Abstract
A markerless genetic exchange system was successfully established in Methanosarcina mazei strain Gö1 using the hpt gene coding for hypoxanthine phosphoribosyltransferase. First, a chromosomal deletion mutant of the hpt gene was generated conferring resistance to the purine analog 8-aza-2,6-diaminopurine (8-ADP). The nonreplicating allelic exchange vector (pRS345) carrying the pac-resistance cassette for direct selection of chromosomal integration, and the hpt gene for counterselection was introduced into this strain. By a pop-in and ultimately pop-out event of the plasmid from the chromosome, allelic exchange is enabled. Using this system, we successfully generated a M. mazei deletion mutant of the gene encoding the regulatory non-coding RNA sRNA154. Characterizing M. mazeiΔsRNA 154 under nitrogen limiting conditions demonstrated differential expression of at least three cytoplasmic proteins and reduced growth strongly arguing for a prominent role of sRNA154 in regulation of nitrogen fixation by posttranscriptional regulation.
1. Introduction
Methanosarcina mazei strain Gö1 belongs to the methylotrophic methanogenic Archaea and, due to its role in methane production, is of high ecological relevance [1]. It serves as an archaeal model for investigating nitrogen stress responses, salt adaptation, methane production from different substrates, energy metabolism, as well as analyzing the role of small RNAs as regulatory elements in stress responses [2–7]. Although it only grows under strictly anaerobic conditions, the organism is genetically tractable and single colonies can be obtained on agar plates, a general requirement for genetic studies [8, 9]. However, genetic manipulation is restricted due to the fact that puromycin is the only selectable marker commercially available for methanoarchaea, which complicates generation of multiple mutations or even complementation experiments. Using Methanosarcina acetivorans, Metcalf and coworkers developed a so-called markerless exchange method using the hpt gene encoding hypoxanthine phosphoribosyltransferase as a counterselectable marker [10]. A Δhpt strain which shows resistance towards the toxic purine analog 8-aza-2,6-diaminopurine (8-ADP) can be used for counterselection following integration of an nonreplicable plasmid containing the wild-type hpt gene and the desired mutation with flanking regions for recombination. The complete plasmid is integrated into the site of the desired mutation (pop-in) in the chromosome by a single homologous recombination event, making the strain sensitive to 8-ADP and allowing selection for puromycin resistance. The presence of 8-ADP permits selection for removal of the plasmid-based hpt gene (in concert with the vector backbone) by another single homologous recombination (pop-out) event. During this latter event, the gene of interest can be exchanged by the mutant construct [10]. Theoretically, allelic exchange takes place with a chance of 50% resulting in the desired mutant strain.
The goal of this study was to establish this method for M. mazei in order to allow markerless chromosomal deletion or point mutations of small regulatory RNA genes. To set up the system, a Δhpt strain as well as the allelic exchange vector containing the wild-type hpt gene for counterselection was generated. To validate the method, we deleted the small noncoding RNA sRNA154. This sRNA has been identified in a genome wide RNA-seq screen and shown to be differentially transcribed dependent on nitrogen availability [7]. We suggest that sRNA154 plays a central role in nitrogen regulation in M. mazei, potentially adding another level of regulation to the known regulatory mechanism via the general nitrogen transcriptional repressor NrpR [11, 12]. sRNA154 is located in the intergenic region of MM3337 and MM3338 encoding a conserved and a hypothetical protein, respectively [7, 13]. A potential NrpRI operator (GGTA-N6-TACC) has been identified in the promoter region of sRNA154 gene implying that this small RNA is under direct control of the global nitrogen regulator NrpRI [7].
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
Strains and plasmids used in this study are listed in Table 1. Plasmid DNA was transformed into E. coli according to the method of Inoue et al. [14] and into M. mazei using liposome-mediated transformation as described recently [8, 15].
Table 1.
Strains and plasmids used in this study.
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
|
| ||
| Methanosarcina mazei strain Gö1 | Wild type | DSM No. 3647 |
| M. mazei Δhpt | M. mazei, with hpt deletion | This study |
| M. mazei ΔsRNA154 | M. mazei, with sRNA154 deletion | This study |
| E. coli DH5α | general cloning strain | Stratagene, La Jolla, US |
| E. coli DH5α/λpir | general cloning strain | [16] |
|
| ||
| Plasmids | ||
|
| ||
| pMCl210 | general cloning vector | [17] |
| pBSK+ | general cloning vector | Stratagene, La Jolla, US |
| pDRIVE | general cloning vector | Qiagen, Hilden, Germany |
| pRS207 | pac-resistance cassette in pSL1180 | [8] |
| pRS269 | pmcr of M. voltae in pDRIVE | This study |
| pRS283 | M. mazei hpt deletion construct in pBSK+ | This study |
| pRS311 | pBSK+ plus M. mazei hpt gene | This study |
| pRS320 | pRS311 with pmcr upstream of hpt | This study |
| pRS345 | pRS311 with pac-resistance cassette | This study |
| pRS606 | pMCL210 with 930 bp of sRNA154 upstream region | This study |
| pRS631 | pMCl210 plus sRNA154 deletion construct | This study |
| pRS632 | Δ154 construct (pRS631) inserted into the ApaI site of pRS345 | This study |
2.2. Growth
M. mazei wild-type and mutant strains were grown in minimal medium under a nitrogen gas atmosphere in 5 or 50 mL closed growth tubes, which were incubated at 37°C without shaking [18, 19]. To screen on 8-ADP, however, the concentration of yeast extract in the minimal medium was reduced from 2 g/L to 0.5 g/L. In general, the medium was supplemented with 150 mM methanol or 25 mM trimethylamine (TMA) and 40 mM acetate as carbon sources and reduced with 2 mM cystein and 1 mM sodium sulfide. For nitrogen limited growth, ammonium was omitted from the media; molecular nitrogen in the gas phase served as sole nitrogen source [19]. In general, the Methanosarcina cultures were supplemented with 100 μg/mL ampicillin to prevent bacterial contamination. For mutant selection, puromycin (5 μg/mL) was added to the medium, for counterselection during markerless exchange the medium was supplemented with 8-ADP (20 μg/mL). Growth was monitored by determining the optical density of the cultures at 600 nm (O.D.600). M. mazei wild-type and mutant strains were grown on solid medium by carefully spreading the cells on 1.5% bottom agar containing 25 mM TMA as carbon source and incubated in an intrachamber incubator under a gas atmosphere consisting of 79.9% N2, 20% CO2, and 0.1% H2S. Mutants were selected by adding 5 μg/mL puromycin or 20 μg/mL 8-ADP (final concentration) to the agar. To identify positive pop-out mutants, single colonies derived in the presence of 8-ADP were streaked in parallel on plates complemented with puromycin and 8-ADP, respectively, to screen for puromycin sensitivity and 8-ADP resistance.
2.3. Construction of Plasmids
All primers used in this study are listed in supplementary Table 1. The plasmid for generating an M. mazei htp null mutant was constructed as follows: the sequences 800 bp down- and upstream of the hpt gene were amplified using chromosomal M. mazei DNA and the primer sets Mm 201 800 up.for/Mm 201 800 up/rev and Mm 201 800 down.for/Mm 201 800 down.rev, respectively. The PCR products obtained contained additional synthetic primer-mediated restriction sites which, for the 800 up stream product included a BamHI at the 5′ end and EcoRI site at the 3′ end and for the 800 downstream fragment an EcoRI site at the 5′ end and KpnI site at the 3′ end. Both fragments were restricted using BamHI/EcoRI and EcoRI/KpnI, respectively, and cloned into pBSK+ (Stratagene, La Jolla, Calif, USA) yielding plasmid pRS283. The allelic exchange vector for the markerless exchange was generated by amplifying the hpt gene from chromosomal M. mazei DNA using the primers Mm hpt for and Mm hpt rev with additional BamH1 and XhoI sites, respectively. The PCR fragment was digested using BamHI and XhoI and ligated to BamHI and XhoI linearized pBSK+ to generate plasmid pRS311. In order to provide the hpt gene of pRS311 with a strong archaeal promoter, the known pmcr promoter of Methanococcus voltae [9] was cloned upstream of the gene. This was achieved by amplifying pmcr with the primers pmcr BamHI and pmcr XhoI using pRS207 [8] as template. The PCR product was cloned into TOPO-TA-cloning vector pDRIVE (Qiagen, Hilden, Germany) yielding plasmid pRS269. Digestion of pRS269 with BamHI resulted in excision of the pmcr promoter that was cloned into the BamHI site located directly upstream of the hpt gene of plasmid pRS311, resulting in pRS320. Finally, the 1.7 kbp EcoRI fragment from pRS204 containing the pac-cassette under the control of the constitutive promoter (pmcr) and terminator (tmcr) from the mcr-gene of M. voltae was cloned into the unique NotI site of pRS320, generating plasmid pRS345. This plasmid was used for markerless allelic exchanges by cloning the desired mutation into its unique ApaI site. To construct the M. mazei sRNA154 deletion mutant, approximately 1000 bp of the upstream-flanking region of the small RNA was amplified using the primer pair Mm s154_1 for and Mm s154_1 rev using genomic M. mazei DNA as template. The PCR product (937 bp) was digested with ApaI and XhoI (restriction sites provided by the primers; see underlined sequences) and ligated into the ApaI/XhoI-opened pMCL210 vector resulting in plasmid pRS606. A 1364 bp PCR product of the downstream region was generated by primers Mm s154_2 for and Mm s154_2 rev introducing an XhoI and a SmaI restriction site, respectively, which was subsequently cloned into an XhoI/SmaI-linearized pRS606, fusing the sRNA154 flanking region together and thereby deleting sRNA154. The plasmid was designated pRS631. The complete deletion construct was excised using ApaI and SmaI, treated with Mung Bean nuclease, and ligated into pRS345, which was linearized with ApaI followed by a Mung Bean nuclease treatment yielding plasmid pRS632. All constructs were verified by sequence analysis.
2.4. PCR Analysis for Mutant Strain Confirmation
Verification of sRNA154 deletion was performed using the primer pair Mm s154_1 and Mm s154_seq rev A ~250 bp product of the bla gene was amplified using the primers bla rev. and bla for. The primer pair pac1 and pac2 was used to generate a ~300 bp product of the pac-cassette. Generally, 2 ng of chromosomal DNA was used as template.
2.5. RNA Preparation and Northern Blot Analysis
Total RNA isolations and Northern blot analyses were performed essentially as described before [7], except that Isol-RNA Lysis Reagent (5 PRIME GmbH, Hamburg, Germany) was used for total RNA preparation.
2.6. Cell Extracts
M. mazei cell extracts were prepared as described previously [20]. 65 μg of M. mazei wild type, Δhpt and ΔsRNA154 crude extracts were separated by 12.5% SDS-PAGE.
3. Results and Discussion
As mentioned above, markerless exchange of alleles originally developed for M. acetivorans was applied for M. mazei using the hpt gene as counterselection marker [10] and successfully generated a null mutant of the gene encoding sRNA154.
3.1. Settingup a Markerless Exchange System for M. mazei
Members of the methanoarchaea become regularly resistant to the purine analog 8-ADP (2.9 × 10−5) [10], possibly by developing spontaneous mutations in the hpt gene that encodes a hypoxanthine phosphoribosyltransferase. Nevertheless, we decided to construct a Δhpt mutant by applying the markerless exchange method of Pritchett et al. [10] rather than screening for a naturally occurring hpt-deficient strain. A pKS bluescript derivative was constructed carrying 800 bp of both the 5′ and 3′ flanking chromosomal region of the M. mazei hpt gene fused together, thereby creating an hpt deletion construct (pRS283). The nonreplicating plasmid pRS283 was transformed into M. mazei* [8], which will be referred to as wild type, and successful integration into the chromosome via a single homologous recombination event was confirmed by the gain of puromycin resistance (Figure S1A). Single colonies were then inoculated into liquid medium containing 20 μg/mL 8-ADP. Cells that carry the wild-type hpt gene on the chromosome are sensitive to 8-ADP unless hpt is obliterated by a pop-out event, removing both the hpt gene and the plasmid backbone (Figure S1B). Unfortunately, the standard minimal medium used for M. mazei [18] cannot be used for this approach as 8-ADP had little effect on growth of the cells (Figure 1(a)).Yeast extract, which is presumably rich in purines and pyrimidines, might affect uptake of 8-ADP. Growth in media with significantly reduced yeast extract (0.5 g/L) clearly demonstrated that 20 μg/mL 8-ADP was inhibitory (Figure 1(b)). As expected, the M. mazei Δhpt grew in the presence of 8-ADP on standard and on yeast-reduced medium (Figures 1(a) and 1(b)). Single colonies of the M. mazei Δhpt mutant strain were obtained by plating on solid medium containing 8-ADP, which were subsequently tested for puromycin sensitivity and simultaneous 8-ADP resistance by streaking on the respective plates. To confirm deletion of hpt, colonies that showed the desired phenotype were subjected to Southern blot analysis (data not shown).
Figure 1.
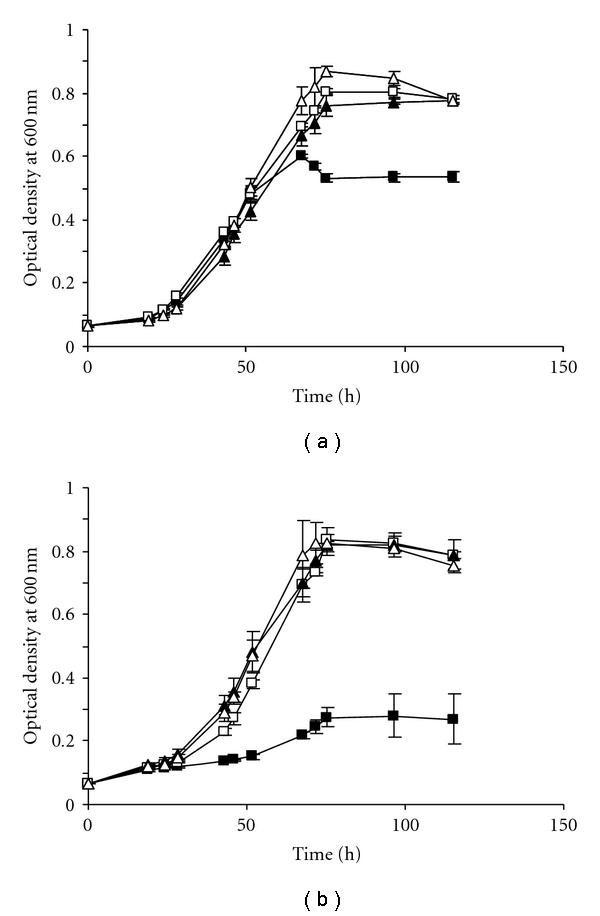
Growth analysis of M. mazei Δhpt versus wild type in the presence of 8-ADP. Two different media were tested, (a) standard minimal medium [18] and (b) minimal medium with significantly reduced yeast extract (0.5 g/L). M. mazei strains were grown in 50 mL of the respective medium complemented with 150 mM methanol as carbon source under a gas atmosphere of N2/CO2 (80 : 20). Strains were incubated without additives (open symbols) or in the presence of 20 μg/mL 8-ADP (closed symbols). Squares: wild type; triangles: M. mazei Δhpt. Standard deviations of three replicates for each strain are indicated.
In a second step, the allelic exchange vector pR345 was constructed containing the bla gene and pac-resistance cassette for selection in E. coli and M. mazei, respectively, as well as the hpt gene as counterselectable marker. To provide the hpt gene with a strong promoter, the native promoter was exchanged with promoter pmcr of M. voltae [9]. The unique ApaI site in pRS345 provided an insertion site for the mutant construct of interest.
3.2. Generation of a M. mazei ΔsRNA154 Chromosomal Mutant
To validate the system, we deleted the gene encoding the small RNA154 which is transcribed exclusively under nitrogen limitation and supposedly plays a central role in nitrogen stress responses [7]. A deletion construct generated by fusing the flanking regions of sRNA154 together was inserted into the ApaI site of the allelic exchange vector pRS345. The resulting plasmid (pRS632) was transformed into the M. mazei Δhpt strain followed by selection for pop-in/pop-out events as described above. Successful deletion of the sRNA154 gene was evaluated by PCR. PCR verification with primers binding up- and downstream of sRNA154 was performed yielding a PCR product of ~1,100 bp for wild type and ~940 bp for M. mazei ΔsRNA154. The PCR product representing the wild type was detected as expected in M. mazei wild type and the diploid strain with plasmid pRS632 inserted into the chromosome (Figure 2(a)). The respective amplicon for ΔsRNA154 was clearly detected in the control (pRS632), in the diploid strain and was very prominent in all eight potential M. mazei ΔsRNA154 mutants analyzed (Figure 2(b)). However, in seven out of the eight putative ΔsRNA154 mutants, traces of PCR products corresponding to the product derived from sRNA154 wild type were also observed. This might be explained by the fact that several archaea have been demonstrated to possess multiple genome copies, as has been recently described by Soppa and coworkers [21]. They showed that M. acetivorans contains up to 17 copies dependent on the growth phase [21]. This polyploidy might result in incomplete allelic exchange with some of the chromosome copies remaining wild type. Since we could only confirm one out of eight mutant candidates, it appears that this difficulty occurs more often than anticipated when generating chromosomal mutants of M. mazei.
Figure 2.
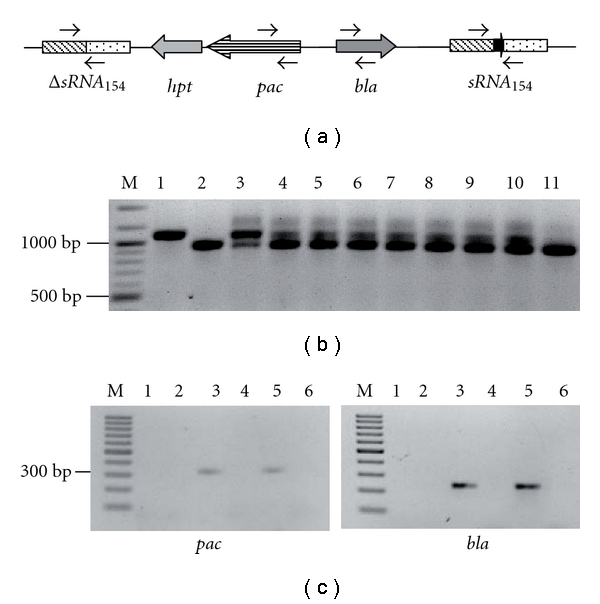
Verification of ΔsRNA154 strains by PCR. (a) Cartoon of plasmid pRS623 integration into the M. mazei chromosome. Primers for PCR verification are shown by arrows (sizes do not correspond to actual proportions). (b) M. mazei wild-type and mutant strains ΔsRNA154, Δhpt, and a diploid strain with pRS632 integrated into the chromosome were tested by PCR analysis for presence of the sRNA154 gene and ΔsRNA154 deletion construct. Lane M, 100 bp marker, Fermentas; lane 1: M. mazei wild type; lane 2: pRS623 carrying ΔsRNA154 deletion; lane 3: diploid strain; lanes 4–11: different ΔsRNA154 candidate strains, whereas only the ΔsRNA154 clone in lane 11 shows the unique genotype and was further analyzed by PCR analysis. (c) M. mazei wild-type and the same mutant strains were analyzed for presence of pRS632 backbone by PCR using primers for amplifying parts of the bla and pac genes, respectively. Lane M, 100 bp marker (Fermentas); lane 1: M. mazei WT; lane 2: Δhpt; lane 3: diploid strain; lane 4: Δ154. lane 5: pRS632; lane 6: water control.
The mutant depicted in lane 11 (Figure 2(b)) showing the ΔsRNA154 PCR product was further examined for plasmid removal. PCR analyses using chromosomal DNA from M. mazei wild type, the Δhpt mutant, the diploid strain, and ΔsRNA154 as well as pRS632 as positive control clearly demonstrated the presence of the bla and pac genes exclusively in the diploid strain and the plasmid control, whereas for ΔsRNA154, both genes were not detectable. As a second line of evidence, Northern blot analyses were performed with total RNA derived from the wild type, Δhpt and ΔsRNA154 strains grown under nitrogen limitation and using a radioactively labelled oligonucleotide probe against sRNA154. Consistent with the previous data, Northern blot analyses clearly demonstrated that sRNA154 with a size of 130 nucleotides (nct) is present in the wild type and Δhpt strains under nitrogen limitation but is not detectable in the sRNA154 deletion strain, further confirming successful markerless allelic exchange (Figure 3). By generating this ΔhptΔsRNA154 mutant, which will be referred to as ΔsRNA154 strain, we have effectively established the markerless exchange system in M. mazei.
Figure 3.
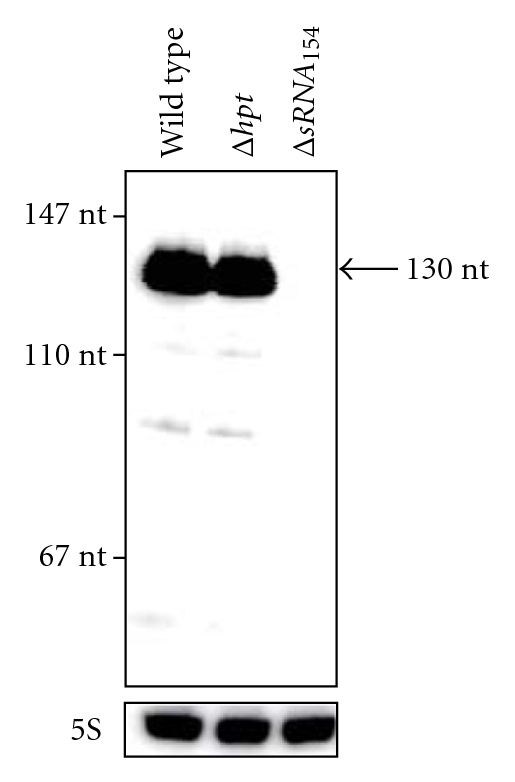
Northern blot analysis of M. mazei ΔsRNA154 strain. Total RNA was purified from the respective M. mazei strains (ΔsRNA154, Δhpt, and wild type) all grown under nitrogen limiting conditions. 10 μg of each RNA were separated by a denaturing 6% PA gel and subsequently analyzed by Northern blot using a 32P-ATP-labelled oligonucleotide homologous to sRNA154. For each sample, the abundance of 5S rRNA was determined to exclude variations in RNA amounts.
3.3. Characterization of the ΔsRNA154 Mutant Strain
To analyze the functional role of sRNA154 in nitrogen metabolism, we characterized the M. mazei ΔsRNA154 mutant growing under conditions of nitrogen limitation, in which the sRNA is strongly expressed. Growth analyses demonstrated reduced growth of M. mazei ΔsRNA154 with a growth rate of μ = 0.02 h−1 compared to μ = 0.03 h−1 obtained for the wild type (Figure 4(a)). Nevertheless, ΔsRNA154 did not reach the same final cell densities as the wild type. Negative effects on nitrogen fixation due to the absence of the hpt gene were excluded by analysing growth behaviour of the parental strain (M. mazei Δhpt) (Figure 4(a)). As expected, no different growth phenotype of these three M. mazei strains was observed under nitrogen sufficiency as under this condition the sRNA154 is not transcribed (Figure 4(b)).
Figure 4.
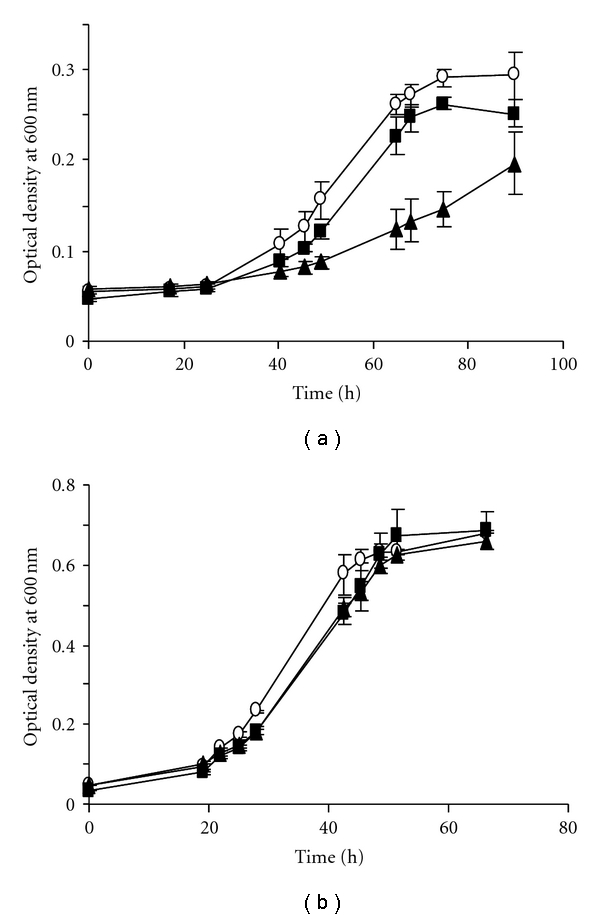
Growth analysis of ΔsRNA154 versus wild type under nitrogen limitation (a) and under nitrogen sufficiency: (b) M. mazei strains were grown in 50 mL liquid minimal medium complemented with 150 mM methanol as carbon source under nitrogen limiting conditions (a) and with 10 mM NH4 + as nitrogen source (b) under a gas atmosphere of N2/CO2 (80 : 20). Open circles: wild type; closed squares: M. mazei Δhpt; closed triangles: M. mazei ΔsRNA154. Standard deviations of five replicates for each strain are indicated.
Characterizing the protein expression patterns of ΔsRNA154 under nitrogen limitation and nitrogen sufficiency by one-dimensional SDS-PAGE clearly demonstrated differences in the protein patterns only under nitrogen depletion (Figure 5). At least three different proteins were differentially synthesized under nitrogen limitation in the absence of sRNA154 in comparison to the wild type (Figure 5(a)). Two proteins (1 and 2) with the molecular mass of approximately 66 and 40 kDa were exclusively or significantly more strongly expressed in the mutant, whereas a 35 kDa protein (3) was present in the wild type but appears to be absent in the mutant. These findings indicate that sRNA154 controls the protein expression either directly or indirectly and again strongly support a prominent function of the sRNA154 in nitrogen regulation.
Figure 5.
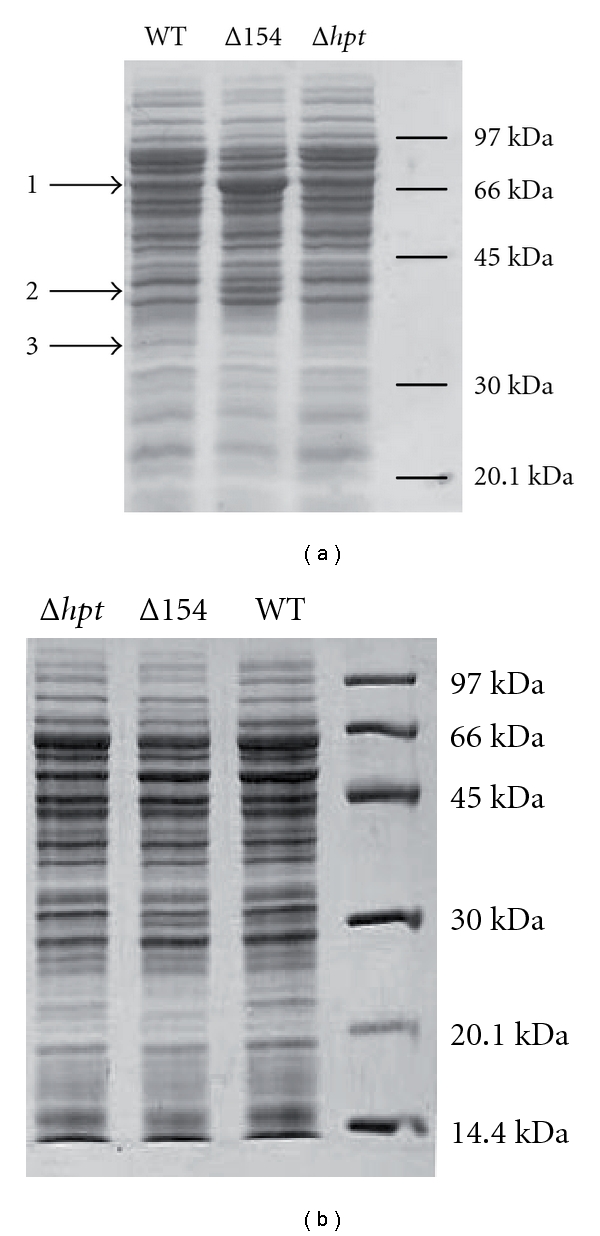
Analysis of protein expression pattern of ΔsRNA154 versus wild type (a) under nitrogen limitation and (b) under nitrogen sufficiency. The M. mazei strains ΔsRNA154, Δhpt, and wild type were grown in 50 mL medium under standard nitrogen-limiting conditions or under nitrogen sufficiency (see Figure 4). Cells were harvested in exponentially phase. Equal amounts of cell extracts (65 μg) were applied to a 12% SDS-PA gel, which was subsequently stained with Coomassie blue. Arrows 1–3 indicate protein bands with differential expression in M. mazei ΔsRNA154 and wild-type strains. Marker: LMW marker GE healthcare.
The ΔsRNA154 mutant represents the first chromosomal deletion mutant of a small RNA in M. mazei. As it is only transcribed under nitrogen fixing conditions, presumably under the control of the global nitrogen regulator NrpRI [7], we suggest that sRNA154 plays a central role in regulation of nitrogen metabolism. The differences in the cytoplasmic protein patterns that result in reduced growth of ΔsRNA154 under nitrogen fixing conditions argue for a prominent role of sRNA154 in regulation of nitrogen fixation. Posttranscriptional regulation by sRNA154 would add another level of regulation of nitrogen metabolism in M. mazei possibly resulting in tighter control or fine tuning of translation of the target mRNAs.
4. Conclusion
By generating a Δhpt strain and a plasmid for allelic replacements, we successfully applied the markerless exchange system to M. mazei. The method was further optimized by using medium with reduced yeast extract, thereby enhancing the toxic effect of 8-ADP during counterselection. Generation of ΔsRNA154 revealed the role of sRNA154 in nitrogen metabolism as demonstrated by reduced growth as well as differential synthesis of at least three proteins under nitrogen fixing conditions in the absence of sRNA154.
Acknowledgments
This paper was financially supported by the Deutsche Forschungsgemeinschaft (DFG) as part of the priority program SPP 1258 “Sensorische und regulatorische RNAs in Prokaryoten.”
References
- 1.Rogers JE, Whitman WB, editors. Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides and Halomethanes. Washington, DC, USA: ASM Press; 1991. [Google Scholar]
- 2.Ehlers C, Grabbe R, Veit K, Schmitz RA. Characterization of GlnK1 from Methanosarcina mazei strain Gö1: Complementation of an Escherichia coli glnk mutant strain by G1nK1. Journal of Bacteriology. 2002;184(4):1028–1040. doi: 10.1128/jb.184.4.1028-1040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflüger K, Ehrenreich A, Salmon K, et al. Identification of genes involved in salt adaptation in the archaeon Methanosarcina mazei Gö1 using genome-wide gene expression profiling. FEMS Microbiology Letters. 2007;277(1):79–89. doi: 10.1111/j.1574-6968.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Deppenmeier U, Muller V. Life close to the thermodynamic limit: how methanogenic archaea conserve energy. Results & Problems in Cell Differentiation. 2008;45:123–152. doi: 10.1007/400_2006_026. [DOI] [PubMed] [Google Scholar]
- 5.Hovey R, Lentes S, Ehrenreich A, et al. DNA microarray analysis of Methanosarcina mazei Gö1 reveals adaptation to different methanogenic substrates. Molecular Genetics and Genomics. 2005;273(3):225–239. doi: 10.1007/s00438-005-1126-9. [DOI] [PubMed] [Google Scholar]
- 6.Grüber G, Svergun DI, Coskun U, et al. Structural insights into the A1 ATPase from the archaeon, Methanosarcina mazei Gö1. Biochemistry. 2001;40(7):1890–1896. doi: 10.1021/bi002195t. [DOI] [PubMed] [Google Scholar]
- 7.Jäger D, Sharma CM, Thomsen J, Ehlers C, Vogel J, Schmitz RA. Deep sequencing analysis of the Methanosarcina mazei Gö1 transcriptome in response to nitrogen availability. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(51):21878–21882. doi: 10.1073/pnas.0909051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlers C, Weidenbach K, Veit K, Deppenmeier U, Metcalf WW, Schmitz RA. Development of genetic methods and construction of a chromosomal glnK 1 mutant in Methanosarcina mazei strain Gö1. Molecular Genetics and Genomics. 2005;273(4):290–298. doi: 10.1007/s00438-005-1128-7. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf WW, Zhang JK, Apolinario E, Sowers KR, Wolfe RS. A genetic system for Archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2626–2631. doi: 10.1073/pnas.94.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett MA, Zhang JK, Metcalf WW. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Applied and Environmental Microbiology. 2004;70(3):1425–1433. doi: 10.1128/AEM.70.3.1425-1433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weidenbach K, Ehlers C, Kock J, Ehrenreich A, Schmitz RA. Insights into the NrpR regulon in Methanosarcina mazei Gö1. Archives of Microbiology. 2008;190(3):319–332. doi: 10.1007/s00203-008-0369-3. [DOI] [PubMed] [Google Scholar]
- 12.Weidenbach K, Ehlers C, Kock J, Schmitz RA. NrpRII mediates contacts between NrpRI and general transcription factors in the archaeon Methanosarcina mazei Gö1. FEBS Journal. 2010;277(21):4398–4411. doi: 10.1111/j.1742-4658.2010.07821.x. [DOI] [PubMed] [Google Scholar]
- 13.Deppenmeier U, Johann A, Hartsch T, et al. The genome of Methanosarcina mazei: evidence for lateral gene transfer between bacteria and archaea. Journal of Molecular Microbiology and Biotechnology. 2002;4(4):453–461. [PubMed] [Google Scholar]
- 14.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96(1):23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 15.Weidenbach K, Glöer J, Ehlers C, Sandman K, Reeve JN, Schmitz RA. Deletion of the archaeal histone in Methanosarcina mazei Gö1 results in reduced growth and genomic transcription. Molecular Microbiology. 2008;67(3):662–671. doi: 10.1111/j.1365-2958.2007.06076.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR . Journal of Bacteriology. 1988;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano Y, Yoshida Y, Yamashita Y, Koga T. Construction of a series of pACYC-derived plasmid vectors. Gene. 1995;162(1):157–158. doi: 10.1016/0378-1119(95)00320-6. [DOI] [PubMed] [Google Scholar]
- 18.Deppenmeier U, Blaut M, Mahlmann A, Gottschalk G. Reduced coenzyme F420: heterodisulfide oxidoreductase, a proton-translocating redox system in methanogenic bacteria. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(23):9449–9453. doi: 10.1073/pnas.87.23.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlers C, Veit K, Gottschalk G, Schmitz RA. Functional organization of a single nif cluster in the mesophilic archaeon Methanosarcina mazei strain Gö1. Archaea. 2002;1(2):143–150. doi: 10.1155/2002/362813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers C, Weidenbach K, Veit K, Forchhammer K, Schmitz RA. Unique mechanistic features of post-translational regulation of glutamine synthetase activity in Methanosarcina mazei strain Gö1 in response to nitrogen availability. Molecular Microbiology. 2005;55(6):1841–1854. doi: 10.1111/j.1365-2958.2005.04511.x. [DOI] [PubMed] [Google Scholar]
- 21.Hildenbrand C, Stock T, Lange C, Rother M, Soppa J. Genome copy numbers and gene conversion in methanogenic archaea. The Journal of Bacteriology. 2011;193(3):734–743. doi: 10.1128/JB.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


