Abstract
Background
Ubiquitin carboxyl-terminal esterase-L1 (UCHL1) is a protein highly selectively expressed in neurons and has been linked to neurodegenerative disease in humans. We hypothesize that UCHL1 would be an effective serum biomarker for brain injury as tested in canine models of hypothermic circulatory arrest (HCA) and cardiopulmonary bypass (CPB).
Methods
Canines were exposed to CPB (n=14), 1 hour(h) HCA (n=11), or 2h-HCA (n=20). Cerebrospinal fluid (CSF) and serum were collected at baseline, 8h, and 24h post-treatment. UCHL1 levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA). Neurological function and histopathology were scored at 24h, and UCHL1 immunoreactivity was examined at 8h.
Results
Baseline UCHL1 protein levels in CSF and serum were similar for all groups. In serum, UCHL1 levels were elevated at 8h post-treatment for 2h-HCA subjects compared to baseline values (p<0.01), and also compared to CPB canines at 8h (p<0.01). A serum UCHL1 level above 3.9ng/(mg total protein) at 8h had the best discriminatory power for predicting functional disability. In CSF, UCHL1 was elevated in all groups at 8h post-treatment compared to baseline (p<0.01). However, UCHL1 levels in CSF remained elevated at 24h only in 2h-HCA subjects (p<0.01). Functional and histopathology scores were closely correlated (Pearson’s coefficient: 0.66; p<0.01), and were significantly worse in 2h-HCA animals.
Conclusions
This is the first report associating elevated serum UCHL1 with brain injury. The novel neuronal biomarker UCHL1 is increased in serum 8h after severe neurological insult in 2h-HCA animals compared with CPB animals. These results support the potential for use in cardiac surgery patients, and form the basis for clinical correlation in humans.
Keywords: Animal Model, Cardiopulmonary bypass (CPB), Biomarker, Hypothermia/circulatory arrest, Neurology/Neurologic injury
Introduction
Despite advances in operative and anesthetic techniques, neurological injury remains a devastating problem following cardiac surgery, with stroke rates between 1–6%.1,2 Furthermore, delirium after coronary artery bypass increases the risk of long-term mortality,3 and cognitive dysfunction is present in nearly one-quarter of patients at one month.4 Patients with neurologic injury have increased hospital length of stay, cost, and need for inpatient rehabilitation.2 Although the risk of stroke remains relatively low, with nearly 300,000 cardiac surgical procedures performed annually in the United States,5 neurological injury is a significant burden to patients, their families, and the health care system.
Nearly 35 years have elapsed since hypothermic circulatory arrest (HCA) was first introduced for cerebral protection during aortic arch reconstructions.6 Yet, end organ damage remains a significant problem in the setting of HCA, and there are no currently proven strategies to prevent brain injury.7,8 In the evolution of the surgical approach to conditions that require temporary cerebral blood flow exclusion, some centers have studied selective antegrade perfusion or retrograde cerebral perfusion.9,10 However, HCA remains an established and viable strategy for complex aortic arch repairs and congenital cardiac lesions, and is still widely used at many centers.8,11,12 Experimental evidence has implicated post-HCA glutamate excitotoxicity as an important mediator of this injury.13 Differential gene regulation has been detected in canines undergoing HCA as compared to cardiopulmonary bypass (CPB) only, further elucidating the mechanisms in HCA-related brain injury.14
Brain injury is often diagnosed several hours to days after surgery; detecting neurologic injury early after the initial insult can enable clinicians to diagnose and implement treatments to reverse or mitigate the damage. Thus, the development of readily obtainable biomarkers that correlate with early brain injury harbors much promise for improving neurologic-related morbidity after cardiac surgery. Several biomarkers for brain injury have been widely studied, such as neuron-specific enolase (NSE), glial protein S-100 beta (S-100β), and myelin basic protein (MBP); however, none has been definitively correlated with clinical outcomes and studies relating these proteins to pathophysiological parameters are lacking.15 Our laboratory detected spectrin breakdown products (SBDP) as biomarkers for central nervous system (CNS) injury, although these findings were confined to the cerebrospinal fluid (CSF).16
Because of abundant expression in neurons, ubiquitin carboxyl-terminal esterase L1 (UCHL1) (first recognized as neuronal-specific gene product 9.5)17 has garnered enthusiasm as a potential CNS biomarker. UCHL1 accounts for nearly 5% of total soluble brain protein, is involved in ubiquitination pathways, and has been linked with neurodegenerative disorders in humans such as Parkinson’s disease.18 Recent evidence supports the detectable presence of UCHL1 in CSF in animal and human studies of subarachnoid hemorrhage, and ischemic and traumatic brain injury.19–22 To date, there is only one study examining UCHL1 elevations following cardiac surgery, but those results were limited to CSF samples.23 Given the mounting evidence for UCHL1 as a biomarker for neuronal injury, we tested the hypothesis that UCHL1 is an effective serum biomarker for brain injury in a canine model of CPB and HCA.
Methods
Animals
We used our clinically relevant canine model of HCA and CPB.13,14,16,24,25 Six to 12-month-old, 30-kg male class-A dogs were used (Marshal Bioresources, North Rose, NY). The Johns Hopkins Animal Care and Use Committee approved the experimental protocols, which complied with the “Guide for the Care and Use of Laboratory Animals” (1996, U.S. National Institutes of Health).
Experimental Design
Canines were randomly exposed to 2 hours(h) of HCA (n=20), 1h-HCA (n=11), or CPB alone (n = 14) and survived to either 8h (2h-HCA, n=10; 1h-HCA, n=5; and CPB, n=8) or 24h (2h-HCA, n=10; 1h-HCA, n=6; and CPB, n=6) after treatment. CSF and serum samples were collected at baseline (prior to the surgical incision but after induction of anesthesia), at 8h, and 24h. For both baseline and subsequent CSF collection, under sedation and in a routine sterile fashion, the spinal canal is entered with a 22G needle through the cisterna magnum (at the base of the skull posteriorly). Samples are immediately frozen in a −80°C freezer. Blood samples are obtained through previously placed peripheral intravenous catheters, cold centrifuged to collect serum, and frozen at −80°C. At the conclusion of the experiment, all subjects were euthanized by exsanguination, and brains harvested for analysis.
Surgical hypothermic circulatory arrest procedure
Anesthesia was induced with methohexital sodium 9mg/kg. Animals were endotracheally intubated and maintained on inhaled isoflurane (0.5%–2%), 100% oxygen, and intravenous fentanyl (150–200 μg/dose). Tympanic membrane, esophageal, and rectal probes monitored temperatures throughout the experiment. A left femoral artery cannula was placed for arterial blood gas and hemodynamic monitoring.
Standard CPB circuits with a40-μm arterial filter (Sorin Group, Arvada, CO) were used in all experiments. Intravenous heparin (300U/kg) was administered and the right femoral artery cannulated (12F–14F), advancing the cannula into the abdominal aorta. Two separate venous cannulas (18F–20F) were advanced to the right atrium via the right femoral and external jugular veins. Vessels were cannulated by an open cutdown technique. Closed-chest CPB was initiated using pump flows of 60–80mL · kg−1 · min−1 to maintain mean arterial pressure of 60–80mmHg, and activated clotting times were maintained >500 seconds. For animals in the 1h- or 2h-HCA groups, the pump was stopped when tympanic temperatures reached 18°C (approximately 30 minutes).
Animals underwent 1h- or 2h-HCA with alpha-stat regulation of arterial blood gases (pH, 7.3–7.4; arterial partial pressure of oxygen >300mmHg and carbon dioxide 30–40mmHg). Once HCA finished, CPB was resumed, and rewarming commenced (5°C temperature gradient every 15 minutes to a core temperature of 37°C for 2h). Intravenous phenylephrine was used when necessary to maintain mean arterial pressure >75mmHg. External defibrillation was performed when temperatures were 32°C. At 37°C, animals were separated from CPB, decannulated, and reversed with protamine (3mg/kg intravenous).
Animals recovered from anesthesia while intubated, with frequent monitoring of vital signs, arterial blood gases, and urine output. Once hemodynamically and clinically stable, they were extubated and transferred to their crate for recovery. Analgesics were administered per protocol after the procedure.
Cardiopulmonary bypass only
After induction and cannulation, animals underwent 2h of CPB without HCA. Animals were cooled to 32°C, and the heart continued to beat during this operation. Animals were recovered from anesthesia as described above.
Neurologic assessment
The University of Pittsburgh Canine Neurological Score was independently determined at 24h by two non-blinded study team members.26 The score includes 22 clinical questions relating to level of consciousness, respiration, cranial nerve function, reflexes, behavior, and motor and sensory function. Animals had normal neurologic function before experimentation. No additional sedation was given within 12h of neurologic assessment.
Euthanasia and tissue procurement
Animals were sedated, intubated, and anesthetized. A sternotomy was performed, 300units/kg of heparin given, and the ascending aorta cannulated (22F). Cold perfusion via the aortic cannula flushed the brain with 12L of saline solution (4°C) at 60mmHg. The right atrial appendage was transected, and venous return suctioned into a reservoir. Brains were harvested by means of a wide craniectomy.
Tissue preparation and UCHL1 Immunohistochemistry
Brains were immersion-fixed for one week in 4% paraformaldehyde, cut into 2mm coronal blocks, embedded in paraffin and sectioned at 6 μm. For immunohistochemistry, endogenous peroxidase was quenched (3% hydrogen peroxide in methanol,10 min, RT), slides were blocked (phosphate buffered saline (PBS) with 5% normal goat serum (NGS), 0.2%triton X-100, and 0.2%gelatin, 60 min, RT) and incubated in rabbit anti-UCHL1 (Novus Biologicals NB100-65827, 1:1600 in PBS with 1.5%NGS, 0.2% Triton X-100, 0.2%gelatin and 1 μl/ml sodium azide, 48 h, 4°C). The primary antibody was visualized using the avidin-biotin-peroxidase complex method (ABC-Elite, Vector Laboratories).
Histologic Analysis
Hematoxylin and eosin staining was performed for blinded histologic evaluation by a single neuropathologist (J.C.T.). Eleven distinct regions of the canine brain were evaluated for the presence of apoptosis and necrosis. These regions include midfrontal cortex, superior parietal cortex, basal ganglia, hippocampus (dentate gyrus and CA regions), entorhinal cortex, amygdala, cerebellum (molecular layer, Purkinje layer, and granule layer), and brainstem. A semiquantitative scale was used to assess the degree of necrosis and apopstosis in each region. These scores were summed to obtain the neuronal cell death score, ranging from a minimum of 0 (no damage) to a maximum of 99 (extreme neuronal damage).
ELISA analysis
UCHL1 levels in CSF and serum were measured using a UCHL1 sandwich enzyme-linked immunosorbent assay (ELISA) modified from a protocol previously reported.20,21 Mouse monoclonal anti-human UCHL1 antibody and rabbit polyclonal anti-human UCHL1 antibody were made in-house against recombinant human UCHL1 full length protein and partial protein, respectively. Both were affinity purified and specificity was confirmed by immunoblotting. Reaction wells were coated with capture antibody (purified mouse monoclonal anti-human UCHL1) in 0.05M sodium bicarbonate, pH 9.6 and incubated overnight at 4°C. Plates were then washed with blocking buffer (Tris buffer saline with 0.02% Tweeen-20 (v/v);[TBST]), and further incubated for 30 minutes at ambient temperature with gentle shaking. Antigen standard (UCHL1 standard curve:0, 0.06–15 ng/mL), unknown samples (5 μL of CSF; 20 μL of serum), or assay internal control samples were incubated overnight with detection antibody (rabbit polyclonal anti-human UCHL1, 100 μL total volume). The capture antibody coated plate was then incubated with detection antibody-sample mixture for 1.5h at room temperature, and washed using an automatic plate washer (each well rinsed with 350 μL wash buffer [TBST]). The plate was then incubated with anti-rabbit-IgG-HRP (Amersham Biosciences) at room temperature for 1h and developed with Ultra-TMB ELISA substrate (Pierce# 34028) for 10 minutes. The plate was read at 450nm with a Molecular Devices Spectramax 190 spectrophotometer. The intra-assay coefficient of variance (CV)=2.1%–7.9% while interassay CV=0.9%–10.6 % within the assay dynamic range. The limit of detection (LOD) was 0.030 ng/mL; samples with undetectable levels were assigned 50% of the LOD (i.e.,0.015ng/mL). If samples yielded a signal above the quantification range, samples were diluted and re-assayed. For all serum samples, concentrations were normalized against total serum protein concentration for the same sample (ng/mg protein).
Genomic Analysis
Canine microarray analysis was performed in a blinded fashion at the Johns Hopkins Deep Sequencing and Microarray Core facility. Detailed methods of this protocol have been described previously.14 Gene expression profiles in samples from ventral anterior hippocampus were compared between 2h-HCA, 1h-HCA, CPB and untreated normal dogs (each treatment group was compared to normal controls). Exploratory data analysis was performed on normalized data with a false discovery rate (FDR)<0.10 considered significantly regulated.27 To evaluate gene function, Entrez Gene IDs for human orthologs were assigned, and Ingenuity Pathways Analysis software (Ingenuity Systems, Inc., http://www.ingenuity.com, Redwood City, CA) was used to identify proteins that interact directly with UCHL1 or are part of canonical pathways that include UCHL1. For UCHL1-related genes that were significantly regulated in one or more treatment groups, fold-change vs. normal was determined in all groups.
Statistical Analysis
Neurologic scores are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) compared neurologic and histology scores among groups. For all subjects, the paired comparisons t-test examined differences in levels of UCHL1 from baseline to 8h after treatment. For 24h survival animals, repeated-measures ANOVA was used to account for the repeated serum samples from within the same canine subject over time. Post-hoc pairwise comparisons were conducted using the Tukey-Kramer method. Correlations between functional and histopathological scores were assessed using linear regression and Pearson’s correlation coefficient. Threshold values for UCHL1 levels that predict severe functional impairment (neuro score >100) were determined using unadjusted logistic regression and receiver operating characteristic (ROC) curves (area under the curve >0.7 considered significant). P-values <0.05 were considered significant, and analysis was performed using STATA software (v9.2, StataCorp-LP, College Station, TX).
Results
Subjects
Twenty dogs underwent 2h-HCA, 11 had 1h-HCA, and 14 had CPB. Roughly half of the dogs in each group (ten 2h-HCA, five 1h-HCA and eight CPB) were sacrificed at 8h and the other half at 24h.
Neurologic Scores
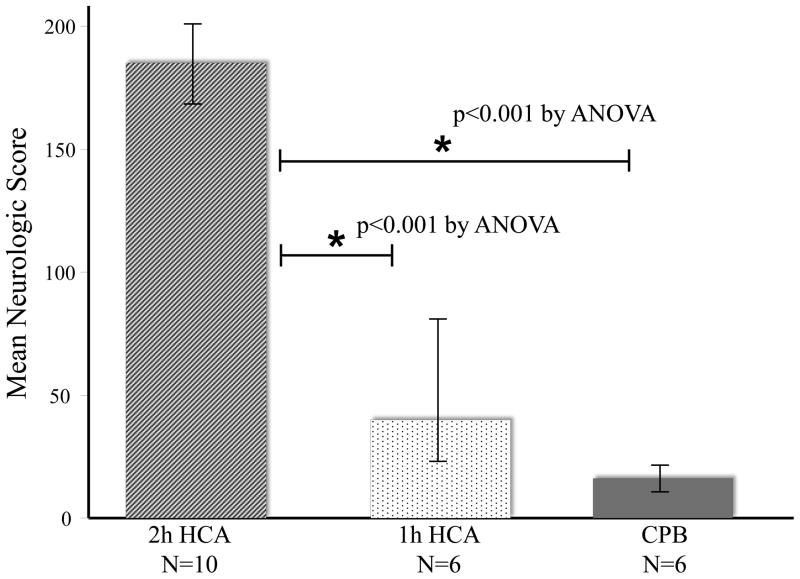
Using the neurologic scoring system (0 to 480), higher scores indicate worse neurologic function. Scores at 24h were significantly different among groups, with lower scores observed in groups subjected to milder insult (Figure 1). For 2h-HCA animals, the mean neurologic score was 185±28. In 1h-HCA animals, the mean score was 40±50. Two 1h-HCA dogs were effectively normal (scores < 20); in contrast, no 2h-HCA dog had a score less than 130—all were severely impaired. CPB animals demonstrated negligible change in neurologic function (mean score:16±16).
Figure 1.
Average neurologic scores (based on two independent observers) for animals 24 hours (h) after 2h-HCA, 1h-HCA, or CPB, using the University of Pittsburgh Canine Neurological Score (0 to 480, with higher scores corresponding with greater functional neurologic impairment). *Indicates p<0.05 by Tukey-Kramer method post-hoc pairwise comparisons.
Histopathology Scores
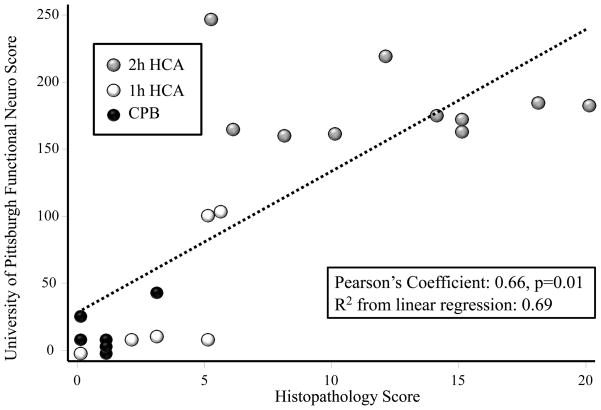
Dogs exposed to 2h-HCA had the highest levels of cellular injury on histologic examination (mean neuronal cell death score 9.6±5.5; Table 1). This level was different when compared to 1h-HCA and CPB groups. Most brain regions showed minimal neuronal death, but at 24h in the 2h-HCA group the hippocampus was severely affected (up to 75% apoptotic neurons in the dentate gyrus). Linear regression analysis showed a strong correlation between histopathology scores and University of Pittsburgh Functional Assessment scores (Figure 2).
Table 1.
Average Histology Scores for Neuronal Cell Death (Total and by Brain Region)
| Brain Region | Potential Score | 2h-HCA (n = 10) | 1h-HCA (n = 6) | CPB (n = 6) | p-Valuea |
|---|---|---|---|---|---|
| Composite Score of all Brain Regions Examined | 99 | 9.6 (±5.5)b,c | 4.1 (±2.2) | 0.7 (±1.3) | <0.01 |
|
| |||||
| Notable regions | |||||
| Cortex | 18 | 1.3 (±1.4)b,c | 0.5 (±0.7) | 0 (±0) | 0.02 |
| Basal ganglia | 9 | 1.0 (±0.5)b,c | 0.6 (±0.6) | 0.1 (±0.3) | <0.01 |
| Hippocampus | 18 | 3.2 (±0.9)b,c | 1.3 (±0.9)b | 0.1 (±0.3) | <0.01 |
| Cerebellum | 27 | 0.9 (±0.9) d | 0.9 (±0.8) | 0.1 (±0.4) | 0.14 |
CPB = cardiopulmonary bypass; HCA = hypothermic circulatory arrest.
Eleven distinct brain regions examined included: midfrontal cortex, superior parietal cortex, basal ganglia, hippocampus (dentate gyrus and CA region), entorhinal cortex, amygdala, cerebellum (molecular layer, Purkinje layer, and granule layer), and brainstem
P-value corresponds to one-way analysis of variance
Significantly different from reference (CPB) on post-hoc pairwise comparisons using Tukey-Kramer test
Significantly different from 1h-HCA on post-hoc pairwise comparisons using Tukey-Kramer test
2h-HCA subjects demonstrate excessive hemorrhage on histology analysis
Figure 2.
Linear regression analysis depicting correlation between Histopathology scores and University of Pittsburgh Functional Assessment Scores. Strength of correlation was determined using linear regression analysis with Pearson’s coefficient and R2 value.
Immunohistochemistry Results
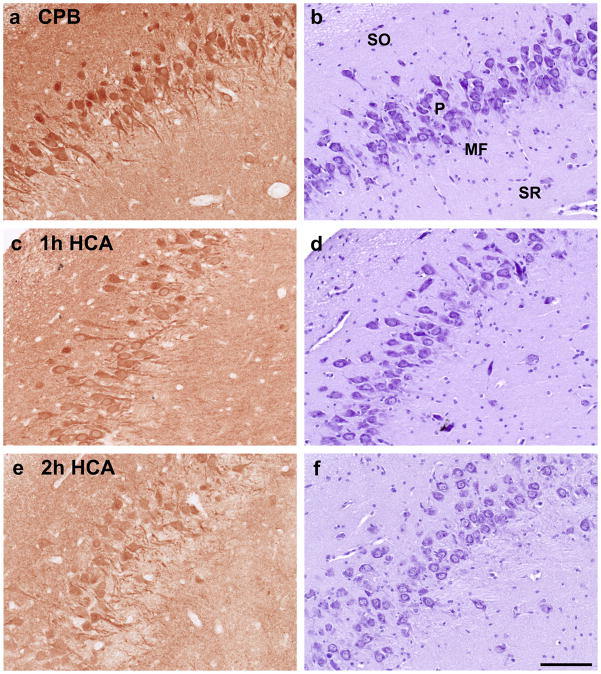
UCHL1 staining in the hippocampus was less in 2h-HCA animals than in 1h-HCA and CPB subjects. In the dentate gyrus, there is moderate expression of UCHL1 in the CPB group, but almost complete loss of protein expression in the 2h-HCA group. In the CA3 region, there is a change from high expression in the CPB group to moderate expression in pyramidal neurons after 2h-HCA. However, there is near-complete loss of UCHL1 in the mossy fiber layer after 2h-HCA, where axon terminals from the depleted dentate granule neurons are located (Figure 3).
Figure 3.
UCHL1 immunoreactivity (left panel) and corresponding Nissl-stained sections (right panel) in CA3 region of hippocampus for CPB (a, b), 1h-HCA (c, d) and 2h-HCA (e, f). SO=stratum oriens, P=pyramidal neurons, MF=mossy fiber layer, SR=stratum radiatum.
ELISA results
Serum
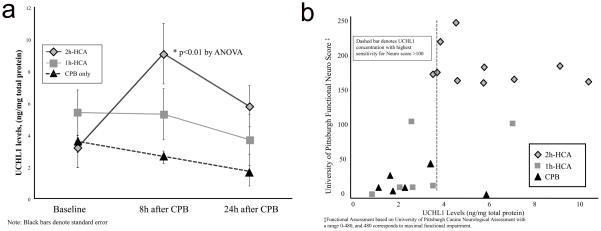
In 2h-HCA animals, serum UCHL1 levels were increased from baseline 8h after treatment (3.2±0.3ng/mg protein to 9.3±2.0ng/mg protein, p=0.01), but were similar to baseline by 24h (Figure 4a). In 1h-HCA and CPB animals, serum levels of UCHL1 were not elevated at either time point. Comparison of the three groups revealed that 2h-HCA animals had significantly elevated UCHL1 in serum at 8h compared to CPB (p=0.01), but by 24 hours there were no longer any detectable differences among the groups. Correlation of serum UCHL1 levels for the three groups against University of Pittsburgh Functional Assessment scores revealed that 3.9ng/mg total protein appeared the most sensitive break point for predicting functional impairment in an unadjusted logistic regression analysis (area under receiver operating characteristic (ROC) curve:0.9, p=0.02) (Figure 4b).
Figure 4.
Line graph (a) showing serum levels of UCHL1 by treatment groups across three time points. Scatter plots (b) for serum UCHL1 levels correlated with functional assessment score. Break point denoted by dashed line indicates highest sensitivity for predicting outcome of interest (Neuro Score >100) by univariate logistic regression analysis.
CSF
In 2h-HCA animals, UCHL1 protein in CSF was significantly increased from baseline 8h after HCA (11.7±6.0ng/ml to 144.5±21.7ng/ml, p<0.01) and remained elevated 24h after HCA (p<0.01). In 1h-HCA and CPB animals, UCHL1 levels were elevated at 8h compared to baseline (1h-HCA,5.4±2.4ng/ml to 56.2±18.6ng/ml, p=0.01; CPB, 12.4±6.1 ng/ml to 68.1±21.0ng/ml, p=0.02), but returned to baseline levels at 24 hours. Comparison among the three groups at 8h and 24h revealed that 2h-HCA animals had elevated levels of UCHL1 compared to 1h-HCA and CPB at both time points (Figure 5a-online). CSF levels of UCHL1 for the three groups were correlated with University of Pittsburgh Functional Assessment scores. The most sensitive break point for predicting functional impairment in an unadjusted logistic regression analysis was 61ng/ml (ROC curve area:0.77, p=0.09)(Figure 5b-online).
Genomic results
In 2h-HCA animals, three genes were significantly regulated at 8h, and eight genes were significantly regulated at 24h. In the 1h-HCA group, one gene was significantly regulated at 8h, and none at 24h. No genes were significantly regulated in the CPB group. Among the regulated genes, three mRNAs for proteins that directly interact with UCHL1 and two mRNAs for proteins in the Parkin pathway were significantly downregulated, but six mRNAs for proteasome subunits were significantly upregulated. Detailed information regarding regulated genes is shown in Table 2 (online).
Table 2.
(online). Numbers of Significantly Regulated Genes at 8 and 24 Hours After 2h-HCA, 1h-HCA, and CPB
| Gene | Fold-change vs. Normal group (Bold = FDR<0.10)
|
Gene Name | |||||
|---|---|---|---|---|---|---|---|
| 2h-HCA | 2h-HCA | 1h-HCA | 1h-HCA | CPB | CPB | ||
| 8h Surv | 24h Surv | 8h Surv | 24h Surv | 8h Surv | 24h Surv | ||
| USP21 | −1.38 | −2.05 | 1.04 | 1.14 | −1.05 | 1.06 | ubiquitin specific peptidase 21 |
| PSMB8 | 1.12 | 2.22 | −1.13 | 1.18 | 1.12 | 1.11 | proteasome (prosome, macropain) subunit, beta type, 8 (large multifunctional peptidase 7) |
| PSME2 | 1.17 | 1.69 | 1.02 | 1.19 | 1.10 | −1.07 | proteasome (prosome, macropain) activator subunit2 (PA28 beta) |
| PSMB10 | 1.16 | 1.90 | 1.03 | 1.45 | 1.31 | 1.07 | proteasome (prosome, macropain) subunit, beta type, 10 |
| PSMB9 | −1.16 | 1.58 | −1.57 | −1.19 | −1.52 | −1.16 | proteasome (prosome, macropain) subunit, beta type, 9 (large multifunctional peptidase 2) |
| PSMA6 | 1.07 | 1.27 | −1.02 | 1.13 | 1.06 | −1.08 | proteasome (prosome, macropain) subunit, alpha type, 6 |
| SEPT5 | −1.24 | −1.46 | 1.21 | −1.02 | 1.04 | 1.02 | septin 5 |
| PSMB3 | 1.16 | 1.27 | 1.05 | 1.23 | 1.10 | 1.13 | proteasome (prosome, macropain) subunit, beta type, 3 |
| VHL | −1.17 | 1.04 | −1.11 | 1.01 | 1.04 | 1.01 | von Hippel-Lindau tumor suppressor |
| SNCAIP | −1.25 | −1.04 | −1.20 | −1.04 | −1.25 | −1.08 | synuclein, alpha interacting protein |
| MYCN | −1.25 | −1.01 | −1.41 | −1.17 | −1.36 | 1.02 | v-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian) |
HCA = hypothermic circulatory arrest; CPB = cardiopulmonary bypass
Bold numbers indicate gene terms significantly regulated for each treatment group with a false discovery rate < 0.1.
Discussion
The promising field of proteomics has facilitated the development of new biomarkers specific for organ injury. The advantages of neuron-specific biomarkers are threefold: Earlier detection of brain injury may 1) allow for therapeutic, pharmacologic and hemodynamic interventions; 2) improve prognostic abilities; and 3) aid in future research. UCHL1 is a candidate serum biomarker known to be abundant with high specificity in neurons.17,26 It has further been linked with several neurodegenerative diseases in humans, including Parkinson’s disease.18 Canines undergoing prolonged HCA (2h) had significant increases in serum UCHL1 protein at 8h compared to baseline, and compared with the CPB group at 8h. The 2h-HCA canines also had greater neurological impairment and histological damage. IHC analysis elucidated plausible biologic mechanisms to support the ELISA results, and genomic analysis suggests downregulation of proteins that bind to UCHL1 and possible compensatory upregulation of proteasomal subunits. Taken collectively, these findings support the use of UCHL1 as a serum biomarker specific for neurologic injury after HCA.
Consistent with our earlier work, dogs subjected to 2h-HCA sustained significantly greater histologic damage (most pronounced in the hippocampus) compared to 1h-HCA and CPB only. Because our purpose was to assess the utility of the UCHL1 biomarker as an indicator of clinical brain injury, we used the University of Pittsburgh Functional Assessment score (cut-off>100) as our dependent variable in unadjusted logistic regression analysis. This scoring system is a validated instrument for this purpose, and regression analysis confirmed a reliable correlation between this measure of neurological impairment and histopathology scores (Figure 2).26 This supports our decision to use the functional assessment score as the outcome measure, however this outcome measure was determined only at 24h survival.
The IHC results substantiate the elevated levels of UCHL1 detected in the CSF and serum. ICH staining revealed a progressive loss of neuronal UCHL1 protein with increasing severity of treatment, which is consistent with the progressive increase observed in CSF. The brains of animals subjected to CPB had preserved UCHL1 immunoreactivity within intact neurons. This staining was less intense for 1h-HCA canines, and greatly diminished in the 2h-HCA group. Consistent with this finding, 11 genes intimately related to UCHL1 were up or down-regulated after 2h-HCA; in comparison, only 1 gene was significantly regulated in 1h-HCA and none after CPB only. This transcriptional profile across the 3 treatment groups mirrors our earlier findings on transcriptional regulation among a broad array of gene categories, and supports the safety of CPB.14 In this study, we elected to use Ingenuity Pathways Analysis to identify genes related to UCHL1. This system is human-curated, and therefore only validated interactions and plausible mechanistic pathways are considered.
We detected elevations of UCHL1 in the CSF at 8h, among all three study groups. However, serum elevations of UCHL1 were observed only in the 2h-HCA group at 8h. To enhance sensitivity and specificity in clinical use, a panel of biomarkers with different time course profiles for release and degradation will be important, akin to the use of serum markers for myocardial infarction. Although the sandwich ELISA yields highly quantitative results, we applied rigorous methods to standardize the serum concentrations by using the total protein concentration for the respective sample. Thus, we believe the serum results are robust and valid.
The IHC analysis supports the serum findings, demonstrating release of UCHL1 protein from injured neurons in the 2h-HCA group. Although UCHL1 release is believed to be associated with neuronal injury/death, we believe that the early presence of this biomarker may aid in identification of threatened yet salvageable neurons in adjacent regions of the brain (after intraoperative or perioperative hypoperfusion or watershed infarctions). Pharmacologic interventions as well as aggressive treatment of hypoxia and hypotension may spare these threatened neurons. Future clinical studies are needed to fully assess the ability of this marker to influence such therapies.
Previous Work
Our laboratory has previously reported the elevations of Spectrin breakdown products in the CSF following 1h-HCA.16 A novel contribution of the current study is the detection of a novel biomarker specific for CNS injury in serum. Additional studies have investigated the role of UCHL1 as a biomarker in the setting of traumatic brain injury, subarachnoid hemorrhage, and ischemia-reperfusion injury.19–21,23 Papa et al. detected higher CSF levels of UCHL1 in 44 human patients who had sustained traumatic brain injury.21 That study examined additional time points, with detailed characterization of the time course of CSF UCHL1 protein levels; however, the mechanism of neurologic injury differs from HCA. Lewis et al. reported 30 patients with elevations of CSF UCHL1 after subarachnoid hemorrhage, and observed higher mortality rates among patients with the highest levels of UCHL1.19 Siman et al. conducted the only study to date conducted in cardiac surgery patients, with all 19 patients undergoing thoracic aortic aneurysm repair, 7 of whom required HCA.23 Among the 12 patients not requiring HCA (aortic cross-clamping only), patients with acute neurological complications had higher CSF UCHL1 levels, suggesting UCHL1 release in response to warm ischemia and reperfusion injury. This study did not, however, examine serum levels of UCHL1. Our study builds on these existing data by identifying serum elevations of UCHL1 associated with neurologic injury in the setting of HCA.
Limitations
This study is limited with respect to the temporal pattern of biomarker release into the CSF and serum. The current data suggest that UCHL1 peaks in the serum 8h after HCA and begins to lower by 24h. However, it is possible that there is an earlier peak. We acknowledge this limitation, however we attempted to balance study feasibility with prior experience regarding the temporal release of UCHL1 after neurologic injury. Additional studies with shorter survival groups are currently being conducted to address this important issue. This study also does not provide data regarding long-term or late release of this biomarker; such information can only be gleaned from longer survival models, which pose additional logistical obstacles. There are outliers within the data points, but these were not excluded to preserve the purity of the dataset. Also, when examining the relationship between functional impairment and serum UCHL1 concentrations within individual treatment groups (Figure 4b), there does not appear to be a positive correlation. We speculate that a larger sample size and more sensitive functional assessment (as can be performed in humans) would address these limitations.
We were unable to control for the effects of hypothermia on UCHL1 release. Hypothermia may mediate pathways involved in the release of UCHL1. However, given prior experience suggesting a protective effect of hypothermia on neurologic function28 and the preponderance of evidence suggesting that UCHL1 is released in response to neurologic injury, it is unlikely that the serum presence of UCHL1 observed in this study is due purely to hypothermia. Furthermore, it would be difficult to achieve such an experimental condition, as all animals experience spontaneous ventricular fibrillation when undergoing controlled hypothermia.
From a logistical standpoint, if we were to have designed a comparison control group that was progressively cooled for 30 minutes to deep hypothermia and rewarmed afterward, our past experience suggests nearly all of these canines would have experienced a period of ventricular fibrillation. The HCA groups are not subjected to a prolonged duration of ventricular fibrillation because the pump is turned off during the arrest phase. The groups would have been better matched with respect to the incidence of fibrillation (and the requirement of defibrillation), however this hypothetical control group would have been exposed to a longer period of fibrillation.
Additionally, we acknowledge that a 2-hour period to induce injury is quite severe and does not mirror clinical practice. However, the canines used in this study were all young, healthy animals without cerebrovascular disease or prior stroke. In the interest of efficient use of animals and resources, we chose to study an HCA duration that exceeded clinical practice, but would ensure histologic damage and maximize the likelihood of detecting biomarker elevations in the serum.
Conclusion
In summary, this is the first study to document serum elevation of UCHL1 following neurologic injury. The novel neuronal biomarker UCHL1 is increased in serum 8h after severe neurological insult in 2h-HCA animals compared with CPB. These results support the potential for practical use in cardiac surgery patients, and form the basis for clinical correlation in human studies.
Supplementary Material
Line graph (a) depicting CSF levels of UCHL1 by treatment groups across three time points. Scatter plots (b) for CSF UCHL1 concentration correlated with functional assessment score. Break point denoted by dashed line indicates highest sensitivity for predicting outcome of interest (Neuro Score >100) by univariate logistic regression analysis.
Acknowledgments
This study was supported by the National Institutes of Health (NIH RO1 HL091541-18 WAB and 1T32 CA126607-01A2 GJA). Drs. Arnaoutakis and Weiss are the Irene Piccinini Investigators in Cardiac Surgery and Drs. George and Allen are the Hugh R. Sharp Cardiac Surgery Research Fellows. The authors would like to thank Ms. Jennifer Berrong for her expertise in brain tissue preparation. We also express profound gratitude to Mr. Jeffrey Brawn and Mrs. Melissa Jones for their invaluable technical assistance and support. Their decades-long experience with the experimental protocol was vital to completion of this study.
Footnotes
Presented in the C. Walton Lillehei Resident Award Forum at the 91st annual meeting of the American Association for Thoracic Surgery, May 7–11, 2011, Philadelphia, PA.
Disclosures: Kevin Wang, PhD, owns stock and is an executive officer of Banyan Biomarkers, Inc, and as such may benefit financially as a result of the outcomes of this research or the work reported in this publication.
References
- 1.McKhann GM, Grega MA, Borowicz LM, Jr, Bechamps M, Selnes OA, Baumgartner WA, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol. 2002;59:1422–1428. doi: 10.1001/archneur.59.9.1422. [DOI] [PubMed] [Google Scholar]
- 2.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. The New England journal of medicine. 1996;335:1857–1863. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman RF, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKhann GM, Goldsborough MA, Borowicz LM, Jr, Selnes OA, Mellits ED, Enger C, et al. Cognitive outcome after coronary artery bypass: a one-year prospective study. Ann Thorac Surg. 1997;63:510–515. doi: 10.1016/s0003-4975(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 5.STS. Society of Thoracic Surgeons National Database Executive Summary. 2009. [Google Scholar]
- 6.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–1063. [PubMed] [Google Scholar]
- 7.Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ, Jr, Klodell CT, Ejaz AA, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007;134:1554–1560. doi: 10.1016/j.jtcvs.2007.08.039. discussion 1560–1551. [DOI] [PubMed] [Google Scholar]
- 8.Augoustides JG, Floyd TF, McGarvey ML, Ochroch EA, Pochettino A, Fulford S, et al. Major clinical outcomes in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest: quantification of organ-based perioperative outcome and detection of opportunities for perioperative intervention. Journal of cardiothoracic and vascular anesthesia. 2005;19:446–452. doi: 10.1053/j.jvca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Di Eusanio M, Schepens MA, Morshuis WJ, Di Bartolomeo R, Pierangeli A, Dossche KM. Antegrade selective cerebral perfusion during operations on the thoracic aorta: factors influencing survival and neurologic outcome in 413 patients. J Thorac Cardiovasc Surg. 2002;124:1080–1086. doi: 10.1067/mtc.2002.124994. [DOI] [PubMed] [Google Scholar]
- 10.Lytle BW, McCarthy PM, Meaney KM, Stewart RW, Cosgrove DM., 3rd Systemic hypothermia and circulatory arrest combined with arterial perfusion of the superior vena cava. Effective intraoperative cerebral protection. J Thorac Cardiovasc Surg. 1995;109:738–743. doi: 10.1016/S0022-5223(95)70356-X. [DOI] [PubMed] [Google Scholar]
- 11.Elefteriades JA. What is the best method for brain protection in surgery of the aortic arch? Straight DHCA. Cardiol Clin. 2010;28:381–387. doi: 10.1016/j.ccl.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Gega A, Rizzo JA, Johnson MH, Tranquilli M, Farkas EA, Elefteriades JA. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg. 2007;84:759–766. doi: 10.1016/j.athoracsur.2007.04.107. discussion 766–757. [DOI] [PubMed] [Google Scholar]
- 13.Williams JA, Barreiro CJ, Nwakanma LU, Lange MS, Kratz LE, Blue ME, et al. Valproic acid prevents brain injury in a canine model of hypothermic circulatory arrest: a promising new approach to neuroprotection during cardiac surgery. Ann Thorac Surg. 2006;81:2235–2241. doi: 10.1016/j.athoracsur.2005.12.060. discussion 2241–2232. [DOI] [PubMed] [Google Scholar]
- 14.Allen JG, Weiss ES, Wilson MA, Arnaoutakis GJ, Blue ME, Talbot CC, Jr, et al. Hawley H. Seiler Resident Award. Transcriptional profile of brain injury in hypothermic circulatory arrest and cardiopulmonary bypass. Ann Thorac Surg. 2010;89:1965–1971. doi: 10.1016/j.athoracsur.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, et al. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–1721. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- 16.Weiss ES, Wang KK, Allen JG, Blue ME, Nwakanma LU, Liu MC, et al. Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest. Ann Thorac Surg. 2009;88:543–550. doi: 10.1016/j.athoracsur.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9. 5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40:1542–1547. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, et al. Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proc Natl Acad Sci U S A. 2009;106:4635–4640. doi: 10.1073/pnas.0806474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis SB, Wolper R, Chi YY, Miralia L, Wang Y, Yang C, et al. Identification and preliminary characterization of ubiquitin C terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J Neurosci Res. 2010;88:1475–1484. doi: 10.1002/jnr.22323. [DOI] [PubMed] [Google Scholar]
- 20.Liu MC, Akinyi L, Scharf D, Mo J, Larner SF, Muller U, et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur J Neurosci. 2009;31:722–732. doi: 10.1111/j.1460-9568.2010.07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, 3rd, Oli MW, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svetlov SI, Prima V, Kirk DR, Gutierrez H, Curley KC, Hayes RL, et al. Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. J Trauma. 2010;69:795–804. doi: 10.1097/TA.0b013e3181bbd885. [DOI] [PubMed] [Google Scholar]
- 23.Siman R, Roberts VL, McNeil E, Dang A, Bavaria JE, Ramchandren S, et al. Biomarker evidence for mild central nervous system injury after surgically-induced circulation arrest. Brain Res. 2008;1213:1–11. doi: 10.1016/j.brainres.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redmond JM, Gillinov AM, Zehr KJ, Blue ME, Troncoso JC, Reitz BA, et al. Glutamate excitotoxicity: a mechanism of neurologic injury associated with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1994;107:776–786. discussion 786–777. [PubMed] [Google Scholar]
- 25.Barreiro CJ, Williams JA, Fitton TP, Lange MS, Blue ME, Kratz L, et al. Noninvasive assessment of brain injury in a canine model of hypothermic circulatory arrest using magnetic resonance spectroscopy. Ann Thorac Surg. 2006;81:1593–1598. doi: 10.1016/j.athoracsur.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Tisherman SA, Safar P, Radovsky A, Peitzman A, Sterz F, Kuboyama K. Therapeutic deep hypothermic circulatory arrest in dogs: a resuscitation modality for hemorrhagic shock with ‘irreparable’ injury. J Trauma. 1990;30:836–847. [PubMed] [Google Scholar]
- 27.Benjamini YaH. Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 28.Coselli JS, Crawford ES, Beall AC, Jr, Mizrahi EM, Hess KR, Patel VM. Determination of brain temperatures for safe circulatory arrest during cardiovascular operation. Ann Thorac Surg. 1988;45:638–642. doi: 10.1016/s0003-4975(10)64766-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Line graph (a) depicting CSF levels of UCHL1 by treatment groups across three time points. Scatter plots (b) for CSF UCHL1 concentration correlated with functional assessment score. Break point denoted by dashed line indicates highest sensitivity for predicting outcome of interest (Neuro Score >100) by univariate logistic regression analysis.