMed] [Google Scholar]
- 16.DeBoer MD, Marks DL. Therapy insight: use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2(8):459–466. doi: 10.1038/ncpendmet0221. [DOI] [PubMed] [Google Scholar]
- 17.Markison S, Foster AC, Chen C, Brookhart GB, Hesse A, Hoare SR, et al. The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology. 2005;146(6):2766–2773. doi: 10.1210/en.2005-0142. [DOI] [PubMed] [Google Scholar]
- 18.Marks DL, Butler AA, Turner R, Brookhart G, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144(4):1513–1523. doi: 10.1210/en.2002-221099. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Jiang W, Tucci F, Tran JA, Fleck BA, Hoare SR, et al. Discovery of 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J Med Chem. 2007;50(22):5249–5252. doi: 10.1021/jm070806a. [DOI] [PubMed] [Google Scholar]
- 20.Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61(4):1432–1438. [PubMed] [Google Scholar]
- 21.Foster AC, Chen C. Melanocortin-4 receptor antagonists as potential therapeutics in the treatment of cachexia. Curr Top Med Chem. 2007;7(11):1131–1136. doi: 10.2174/156802607780906663. [DOI] [PubMed] [Google Scholar]
- 22.Chaki S, Okubo T. Melanocortin-4 receptor antagonists for the treatment of depression and anxiety disorders. Curr Top Med Chem. 2007;7(11):1145–1151. doi: 10.2174/156802607780906618. [DOI] [PubMed] [Google Scholar]
- 23.Weyermann P, Dallmann R, Magyar J, Anklin C, Hufschmid M, Dubach-Powell J, et al. Orally available selective melanocortin-4 receptor antagonists stimulate food intake and reduce cancer-induced cachexia in mice. PLoS ONE. 2009;4(3):e4774. doi: 10.1371/journal.pone.0004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Canc. 2010;102(11):1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9(4):310–322. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- 26.Walsky RL, Obach RS. Validated assays for human cytochrome P450 activities. Drug Metabol Dispos: The Biological Fate of Chemicals. 2004;32(6):647–660. doi: 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- 27.Lu C, Li AP. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague–Dawley rat, minipig, and beagle dog. Chem Biol Interact. 2001;134(3):271–281. doi: 10.1016/S0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 28.Farkas D, Tannenbaum SR. In vitro methods to study chemically induced hepatotoxicity: a literature review. Curr Drug Metab. 2005;6(2):111–125. doi: 10.2174/1389200053586118. [DOI] [PubMed] [Google Scholar]
- 29.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113(3–4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 30.Kariv I, Cao H, Oldenburg KR. Development of a high throughput equilibrium dialysis method. J Pharm Sci. 2001;90(5):580–587. doi: 10.1002/1520-6017(200105)90:5<580::AID-JPS1014>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 32.Papp M, Nalepa I, Antkiewicz-Michaluk L, Sanchez C. Behavioural and biochemical studies of citalopram and WAY 100635 in rat chronic mild stress model. Biochem Behav. 2002;72:465–474. doi: 10.1016/S0091-3057(01)00778-X. [DOI] [PubMed] [Google Scholar]
- 33.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97(22):12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaki S, Oshida Y, Ogawa S, Funakoshi T, Shimazaki T, Okubo T, et al. MCL0042: a nonpeptidic MC4 receptor antagonist and serotonin reuptake inhibitor with anxiolytic- and antidepressant-like activity. Pharmacol Biochem Behav. 2005;82(4):621–626. doi: 10.1016/j.pbb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Peter JC, Lecourt AC, Weckering M, Zipfel G, Niehoff ML, Banks WA, et al. A pharmacologically active monoclonal antibody against the human melanocortin-4 receptor: effectiveness after peripheral and central administration. J Pharmacol Exp Ther. 2010;333(2):478–490. doi: 10.1124/jpet.109.163279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergoni AV, Bertolini A, Wikberg JE, Schioth HB. Selective melanocortin MC4 receptor blockage reduces immobilization stress-induced anorexia in rats. Eur J Pharmacol. 1999;369(1):11–15. doi: 10.1016/S0014-2999(99)00045-X. [DOI] [PubMed] [Google Scholar]
- 37.Ripperger JA, Jud C, Albrecht U. The daily rhythm of mice. FEBS Letters. 585:1384–92. [DOI] [PubMed]
- 38.Foster AC, Chen C, Markison S, Marks DL. MC4 receptor antagonists: a potential treatment for cachexia. IDrugs. 2005;8(4):314–319. [PubMed] [Google Scholar]
- 39.Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, et al. Identification of 2-[2-[2-(5-bromo-2- methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47(7):1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. 2006;317(2):771–777. doi: 10.1124/jpet.105.097725. [DOI] [PubMed] [Google Scholar]
- 41.Cong H, Sun L, Liu C, Tien P. Inhibition of atrogin-1/MAFbx expression by adenovirus-delivered small hairpin RNAs attenuates muscle atrophy in fasting mice. Hum Gene Ther. 2011;22(3):313–324. doi: 10.1089/hum.2010.057. [DOI] [PubMed] [Google Scholar]
- 42.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21(1):140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 43.Raehl CL, Patel AK, LeRoy M. Drug-induced torsade de pointes. Clin Pharm. 1985;4(6):675–690. [PubMed] [Google Scholar]
- 44.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32–45. doi: 10.1016/S0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 45.Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther. 2011;129(2):109–119. doi: 10.1016/j.pharmthera.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Shoemaker LK, Estfan B, Induru R, Walsh TD. Symptom management: an important part of cancer care. Cleve Clin J Med. 2011;78(1):25–34. doi: 10.3949/ccjm.78a.10053. [DOI] [PubMed] [Google Scholar]
- 47.Shimm DS, Logue GL, Maltbie AA, Dugan S. Medical management of chronic cancer pain. JAMA. 1979;241(22):2408–2412. doi: 10.1001/jama.241.22.2408. [DOI] [PubMed] [Google Scholar]
- 48.Reich M. Depression and cancer: recent data on clinical issues, research challenges and treatment approaches. Curr Opin Oncol. 2008;20(4):353–359. doi: 10.1097/CCO.0b013e3282fc734b. [DOI] [PubMed] [Google Scholar]
- 49.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 50.Goodnick PJ, Hernandez M. Treatment of depression in comorbid medical illness. Expert Opin Pharmacother. 2000;1(7):1367–1384. doi: 10.1517/14656566.1.7.1367. [DOI] [PubMed] [Google Scholar]
- 51.Vrinten DH, Gispen WH, Groen GJ, Adan RA. Antagonism of the melanocortin system reduces cold and mechanical allodynia in mononeuropathic rats. J Neurosci. 2000;20(21):8131–8137. doi: 10.1523/JNEUROSCI.20-21-08131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B. The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Res. 2003;990(1–2):113–119. doi: 10.1016/S0006-8993(03)03444-9. [DOI] [PubMed] [Google Scholar]
- 53.Mak RH, Cheung W. Energy homeostasis and cachexia in chronic kidney disease. Pediatr Nephrol. 2006;21(12):1807–1814. doi: 10.1007/s00467-006-0194-3. [DOI] [PubMed] [Google Scholar]
- 54.Plauth M, Schutz ET. Cachexia in liver cirrhosis. Int J Cardiol. 2002;85(1):83–87. doi: 10.1016/S0167-5273(02)00236-X. [DOI] [PubMed] [Google Scholar]
- 55.Koehler F, Doehner W, Hoernig S, Witt C, Anker SD, John M. Anorexia in chronic obstructive pulmonary disease—association to cachexia and hormonal derangement. Int J Cardiol. 2007;119(1):83–89. doi: 10.1016/j.ijcard.2006.07.088. [DOI] [PubMed] [Google Scholar]
- 56.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121(3):227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Von Roenn JH, Roth EL, Craig R. HIV-related cachexia: potential mechanisms and treatment. Oncology. 1992;49(Suppl 2):50–54. doi: 10.1159/000227129. [DOI] [PubMed] [Google Scholar]
- 58.DeBoer MD. Update on melanocortin interventions for cachexia: progress toward clinical application. Nutrition. 2010;26:146–151. doi: 10.1016/j.nut.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C, Tucci FC, Jiang W, Tran JA, Fleck BA, Hoare SR, et al. Pharmacological and pharmacokinetic characterization of 2-piperazine-alpha-isopropyl benzylamine derivatives as melanocortin-4 receptor antagonists. Bioorg Med Chem. 2008;16(10):5606–5618. doi: 10.1016/j.bmc.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 60.Haehling S, Morley J, Coats A, Anker S. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]