Abstract
Cancer stem cells (CSCs), or cancer cells with stem cell properties, have been reported in many human tumors and are thought to be responsible for tumor initiation, therapy resistance, progression, relapse, and metastasis. Despite their potential clinical importance, how CSCs are regulated at the molecular level is not well understood. MicroRNAs (miRNAs), small non-coding RNAs that play critical roles in normal stem cell functions during development, have emerged as important regulators of CSCs as well. In this review, we summarize the current major findings of miRNA regulation of various CSCs and discuss our recent findings that miR-34a suppresses prostate CSCs and metastasis by directly repressing CD44. These recent progresses have important implications for understanding how CSCs are intricately regulated by networks of miRNAs and for developing novel mechanism-based miRNA therapeutics that specifically targets CSCs.
Keywords: miRNA, cancer stem cells, microRNA, let-7, miR-200, miR-34
Introduction
Research in the last decade suggests the presence of cancer stem cells (CSCs), which can both self-renew to regenerate themselves and differentiate into a spectrum of maturing daughter cells that create the cellular heterogeneity of cancer. CSCs were first discovered in acute myeloid leukemia and, since 2003, have also been reported in most solid tumors (1). Emerging evidence indicates that CSCs may be involved in tumor maintenance, therapy resistance, tumor progression, and distant metastasis. Despite their potential clinical significance, how intrinsic CSC properties are regulated at the molecular level is poorly understood. Recent discoveries of microRNAs (miRNAs) have provided a new avenue in understanding the regulatory mechanisms in CSCs.
miRNAs are 21-25 nucleotide (nt) long, non-coding RNAs that induce the target mRNA degradation or repress mRNA translation by imperfect binding to their 3′-untranslated region (2). The miRNA gene is first transcribed by RNA polymerase II into primary transcript (pri-miRNA) in the nucleus, where the hairpin stem-loop structure is processed into precursor miRNA (pre-miRNA) by a micro-processing complex including Drosha and DGCR8. The ~70 nt long pre-miRNA is then exported into cytoplasm where it undergoes a second processing by Dicer, in which one strand of the hairpin is incorporated into the ribonucleoprotein complex called miRNA-induced silencing complex (2). A single miRNA may target dozens of mRNAs and one mRNA can be regulated by multiple miRNAs. Although small, miRNAs play a powerful role in biological processes including development, proliferation, and apoptosis. Early studies have linked miRNAs to controlling the self-renewal and differentiation of embryonic stem cells (ESCs) and later, aberrant expression/functions of miRNAs are implicated in tumorigenesis (3). More recent studies suggest that miRNAs may also regulate CSC properties.
miRNA regulation of development and ESCs
The first two miRNAs, lin-4 and let-7, were both discovered during C. elegans development. Since then, miRNAs have emerged as important regulators of embryonic development and stem cell functions in mammals. The overall roles of miRNAs in both mouse and human ESCs have been evaluated by analyzing the phenotypes of Dicer and DGCR8 mutants. Deletion of Dicer in mouse causes embryonic lethality (4) and Dicer-deficient mouse ESCs exhibit defects in differentiation and G1 cell-cycle arrest (5). Similarly, DGCR8-deficient mouse ESCs demonstrate problems in cell-cycle progression and differentiation evidenced by failing to silence self-renewal genes such as OCT4, REX1, NANOG and SOX2 as well as delayed expression of differentiation markers (6). Other studies have also revealed specific expression and functions of individual miRNAs in ESCs (7).
A regulatory circuitry between miRNAs and ‘pluripotency’ genes required for maintaining ESC stemness has been identified. On one hand, the master regulators of stem cell pluripotency, including OCT-4, NANOG, SOX2, and TCF3, all directly regulate ESC-specific miRNAs by binding to their promoter regions (8). On the other hand, some of these pluripotency genes are also regulated by miRNAs at the post-transcriptional level. Thus, miR-134, miR-296, and miR-470 suppress the expression of NANOG, OCT4 and SOX2 by binding to their coding regions (9). Lin-28, a marker of undifferentiated ESCs and used to generate induced pluripotent stem cells, also forms a negative feedback loop with the let-7 family miRNAs to precisely control each other’s levels. Lin-28 regulates the expression of let-7 by binding to the precursors and blocking their maturation, whereas in differentiated cells where let-7 levels are increased let-7 miRNAs in turn target the Lin-28 mRNA (10).
miRNA regulation of cancer and CSCs
Interestingly, the miRNA expression patterns in tumor cells often bear resemblance to those in ESCs. Let-7, for instance, is excluded in ESCs and often lost in cancers including breast, lung and ovarian cancers. Such cancer-specific miRNA expression signature(s) may become very informative for diagnostic and prognostic purposes. Functional studies of the dysregulated miRNAs indicate that they regulate molecular pathways in cancer via targeting different oncogenes and/or tumor suppressors. More recent evidence suggests that miRNAs may also be involved in tumor development by critically regulating CSCs. Here we discuss the major findings of some recent studies highlighting the roles of certain ‘CSC-specific’ miRNAs in several representative cancer types. From these discussions, we present an emerging theme that several miRNAs may distinctively and concertedly (coordinately) regulate the key biological properties of CSCs.
Differential expression of miRNAs in CSCs
Yu and colleagues were the first to examine the miRNA expression in breast CSCs (BCSCs) (11). The authors enriched BCSCs by consecutively passaging breast cancer cells SKBR3 in mice treated with chemotherapy. The tumors were shown to contain a high percentage of CD44+CD24−/lo cells and high ability to form mammospheres in vitro and tumors in vivo. Importantly, the BCSC-enriched cells expressed much lower levels of let-7 as well as a number of other miRNAs including miR-16, miR-107, miR-128 and miR-20b than the parental cells and the in vitro differentiated progeny (11). Later, Shimono et al identified 37 miRNAs to be differentially expressed in CD44+CD24−/lo BCSCs, in which three clusters, i.e., miR-200c-141, miR-200b-200a-429, and miR-183-96-182 were significantly down-regulated (12). Notably, these miRNAs were markedly reduced in normal mammary stem/progenitor cells as well. In glioblastoma multiforme (GBM), some miRNAs including miR-451, miR-486, miR-425, miR-16, miR-107 and miR-185 were decreased in the CD133+ population (13). In hepatocellular carcinoma (HCC), EpCAM+AFP+ CSCs expressed a unique miRNA signature with upregulation of miR-181 family members and several miR-17-92 cluster members (14). Through unbiased miRNA expression profiling, our group recently demonstrated that prostate cancer stem/progenitor cell populations enriched with surface markers CD44, CD133, or α2β1 prominently and commonly under-express miR-34a and let-7b (15).
BCSCs
BCSCs were the first CSCs to be reported and are among the best characterized in all CSCs in solid tumors. BCSCs are most commonly enriched using the CD44+CD24−/lo marker profile (12) or Aldefluor assays (16). Because of the early discovery and better understanding of BCSCs, miRNA studies in these cells are also more advanced than in other CSCs. Based on profiling results that let-7 was significantly reduced in BCSCs (11), Yu and colleagues further unraveled that let-7 regulated the stem cell properties, i.e., self-renewal and differentiation. Lentiviral-mediated over-expression of let-7a inhibited cell proliferation, mammosphere formation, tumor formation and metastasis in NOD/SCID mice and reduced the proportion of undifferentiated cells in vitro. In contrast, antagonizing let-7 by antisense oligonucleotides enhanced in vitro propagation of non-CSCs. H-RAS and HMGA2 were identified as the direct downstream targets that partially mediated the let-7 effects (11).
Interestingly, a recent study from the same group suggested that other miRNAs besides let-7 might also play a role in regulating BCSCs since over-expression of let-7 alone was not sufficient to completely block the tumor formation and progression (17). Subsequently, miR-30 was found to be one of the miRNAs markedly reduced in BCSCs and to negatively modulate the stemness of BCSCs. Over-expression of miR-30 in BCSCs not only diminished their self-renewal ability but also reduced anoikis resistance and increased apoptosis by directly targeting UBC9 (ubiquitin-conjugating enzyme 9) and ITGB3 (intergrin β3). Conversely, knocking down endogenous miR-30 with antagomirs enhanced self-renewal, tumor regeneration and metastasis in differentiated breast cancer cells. Impressively, a more complete inhibition of self-renewal and mammospheres in BCSCs was observed when both let-7 and miR-30 were introduced at the same time compared with transfecting either miRNA alone (17). The synergistic BCSC-inhibitory effects of let-7 and miR-30 on BCSC self-renewal suggest that multiple miRNAs may distinctively and concertedly regulate CSC properties (Fig. 1A).
Figure 1. miRNAs distinctively and concertedly regulating key properties of CSCs.
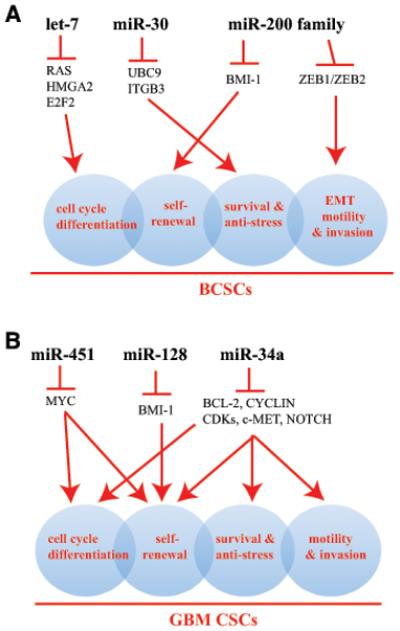
(A) let-7, miR-30, and miR-200 family miRNAs, via targeting critical downstream signaling molecules, regulate several fundamental properties of BCSCs including cell-cycle exit and differentiation, self-renewal, EMT, migration and invasion, and cell survival (represented by 4 shaded circles that overlap with each other). (B) miR-451, miR-128, and miR-34a distinctively and concertedly regulate the key biological properties of CSCs in GBM. Depicted in both (A) and (B) are representative miRNAs that are under-expressed in tumorigenic subpopulations.
miRNA expression profiling in purified CD44+CD24−/lo BCSCs identified 37 miRNAs to be differentially expressed in these cells with miR-200 family significantly down-regulated in both BCSCs and normal mammary stem/progenitor cells (12). Functional studies showed that over-expression of miR-200c reduced the clonogenic and tumor-initiation activities in BCSCs and suppressed formation of mammary ducts by normal mammary stem cells. The stem cell factor BMI-1 was directly modulated by miR-200c. This work (12) thus provides a molecular link between normal breast stem cells and BCSCs.
Recently, ALDH (aldehyde dehydrogenase) has emerged as a functional marker for both normal and malignant stem/progenitor cell populations in various tissues including human (16) and mouse (18) mammary grand. In human mammary epithelial cells, for example, ALDH+ cells were shown to possess high proliferative and broad lineage differentiation potential, and were able to regenerate mammary ductal structures in vivo. Lisewise, breast cancer cells with high ALDH activity were capable of self-renewal and generating tumors in mouse models (16). miRNA expression profiling revealed that miR-205 and miR-22 were most abundant whereas let-7 family members and miR-93 were depleted in ALDH+, Sca-1+ mouse mammary epithelial cells (18). Interestingly, although miR-205 was most abundant in ALDH+ normal mouse mammary progenitor cells, its expression in breast cancer cells remains heterogeneous, varying in different subtypes of breast cancer and at different stages of tumor progression. One group reported high levels of miR-205 in ER+PR+Her2+ breast cancers whereas others reported both high miR-205 expression in triple-negative tumors and low miR-205 levels in metastatic breast cancer cell lines and clinical samples (19).
CSCs are morphologically and phenotypically plastic and possess high migratory and invasive capacities. Several groups have observed that miR-205 and miR-200 family members regulate epithelial-mesenchymal transition (EMT), a process thought to be critical in the metastatic cascade. For example, miR-200 miRNAs and miR-205 are significantly down-regulated in cancer cells undergoing EMT and in metastatic breast cancer specimens (20,21). Over-expression of miR-200 miRNAs prevents TGFβ-induced EMT by negatively regulating the expression of EMT activator ZEB1 (also known as TCF8) and ZEB2 (also known as ZFXH1B and SMAD interacting protein 1 or SIP1). Interestingly, ZEB1 and ZEB2 can also tanscriptionally repress the expression of miR-200 miRNAs by binding to their promoter regions, leading to strong activation of EMT. These findings (20,21) establish a double negative feedback loop between ZEB1/ZEB2 and miR-200 family miRNAs that, together, regulate an important biological process in tumor development and cancer metastasis.
The studies on miRNAs and BCSCs suggest an emerging theme that may also be applicable to understanding how miRNAs regulate other CSCs. BCSCs possess several fundamental biological properties including self-renewal, quiescence associated with slow cell-cycle kinetics or differentiation associated with cell-cycle exit, prosurvival and anti-stress mechanisms (e.g., resistance to anoikis), and high capacities to undergo EMT and to invade, all of which likely contribute to their resistance to anti-cancer therapies and enhanced tumor-initiating and metastatic potential (Fig. 1A). Distinct miRNAs, via their respective downstream targets, distinctively and concertedly regulate these critical CSC properties. Thus, let-7 mainly restricts cell-cycle progression by targeting RAS, HMGA2, and E2F2, miR-30 may preferentially be involved in modulating the survival and stress responses, miR-200 miRNAs negatively regulate the self-renewal by targeting molecules such as BMI-1, and miR-200 (and miR-205) may regulate EMT, migration, and invasiveness in BCSCs (Fig. 1A).
GBM and other brain CSCs
Specific miRNA dysregulation in GBM and other brain CSCs has recently been reported in several studies. By comparing miRNA expression in CD133+ versus CD133− GBM cells, one group identified under-expression of tumor-suppressor miR-451 in the CD133+ population (13). miR-451 is well known to repress Myc expression. Another miRNA expression profiling in human GBM specimens revealed a significant reduction of miR-128 compared to adjacent normal brain tissue (22). Subsequently, miR-128 was shown to inhibit glioma stem cell proliferation in vitro and glioma xenograft growth in vivo. Over-expression of miR-128 significantly blocked glioma CSC self-renewal by directly targeting BMI-1 (22). Finally, miR-34a was found to be down-regulated in human glioblastomas (23). Transfection of miR-34a into bulk GBM cells or GBM CSCs caused cell-cycle arrest or apoptosis and also inhibited xenograft growth, mediated by down-regulation of multiple oncogenic targets including c-MET, Notch-1/2, and CDK6 (23). These studies in GBM (13,22,23) support the concept that several major miRNAs may distinctively and concertedly act together to restrict the key GBM CSC properties (Fig. 1B).
miR-199-5p was down-regulated in medulloblastoma and over-expression of miR-199-5p inhibited proliferation and anchorage-independent growth of medulloblastoma cells by targeting HES-1 (24), a transcription factor of the Notch signaling pathway. Significantly, over-expression of miR-199-5p decreased the CD133+ subpopulation of cells and inhibited tumor development of medulloblastoma cells.
Prostate CSCs
Our group was the first to profile miRNA expression in prostate cancer (PCa) stem/progenitor cells (15). Prostate CSCs (PCSCs) with high tumor-initiating and metastatic potential are enriched in the side population (25), CD44+ (26), and CD44+α2β1+ (27) subpopulations. PCa cells with CD133+CD44+α2β1+ phenotype also demonstrate enhanced clonogenic potential in vitro (28). Through an unbiased miRNA expression profiling in five PCa stem/progenitor cell populations purified from prostate cancer xenografts, including three CD44+ populations from the LAPC9, LAPC4 and Du145 tumors, CD133+ cells from LAPC4 tumors, and α2β1+ cells from Du145 tumors, we identified miR-34a, together with let-7b, to be commonly under-expressed in all marker-positive cell populations (15). The under-expression of miR-34a was subsequently corroborated in CD44+ PCa cells purified from ~20 patient prostate tumors. Over-expression of miR-34a in bulk PCa cells or purified CD44+ cells by transfecting with mature oligonucleotide mimics or infecting with lentiviral vectors encoding pre-miR-34a exerted pronounced inhibitory effects on tumor growth and metastasis in vivo. In contrast, neutralizing endogenous miR-34a using antagomirs in bulk or CD44− PCa cells promoted tumor regeneration and metastasis. Strikingly, delivery of miR-34a oligos systemically through tail vein inhibited metastasis to the lung and other organs and prolonged the survival of animals bearing orthotopic human PCa, indicating the therapeutic potential of this miRNA. Mechanistically, miR-34a suppressed PCSC properties as it inhibited prostasphere establishment, migration and invasiveness of CD44+ PCa cells, and serial prostasphere passaging and serial tumor transplantation. Of significance, we demonstrated that CD44 itself represented a direct and relevant downstream target of miR-34a. Hence, the CD44 protein levels decreased in cells over-expressing miR-34a and knocking down of CD44 functionally phenocopied the miR-34a effects in inhibiting tumor development and metastasis. Our findings (15) shed new light on the mechanisms of miRNA regulation of PCSCs.
Other CSCs
Interestingly, miR-34, a transcriptional target of p53, not only inhibits the GBM CSCs (23) and PCSCs (15) but also restrains the biological properties of pancreatic and gastric CSCs (29,30). Restoration of miR-34 expression in these latter CSCs inhibits sphere formation in vitro and tumor regeneration in vivo (29,30). HCC CSCs identified by EpCAM+AFP+ marker profile overexpressed the miR-181 family and several miR-17-92 cluster members (14). Inhibition of miR-181 led to a reduction in the number of EpCAM+ HCC cells and in tumor-initiating ability whereas over-expression of miR-181 increased the EpCAM+ cells. The biological effects of miR-181 might be mediated via targeting CDX2 (caudal type homeobox transcription factor 2), GATA6, and NLK (nemo-like kinase), a Wnt/beta-catenin pathway inhibitor (14).
Therapeutic implications and perspectives
Dysregulation of miRNAs has been intimately implicated in tumor development and miRNAs may regulate tumorigenesis via modulating CSC properties. Thus, let-7 miRNAs control the cell-cycle and differentiation properties of BCSCs, miR-200c modulates the self-renewal of BCSCs by targeting Bmi-1, and miR-34a restricts the migratory and invasive properties of PCSCs by directly repressing CD44. The new findings discussed above better our understanding of CSC regulation and provide novel insight on developing new strategies to target therapy-resistant cancer cells. Given that CSCs appear to be involved in multiple steps of tumorigenesis including tumor initiation, tumor maintenance, metastasis, and therapy resistance, and that miRNAs exert a broad regulatory role on tumor development, miRNA based therapeutics that specifically targets CSCs may add novel firepower to the anti-cancer arsenal, as exemplified by our recent demonstrations of the impressive therapeutic efficacies of systemically delivered miR-34a on pre-established human prostate cancers. As distinct miRNAs seem to distinctively and concertedly regulate key and interconnected biological properties of CSCs (Fig. 1), complete eradication of CSCs and residual tumors may entail manipulations or targeting of multiple miRNAs. In addition to developing miRNAs as anti-CSC therapeutics, miRNA expression profiling in CSCs or specific subtypes of cancer and at various clinical stages may have diagnostic and prognostic values.
Acknowledgments
Grant Support
Work in the authors’ lab was supported, in part, by grants from the National Institutes of Health (R01-ES015888, R21-ES015893, R21-CA150009), Department of Defense (W81XWH-08-1-0472), and Elsa Pardee Foundation (D.G.T) and by two Center Grants (CCSG-5 P30 CA016672-34 and ES007784). C. Liu was supported in part by a predoctoral fellowship from DOD. We apologize to the colleagues whose original work could not cited due to space constraint.
Footnotes
Disclosure of Potential Conflict of Interest
The authors disclose no potential conflict of interest.
References
- 1.Visvader JE, Linderman GJ. Cancer stem cells in solid tumors: accumulating evidence and unresolved question. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. microRNA: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–7. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 5.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–5. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese JM, Seila AC, Yeo GW, Sharp PA. RNA sequence analysis defines Dicer’s role in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:18097–102. doi: 10.1073/pnas.0709193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–33. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 10.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;15:843–52. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, et al. MIR-451 and Imatinib mesylate inhibit tumor growth of glioblastoma stem cells. Biochem Biophys Res Commun. 2008;376:86–90. doi: 10.1016/j.bbrc.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 14.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–80. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. MiR-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;9:4194–204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 18.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21:3238–43. doi: 10.1101/gad.1616307. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene SB, Herschkowitz JI, Rosen JM. The ups and downs of miR-205: identifying the roles of miR-205 in mammary gland development and breast cancer. RNA Biol. 2010;7:300–4. doi: 10.4161/rna.7.3.11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 21.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godlewski J, Nowicki MO, Bronisz A, Willians S, Otsuki A, Nuovo G, et al. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–30. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–76. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garzia L, Andolfo I, Cusanelli E, Marino N, Petrosino G, De Martino D, et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS One. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 26.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 27.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+ α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 28.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 29.Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]


