Abstract
In psychophysical experiments, humans use different verbal responses to pruritic and algesic chemical stimuli to indicate the different qualities of sensation they feel. A major challenge for behavioral models in the mouse of chemical itch and pain in humans is to devise experimental protocols that provide the opportunity for the animal to exhibit a multiplicity of responses as well. One basic criterion is that chemicals that evoke primarily itch or pain in humans should elicit different types of responses when applied in the same way to the mouse. Meeting this criterion is complicated by the fact that the type of behavioral responses exhibited by the mouse depends in part on the site of chemical application such as the nape of the neck which evokes only scratching with the hind paw vs. the hind limb which elicits licking and biting. Here, we review to what extent mice behaviorally differentiate chemicals that elicit itch vs. pain in humans.
Introduction
The ideal animal model of stimulus evoked itch or pain in humans is one wherein the animal's responses are analogous to our own. For such a model to be valid there are some basic criteria to be met. First, the animal should be capable of detecting the stimuli in question. Second, the animal should exhibit differential behaviors that distinguish between stimuli that elicit predominantly itch from those that are perceived as primarily nociceptive (or even overtly painful) and do not elicit itch. In this brief review we discuss the applicability of these criteria to the behavior of the mouse – a species commonly used in molecular, cellular and genetic studies of sensory function. We limit our discussion to behaviors directed toward the site of a chemical acutely applied to the skin. The basic question at hand is whether these “site-directed behaviors” differ in relation to whether the chemical evokes predominantly itch or nociceptive sensations in humans.
In humans, a pruritic (itchy) stimulus elicits two types of response, one related to the sensations such as a verbal report (“I have a weak itch”) and the other, a reaction to the sensation, such as a feeling of discomfort and behavior directed toward the stimulus site to reduce or eliminate the sensation and the source of irritation (e.g. scratching). Thus, itch is often defined as a sensation that is unpleasant and evokes the desire to scratch.
An algesic (nociceptive) stimulus also elicits two types of responses, one type related to sensation (“I feel a strong burning sensation”) and the other a set of reactions to the sensation including feelings of discomfort (“it hurts”) and responses directed towards eliminating it. Thus, pain, like itch, is an unpleasant sensation that can evoke site-directed behaviors. For example, these behaviors in animals or humans might be withdrawal, rubbing, shaking and licking/biting of the affected site that, analogous to the responses to itch, are aimed at alleviating or eliminating the effects of the stimulus and/or the ongoing irritation or discomfort.
The disadvantage of using site-directed behaviors as endpoints for sensory events is that they generate additional sensory input that may alter the perceptual experience and thus the behavioral responses the experimenter wishes to measure. For example, rubbing or scratching the site of a painful or itchy stimulus on the skin might alter the ongoing responses of pruriceptive, mechanosensitive peripheral nociceptors to a chemical pruritic or algesic stimulus, and activate low-threshold or nociceptive mechanoreceptors that might exert a segmental afferent inhibition (e.g.1) of the central transmission of itch or pain in the spinal or medullary dorsal horn (2). Thus, the temporal profile of a chemically evoked itch, measured psychophysically in humans who have been instructed not to touch the skin, should not necessarily match the time course of site-directed behaviors such as scratching in animals (or for that matter in humans). But the incidence of different behaviors and the magnitude and duration of behavior might provide information about what amounts of the chemical an animal can detect, the intensity of the sensation and whether the type of behavior differs according to the qualities of sensation evoked in humans.
Psychophysical studies: Sensory qualities elicited by chemicals in humans
When injected intradermally into the volar forearm of human subjects, histamine evokes primarily sensations of itch and not pain (3, 4) whereas capsaicin evokes pain and no reports of itch (5). But in these studies, the definition of “pain” was restricted to those sensations that “hurt”; and, in the capsaicin study, subjects were not asked to judge the intensity of any itch they may have felt. It is well known that some chemical stimuli applied to the skin can evoke nociceptive sensations such as pricking/stinging or burning that are not rated as painful, i.e. they do not hurt (6, 7). Thus, in the earlier histamine and capsaicin injection experiments subjects were asked to focus on a single category of response. That is, they were asked:” is there itch (or pain) and, if so how much”.
In a more recent study (8), subjects were asked to rate the relative magnitude of itch, and the nociceptive sensations of pricking/stinging, and burning every 30 sec in response to different amounts of intradermally injected capsaicin or histamine using the same scale of magnitude, called the “generalized labeled magnitude scale”. The subjects were asked to judge the perceived intensity of each of the nociceptive sensory qualities independently of whether or not it “hurts”.
The area under the function relating perceived intensity to time was plotted for itch and for pricking/stinging (Fig. 1A). In this scattergram, the histamine responses are near the ordinate indicating that histamine produced predominantly itch but an itch that was typically accompanied by burning (and also some pricking/stinging for which the data are not shown). That is, like other pruritic stimuli we have applied in psychophysical studies in humans, including native cowhage (9) and heat-inactivated spicules soaked in either mucunain (10), cathepsin S (11), the peptide BAM8-22 (12), histamine, or capsaicin (13), the sensation of itch was rarely a pure sensory quality but more typically a dominant itch with lesser sensations of pricking/stinging and burning.
Figure 1.
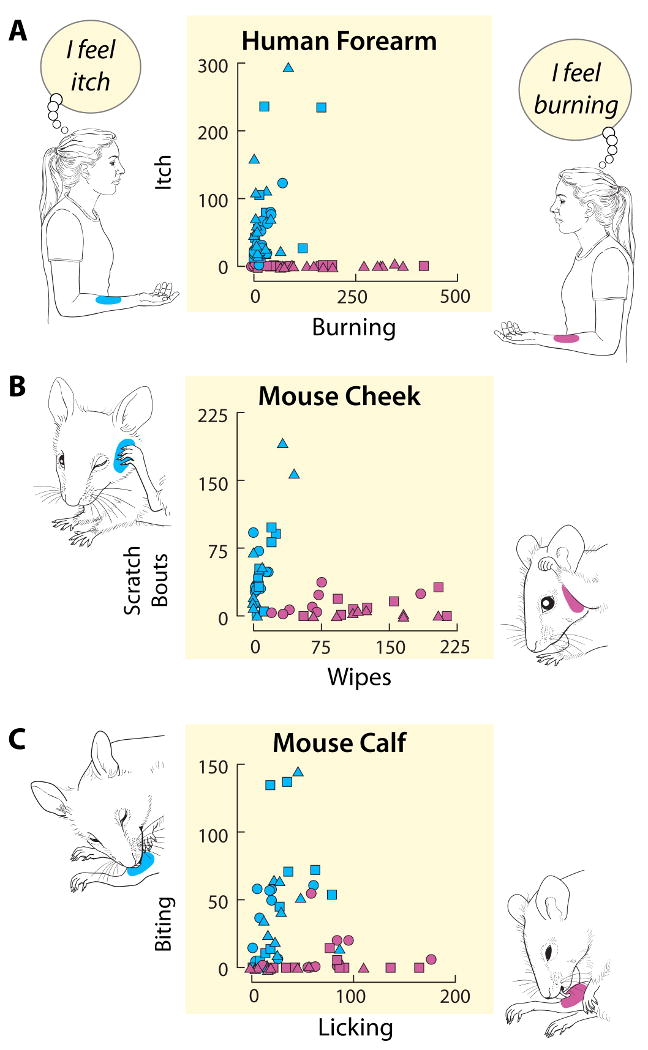
Itch and nociceptive sensations in humans and site-directed behaviors in mice evoked by intradermal injections of capsaicin and histamine. The different symbols represent different doses of histamine (Teal-blue) and capsaicin (Pink). The drawings and adjacent labels of the axes of each graph indicate the dominant qualities of sensation felt by human subjects (A) and the dominant site-directed behaviors exhibited by the mouse evoked by each chemical injected into the specified site on the skin (forearm for humans and either cheek or calf for the mouse). A: The magnitude of itch plotted against the magnitude of burning reported by humans in response to injections in the forearm. Each data point is from one experiment from one of 15 subjects and is the area under the rating curve (intensity vs. time) for itch plotted against the area obtained for burning (similar data for pricking/stinging not shown). For histamine and capsaicin: squares = 0.1 μg in 10 μl, circles = 1.0, triangles = 10. B: The amount of scratching and wiping directed toward the site of a histamine or capsaicin injection into the cheek of the mouse. Each point represents the number of wipes plotted against the number of scratching bouts observed in one mouse for the 20 min following the injection (8 mice at each dose, except n = 10 for histamine = 10 μg) Histamine: squares = 10 μg in 10 μl, circles = 20, triangles = 50; capsaicin: squares = 1 μg in 10 μl, circles = 10, triangles = 40 (modified from Fig. 5, Shimada and LaMotte 2008 (27). C: Total time mice spent licking vs. biting the site of an injection of histamine or capsaicin into the calf of the hind leg. Each data point represents the cumulative amount of time spent biting and licking observed for one mouse for the period of 30 min after it received a particular dose of either capsaicin or histamine. Data for 10 mice at each dose are shown. Histamine: squares = 10 μg in 10 μl, circles = 20, triangles = 50; capsaicin: squares = 4 μg in 10 μl, circles = 10, triangles = 40. All animals were tested under protocols approved by the Institutional Animal Care and Use Committee, and all humans tested with informed consent and protocols approved by the Human Investigation Committee, at Yale University School of Medicine.
In contrast to the effects of histamine, the responses to intradermal injection of capsaicin (Fig. 1A) are clustered nearer the abscissa indicating that capsaicin elicited predominantly nociceptive sensations of burning (also pricking/stinging but these data are not shown). However, capsaicin can produce significant itch if applied topically by soaked filter paper (14) or by capsaicin-soaked, inactivated cowhage spicules (13). Thus, the quality of a chemically evoked sensation may depend in part on how the chemical is delivered to the skin.
Do mice respond to the chemical stimuli that produce itch (or pain) in humans?
The answer to this question depends on many factors, such as the nature of the sensory experience in humans, and genetic differences not only between humans but also between humans and mice. For example, chloroquine, an anti-malarial drug given orally, can elicit an intense, sometimes debilitating itch with pricking/stinging in darker skinned people but rarely in those with lighter skin (15). Though itch is not reported for intradermal injections in dark skinned people, chloroquine can elicit site-directed scratching in mice depending to some degree on the strain of mouse tested (16). Other pruritogens injected intradermally, including histamine, differ in the degree of scratching elicited in different strains of mice (17). Moreover, there are pruritogens such as serotonin that are highly effective in eliciting scratching in some strains of mice (18-20) but produce at best a weak itch in humans (21) and may do so partly by releasing histamine from mast cells (22). These observations underscore the importance of studying chemicals both in animals and in humans. Additionally, it is critical to test novel pruritic agents in humans and/or in animals following pretreatment with an antihistamine and/or in mast cell-deficient mice (SASH mice) to rule out the involvement of histamine that may be released by these chemicals from degranulating mast cells, as was recently reported with chloroquine (23).
Do mice differentiate stimuli that evoke itch or pain in humans?
Different types of site-directed behavior are typically video recorded for subsequent analysis by human observers who are blinded to the condition of the experiment. (Although there are automated systems that can be used to detect different types of responses such as scratching (24, 25) they generally have the inability to determine the location on the skin to which a response is directed).
Mouse models of chemical cutaneous itch and pain can be classified according to the site-directed behaviors measured each with advantages and disadvantages for the experimenter (summarized in the following and in Table 1). Most models have used only one type of behavior such as hind-limb scratching directed toward an injection into the nape of the neck or either licking or biting directed toward an injection into the hind paw. The use of a single category of response to a stimulus is relatively easy to assess but open to multiple interpretations. For example, for a nape injection in the ddY strain of mouse, site-directed scratching was interpreted as itch whereas the lack of response was interpreted as the absence of itch (26). But the absence of response could mean the presence of pain or some other sensory quality or a failure to detect the stimulus. On the other hand, in the ICR or genetically similar CD1 mouse, site-directed scratching with the hind-limb occurs both to a painful (capsaicin) and itchy (histamine) chemical injected into the nape (27). In fact, for the nape, this is the only type of site-directed behavior that is possible. Thus, it is not clear whether prior findings of our group and others based on nape scratching alone indicate the presence of itch and/or nociceptive sensations in response to such candidate pruritogens as SLIGRL (28), substance P, compound 48/80, serotonin, thromboxane A2 (26, 29, 30) and BAM8-22 (23).
Table 1. Site-directed behaviors used to assess cutaneous, chemical itch and pain in the mouse.
| Mouse Model (site-directed behaviors used) | Advantages | Disadvantages | Exemplary references |
|---|---|---|---|
| Scratching of back of neck (one category of response) | Easy for experimenter To measure scratching behavior | Cannot distinguish itch-from pain-associated behavior; Some “spontaneous” scratching exists in absence of stimulus | 16, 17, 33, 38, 39, 45-50 |
| Biting, licking, or flinching of hind paw; (one or more behaviors taken as one category of response | Easy to measure | Cannot distinguish itch-from pain-associated behavior | 40, 41, 51, 52, 53 |
| Wiping vs. scratching of cheek (two categories of response) | Easy to distinguish between forelimb wiping and hind limb scratching (i.e. easy to separate itch- from pain-associated behaviors | Itch and pain mechanisms might differ for face and body; few human comparative studies for the face | 19, 27, 35, 36, 37, 42, 54, 55, 56, 57 |
| Biting vs. licking of hind paw (two categories of response) | Can distinguish itch-from pain-associated behaviors | Discrimination of behaviors is labor intensive; Much spontaneous biting/licking of foot | 43, 44 |
| Licking vs. biting of calf of hind limb (two categories of Response) | Can distinguish itch- from pain- associated behaviors; no spontaneous biting or licking of calf | Discrimination of behaviors is labor intensive | Data in present article |
| Automated Systems | Easy to identify different behaviors | Cannot identify sites of directed behaviors. | 24, 25 |
Because humans often report multiple sensory qualities in response to a pruritic stimulus, an alternate approach to using a single category of mouse behavior is to inject a mouse at a locus where multiple site-directed behaviors are both possible (unlike the nape) and then separately measured. For example, the cheek provides two or more different site directed behaviors: In response to different amounts of chemical intradermally injected, histamine evoked primarily scratching with the hind limb and capsaicin elicited mostly wiping with the forepaw (27). As with human responses, a scattergram is useful in comparing the amount of each type of behavior evoked by the two chemical stimuli (Fig. 1B). The responses to capsaicin fall along the abscissa indicating that mice mainly wiped, a nociceptive response, while the responses to histamine are more mixed but are aligned closer to the ordinate, indicating somewhat more itching than nociception. Thus, there was relatively little scratching to capsaicin just as there was mainly pricking/stinging and burning and essentially no itch in humans. Histamine elicited more of a mixture of responses in the mouse just as itch in humans was often associated with nociceptive sensations.
Opioid agonists can produce itch, and opioid antagonists can block many types of itch, for example, experimentally induced histamine itch (31), cholestatic itch (32), mouse dry skin model (33), and the spontaneous itch produced in NC mice (34). Akiyama et al. (35) have provided additional confirmation of the cheek model by showing that pruritic responses are blocked by naltrexone: scratching of the cheek induced by i.d. injection of histamine and the PAR2 agonist peptide, SLIGRL-NH2, was nearly abolished, without any effect on the wiping caused by these pruritogens or by the algogen, capsaicin. On the other hand, morphine blocked the wiping evoked by capsaicin, but not the scratching induced by either histamine or SLIGRL-NH2. These results are consistent with the notion of a correspondence between scratching and wiping in mice and itch and nociceptive sensations, respectively, in humans.
Studies of other chemical stimuli injected into the cheek of the mouse also provide some evidence for differential site-directed behaviors. For example, histamine, imiquimod, PAR2 & PAR4 activating peptides, formalin and 5-HT elicit mainly scratching of the injected site indicating itch (19, 27, 35-37) whereas, capsaicin, bradykinin and AITC (mustard oil) produced predominant wiping of the face indicating nociceptive sensations (19, 27, 35). Other pruritic chemicals, like chloroquine, and cowhage spicules evoked both scratching and rubbing of the face indicating a mixture of itch and nociceptive sensations after the application of these stimuli (35, 37). Thus, the “cheek model” allows the animal to report differential responses to the application of a stimulus similar to the multiple choices available to humans. This could be advantageous in evaluating whether candidate therapeutic drugs applied to mice will be selective for blocking itch or pain in humans. The cheek model might also be useful in determining whether an agonist selective for a specific isoform of a receptor elicits one type of site-directed behavior rather than a mixture of behaviors that might be evoked by a less selective chemical that activates multiple isoforms. For example, scratching the site of a histamine injection (38) or an allergic contact dermatitis (39) on the rostral back of the mouse was reduced but not eliminated by either an H1 or an H4 antagonist. If the experiment were repeated on the cheek it might be possible to determine whether the reduction produced by each antagonist was more related to pain, to itch or to both.
Another locus on the body to which multiple behaviors can be directed, one commonly used in behavioral studies in the mouse, is the hind paw. Aside from flinching or shaking of the paw, biting and licking appear to be the commonly reported behaviors directed toward the cutaneous site of an injected chemical that evokes itch or nociceptive sensations in humans. For some studies the two behaviors are treated together as one category of response (e.g. 40, 41). In many others, the frequency of only one type of behavior is reported without reporting any measurements of the other but interpreted in terms of the particular type of sensory behavior under investigation. For example, licking has often been interpreted as nociceptive in response to putative algogens, such as capsaicin or formalin (40), but also accepted as indicating the presence of itch in response to candidate pruritogens, such as α-Me-5-HT and ET-1, because the behavior was more prolonged than the licking reported to occur in response to the putative algogens, capsaicin and bradykinin (42). However, in humans, chemical stimuli will sometimes elicit itch of short duration or nociceptive sensations of long duration depending, for example, not only on the type of chemical but the dose as well. Thus, with only one type of behavioral response in an animal, it is difficult to make any specific hypotheses about the corresponding quality of sensation. In a study in which biting and licking behaviors were separately measured (43) serotonin (5-HT) evoked site-directed biting and licking when injected into the hind paw. But only the biting was suppressed in response to either naloxone or a 5-HT antagonist, suggesting that the biting was a pruritic response. In contrast, an injection of formalin into the hind paw produced mainly licking with a little biting. The results seem consistent with the interpretation that licking is mainly associated with pain and biting is associated with itch. In a recent study, Akiyama et al. (44) observed licking and biting as separated variables in a mouse model of dry skin produced by topical acetone applied to the hind paw. They found that spontaneous biting was greater on the acetone-treated vs. the control-treated paw whereas there was no significant difference in the high level of licking observed for both paws. The biting was reduced by the opioid antagonist, naltrexone, but not altered by morphine whereas the licking was unaffected. It was suggested that the biting indicated itch, but that the licking represented normal grooming behavior, not pain.
In a recent study in our laboratory, behavior directed toward the hind paw was videotaped using a high-definition camcorder and played back on a 46″ high-definition television. It was determined that when the recording was played back in real time, biting and licking are indistinguishable. But when played back in slow motion, at ¼ normal speed, licking could be identified as a series of long-stroke head bobs that occurred at about 4 Hz. Occasionally, the tongue was observed being dragged across the skin. In contrast, biting was characterized by little movement of the head, but rather gnawing-like mandible movements near 15 Hz, interspersed with an occasional head “jerk”, possibly for the purpose of wiping away any debris generated by the biting or, alternatively, scratching with the teeth. A drawback in choosing the hind paw as the locus of an injection was that, in the absence of any stimulus, there was ongoing biting and licking due to grooming/cleaning of the hind paw after its use in scratching other body parts, thereby providing a noisy base level of “spontaneous” site-directed behaviors. In addition, it was determined, in confirmation of previously reported observations (42) that there were rather weak responses to an intraplantar injection of histamine possibly because any itch that occurred was relieved by the constant mechanical stimulation of the paw produced by walking and standing on the bedding.
Instead, an alternate site, the calf, was chosen as the site of an injection of different doses of histamine or capsaicin. This area normally receives little or no incidental behaviors such as the grooming that is often directed toward the hind paw (e.g. 44). Also, the experimenter can discriminate licking from biting more easily on the calf than on the hind paw because the former area is larger and evokes longer strokes of licking.
Site-directed behaviors to the calf are illustrated in Fig. 1C. These behaviors differed according to which chemical was intradermally injected. Capsaicin produced mainly licking whereas histamine elicited more of a mixture of responses with more biting than licking for most animals. If we assume that licking is analogous to wiping of the cheek or rubbing of an area that burns or stings by humans, then this behavior is indicative of one or more nociceptive sensations. Similarly, we can think of biting as analogous to scratching with the hind limb and thus, indicative of a pruritic sensation. In fact, perhaps the mice are actually scratching the skin by scraping with their incisors rather than actually biting. Given these assumptions, the responses of the mouse to injections into the calf or the cheek (Fig. 1B and C) correspond well with the sensory responses of humans (Fig. 1A). For both species, capsaicin injection almost always produced a nociceptive response and histamine a mixture of mainly pruritic but also nociceptive responses.
Conclusion
We examined the correspondence between site-directed behaviors in the mouse and the qualities of sensations that humans report when a chemical irritant is applied to the skin. The assumption is that for mouse behavior to model human sensation, the mouse should be able to detect and behaviorally differentiate chemical stimuli that evoke itch vs. pain in humans. There are some caveats. The site-directed behavior that is measured in the mouse is not a direct measure of sensation but rather a reaction to the sensation; it can also modify the sensation. Furthermore, the type of behavior and the number of different behaviors that occur will depend on the site of chemical application. For example, when intradermally injected into the nape of the neck, capsaicin (nociceptive not itchy) and histamine (itchy but with lesser nociceptive sensations) evokes the same response: scratching with the hind paw. In contrast, when applied to the cheek or to the calf, the two stimuli are behaviorally differentiated in a manner corresponding to the sensory responses in humans: Wiping with the forepaw (cheek) or licking (calf) is the major response to capsaicin whereas scratching with the hind paw with some wiping with the forepaw (cheek) and biting accompanied by some licking (calf) occur in response to histamine.
The incidence and magnitude of spontaneous or stimulus evoked site-directed behaviors are potentially useful in mouse models of chronic itch and pain. For example, in a mouse model of itch from dry skin produced by repeated applications of acetone on the hind paw, increased site-directed biting predominated over licking (44). In the future, comparisons of psychophysical measurements of itch and nociceptive sensations in humans with appropriate site-directed behaviors in mice may be useful in a variety of mouse models of chronic itch and pain in humans.
Acknowledgments
Our research was supported by NIH grants NS014624 and NS047399 (R. LaMotte).
The Yale University Human Investigation Committee (HIC) approved the human studies protocol HIC#12780, titled “Psychophysical studies of histamine-induced itch” on December 14th, 2010 and the Yale University Institutional Animal Care and Use Committee (IACUC) last re-approved the animal protocol #7880 titled “Pain adaptation and hyperalgesia: The effects of chronic compression and inflammation of the dorsal root ganglia” on May 16th, 2011. We state that our research as described in this article is in compliance with the ethical policies of Experimental Dermatology which in turn have been adapted (with permission) from the guidelines published by Blackwell Publishing Ltd and the guidelines adopted by the British Medical Association, based in turn on the Committee on Publication Ethics (COPE) guidelines on good publication, and in compliance with their Code of Conduct.
R. LaMotte and S. Shimada designed the research study, S. Shimada and P. Sikand performed the research and analyzed the data, and R. LaMotte, S. Shimada and P. Sikand wrote the paper.
Footnotes
The authors each state that they have no conflicts of interest to declare.
References
- 1.Sandkuhler J. The organization and function of endogenous antinociceptive systems. Prog Neurobiol. 1996;50:49–81. doi: 10.1016/0301-0082(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 2.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atanassoff PG, Brull SJ, Zhang J, Greenquist K, Silverman DG, Lamotte RH. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res. 1999;16:291–298. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- 4.Simone DA, Ngeow JY, Whitehouse J, Becerra-Cabal L, Putterman GJ, LaMotte RH. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosens Res. 1987;5:81–92. doi: 10.3109/07367228709144620. [DOI] [PubMed] [Google Scholar]
- 5.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 6.Green BG, Flammer LJ. Capsaicin as a cutaneous stimulus: sensitivity and sensory qualities on hairy skin. Chem Senses. 1988;13:367–384. [Google Scholar]
- 7.Green BG, Schoen KL. Thermal and nociceptive sensations from menthol and their suppression by dynamic contact. Behav Brain Res. 2007;176:284–291. doi: 10.1016/j.bbr.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011 doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101:1430–1443. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy VB, Shimada SG, Sikand P, LaMotte RH, Lerner EA. Cathepsin S elicits itch and signals via protease-activated receptors. J Invest Dermatol. 2010;130:1468–1470. doi: 10.1038/jid.2009.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikand P, Dong X, LaMotte RH. BAM8-22 Peptide Produces Itch and Nociceptive Sensations in Humans Independent of Histamine Release. J Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green BG. Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept Psychophys. 1990;48:12–18. doi: 10.3758/bf03205007. [DOI] [PubMed] [Google Scholar]
- 15.Bussaratid V, Walsh DS, Wilairatana P, Krudsood S, Silachamroon U, Looareesuwan S. Frequency of pruritus in Plasmodium vivax malaria patients treated with chloroquine in Thailand. Trop Doct. 2000;30:211–214. doi: 10.1177/004947550003000410. [DOI] [PubMed] [Google Scholar]
- 16.Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Inagaki N, Nagao M, Igeta K, Kawasaki H, Kim JF, Nagai H. Scratching behavior in various strains of mice. Skin Pharmacol Appl Skin Physiol. 2001;14:87–96. doi: 10.1159/000056338. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther. 2009;329:945–951. doi: 10.1124/jpet.109.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol. 2010;104:2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 22.Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res. 1997;46:412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki N, Igeta K, Kim JF, et al. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. Eur J Pharmacol. 2002;448:175–183. doi: 10.1016/s0014-2999(02)01933-7. [DOI] [PubMed] [Google Scholar]
- 25.Elliott GR, Vanwersch RA, Bruijnzeel PL. An automated method for registering and quantifying scratching activity in mice: use for drug evaluation. J Pharmacol Toxicol Methods. 2000;44:453–459. doi: 10.1016/s1056-8719(01)00111-3. [DOI] [PubMed] [Google Scholar]
- 26.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 27.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 30.Andoh T, Nishikawa Y, Yamaguchi-Miyamoto T, Nojima H, Narumiya S, Kuraishi Y. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J Invest Dermatol. 2007;127:2042–2047. doi: 10.1038/sj.jid.5700810. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein JE, Swift RM, Soltani K, Lorincz AL. Antipruritic effect of an opiate antagonist, naloxone hydrochloride. J Invest Dermatol. 1982;78:82–83. doi: 10.1111/1523-1747.ep12497974. [DOI] [PubMed] [Google Scholar]
- 32.Bergasa NV, Alling DW, Talbot TL, et al. Effects of naloxone infusions in patients with the pruritus of cholestasis A double-blind, randomized, controlled trial. Ann Intern Med. 1995;123:161–167. doi: 10.7326/0003-4819-123-3-199508010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Bigliardi-Qi M, Gaveriaux-Ruff C, Pfaltz K, et al. Deletion of mu- and kappa-opioid receptors in mice changes epidermal hypertrophy, density of peripheral nerve endings, and itch behavior. J Invest Dermatol. 2007;127:1479–1488. doi: 10.1038/sj.jid.5700661. [DOI] [PubMed] [Google Scholar]
- 34.Maekawa T, Yamaguchi-Miyamoto T, Nojima H, Kuraishi Y. Effects of naltrexone on spontaneous itch-associated responses in NC mice with chronic dermatitis. Jpn J Pharmacol. 2002;90:193–196. doi: 10.1254/jjp.90.193. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and micro-opioid modulation in mice. Acta Derm Venereol. 2010;90:575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- 36.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SJ, Park GH, Kim D, et al. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc Natl Acad Sci U S A. 2011;108:3371–3376. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunford PJ, Williams KN, Desai PJ, et al. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Rossbach K, Wendorff S, Sander K, et al. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsuda M, Ueno S, Inoue K. Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice. Br J Pharmacol. 1999;128:1497–1504. doi: 10.1038/sj.bjp.0702960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakurada T, Mizoguchi H, Kuwahata H, et al. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol Biochem Behav. 2011;97:436–443. doi: 10.1016/j.pbb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagiwara K, Nojima H, Kuraishi Y. Serotonin-induced biting of the hind paw is itch-related response in mice. Pain Research. 1999;14:53–59. [Google Scholar]
- 44.Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett. 2010;484:62–65. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuraishi Y, Yageta Y, Konno M, Andoh T, Yamaguchi-Miyamoto T, Nojima H. Intracisternal, but not intrathecal, injection of naloxone inhibits cutaneous itch-related response in mice. Biol Pharm Bull. 2008;31:2143–2145. doi: 10.1248/bpb.31.2143. [DOI] [PubMed] [Google Scholar]
- 46.Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001;117:1621–1626. doi: 10.1046/j.0022-202x.2001.01585.x. [DOI] [PubMed] [Google Scholar]
- 47.Ohtsuka E, Kawai S, Ichikawa T, et al. Roles of mast cells and histamine in mosquito bite-induced allergic itch-associated responses in mice. Jpn J Pharmacol. 2001;86:97–105. doi: 10.1254/jjp.86.97. [DOI] [PubMed] [Google Scholar]
- 48.Kamei J, Nagase H. Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol. 2001;418:141–145. doi: 10.1016/s0014-2999(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 49.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 50.Trentin PG, Fernandes MB, D'Orleans-Juste P, Rae GA. Endothelin-1 causes pruritus in mice. Exp Biol Med (Maywood) 2006;231:1146–1151. [PubMed] [Google Scholar]
- 51.Sorkin LS, Boyle DL, Hammaker D, Herman DS, Vail E, Firestein GS. MKK3, an upstream activator of p38, contributes to formalin phase 2 and late allodynia in mice. Neuroscience. 2009;162:462–471. doi: 10.1016/j.neuroscience.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fioravanti B, De Felice M, Stucky CL, et al. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J Neurosci. 2008;28:11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- 54.Ross SE, Mardinly AR, McCord AE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson SR, Gerhold KA, Bifolck-Fisher A, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inan S, Dun NJ, Cowan A. Nalfurafine prevents 5′-guanidinonaltrindole- and compound 48/80-induced spinal c-fos expression and attenuates 5′-guanidinonaltrindole-elicited scratching behavior in mice. Neuroscience. 2009;163:23–33. doi: 10.1016/j.neuroscience.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Abdel Samad O, Zhang L, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


