Abstract
Neural progenitor cells must be maintained during development in order to produce the full complement of neuronal and glial derivatives. While molecular pathways have been identified that inhibit progenitor differentiation, it is unclear whether the progenitor state itself is actively maintained. In this study we have investigated the role of Tcf7l1 (formerly named Tcf3) in maintaining spinal progenitor characteristics and allowing the continued production of neurons and glia following primary neurogenesis. We find that spinal cord progenitor markers are progressively lost in embryos lacking Tcf7l1, and that the number of proliferative progenitors decreases accordingly. Furthermore, we show that the production of both neuronal and glial secondary derivatives of the pMN progenitor pool requires Tcf7l1. Together, these results indicate that Tcf7l1 plays an important role in spinal cord progenitor maintenance, indicating that this core function is conserved throughout multiple epithelial cell populations.
Keywords: zebrafish, Tcf7l1, spinal cord, neurogenesis
Introduction
During embryonic development, the rate of neurogenesis is tightly regulated by extrinsic and intrinsic factors. In this process, a balance must be achieved between the maintenance of proliferative progenitors and neuronal or glial differentiation. The vertebrate spinal cord has been well established as a model for CNS neurogenesis, due to its relatively simple anatomy and orderly progression of neuronal and glial differentiation. In the spinal cord, distinct dorso-ventral subregions of progenitors are established by extrinsic signals such as Shh, Bmp, Notch and Wnt (Artavanis-Tsakonas et al., 1999; Jessell, 2000; Briscoe and Ericson, 2001). Over time these specified progenitors undergo differentiation, first producing distinct populations of neurons, then producing glia. Previous work has shown that Notch signaling is required to prevent spinal progenitors from differentiating at the same time, and thus regulates cell diversity (Peng et al., 2007; Shin et al., 2007; Kim et al., 2008; Kimura et al., 2008). However, while Notch signaling primarily acts to block differentiation by repressing proneural gene expression (Holmberg et al., 2008), it is still unclear whether other factors actively maintain the progenitor pool. Furthermore, it is unknown how different aspects of the progenitor state, such as cell cycle progression, progenitor gene expression, and morphology, are coordinated.
One candidate regulator of the CNS progenitor state is the transcription factor Tcf7l1 (formerly known as Tcf3). The vertebrate Tcf/Lef family of molecules mediate canonical Wnt signaling by regulating downstream target gene expression (Behrens et al., 1996; Molenaar et al., 1996; Brannon et al., 1997; Lee et al., 2006). Vertebrates have four Tcf/Lef family members, Lef1, Tcf7, Tcf7l1 and Tcf7l2, which all contain highly conserved β-catenin binding domains and HMG DNA binding domains. These transcription factors function alternately as activators or repressors, depending on the presence or absence, respectively, of β-catenin (Gradl et al., 2002; Houston et al., 2002; Standley et al., 2006). Among the known roles of Tcf/Lef factors, Tcf7l1 is unique in that it acts to maintain progenitor cells in the absence of Wnt signaling. Tcf7l1 is expressed in multipotent embryonic epidermal progenitors and is down-regulated as these cells differentiate during development (Nguyen et al., 2006). In addition, Tcf7l1 expression is maintained in stem cells of the epidermal hair follicle bulge, which contribute to the normal hair cycle as well as wound repair (DasGupta and Fuchs, 1999). Identified transcriptional targets of Tcf7l1 match the gene signature of stem or progenitor cells (Nguyen et al., 2009), and loss of function analyses suggest that Tcf7l1 acts to maintain progenitor multipotency (Nguyen et al., 2009). Finally, Tcf7l1 is an integral component of the core regulatory circuitry of embryonic stem cells acting to influence the balance between pluripotency and differentiation (Cole et al., 2008; Yi et al., 2008).
Wnt signaling, mediated by Tcf/Lef factors, plays well characterized roles in early neural tube proliferation and patterning. The role of Wnt as a mitogen, directly regulating cell cycle control genes, has been confirmed in the CNS using multiple experimental systems (Megason and McMahon, 2002; Zechner et al., 2003; Chesnutt et al., 2004), leading to models in which the Wnt pathway acts to expand the neural progenitor population. Activation of targets that positively specify dorsal fates in concert with BMP signaling, and negatively influence ventral signals such as Hedgehog, support an important function for Wnt signaling in dorsal specification throughout the CNS (Zechner et al., 2007; Alvarez-Medina et al., 2008; Yu et al., 2008). Our previous work showed that Tcf7l1 plays an additional role as a repressor to inhibit precocious progenitor differentiation in the zebrafish spinal cord (Gribble et al., 2009). In zebrafish, Tcf7l1 activity is encoded by two orthologous genes, tcf7l1a and tcf7l1b. While both maternal and zygotic function of tcf7l1a is required for normal embryonic patterning(Kim et al., 2000), zygotic function of the tow genes is redundant in the embryo, as single homozygous mutants for each gene are viable and fertile as adults, and have no developmental phenotypes (Dorsky et al., 2003; Gribble et al., 2009). Interestingly, among tcf/lef genes, only the expression of these family members correlates with the presence of neural progenitors (Gribble et al., 2009). Earlier studies have demonstrated that Tcf7l1 functions primarily as a transcriptional repressor in the absence of Wnt signaling based on both its expression and biochemical activity (DasGupta and Fuchs, 1999; Dorsky et al., 2003; Gribble et al., 2009), as well as loss-of-function phenotypes (Dorsky et al., 2003; Merrill et al., 2004). We found that sox4a, a gene required for neuronal differentiation (Bergsland et al., 2006), is directly repressed by Tcf7l1 (Gribble et al., 2009). However, the full role of Tcf7l1 in spinal progenitors remains unclear. Specifically, it is unknown whether Tcf7l1 functions to generally maintain the progenitor state, in addition to specifically repressing genes associated with differentiation.
Here, we use a combination of loss-of-function techniques to examine the role of Tcf7l1 in spinal progenitor maintenance. By analyzing the expression of multiple spinal progenitor markers, we demonstrate that cells lacking Tcf7l1 lose their progenitor identity while continuing to proliferate. We next characterize the production of later-generated fates known to arise from a single progenitor pool, and find that both secondary motoneuron and oligodendrocyte precursor production requires Tcf7l1 function. Together, these data support a model in which the spinal progenitor population is actively maintained by Tcf7l1, and in its absence this population is gradually depleted, leading to a decreased ability to generate later derivatives..
Results
tcf7l1 genes are expressed in the zebrafish beyond primary neurogenesis
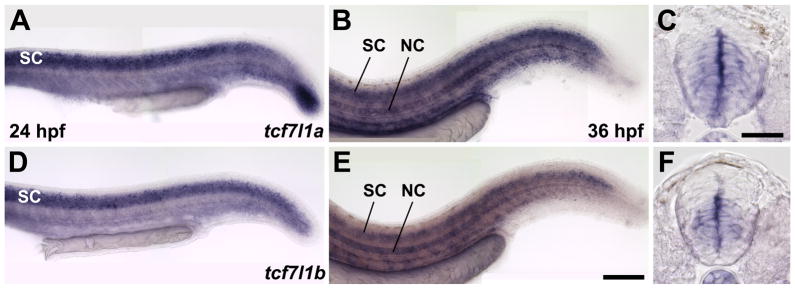
Zebrafish have two tcf7l1 genes, tcf7l1a and tcf7l1b (Dorsky et al., 2003). In previous studies, we showed that the zygotic function of these two genes is redundant during embryonic development and early spinal cord neurogenesis (Dorsky et al., 2003; Gribble et al., 2009). To determine whether these genes may play a continuing role in spinal progenitor maintenance, we examined their expression at later stages. At 24 hours post-fertilization (hpf), tcf7l1a and tcf7l1b are mainly expressed in the developing spinal cord, however tcf7l1a also exhibits strong expression in the last developing somites at the tail tip (Fig. 1A,D). By 36 hpf, both tcf7l1a and tcf7l1b are expressed in the spinal cord, notochord, urogenic duct and blood vessels (Fig. 1B,E). Cross-section analysis reveals that both genes are specifically expressed in spinal cord progenitors at 36 hpf (Fig. 1C,F). The two patterns are not completely identical, as tcf7l1a is expressed throughout the ventral spinal cord while tcf7l1b is restricted from the most ventral region. Nevertheless, these expression patterns suggest that expression of tcf7l1a and tcf7l1b overlaps significantly and the two genes may be functionally redundant through 36 hpf in the spinal cord.
Figure 1. Expression of tcf7l1a and tcf7l1b is maintained in the zebrafish trunk following primary neurogenesis.
(A–C) Expression of tcf7l1a mRNA at 24 hpf (A), and 36 hpf (B,C). (D–F) Expression of tcf7l1b mRNA at 24 hpf (D), and 36 hpf (E,F). Lateral whole-mount views are shown in (A,B,D,E) and cross-sections through the spinal cord are shown in (C,F) SC: spinal cord; NC: notochord. Scale bars = 20μM in C, 80μM in E.
Tcf7l1 is required for maintenance of progenitor markers in the developing spinal cord
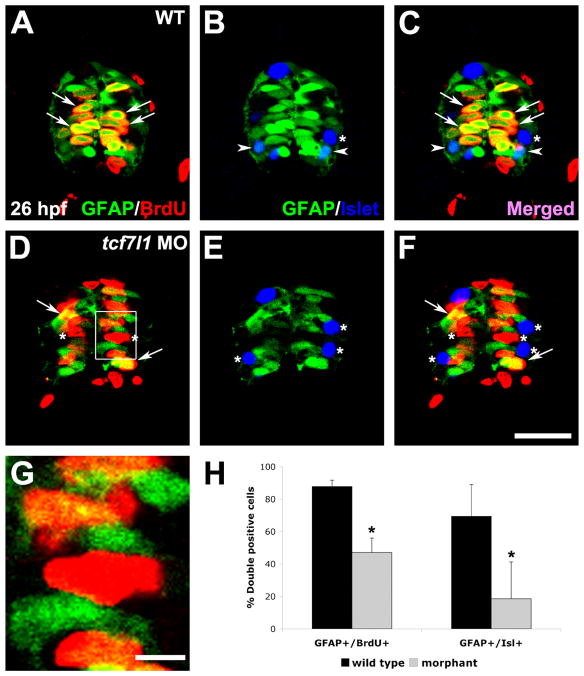
Our previous work showed that Tcf7l1 inhibits precocious neurogenesis in the developing spinal progenitors, and specific neuronal subpopulations, such as isl1-positive motoneurons, were reduced in Tcf7l1-deficient embryos (Gribble et al., 2009). To address whether these phenotypes were related to progenitor maintenance defects, we performed immunohistochemistry for GFP on Tg(gfap:GFP)mi2001 transgenic zebrafish. This transgene has been previously shown to label proliferating spinal radial glial progenitors, which endogenously express GFAP (Bernardos and Raymond, 2006). We performed BrdU labeling for 20 minutes in wild-type and tcf7l1 splice-blocking morpholino-injected embryos, followed by fixation and cryosectioning at 26 hpf. The overall expression level of gfap:gfp was drastically reduced in tcf7l1 morphant spinal cords compared to uninjected controls (Fig. 2A–F). The number of GFP+ cells was decreased at 26 hpf to 20.78±3.10 per section in tcf7l1 morphants, from 29.56±2.34 per section in wild-type embryos (±SD, n=9 sections, p<0.05). Strikingly, in tcf7l1 morphants many BrdU+ cells located in the medial spinal cord failed to express gfap:gfp, while nearly all BrdU+ cells in controls were GFP+ (Fig. 2A,D,G,H). At 26 hpf, secondary motoneurons marked by Isl1/2 continue to be produced from gfap:gfp-expressing progenitors. In control embryos, these cells are labeled by gfap:gfp and Isl1/2 co-expression likely due to GFP perdurance (Fig. 2B). In tcf7l1 morphants, we observed a significant decrease in gfap:gfp/Isl double-positive cells (Fig. 2E,H), suggesting that the motoneuron progenitor pool may be decreased at this timepoint. Consistent with this possibility, the total number of ventral Isl+ cells was decreased to 2.00±0.79 per section in tcf7l1 morphants, from 3.33±0.79 per section in wild-type embryos (±SD, n=9 sections, p<0.05). Together, these results indicate that Tcf7l1 may be required for maintenance of the progenitor state, and that the majority of Isl+ cells produced in morphants may be primary motoneurons.
Figure 2. gfap:gfp expression is lost in tcf7l1 morphants.
(A–F) Cross-sections through the spinal cord at 26 hpf. (A–C) Tg(gfap:GFP)mi2001 embryo co-labeled for BrdU and Isl1/2, following 20 minutes of BrdU incubation. (D–F) tcf7l1a/b morpholino-injected Tg(gfap:GFP)mi2001 embryo labeled as described above. Arrows indicate GFP+/BrdU+ cells, arrowheads indicate GFP+/Isl+ cells, and asterisks indicate GFP−/BrdU+ or GFP−/Isl+ cells. (G) Higher magnification view of box in (D). (H) Percent of double-positive cells/section in wild-type and tcf7l1 morphant spinal cords. Error bars indicate SD and asterisks indicate statistical significance. p < 0.001 by Student’s unpaired t-test. n=9 sections from 3 individual embryos for each bar. Scale bars = 20μM in F, 5μM in G.
It is possible that Tcf7l1 specifically regulates the expression of gfap:gfp, rather than progenitor identity in general. We therefore examined the expression of Sox3, which also marks spinal progenitors and may play a role in the regulation of progenitor identity (Bylund et al., 2003; Harrington et al., 2010). As before, control and tcf7l1 morphant gfap:gfp embryos were labeled with BrdU for 20 minutes. We found that the overall expression level of Sox3 protein was reduced in tcf7l1a morphants at both 26 and 36 hpf (Fig. 3A–D). In addition, the number of Sox3+ cells was decreased at 26 hpf to 22.78±1.50 per section in tcf7l1 morphants, from 29.11±1.17 per section in wild-type embryos (±SD, n=9 sections, p<0.005). By 36 hpf fewer BrdU+ cells in morphants expressed Sox3 than in wild-type controls (Fig. 3C–E). These results indicate that Tcf7l1 is required for the continued expression of multiple spinal progenitor markers. As we reported previously (Gribble et al., 2009), some BrdU+ cells also ectopically expressed HuC/D, which marks differentiating neurons (not shown).
Figure 3. Sox3 expression is lost in tcf7l1 morphants.
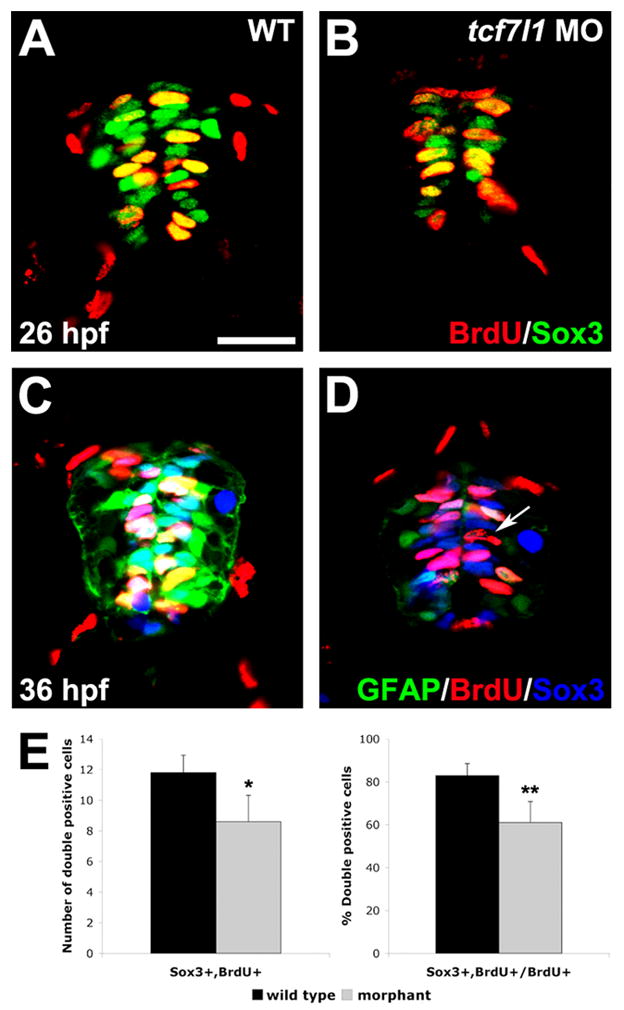
(A,B) BrdU and Sox3 co-immunohistochemistry in spinal cross-sections of wild-type and tcf7l1 morphant embryos. Sox3 expression is reduced in tcf7l1 morphants at 26 hpf. (C,D) BrdU, Sox3 and gfap:GFP triple-labeling at 36 hpf. Expression of Sox3 and GFAP are greatly reduced in tcf7l1 morphants (D) compared to wild-type embryos (C). Arrow indicates a BrdU+ cell without expression of GFAP or Sox3. (E) Number and percent of BrdU/Sox3 double-positive cells/section in wild-type and tcf7l1 morphant spinal cords. Error bars indicate SD and asterisks indicate statistical significance. *p < 0.05, **p<.0.001 by Student’s unpaired t-test. n=12 sections from 5 individual embryos for each bar. Scale bar = 20μM.
Tcf7l1 is necessary for generation of secondary motoneurons in the ventral spinal cord
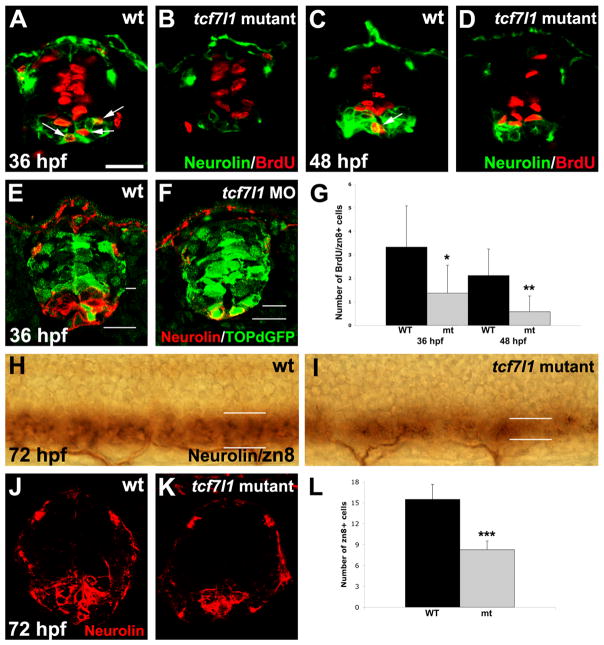
Because morphants exhibited decreased Isl+ motoneuron generation from gfap:gfp-expressing cells at 26 hpf (Fig. 2), we hypothesized that secondary motoneurons may be affected by the loss of Tcf7l1 function. We analyzed secondary motoneuron formation using the Neurolin (zn8) antibody, which specifically marks these cells (Kim et al., 2008). To allow phenotypic analysis beyond 36 hpf, we examined tcf7l1a/b double mutant embryos (Gribble et al., 2009) instead of morphants for these experiments. At both 36 and 48 hpf, some Neurolin-positive cells were double-labeled with BrdU in control embryos (Fig. 4A–D), suggesting that secondary motoneurons were still being produced from ventral progenitors. However, as with Isl1/2 labeling, Neurolin/BrdU double-positive cells were significantly reduced in tcf7l1 mutants compared to wild-type embryos (Fig. 4A–D,G). Single mutants for tcf7l1a and tcf7l1b had no significant phenotype (not shown), suggesting that the two genes act redundantly in the spinal cord at this stage of development.
Figure 4. Production of secondary motoneurons is decreased in tcf7l1 morphants and mutants.
(A–D) BrdU and Neurolin double immunohistochemistry on cryosections of wild-type and tcf7l1 mutant embryos. Arrows indicate double-labeled cells. BrdU+/Neurolin+ secondary motoneurons are reduced in tcf7l1 mutants compared to wild-type embryos at 36 and 48 hpf. (E,F) GFP and Neurolin double immunohistochemistry on cryosections of wild-type and tcf7l1 morphants expressing the top:dgfp Wnt reporter. In morphants, GFP expression is extended ventrally into the zone of decreased Neurolin expression. (G) Number of BrdU/Neurolin double-positive cells per cryosection in wild-type and tcf7l1 mutant embryos. Error bars indicate SD and asterisks indicate statistical significance. *p < 0.05, **p < 0.005 by Student’s unpaired t-test. n=12 sections from 5 individual embryos for each bar. (H–K) Whole-mount (H,I) and cryosection (J,K) immunostaining for Neurolin, showing reduction of secondary motoneurons in tcf7l1 mutants compared to wild-type controls at 72 hpf. (L) Number of Neurolin double-positive cells per cryosection in wild-type and tcf7l1 mutant embryos. Error bars indicate SD and asterisks indicate statistical significance. ***p < 0.001 by Student’s unpaired t-test. n=12 sections from 5 individual embryos for each bar. Scale bar = 20μM.
Interestingly, the zone of Neurolin expression in wild-type embryos normally coincides with low expression of the Wnt activity reporter topd:gfp (Dorsky et al., 2002). At 36 hpf, top:dgfp is normally expressed at low levels in intermediate spinal progenitors, and excluded from the ventral Neurolin-positive zone (Fig. 4E). However in tcf7l1 morphants, top:dgfp expression is increased overall and expanded into the most ventral region of the spinal cord, concomitant with decreased Neurolin expression (Fig. 4F). These results indicate that Tcf7l1-mediated repression of Wnt target genes may function to maintain the progenitor population long enough to allow secondary motoneuron formation.
To test whether Tcf7l1 function is specifically required for late-born secondary motoneuron formation rather than for all ventral neurogenesis, we examined the expression of GABA, which marks earlier-born ventral interneurons (Bernhardt et al., 1992). We found that unlike the decrease in Neurolin-positive secondary motoneurons, ventral GABA-positive cells were present in a normal distribution at 36 hpf (Supplementary Fig. 1A–D). Finally, to rule out the possibility that Neurolin expression was reduced by general developmental delay, we performed Neurolin whole mount immunostaining on wild-type and tcf7l1a/b mutants at 72 hpf. The number of Neurolin-positive cells located in the ventral spinal cord was reduced in the mutant embryos even at this later stage (Fig. 4H–L), indicating that Tcf7l1 function is required for the continued generation of secondary motoneurons by spinal progenitors.
Tcf7l1 is required for normal oligodendrocyte precursor cell development
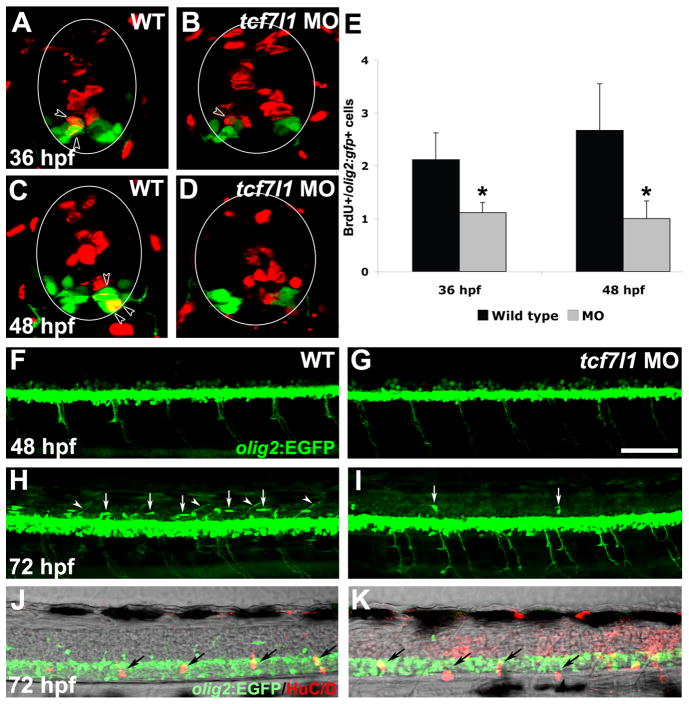
In addition to secondary motoneurons, oligodendrocytes are also derived from olig2-positive progenitor cells in the pMN domain of the developing zebrafish spinal cord following primary neurogenesis (Park et al., 2007). Our previous data showed that olig2 expression was not affected in tcf7l1 morphants at early embryonic stages (Gribble et al., 2009). However since tcf7l1 genes are strongly expressed throughout the ventral spinal cord through at least 36 hpf, and secondary motoneuron production from the pMN domain is dependent on Tcf7l1, we hypothesized that oligodendrocyte formation might be similarly affected. To test whether Tcf7l1 is required for olig2-expressing progenitor proliferation, we performed short-pulse BrdU labeling in tcf7l1 MO-injected Tg(olig2:EGFP)vu12 embryos (Park et al., 2007), at 36 and 48 hpf. The number of GFP and BrdU double-positive cells were reduced in tcf7l1 morphants compared to controls at both stages (Fig. 5A–E).
Figure 5. OPC development is reduced in tcf7l1 morphants.
(A–D) BrdU and GFP double immunohistochemistry on cryosections of wild-type and tcf7l1 morphant Tg(olig2:EGFP)vu12 embryos. Spinal cord is outlined in circles and arrowheads indicate double-labeled cells. In tcf7l1 morphants, fewer BrdU+ cells are observed within the GFP+ population. (G) Number of BrdU+/olig2:gfp+ cells per cryosection in wild-type embryos and tcf7l1 morphants at 36 and 48 hpf. Error bars indicate SD and asterisks indicate statistical significance. *p < 0.05 by Student’s unpaired t-test. n=9 sections from 3 different embryos for each bar. (F–K) Confocal projection images showing lateral views of the spinal cord in Tg(olig2:EGFP)vu12 embryos. (F,G) GFP-positive cells are reduced in tcf7l1 morphants at 48 hpf. (H,I) At 72 hpf, dorsally migrating OPCs (arrows) and dorsally projecting motoneurons (arrowheads) are absent in tcf7l1 morphants. (J,K) The decrease in OPCs is not due to a general developmental delay, as normal dorsal root ganglion development (arrows) is observed in tcf7l1 morphants. Scale bars = 20μM in D, 40μM in F.
We then examined whether the decrease in proliferation among olig2:gfp expressing cells correlated with a decreased number of oligodendrocyte precursor cells (OPCs). At 72 hpf, dorsally-migrating GFP-positive OPCs were greatly decreased in tcf7l1 morphants (Fig. 5G,H). The reduction in OPC marker expression did not correlate with a general developmental delay, as dorsal root ganglion formation was normal in 72 hpf tcf7l1 morphant embryos (Fig. 5I,J). Together, these results suggest that Tcf7l1 is required for the continued production of multiple late derivatives of ventral spinal progenitors.
Loss of ventral cell types is not due to patterning defects
While our previous study showed that Tcf7l1 is not required for dorsal/ventral patterning in the spinal cord before 24 hpf, our current results indicate that at 36 hpf tcf7l1a deficient embryos have reduced numbers of two ventral spinal cord cell types: sMNs and OPCs. To test whether Tcf7l1 knockdown affects dorsal/ventral patterning of the spinal cord at this embryonic stage, we performed in situ hybridization with several region-specific patterning markers, msxC, pax3, dbx2 and nkx6.1 (listed dorsal to ventral). We found that the expression of all of these markers was unaffected in tcf7l1 morphants (Supplementary fig. 2A–H), suggesting that the reductions in sMNs and OPCs were not a result of patterning defects.
Discussion
Tcf7l1 acts uniquely in CNS development, independent from other characterized functions of Wnt/β-catenin signaling and Tcf/Lef-mediated activation in regulating neural progenitor proliferation and dorsal/ventral neural tube patterning. Our previous work identified a role for Tcf7l1 (formerly named Tcf3) in blocking premature differentiation of spinal progenitors (Gribble et al., 2009). We found that the two zebrafish orthologs tcf7l1a/b are expressed in cells with low levels of Wnt activity, where their gene products function primarily as transcriptional repressors. In the absence of Tcf7l1 function, a direct target gene, sox4a, is ectopically expressed and promotes neuronal differentiation. While these data provided a partial mechanism for the tcf7l11 morphant phenotype, they left some key questions unaddressed. First, it was not clear whether Tcf7l1 plays an additional role in the maintenance of progenitor markers. This possibility was raised by our observations that morphant cells sometimes remained proliferative even while they expressed differentiated neuronal markers, suggesting that they might have a dual progenitor/neuron identity. Second, the effects on successive phases of spinal progenitor derivatives were unexplored. While we had observed a general decrease in spinal interneuron differentiation, it was not clear whether this was due to a failure in fate specification or rather a loss of progenitor maintenance. Answering these questions would lead to a more complete understanding of how spinal progenitors are maintained throughout development and even into adult stages.
Tcf7l1 maintains the spinal progenitor state
In this study we used several approaches to determine the role of Tcf7l1 in spinal progenitor maintenance. We first demonstrated that tcf7l1a/b gene expression in the spinal cord persists beyond primary neurogenesis, as progenitors continue to proliferate and generate differentiated neurons (Fig. 1). While the expression patterns of the two genes are not completely identical, all our phenotypic analyses suggest that their zygotic function is redundant. Single homozygous mutants for each gene are viable and fertile, indicating that any non-redundant functions throughout life of the animal are not essential. Next, we used transgenic and immunohistochemical markers to show that Tcf7l1 is required for maintenance of multiple spinal progenitor phenotypes (Figs. 2–3). Our data also indicate that the proliferative state can be uncoupled from progenitor marker expression, as cells lacking gfap:gfp and Sox3 are still able to incorporate BrdU. Together, these results suggest that Tcf7l1 is required to simultaneously maintain the progenitor state and inhibit differentiation.
Failure of progenitor maintenance results in loss of multiple secondary pMN derivatives
Because the spinal progenitor pool must be maintained in order to produce later-born fates, loss of Tcf7l1 function would be predicted to influence the number of these progeny. Most primary neurons in the zebrafish spinal cord have differentiated by 18 hpf (Bernhardt et al., 1990), and neurons born after this point are secondary derivatives. Because few specific markers exist for secondary interneurons, we focused our analysis on motoneuron progenitors. We found that tcf7l1 mutants and morphants produced significantly fewer secondary motoneurons and OPC’s (Figs 4–5). Both of these cell types arise from an olig2-expressing pMN progenitor population (Park et al., 2007), and we observed a decreased number of proliferating olig2:gfp+ cells in tcf7l1a morphants (Fig. 4). These data suggest that fewer pMN progenitors are present when Tcf7l1 function is lost, resulting in a general decrease in all progeny. Our findings reveal a different role for Tcf7l1 in progenitor maintenance than has been previously found for Notch signaling. Careful analysis of pMN fates following manipulation of the Notch pathway has shown that alternative fates are increased when Notch signaling is lost (Kim et al., 2008). For example, early inhibition of Notch leads to increased GABAergic interneurons at the expense of motoneurons (Shin et al., 2007), while later inhibition results in more secondary motoneurons at the expense of radial glial progenitors and OPC’s. In contrast, we do not observe increases in alternative fates when Tcf7l1 is lost. Rather, progenitors prematurely differentiate as HuC/D+ neurons and presumably die due to lack of proper fate specification. Together, our work suggests a general role for Tcf7l1 in progenitor maintenance, rather than in mediating binary cell fate decisions. We predict that our findings should extend to other secondary neuron populations throughout the expression domain of tcf7l1 genes, but confirmation will require the use of specific markers for these cell types.
A conserved function for Tcf7l1 in epithelial progenitor maintenance
In some epithelial tissues, continued progenitor self-renewal is a necessary part of homeostasis. In epithelial progenitors of the skin and hair follicles, Tcf7l1 plays a critical role in maintaining the progenitor state, as a transcriptional regulator of progenitor-specific genes (Nguyen et al., 2006; Cole et al., 2008; Yi et al., 2008). While progenitor self-renewal is much more limited in tissues of the central nervous system, there are specific instances in which analogous processes may function. During embryogenesis, radial glial neural progenitors proliferate and generate progeny throughout the brain and spinal cord, and these populations must be maintained long enough to generate the full complement of neurons and glia necessary for function. Following embryogenesis, discrete populations of neural stem cells are maintained in the brain, but the mechanisms responsible for promoting self-renewal and multipotency of these progenitors are largely uncharacterized. Our work here suggests that the maintenance of epithelial progenitors in different tissues may require a common gene program, which includes transcriptional repression by Tcf7l1.
Potential implications for maintenance of spinal cord progenitors beyond embryogenesis
Radial glial-like ependymal cells in adult fish and amphibians express GFAP and maintain processes that span the spinal cord to the pial surface (Tomizawa et al., 2000). Because regenerating motoneurons in zebrafish are thought to arise from these persistent radial glial progenitors (Reimer et al., 2009), these cells may represent an endogenous stem cell population capable of post-injury repair in the zebrafish spinal cord. Our data suggest that Tcf7l1 may be a key component of the mechanism underlying the persistence of radial glial progenitors beyond embryogenesis. To test this hypothesis, it will be necessary to determine the expression and functional requirement for Tcf7l1 at larval and adult stages.
Experimental Procedures
Zebrafish maintenance
Embryos were obtained from natural spawning of wild-type (AB*) or transgenic and mutant zebrafish lines listed below, and were staged according to Kimmel et al., (Kimmel et al., 1995). tcf7l1 mutants were generated using tcf7l1am881 and tcf7l1bzd10 alleles and mutant embryos were identified as described previously (Gribble et al., 2009). The Tg(olig2:EGFP)vu12 transgenic line was obtained from Dr. Bruce Appel (Park et al., 2007; Takada et al., 2010). Tg(gfap:GFP)mi2001 transgenic zebrafish were obtained from Dr. Pamela Raymond (Bernardos and Raymond, 2006). Tg(top:dgfp)w25 fish have been described and characterized previously (Dorsky et al., 2002).
Morpholino injections and in situ hybridization
tcf7l1a/b (splice-blocking) + p53 (translation-blocking) morpholino injections were performed as described previously (Gribble et al., 2009). Antisense digoxigenin-labeled RNA probes for tcf7l1a, tcf7l1b, msxC, pax3, dbx2 and nkx6.1 were produced using a DIG-RNA labeling kit (Roche) according to the manufacturer’s instructions. All probes were described previously in (Gribble et al., 2009), except msxC which was described in (Bonner et al., 2008). Whole mount in situ hybridization was performed with digoxigenin labeled probes as described previously (Thisse et al., 1993).
BrdU labeling and immunostaining
26, 36, and 48 hpf zebrafish embryos were incubated in a 10 mM BrdU solution containing 15% DMSO in Ringer’s solution. At 26 and 36 hpf, embryos were fixed after a 20 minute incubation and embryos at 48 hpf were fixed after a 1 hour incubation. All embryos were fixed in 4% paraformaldehyde for 2 hrs in room temperature. For cryosectioning, embryos were cryoprotected in a 30% sucrose/PBS solution, then mounted in OCT compound (Tissue-Tek). Blocks were sectioned at 10μm thickness for immunohistochemistry.
Immunostaining on whole mount embryos and cryosections was performed as described previously (Gribble et al., 2009). For BrdU detection, slides were incubated for 1 hour in 2N HCl. The following primary antibodies were used: rat polyclonal anti-BrdU (AbD Serotec), mouse monoclonal anti-Isl1/2 (DSHB), rabbit polyclonal Sox3 (Zhang et al., 2003), mouse monoclonal anti-Neurolin (zn8, ZIRC), mouse monoclonal anti-HuC/D (Invitrogen). We used secondary antibodies conjugated to Alexa Fluor 568 or Alexa Fluor 647 (Invitrogen). For whole mount DAB immunohistochemistry, we used the Vectastain ABC kit (Vector Laboratories).
Imaging
Fixed whole-mount embryos and sections were mounted in 80% glycerol and Fluoromount-G (Southern Biotech), respectively, and imaged using conventional (bright-field) or confocal (fluorescent) microscopy. Confocal images were acquired using an Olympus FV1000 microscope. For live imaging, embryos were manually dechorionated at appropriate embryonic stages and transferred into embryo media containing tricaine for anesthesia. Embryos were then embedded in 0.8 % low-melting temperature agarose and mounted in 35-mm Petri dishes.
Supplementary Material
(A,B) Lateral whole-mount (A) and cross-section (B) views of 36 hpf wild-type embryos stained with Neurolin and GABA antibodies. Both secondary motoneurons (red) and GABAergic interneurons (green) are present in the ventral spinal cord. (C,D) Lateral whole-mount (C) and cross-section (D) views of 36 hpf tcf7l1 morphants. Despite the decrease in Neurolin-positive cells, ventral GABAergic interneurons are present in a normal distribution. Scale bar = 20μM.
Lateral whole-mount views of 36 hpf wild-type and tcf7l1 morphant embryos following in situ hybridization for spinal progenitor markers. (A,B) msxC labels the roof plate. (C,D) pax3 labels dorsal progenitors. (E,F) dbx2 labels intermediate progenitors. (G,H) nkx6.1 labels ventral progenitors. All markers are expressed in normal domains in tcf7l1 morphants. Scale bar = 80μM.
Acknowledgments
Grant Sponsor: NIH (NINDS); Grant number R01 NS053897
References
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006;20:3475–3486. doi: 10.1101/gad.403406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, Chitnis AB, Lindamer L, Kuwada JY. Identification of spinal neurons in the embryonic and larval zebrafish. J Comp Neurol. 1990;302:603–616. doi: 10.1002/cne.903020315. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, Patel CK, Wilson SW, Kuwada JY. Axonal trajectories and distribution of GABAergic spinal neurons in wildtype and mutant zebrafish lacking floor plate cells. J Comp Neurol. 1992;326:263–272. doi: 10.1002/cne.903260208. [DOI] [PubMed] [Google Scholar]
- Bonner J, Gribble SL, Veien ES, Nikolaus OB, Weidinger G, Dorsky RI. Proliferation and patterning are mediated independently in the dorsal spinal cord downstream of canonical Wnt signaling. Dev Biol. 2008;313:398–407. doi: 10.1016/j.ydbio.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, Chitnis A. Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130:1937–1947. doi: 10.1242/dev.00402. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT. A Transgenic Lef1/beta-Catenin-Dependent Reporter Is Expressed in Spatially Restricted Domains throughout Zebrafish Development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Gradl D, Konig A, Wedlich D. Functional diversity of Xenopus lymphoid enhancer factor/T-cell factor transcription factors relies on combinations of activating and repressing elements. J Biol Chem. 2002;277:14159–14171. doi: 10.1074/jbc.M107055200. [DOI] [PubMed] [Google Scholar]
- Gribble SL, Kim HS, Bonner J, Wang X, Dorsky RI. Tcf3 inhibits spinal cord neurogenesis by regulating sox4a expression. Development. 2009;136:781–789. doi: 10.1242/dev.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington MJ, Chalasani K, Brewster R. Cellular mechanisms of posterior neural tube morphogenesis in the zebrafish. Dev Dyn. 2010;239:747–762. doi: 10.1002/dvdy.22184. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Hansson E, Malewicz M, Sandberg M, Perlmann T, Lendahl U, Muhr J. SoxB1 transcription factors and Notch signaling use distinct mechanisms to regulate proneural gene function and neural progenitor differentiation. Development. 2008;135:1843–1851. doi: 10.1242/dev.020180. [DOI] [PubMed] [Google Scholar]
- Houston DW, Kofron M, Resnik E, Langland R, Destree O, Wylie C, Heasman J. Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development. 2002;129:4015–4025. doi: 10.1242/dev.129.17.4015. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shin J, Kim S, Poling J, Park HC, Appel B. Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos. Dev Dyn. 2008;237:2081–2089. doi: 10.1002/dvdy.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Satou C, Higashijima S. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135:3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]
- Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson Maduro J, Godsave S, Korinek V, Roose J, Destr'ee O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Park HC, Shin J, Roberts RK, Appel B. An olig2 reporter gene marks oligodendrocyte precursors in the postembryonic spinal cord of zebrafish. Dev Dyn. 2007;236:3402–3407. doi: 10.1002/dvdy.21365. [DOI] [PubMed] [Google Scholar]
- Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–827. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Kuscha V, Wyatt C, Sorensen I, Frank RE, Knuwer M, Becker T, Becker CG. Sonic hedgehog is a polarized signal for motor neuron regeneration in adult zebrafish. J Neurosci. 2009;29:15073–15082. doi: 10.1523/JNEUROSCI.4748-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Poling J, Park HC, Appel B. Notch signaling regulates neural precursor allocation and binary neuronal fate decisions in zebrafish. Development. 2007;134:1911–1920. doi: 10.1242/dev.001602. [DOI] [PubMed] [Google Scholar]
- Standley HJ, Destree O, Kofron M, Wylie C, Heasman J. Maternal XTcf1 and XTcf4 have distinct roles in regulating Wnt target genes. Dev Biol. 2006;289:318–328. doi: 10.1016/j.ydbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Takada N, Kucenas S, Appel B. Sox10 is necessary for oligodendrocyte survival following axon wrapping. Glia. 2010;58:996–1006. doi: 10.1002/glia.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Inoue Y, Nakayasu H. A monoclonal antibody stains radial glia in the adult zebrafish (Danio rerio) CNS. J Neurocytol. 2000;29:119–128. doi: 10.1023/a:1007156529390. [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, McDonnell K, Taketo MM, Bai CB. Wnt signaling determines ventral spinal cord cell fates in a time-dependent manner. Development. 2008;135:3687–3696. doi: 10.1242/dev.021899. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zechner D, Muller T, Wende H, Walther I, Taketo MM, Crenshaw EB, 3rd, Treier M, Birchmeier W, Birchmeier C. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–190. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Zhang C, Basta T, Jensen ED, Klymkowsky MW. The beta-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development. 2003;130:5609–5624. doi: 10.1242/dev.00798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A,B) Lateral whole-mount (A) and cross-section (B) views of 36 hpf wild-type embryos stained with Neurolin and GABA antibodies. Both secondary motoneurons (red) and GABAergic interneurons (green) are present in the ventral spinal cord. (C,D) Lateral whole-mount (C) and cross-section (D) views of 36 hpf tcf7l1 morphants. Despite the decrease in Neurolin-positive cells, ventral GABAergic interneurons are present in a normal distribution. Scale bar = 20μM.
Lateral whole-mount views of 36 hpf wild-type and tcf7l1 morphant embryos following in situ hybridization for spinal progenitor markers. (A,B) msxC labels the roof plate. (C,D) pax3 labels dorsal progenitors. (E,F) dbx2 labels intermediate progenitors. (G,H) nkx6.1 labels ventral progenitors. All markers are expressed in normal domains in tcf7l1 morphants. Scale bar = 80μM.