Abstract
We hypothesized that decision-making strategies in juvenile animals, rather than being immature, are optimized to navigate the uncertainty and instability likely to be encountered in the environment at the time of the animal's transition to independence. We tested juvenile and young adult mice on discrimination and reversal of a 4-choice and 2-choice odor-based foraging task. Juvenile mice (P26–27) learned a 4-choice discrimination and reversal faster than adults (P60–70), making fewer perseverative and distraction errors. Juvenile mice had shorter choice latencies and more focused search strategies. In both ages, performance of the task was significantly impaired by a lesion of the dorsomedial frontal cortex. Our data show that the frontal cortex can support highly flexible behavior in juvenile mice at a time coincident with weaning and first independence. The unexpected developmental decline in flexibility of behavior one month later suggests that frontal cortex based executive function may not inevitably become more flexible with age, but rather may be developmentally tuned to optimize exploratory and exploitative behavior for each life stage.
Keywords: Executive function, Adolescence, Mouse, Cortex, Uncertainty, Lesion
1. Introduction
Executive function describes a set of overlapping neurocognitive processes that are essential for the expression of goal-directed behavior. Executive function is thought to mature in parallel with the development and maturation of the frontal cortex (Casey et al., 2008, Gogtay et al., 2004, Paus et al., 2008). Cognitive flexibility, a hallmark of high level executive function, is dependent on appropriate feedback utilization, working memory capacity, and conceptual transfer (Anderson, 2003). The ability to flexibly adjust behavior in response to dynamic environmental contingencies is one of the most important aspects of adaptive goal-directed behavior.
Children show developmental increases in performance on tests of cognitive flexibility, with specific aspects of flexibility maturing at different rates. Response inhibition and the ability to switch between response sets for simple stimuli emerges by age 5 (Espy, 1997). The ability to focus attention on specific dimensions of complex stimuli and shift between attention sets reaches levels of adult-like performance at age 11–12 (Chelune and Baer, 1986, Crone et al., 2004, Luciana and Nelson, 1998, Romine et al., 2004, Somsen, 2007). Although young children can perform shift and reversal tasks at above chance performance, maturation of top-down inhibitory control and working memory likely contribute to increase efficiency and performance through mid- to late adolescence (Crone et al., 2006b, De Luca et al., 2003, Huizinga et al., 2006, Luna et al., 2004). Overall, human executive function is thought to increase from childhood through young adulthood, and to decline only after middle age (Cepeda et al., 2001, De Luca et al., 2003, Rhodes, 2004).
A current model of human brain development proposes that development of the frontal cortex lags in relation to subcortical motivational systems during late childhood and adolescence (Casey et al., 2008). The nucleus accumbens shows exaggerated activation relative to prefrontal cortex activation in adolescents (Eshel et al., 2007, Galvan et al., 2006). This pattern accounts for heightened responsiveness to incentives (Galvan, 2010) with insufficient top-down cognitive control, resulting in risk-taking and poor decision-making in adolescents compared to adults. From a public health perspective, adolescence is a period of vulnerability to suboptimal decision-making with consequences for increased rates of injury, pregnancy, addiction, and other adverse outcomes (Arnett, 1992).
Despite the public health statistics, the unique behavioral characteristics of the late childhood and adolescent period may also be developmentally appropriate, perhaps even optimal in an evolutionary sense (Steinberg and Belsky, 1996). During adolescence, the subjective evaluation of risks inherent to possible outcomes may rely on life stage appropriate drives. For example, adolescents may value peer approval to a greater degree than adults and let this factor play a large role in their decisions (Gardner and Steinberg, 2005). Thus, what adults malign as “risky behavior” in adolescence perhaps is not necessarily driven by impulsivity or the inability to evaluate relative costs and benefits (Steinberg, 2008). To the contrary, adolescents (and even older children) may exhibit high levels of goal-directed decision-making, although it may be motivated by differential evaluation of decision outcomes than in adults (Furby and Beyth-Marom, 1992). Investigation of behavioral development in animal models may provide an informative contrast to human studies.
In altricial animals that become independent early in life, such as rodents, executive function, and the circuits supporting it, may be developmentally specialized for decision-making appropriate for the environmental demands specific to different life stages. For rodents, highly flexible foraging strategies with short temporal integration may be more adaptive during periods of instability and risk such as weaning and natal site dispersal, characteristic of early life (König and Markl, 1987, Laviola et al., 2003). In contrast, exploitative strategies with longer temporal integration may be optimal later in adulthood when a nest site and stable territory have been established.
Specific aspects of decision-making and executive function in rodents can be assessed using digging-based reversal and set-shifting paradigms inspired by the intra-dimensional/extra-dimensional shift task developed for use in primates as well as humans by Roberts and colleagues (Birrell and Brown, 2000, Bissonette et al., 2008, Garner et al., 2006, Roberts et al., 1988). Tasks that use odor and texture cues that predict buried food reward are rapidly learned by rodents and likely tap ethologically relevant foraging strategies. Mice and rats can learn these tasks and reverse a contingency rule within hours, making them useful models to measure developmental changes in decision making.
In order to compare juvenile and adult decision-making strategies in different conditions of uncertainty, we employed both 4-choice and 2-choice versions of a digging task. Reversal of a reward contingency in an odor discrimination 4-choice task requires feedback utilization and behavioral inhibition. Multiple potential choices modulate the load on working memory and attention (D’Cruz et al., 2011, Kim and Ragozzino, 2005), and may better approximate the wide variety of choice in an open environment. A 2-choice digging task which includes reversals and attention shifts has been more commonly studied in rodents, and uses complex multimodal stimuli to create uncertainty. In the 2-choice digging task, only one dimension of a stimulus (e.g. odor) predicts the location of a reward while the other dimension (e.g. texture) provides irrelevant information. An attention set-shift component of the task requires the mice to transfer the rule of which dimension is rewarded to a new set of compound stimuli (intra-dimensional shift; IDS). The strength of learning of the attention set for that dimension can also be challenged when the previously irrelevant dimension is rewarded (extra-dimensional shift; EDS) (Birrell and Brown, 2000). We hypothesized that the presence of multiple choices in the 4-choice task might be a better model for a naturalistic foraging environment and make the task more sensitive to performance errors, and therefore reveal differences in decision-making strategies employed by juvenile mice compared to adults.
Maturation of the frontal cortex is thought to drive changes in behavior during peri-adolescent development. Previous studies have shown that regions of frontal cortex have dissociable roles in cognitive flexibility. Lesion studies in rodents have shown that orbitofrontal lesions impair 2-choice reversal learning while medial prefrontal cortex lesions impair performance on extra-dimensional shifts (Birrell and Brown, 2000, Bissonette et al., 2008, Kim and Ragozzino, 2005, McAlonan and Brown, 2003). Inactivation of the dorsomedial frontal cortex (centered on the cingulate) in rats disrupts reversal performance on a 4-choice reversal task (Ragozzino and Rozman, 2007). Additionally, studies in monkeys have implicated neurons in the cingulate cortex in guiding choice based on the history of actions and outcomes (Kennerley et al., 2006). Cingulate neurons also distinguish between exploratory and exploitative decisions made in a dynamic foraging task, and their firing rates predict the strategy used next (Pearson et al., 2009). To confirm the involvement of the frontal cortex in reversal learning at different ages, we compared performance of juvenile and adult mice with sham operations and dorsomedial frontal cortex lesions targeting the cingulate region in a 4-choice odor discrimination and reversal task.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice were bred in our animal facility and were housed on a 12 h/12 h reverse light-dark cycle (lights on at 10 PM). Mice were weaned on postnatal day (P) 21 and group housed with nesting material and toys. Food restriction began three days before behavioral pre-training. During food restriction, juvenile mice were given 1.5–1.8 g of food per day and continued to gain weight throughout the experiment. Juvenile mouse weights on testing day ranged from 8 to 11.7 g (M = 106 ± 1.41% of weaning weight). Age matched mice that were not food restricted gained a similar percentage of weight by P26 (M = 109 ± 1.18% of weaning weight). Adult mice were moderately food restricted and ranged from 17.5 to 23.4 g at testing (M = 87 ± 0.89% of ad libitum weight). Water was freely available both in the homecage and in the maze during all phases of behavioral testing. All animal procedures were approved by the Ernest Gallo Clinic and Research Center Institutional Animal Care and Use Committee.
2.2. Dorsomedial frontal cortex lesions
Bilateral stereotactic lesions were made in the dorsal prefrontal cortex of juvenile and adult mice three days before behavioral pre-training. Lesions were made under isoflurane anesthesia using established coordinates (Franklin and Paxinos, 2008). Intramuscular dexamethasone (0.06 mg/kg; Vedco, St. Joseph, MD) was administered prior to surgery. Using a Nanojet II injector (Drummond Scientific Company, Broomall, PA), 0.1 μl of NMDA (20 mg/ml in sterile 0.9% saline) was injected at four lesion sites (AP: +1.7 mm or +0.6 mm; ML: ±0.5 mm; V: 0.4 mm at rostral position or 0.8 mm at caudal position). For control sham operations, saline vehicle was injected at the same coordinates. Prior to surgery and during recovery, mice were given access to 0.5 mg/ml cherry-flavored acetaminophen solution (Perrigo, Allegan, MI) and 0.7 mg/ml oral sulfamethoxazole with 0.1 mg/ml trimethoprim antibiotic solution (Hi-Tech Pharmacal, Amityville, NY) in drinking water.
2.3. Apparatus
The 4-choice maze was a square box 12″ × 12″ × 9″ constructed of 0.25″ clear acrylic (Fig. 1a and b). Four internal walls measuring 3″ wide partially divided the four quadrants. Odor stimuli were presented in white ceramic pots measuring 2.88″ diameter and 1.75″ deep. Pots were sham baited with a Honey Nut Cheerio (General Mills, Minneapolis, MN) secured underneath a mesh screen at the bottom. A 6″ diameter removable cylinder fit in the center of the maze and was lowered between trials (after a digging response) to isolate the mouse from the rest of the maze.
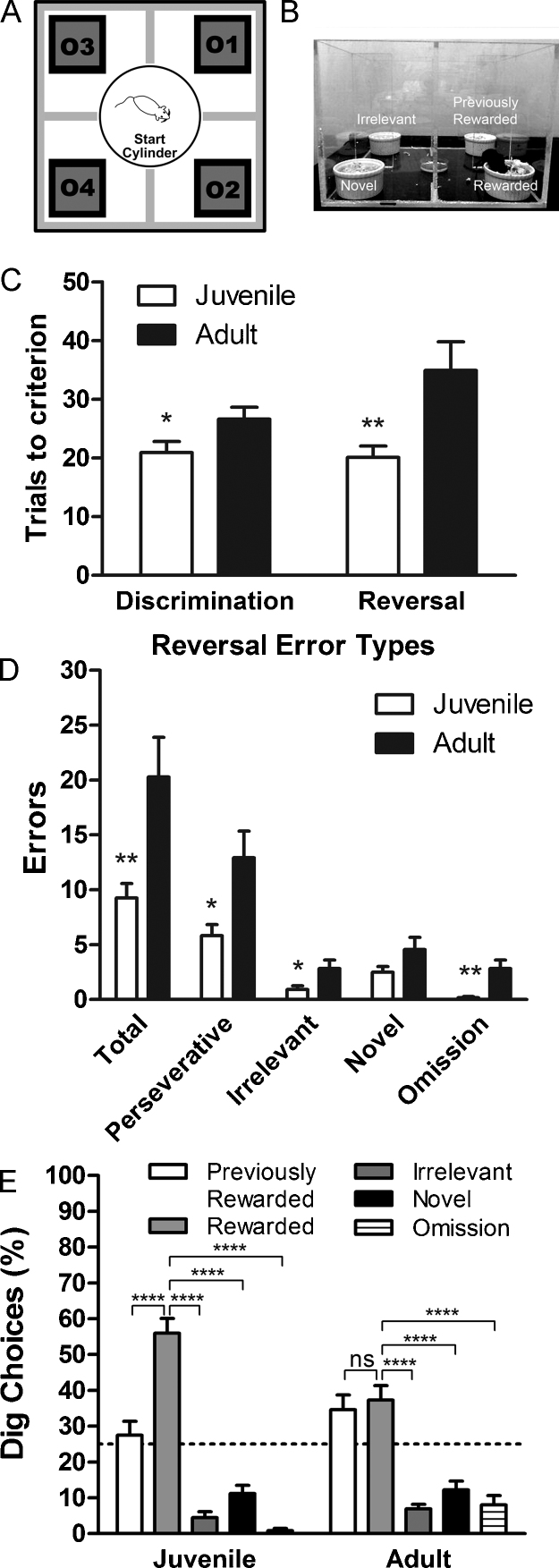
Fig. 1.
Juvenile mice show greater behavioral flexibility in a 4-choice odor discrimination and reversal task. (A and B) Schematic of arena used in 4-choice task. Mice learned to discriminate among four odor choices to find a buried cereal reward. The odor cue-reward contingency was reversed in the same session, and a novel odor was introduced. (C) Juvenile mice required fewer trials to reach criterion in both the discrimination and reversal phase. (D) Analysis of reversal error type showed that juvenile mice made fewer total, perseverative, and irrelevant errors compared to adults. (E) The number of choices to dig in an odor was plotted as a percentage of total trials. Juvenile mice dug in the rewarded odor pot more often than all other choices, while adults dug equally in the previously rewarded and currently rewarded odor pots during the reversal. The dotted line represents chance performance. Bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, uncorrected.
A smaller maze constructed of 0.25″ clear acrylic measuring 7.5″ × 7.5″ × 7.5″ was used in the 2-choice discrimination test (Fig. 4a and b). The maze was divided by a removable gate into a start compartment and two smaller 3″ × 3″ compartments for the digging pots. The digging pots were acrylic boxes measuring 2.5″ × 2.5″ × 2.5″.
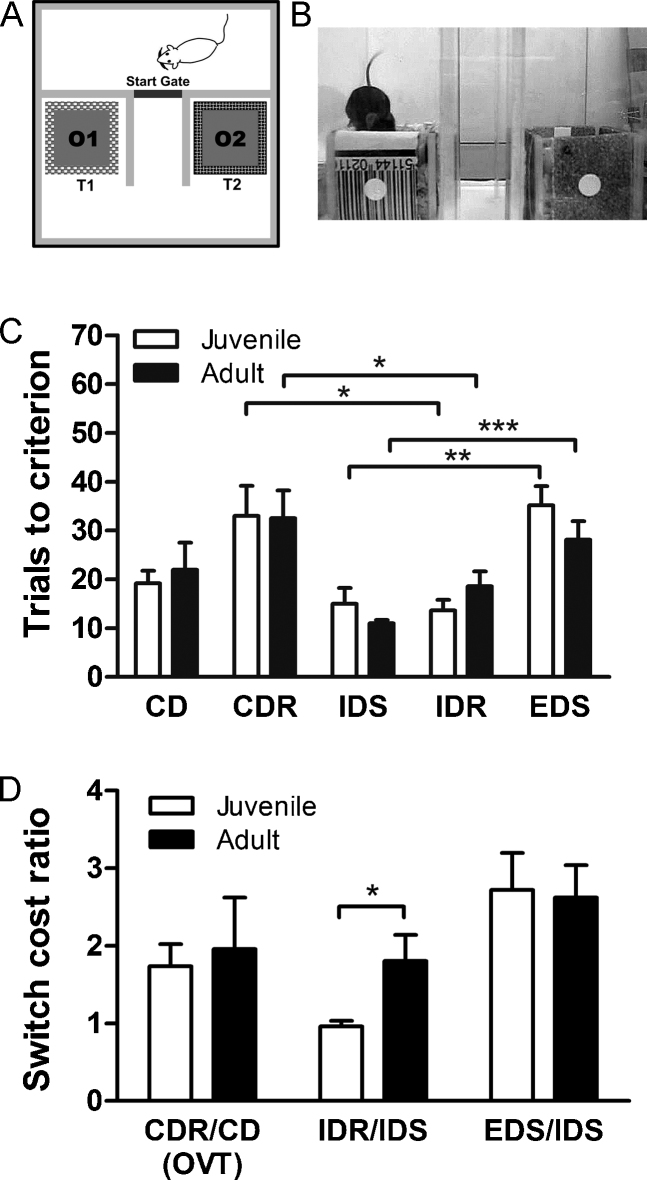
Fig. 4.
Juvenile and adult performance on a 2-choice attention set-shift task. (A and B) Schematic of 2-choice arena. Compound stimuli had an odor dimension (scented digging material) and a texture dimension (texture wrapped around outside of pot). Mice learned a series of compound discriminations (CD; IDS) and reversals (CDR; IDR) over the course of six sessions in which odor was the rewarded dimension and texture was irrelevant. On the final day, the attention set of the mice was challenged by an extra-dimensional shift (EDS) in which texture was now the rewarded dimension. (C) Juvenile and adult mice did not show differences in the number of trials to reach criterion on any task phase. Both age groups required more trials to complete CDR (after overtraining) than IDR. Juvenile and adult mice also required more trials to complete EDS compared to IDS, indicating the formation of an attention set. (D) The switch cost is the ratio between the trials to criterion in a rule switch stage divided by trials to learn the compound discrimination before the switch. Juveniles had a significantly lower switch cost in the IDR phase, but were not different from adults after overtraining in the CDR phase, or in the EDS phase. Bars represent the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, uncorrected.
2.4. Four-choice odor discrimination and reversal
2.4.1. Pre-training procedure
The 4-choice odor discrimination and reversal task was adapted from Kim and Ragozzino (2005). The first day of pre-training was a habituation session to familiarize the mice with the maze and pots. Small pieces of Honey Nut Cheerio (approximately 10 mg each) were placed inside of four empty digging pots, one in each of the four quadrants. The mouse was placed in the start compartment. The cylinder was then lifted and the mouse was allowed to explore the maze and consume the cereal pieces in the pots. After 10 min, the mouse was returned to the start cylinder and the pots were re-baited. This procedure was repeated for a total habituation time of 30 min. The maze was wiped with 70% ethanol between animals.
The second day of pre-training was a shaping session to teach the mice how to dig to find cereal pieces buried in coarse pine wood shavings (Hartz Mountain Corporation, Secaucus, NJ). One pot with increasing amounts of wood shavings covering the cereal reward was used in this shaping phase. The quadrant containing the pot was alternated in each trial (SE to NW to SW to NE) and all quadrants were rewarded equally. Trials were untimed and consisted of two trials with no shavings covering the cereal piece followed by two trials with a dusting of shavings, two trials where the pot was a quarter full, two trials of half full pots, and finally four trials with the cereal piece completely buried. Most animals retrieved the reward in the 12 total shaping trials within 1 h.
2.4.2. Four-choice discrimination and reversal procedure
Wood shavings were scented on the day of testing. Anise extract (McCormick, Hunt Valley, MD) was used undiluted at 0.02 ml/g of shavings. Clove, litsea, and eucalyptus oils (San Francisco Massage Supply Co., San Francisco, CA) were diluted 1:10 in mineral oil and mixed at 0.02 ml/g of shavings. Thymol (“thyme”; Alfa Aesar) was diluted 1:20 in 50% ethanol and mixed at 0.01 ml/g of shavings. The odors used in the initial discrimination and reversal phases are listed in Table 1.
Table 1.
Four-choice odor stimuli.
| O1 | O2 | O3 | O4 | |
|---|---|---|---|---|
| Discrimination | Anise | Clove | Litsea | Thyme |
| Reversal | Anise | Clove | Litsea | Eucalyptus |
During the initial discrimination phase, the animal had to discriminate among four odors and learn which one was associated with a buried Cheerio reward. The stimulus presentation was pseudo-randomized such that an odor was never in the same quadrant two trials in a row. Each trial began with the mouse confined to the central start cylinder, which was equidistant to all the odor pots. Timing began when the cylinder was lifted. Entry to each quadrant was recorded if all four paws crossed beyond the perimeter of the start area. The mouse could freely explore the arena until it chose to dig in a pot. Latency was the time elapsed until a dig. Digging was defined as purposefully moving the shavings with both front paws, but not as superficial sniffing or chewing of the shavings. The cylinder was lowered as soon as the mouse initiated digging to prevent multiple digging choices. If an incorrect choice was made, then the trial was terminated and the mouse was returned to the start cylinder. All pots were removed from the maze and re-baited, if necessary, between trials. A trial was terminated if no choice was made within 3 min and was recorded as an omission. Analysis of entries or latency did not include omission trials. If the animal had two omission trials in a row, digging was reinstated by placing a pot of unscented shavings with a well exposing the cereal piece in the start cylinder. After three pairs of omission trials (unusual), the currently rewarded odor pot was placed in the start cylinder with a well exposing the cereal piece. These hint trials were not included in the final analysis. Criterion was met when the animal completed 8 out of 10 consecutive trials correctly.
Once criterion was met on the discrimination phase, the animal moved on to the reversal phase immediately within the same session. All shavings were replaced with new shavings to prevent discrimination via unintentional cues. Odor four was swapped out for a novel odor. Perseverative errors were choices to dig in the pot with the previously rewarded odor. Irrelevant errors were choices to dig in the pot with the odor that was never rewarded. Novel errors were choices to dig in the pot with the newly introduced odor, which was also never rewarded. To complete the reversal, the mouse had to reach criterion by completing 8 out of 10 consecutive trials correctly. Animals typically completed both discrimination and reversal phases within 3 h. Before being returned to the homecage, the mouse was placed in a container containing shavings bearing all five odors to prevent transmission of an odor preference to its cagemates (Galef, 1977).
2.5. Two-choice reversal and attention set-shift
2.5.1. Pre-training procedure
Mice are able to perform a digging-based attention set-shift task similar to that used in rats (Garner et al., 2006). On the first day of pre-training the mice were habituated to the maze. Mice began in the start compartment with the gate down. The gate then was lifted and the mouse was allowed to explore and consume a piece of cereal from each of two empty pots. The mouse was returned to the start and the pots were re-baited every 5 min for a total habituation time of 30 min.
Mice learned to dig in wood shavings to find a buried cereal piece on the second day of pre-training. Two pots were used and the mouse had to consume cereal pieces from both pots to complete a trial. Shaping trials progressed from one trial with empty pots to one trial with a dusting of shavings, one trial with a quarter full pots and one trial with half full pots. The final three trials consisted of the presentation of two pots full of shavings, each containing a buried piece of cereal.
2.5.2. Two-choice reversal and set-shift procedure
Odor and texture stimuli used in each phase of the 2-choice task are listed in Table 2. Cinnamon, vanilla, almond, and coconut extracts (McCormick, Hunt Valley, MD) were mixed undiluted with wood shavings (0.02 ml/g of wood shavings). Lemongrass and blood orange oil (San Francisco Massage Supply Co., San Francisco, CA) were diluted 1:10 in mineral oil and mixed at 0.02 ml/g with shavings. Each texture pair consisted of reverse sides of the same material to ensure consistent odor cues. Textured materials were wrapped around the outside, rim, and interior of the digging pots. The identity of the rewarded exemplar from each pair was balanced across each group. There were four possible combinations of odor and texture in each phase. These were presented pseudo-randomly and balanced for spatial location.
Table 2.
Two-choice exemplar combinations.
| Day | Phase | Odor |
Texture |
||
|---|---|---|---|---|---|
| O1 | O2 | T1 | T2 | ||
| 1 | CD | Cinnamon | Vanilla | Velvet | Reversed Velvet |
| 2 | OVT | Cinnamon | Vanilla | Velvet | Reversed Velvet |
| 3 | OVT | Cinnamon | Vanilla | Velvet | Reversed Velvet |
| 4 | CDR | Cinnamon | Vanilla | Velvet | Reversed Velvet |
| 5 | IDS | Almond | Coconut | Diaper Paper | Reversed Diaper Paper |
| 6 | IDR | Almond | Coconut | Diaper Paper | Reversed Diaper Paper |
| 7 | EDS | Orange | Lemongrass | Sandpaper | Reversed Sandpaper |
A trial commenced with the animal in the start compartment and timing began when the start gate was lifted. The latency to dig was recorded using the same definition of digging as the 4-choice task. If the mouse did not dig within 5 min, the trial was terminated and recorded as an omission. Omission trials were rare in the 2-choice task (1% of trials). The animal reached criterion when it correctly completed 8 out of 10 consecutive trials.
The first day of training was a compound discrimination (CD) in which the pots could be distinguished by either odor or texture information, but only odor was the relevant rewarded dimension. Days 2 and 3 were overtraining (OVT) on the same stimulus pairs from CD to a criterion of 45 correct trials over two days. On day four, the learned association from CD was reversed (CDR) such that the previously unrewarded odor in the pair became rewarded. Day 5 was an intra-dimensional shift (IDS) that introduced a new set of stimuli in which odor remained the dimension predictive of reward and texture provided irrelevant information. Day 6 reversed (IDR) the rewarded odor from IDS. The final day of training was an extra-dimensional shift (EDS) where a novel compound discrimination was presented, but the texture dimension now predicted in which pot the reward was buried. Thus, after six days of developing an attention set for odor, the animal was required to shift attention to the previously irrelevant texture dimension. Others have shown that the development of an attention set requires several sessions of experience with the initial rewarded dimension, including overtraining on CD, intra-dimensional shift (IDS), and intra-dimensional reversal (IDR) (Bissonette et al., 2008, Garner et al., 2006).
2.6. Histology and immunohistochemistry
Lesion and control operated animals were transcardially perfused with 4% paraformaldehyde in PB (0.1 M, pH 7.4) promptly after training. Coronal sections (50 μm) were cut using a vibratome and adjacent sections were stained using cresyl violet and rabbit anti-GFAP (Chemicon) antibodies using standard methods (Bissonette et al., 2008). To trace the extent of the lesions, cresyl violet and anti-GFAP stained images were overlaid with electronic images from an adult mouse brain atlas (Franklin and Paxinos, 2008) using Adobe Photoshop CS4.
2.7. Statistical analysis
Values are reported as mean (M) ± SEM. Two-tailed t tests were used for all statistical comparisons, unless otherwise noted. An extra sum-of-squares F test was used to compare cumulative performance data models. Statistical significance was set at P < 0.05; analysis and graphing were performed with GraphPad Prism v5.05.
3. Results
3.1. Four-choice odor discrimination and reversal
We compared juvenile (N = 12) and adult (N = 11) mice in a 4-choice odor discrimination and reversal paradigm. Juveniles required fewer trials than adults to reach criterion in the initial discrimination phase [t(21) = 2.08, P < 0.05] (Fig. 1c). In the reversal phase, a previously irrelevant odor (Odor 2) predicted the location of the reward and a novel odor (Odor 4) was introduced (Table 1). During the reversal phase, juvenile mice reached criterion in significantly fewer trials than adults [t(21) = 3.22, P < 0.01] (Fig. 1c) and made significantly fewer errors [t(21) = 2.95, P < 0.01] (Fig. 1d). Further analysis of reversal error type revealed that juveniles made significantly fewer perseverative errors [t(21) = 2.77, P < 0.05], significantly fewer irrelevant errors [t(21) = 2.30, P < 0.05], and had fewer omission trials [t(21) = 3.08, P < 0.01]. There was no significant difference in novel errors [t(21) = 1.70, P = 0.10] (Fig. 1d). We also compared the trial number upon which mice made the first novel error and found no significant difference between age groups [juvenile: M = 5.33 ± 1.51; adult: M = 10.18 ± 3.62; t(21) = 1.28, P = 0.22]. The number of choices to dig in each odor was normalized to the total number of trials completed by the animal during the reversal phase. Juveniles dug in the rewarded odor in a significantly greater percentage of trials [t(22) = 5.00, P < 0.0001]. In contrast, adults dug in the rewarded and previously rewarded odors a similar percentage of trials [t(20) = 0.46, P = 0.65] (Fig. 1e).
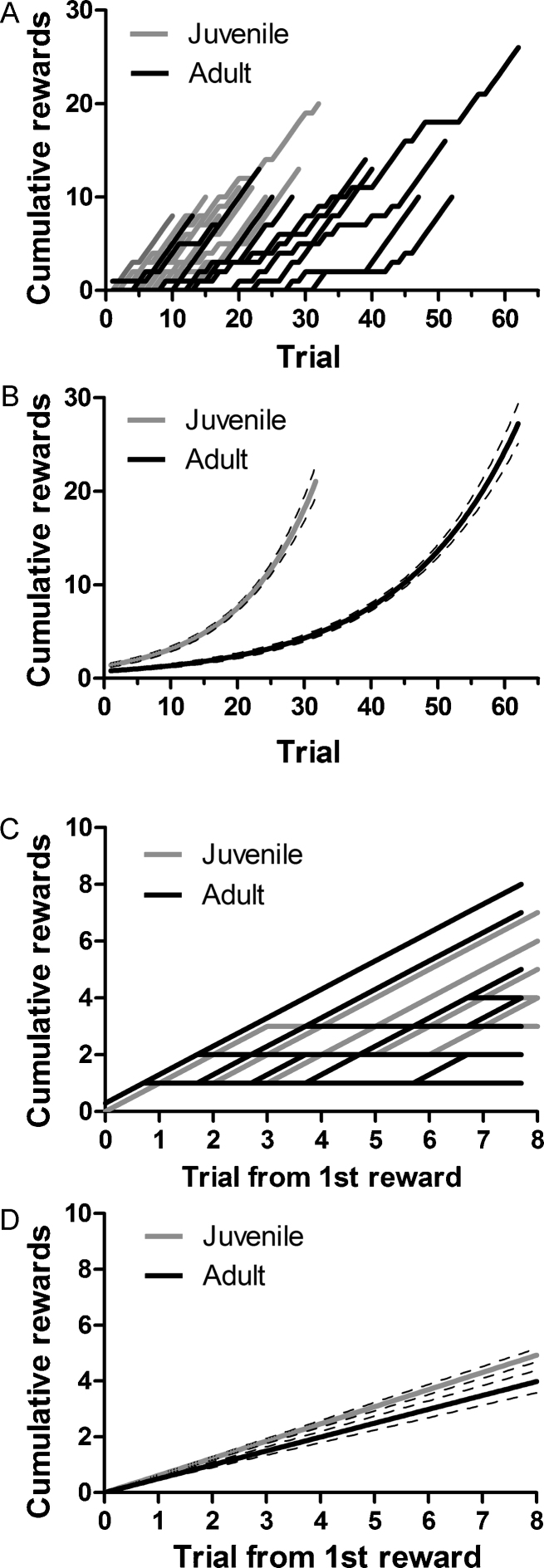
We further explored the time course of reversal learning in juvenile and adult mice during the 4-choice task. The cumulative number of rewards achieved was plotted vs. trial number (Fig. 2a). In this plot, an animal with perfect performance would have a slope of one, whereas errors result in flattening of the slope. If errors were evenly distributed, then the data would be best-fit by a straight line. However, if errors were concentrated at the beginning of the reversal and rewards were accumulated near the session end, then the data would be best-fit by an exponential in the form of Y = Y0*exp(X/τ) where tau (τ) is the time constant. The data were best-fit by exponential growth curves [juvenile: R2 = 0.74, τ = 11.41 trials, 95% confidence interval (CI) = (10.60, 12.35); adult: R2 = 0.70, τ = 17.35 trials, 95% CI = (16.21, 18.67)] (Fig. 2b). Comparison using an extra sum of squares F test showed a significant difference in age groups in reward accumulation [F(1,635) = 49.62, P < 0.0001].
Fig. 2.
The time course of reversal learning following feedback is different between juvenile and adult mice. (A) The cumulative number of rewards achieved was plotted against trial number. Each line represents one animal. Although juvenile and adult mice achieved a similar number of rewards, adult mice required more trials to do so. (B) Exponential growth curves best modeled the course of reversal learning. Juveniles accumulated rewards at a greater rate than adults (P < 0.0001; juvenile: τ = 11.41; adult: τ = 17.35). (C) To investigate changes in behavior after positive feedback, the cumulative number of rewards was aligned for all subjects to the trial the first reward was achieved. Lines represent individual animals, and adult lines are offset when overlapping juveniles to enhance visibility. (D) Straight lines best modeled the rate of reward accumulation after the first correct response in the reversal phase. Juveniles showed a steeper rate of accumulating rewards after positive feedback than adults (P < 0.0001). Dashed lines in B and D show the 95% confidence interval of best-fit lines.
We next examined the accumulation of rewards after the first correct trial during reversal to see if juveniles and adults differed in reward accumulation after receiving positive feedback (Fig. 2c; data are plotted for only eight trials because after that point some animals have reached criterion). These data were best-fit by straight lines [juvenile: R2 = 0.80, slope = 0.62, 95% CI = (0.59, 0.65); adult: R2 = 0.47, slope = 0.50, 95% CI = (0.45, 0.55)]. Again, comparison using an extra sum of squares F test showed that the rate of reward accumulation after the first correct trial in reversal was significantly different between juveniles and adults [F(1,205) = 16.63, P < 0.0001].
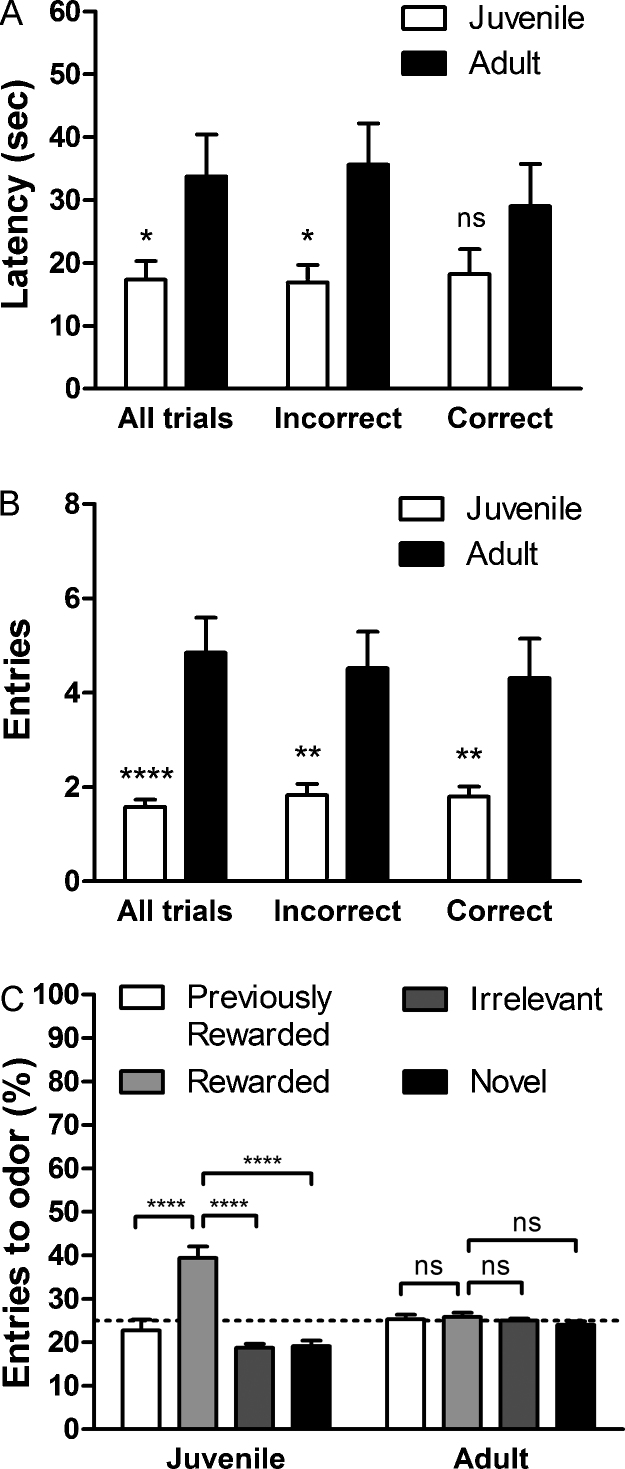
Behavior within each trial during the 4-choice reversal task also differed between adults and juveniles. Juvenile mice showed significantly shorter latency to dig when compared to adults [t(21) = 2.31, P < 0.05]. When trials were separated by performance, juveniles showed significantly shorter latency to dig on incorrect trials [t(21) = 2.69, P < 0.05] but not on correct trials [t(21) = 1.41, P = 0.17] (Fig. 3a). Juveniles also made significantly fewer entries to the four arena compartments in the reversal [t(21) = 4.98, P < 0.0001], as well as when trials were divided into incorrect [t(21) = 3.47, P < 0.01] and correct trials [t(21) = 3.04, P < 0.01] (Fig. 3b). However, there was no difference between juveniles and adults in the rate of entries per minute [juvenile: M = 6.99 ± 1.02; adult: M = 11.60 ± 3.17; t(21) = 1.44, P = 0.17] indicating that speed alone cannot account for the differences in latency or entries. Entries to each odor were plotted as percentages of total entries (Fig. 3c). Juveniles made proportionally more entries to the rewarded odor compared to the other odors [previously rewarded: t(21) = 4.33, P < 0.001; irrelevant: t(21) = 6.97, P < 0.0001; novel t(21) = 6.51, P < 0.0001, uncorrected P value shown]. In contrast, adults made entries to all the odors equally [previously rewarded: t(20) = 0.37, P = 0.71; irrelevant: t(20) = 0.75, P = 0.46; novel t(20) = 1.38, P = 0.18, uncorrected P value shown].
Fig. 3.
Measures of motivation and response planning differ between juvenile and adult mice in 4-choice reversal learning. (A) Latency to dig was significantly shorter in juvenile mice, but there was no difference from adult mice when only correct trials were considered. (B) Juvenile mice made significantly fewer entries within a single trial for both incorrect and correct trials. Bars represent the mean ± SEM. (C) The number of entries in a reversal trial was plotted as a percentage of the total trial entries. Juvenile mice made a significantly greater proportion of entries to the rewarded odor quadrant, while adult mice made entries to all the quadrants equally. The dotted line shows the chance level of encountering the quadrants. Note: the location of the four odors was changed each trial. ns P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, uncorrected.
3.2. Two-choice reversal and attention set-shift
Separate groups of juvenile (N = 6) and adult (N = 7) mice were trained on a 2-choice attention set-shift task. There were no significant differences between juvenile and adult mice in the number of trials to reach criterion on any phase of the task [CD: t(11) = 1.28, P = 0.23; CDR: t(10) = 0.06, P = 0.95; IDS: t(11) = 1.30, P = 0.22; IDR: t(11) = 1.27, P = 0.23; EDS: t(11) = 1.28, P = 0.23] (Fig. 4c). Both juveniles and adults required significantly more trials to complete CDR compared to IDR [juvenile: t(10) = 2.96, P < 0.05; adult: t(11) = 2.24, P < 0.05]. If the mice developed an attention set, then EDS should require more trials to complete than IDS. Both juveniles and adults took significantly more trials to reach criterion on EDS compared to IDS [juvenile: t(10) = 3.93, P < 0.01; adult: t(12) = 4.44, P < 0.001]. Furthermore, there was no difference in the number of errors made during overtraining on CD, suggesting that juveniles and adults showed comparable discrimination learning and set maintenance [juvenile: M = 5.33 ± 1.91; adult: M = 10.14 ± 2.22; t(11) = 1.61, P = 0.14].
We further compared performance on rule switching (reversals and shifts) relative to performance in discrimination learning by comparing switch costs for different phases of the 2-choice task. The switch cost was calculated for each animal as the ratio of trials to criterion in a rule switch stage divided by the trials to learn a new set of stimuli in a compound discrimination. For each subject, we calculated the switch cost ratio for CDR/CD, IDR/IDS, and EDS/IDS (Fig. 4d). A ratio of one meant that the mice did not show a switch cost, while a higher ratio indicated that the new rule required relatively more trials to learn than the initial discrimination. We found that on average juveniles showed lower switch costs than adults in IDR [juvenile: M = 0.96 ± 0.07; adult: M = 1.81 ± 0.34; t(11) = 2.28; P < 0.05]. However, juveniles and adults had similar switch costs following overtraining in CDR [juvenile: M = 1.74 ± 0.29; adult: M = 1.96 ± 0.66; t(10) = 0.31; P = 0.77], and also in EDS [juvenile: M = 2.72 ± 0.48; adult M = 2.62 ± 0.42; t(11) = 0.15; P = 0.88].
3.3. Dorsomedial frontal cortex lesions and 4-choice odor discrimination and reversal
To investigate the contribution of the frontal cortex to cognitive flexibility in the 4-choice reversal task in mice, we made excitotoxic lesions and control vehicle injections in a separate group of juvenile (lesion: N = 14; sham: N = 11) and adult mice (lesion: N = 11; sham: N = 9). In the initial odor discrimination, lesions significantly improved acquisition of the task relative to shams in the adult group [t(18) = 3.13, P < 0.01] but not in juveniles [t(23) = 0.06, P = 0.95] (Fig. 5a and b). During the initial discrimination, adult lesion mice made fewer errors after the first correct response compared to sham operated littermates [sham: M = 11.78 ± 1.05; lesion: M = 7.27 ± 0.95; t(18) = 3.17, P < 0.01]. Juvenile lesion mice made a similar number of errors as shams after the first correct response [sham: M = 11.18 ± 1.80; lesion: M = 10.21 ± 2.47; t(23) = 0.30, P = 0.77] in the discrimination. In the reversal phase, lesions significantly increased the number of trials to reach criterion for both juveniles and adults [juvenile: t(23) = 2.64, P < 0.05; adult: t(18) = 2.49, P < 0.05] (Fig. 5a and b). Fig. 5c shows illustrations of the minimum, representative, and maximum extent of lesions in juveniles and adults. Cell loss was clearly visible with cresyl violet histology in the cingulate cortex, and portions of prelimbic and secondary motor (M2) cortices in some animals (Fig. 5c and d). Immunoreactivity for the glial marker GFAP was observed at the edges of the lesions identified by nissl staining (Fig. 5d and e). We compared the rate of entries per minute between lesion and sham operated mice to test for gross coordination or locomotor deficits resulting from any damage to motor cortex. No significant differences in the rate of entries were found comparing sham and lesion animals in either juveniles [t(23) = 0.20, P = 0.83] or adults [t(18) = 1.94, P = 0.10] (Fig. 5f).
Fig. 5.
Lesions of the dorsomedial frontal cortex impair 4-choice reversal learning in juvenile and adult mice. Trials to reach criterion in the odor discrimination and reversal in 4-choice task for lesion and control sham operated juvenile (A) and adult (B) mice. Juvenile lesioned mice required significantly more trials on the reversal phase compared to age-matched sham controls. Adult lesioned mice required fewer trials to achieve criterion in the discrimination phase and more trials than sham controls to reach criterion during the reversal phase. *P < 0.05; **P < 0.01. (C) The maximum extent of bilateral excitotoxic lesions are shown in the lightest grey; medium grey indicates representative lesion size of the majority of animals; and black illustrates the minimum lesion extent. The middle column shows Nissl staining of a representative lesion. Drawings were adapted from Franklin and Paxinos, 2008. No significant damage was observed in sham operated mice. (D) Low power magnification of Nissl stained section of lesioned brain. (E) Adjacent section showing GFAP immunoreactivity at the border of the lesioned area. Images in D and E were taken at 4× magnification. (F) There was no significant difference in the rate of entries per minute between lesion and sham mice, suggesting there was no gross motor impairment induced by the lesion. Bars represent the mean ± SEM.
4. Discussion
In our study of mice performing a 4-choice reversal task, juvenile mice showed greater efficiency and therefore greater flexibility in learning to reverse an association (Fig. 1, Fig. 2). Juvenile mice more quickly abandoned digging in the previously rewarded odor pot after the reward contingency was changed, committing fewer perseverative errors than adults (Fig. 1d). After the first reward was achieved in the reversal phase, juveniles continued to earn rewards at a higher rate than adults (Fig. 2d). These data suggest that juvenile mice adjust their response to both positive and negative outcomes, and that their adjustment behavior in a 4-choice reversal task is more sensitive to recent outcomes when compared to adults. This difference between juveniles and adults may be due to faster updating of outcome expectancy or action selection (or both), and is likely facilitated by a heightened responsiveness to incentives that emerges in the peri-adolescent period (Galvan, 2010, Sturman et al., 2010, Wilmouth and Spear, 2009).
On the surface, slower reversal in adult mice compared to juveniles in the 4-choice task seems in stark contrast to a large literature showing improving cognitive flexibility with development in young humans (Cepeda et al., 2001, Chelune and Baer, 1986, Crone et al., 2004, Crone et al., 2006a, De Luca et al., 2003, Huizinga and van der Molen, 2007, Luciana and Nelson, 1998, Rhodes, 2004, Romine et al., 2004). However, our results may be consistent with subtle observations of strategy from a developmental study of the Iowa Gambling Task (IGT) (Cassotti et al., 2011). The IGT is intended to model real life decision-making, requiring integration of rewards and costs of different sizes and probabilities, and is sensitive to prefrontal cortex damage (Bechara et al., 1994). Overall, adults showed more optimal card choice than adolescents (10–14) and children (9–10) in the IGT, integrating costs and benefits over the entire task to choose an overall strategy biased towards low risk card decks that have relatively low payout but also low costs (Cassotti et al., 2011; see also Crone and van der Molen, 2004). However, analysis of card choice patterns showed that adults switched less often and perseverated more following a loss compared to children or adolescents. With experience, children and adolescents preferred non-optimal risky decks with high rewards and high costs. Between the two risky decks, they were biased towards the deck that delivered less frequent punishment (Cassotti et al., 2011). The behavior of children and adolescents in the IGT was quantitatively non-optimal in the long run in the stable context of the task. However, it does appear to have been driven by a sensitive behavioral strategy that weighed negative and positive outcomes and their temporal integration in a manner different from adults. We hypothesize that heightened responsiveness to incentives and short temporal integration of outcomes may be adaptive in an uncertain and changing environment, and that this may explain developmental differences in decision-making strategies that emerge in different species depending on their life history and age of independence.
Juvenile mice showed shorter latencies in solving the 4-choice reversal task (Fig. 3a) and less exploratory entries (Fig. 3b). Additionally, analysis of the search pattern of entries showed that juveniles spent proportionally more time near the rewarded odor pot than with irrelevant odor pots, while adults explored all the odor pots equally (Fig. 3c). This is consistent with the idea that motivational systems, response planning, and decision-making strategy under conditions of uncertainty differ between juvenile and adult mice. We hypothesize that this difference is due to either increased sensitivity to anticipated reward and/or the high probability of predation risk that juvenile mice are likely to encounter during trial and error exploration of a new environment at the time of first independence (Mabry and Stamps, 2008). Motivational and cognitive systems may be tuned not only to maximize foraging success, but also to manage alternate risks, like predation (Arcis and Desor, 2003), that may also vary with the lifespan.
In a second experiment, we compared a new set of juvenile and adult mice on a commonly used 2-choice reversal and set-shift task (Fig. 4). We found that there was no difference in reversal or set-shift performance between juveniles and adults (Fig. 4c). However, we did find that switch costs varied as a function of prior experience with a rule (Fig. 4d), in accord with previous studies (Bissonette et al., 2008, Garner et al., 2006). Juvenile mice had lower switch costs than adults in a reversal (IDR), but switch cost for juveniles rose to adult levels after overtraining (CDR) (Fig. 4d). These data suggest that decision-making differences between adults and juveniles are magnified under conditions of uncertainty when they have less experience with a rule and context.
A recent report (Newman and McGaughy, 2011) observed that adolescent rats (P40–53) took more trials to reach criterion in a digging based 2-choice reversal and set-shift task than adult rats (P66–80). The contrast of these results with our finding that juvenile mice performed similarly to adults in the 2-choice task (Fig. 4c) may be explained partially by the difference in species and developmental stage at testing. Our young mice were tested in early adolescence before the onset of puberty, while the rats in the Newman and McGaughy study were tested in mid- to late adolescence. Additionally, the experimental design of the 2-choice digging task differed in several key aspects between the studies. Newman and McGaughy used spatially overlapping cues of odor and digging media as the initial rewarded dimensions. In our 2-choice study, the rewarded dimensions of odor and digging pot texture (pots contained equivalent digging media) were spatially segregated. It is possible that juvenile rodents are impaired at distinguishing spatially overlapping stimulus features (see Barrett and Shepp, 1988), but are not more generally impaired in behavioral flexibility. Furthermore, Newman and McGaughy counterbalanced the initial rewarded dimension between odor and media, while our study initially rewarded the odor dimension and then reversed to the texture dimension for all mice.
Different levels of uncertainty, in terms of chance and risk, also distinguish 4-choice and 2-choice tasks (D’Cruz et al., 2011). Two-choice reversal tasks are more commonly employed and have a chance probability of reward of 50%. When one choice is eliminated, it is certain that the other remaining choice contains the reward. Reversal in a 4-choice task reduces the chance probability of reward to 25%. Even when one choice is eliminated, it impossible to know which of the remaining choices is rewarded. Thus, subsequent decisions involve risky trial and error learning. A recent fMRI study in human subjects showed enhanced activation of ventral striatum, midbrain, and motor cingulate regions during a 4-choice compared to a 2-choice task (D’Cruz et al., 2011). These data suggest that in a juvenile or adolescent animal, developmental differences in both motivational and cognitive systems could have then been magnified by testing with a 4- vs. 2-choice design.
We showed that dorsomedial frontal lesions, centered on the cingulate cortex, impacted reversal performance in a 4-choice task in both juvenile and adult mice (Fig. 5). These data suggest that in mice the frontal cortex contributes to performance in this task, but we do not mean to suggest that changes in this brain region are the sole source of the behavioral difference between juvenile and adult mice. Subcortical motivational systems and cortical association areas connect via cortical basal ganglia loops, and therefore maturation of either or both systems likely impact developmental changes in decision-making. However, a recent study showing “surprise signals” (that encode unsigned reward prediction errors) in the anterior cingulate cortex of macaques suggests that the cingulate may be a critical region for rapid adjustment of behavior in response to both positive and negative feedback, particularly under conditions of novelty and uncertainty (Hayden et al., 2011).
Novelty seeking has been shown to be greater in adolescence in rodent studies of spatial exploration (Laviola et al., 2003, Macrì et al., 2002). However, the juvenile mice in our study showed similar responses to a novel odor when compared to adults (Fig. 1d and e). Our mice (males) were P26–27 when they performed the task, which is three days after the time that mice will naturally wean (König and Markl, 1987). In female mice this is the time of vaginal opening and just before the first estrus (Chehab et al., 1997). It is possible an enhanced interest in novelty does not apply across all behavioral domains or emerges after puberty in mice. The precise temporal changes in reversal learning performance between P26 and P60, and their relationship to puberty have yet to be explored. Furthermore, all the mice in our study were male. Future studies will investigate this behavior in females at different life stages. As females and males typically follow different patterns of natal site dispersal in all species to prevent inbreeding, we predict that female mice may show differences in decision-making with age when compared to males.
5. Conclusions
During the peri-adolescent period, motivational systems show greater sensitivity to salience (Galvan, 2010, Sturman et al., 2010, Wilmouth and Spear, 2009) and measures of flexible goal-directed behavior begin to approach adult levels (Chelune and Baer, 1986, Crone et al., 2004, Crone et al., 2006a, De Luca et al., 2003, Huizinga and van der Molen, 2007, Luciana and Nelson, 1998, Rhodes, 2004, Romine et al., 2004). Yet, decision-making skills in children and adolescents are often compared to decision-making skills in adult patients with frontal lobe damage (Chelune and Baer, 1986, Crone and van der Molen, 2004). These comparisons imply that young brains are operating in an impaired or suboptimal fashion, essentially without a frontal cortex. Our data showed that under conditions that enhanced choice uncertainty, juvenile mice showed more rapid reversal learning than adults. We suggest that the frontal cortex, and decision-making circuits generally, are operative in juveniles and in adults, but that they integrate information and evaluate actions and outcomes differently. Differences in the incentive salience of recent behavioral outcomes, in particular, may drive juveniles to more rapidly update their behavior. We hypothesize that when altricial animals transition to first independence they may engage unique decision-making strategies evolutionarily optimized for the instability of this life stage.
Acknowledgements
The authors thank Denise Niell, Karen Berger, Angela Vandenberg, Natalia Caporale, Lung-Hao Tai, and Patricia Janak for assistance with experiments, analysis and discussion. This project was supported by the State of California, NIMH R01MH087542, NIDA R01DA029150, National Science Foundation Graduate Research fellowship (C.J.), and the M.P. Royer and K. Clayton family.
References
- Anderson P. Assessment and development of Executive Function (EF) during childhood. Child Neuropsychol. 2003;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Arcis V., Desor D. Influence of environment structure and food availability on the foraging behaviour of the laboratory rat. Behav. Process. 2003;60:191–198. doi: 10.1016/s0376-6357(02)00122-5. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev. Rev. 1992;12:339–373. [Google Scholar]
- Barrett S.E., Shepp B.E. Developmental changes in attentional skills: the effect of irrelevant variations on encoding and response selection. J. Exp. Child Psychol. 1988;45:382–399. doi: 10.1016/0022-0965(88)90038-0. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Birrell J.M., Brown V.J. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette G.B., Martins G.J., Franz T.M., Harper E.S., Schoenbaum G., Powell E.M. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassotti M., Houde O., Moutier S. Developmental changes of win-stay and loss-shift strategies in decision making. Child Neuropsychol. 2011;4:1–12. doi: 10.1080/09297049.2010.547463. [DOI] [PubMed] [Google Scholar]
- Cepeda N.J., Kramer A.F., Gonzalez de Sather J.C. Changes in executive control across the life span: examination of task-switching performance. Dev. Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- Chehab F.F., Mounzih K., Lu R., Lim M.E. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Chelune G.J., Baer R.A. Developmental norms for the Wisconsin card sorting test. J. Clin. Exp. Neuropsychol. 1986;8:219–228. doi: 10.1080/01688638608401314. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Donohue S.E., Honomichl R., Wendelken C., Bunge S.A. Brain regions mediating flexible rule use during development. J. Neurosci. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Ridderinkhof K.R., Worm M., Somsen R.J., van der Molen M.W. Switching between spatial stimulus-response mappings: a developmental study of cognitive flexibility. Dev. Sci. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- Crone E.A., van der Molen M.W. Developmental changes in real life decision making: performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Dev. Neuropsychol. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz A.-M., Ragozzino M.E., Mosconi M.W., Pavuluri M.N., Sweeney J.A. Human reversal learning under conditions of certain versus uncertain outcomes. NeuroImage. 2011;56:315–322. doi: 10.1016/j.neuroimage.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C.R., Wood S.J., Anderson V., Buchanan J.A., Proffitt T.M., Mahony K., Pantelis C. Normative data from the CANTAB. I: Development of executive function over the lifespan. J. Clin. Exp. Neuropsychol. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair R.J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy K.A. The shape school: assessing executive function in preschool children. Dev. Neuropsychol. 1997;13:495–499. [Google Scholar]
- Franklin K.B.J., Paxinos G. 3rd ed. Academic Press; Amsterdam, Boston: 2008. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Furby L., Beyth-Marom R. Risk taking in adolescence: a decision-making perspective. Dev. Rev. 1992;12:1–44. [Google Scholar]
- Galef B.G. Social transmission of food preferences – adaptation for weaning in rats. J. Comp. Physiol. Psychol. 1977;91:1136–1140. [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010:4. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev. Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Garner J.P., Thogerson C.M., Würbel H., Murray J.D., Mench J.A. Animal neuropsychology: validation of the intra-dimensional extra-dimensional set shifting task for mice. Behav. Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden B.Y., Heilbronner S.R., Pearson J.M., Platt M.L. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J. Neurosci. 2011;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., van der Molen M.W. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huizinga M., van der Molen M.W. Age-group differences in set-switching and set-maintenance on the Wisconsin card sorting task. Dev. Neuropsychol. 2007;31:193–215. doi: 10.1080/87565640701190817. [DOI] [PubMed] [Google Scholar]
- Kennerley S.W., Walton M.E., Behrens T.E.J., Buckley M.J., Rushworth M.F.S. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kim J., Ragozzino M.E. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol. Learn. Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., Markl H. Maternal care in house mice: I. The weaning strategy as a means for parental manipulation of offspring quality. Behav. Ecol. Sociobiol. 1987;20:1–9. [Google Scholar]
- Laviola G., Macrì S., Morley-Fletcher S., Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Luciana M., Nelson C.A. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mabry K.E., Stamps J.A. Searching for a new home: decision making by dispersing brush mice. Am. Nat. 2008;172:625–634. doi: 10.1086/591682. [DOI] [PubMed] [Google Scholar]
- Macrì S., Adriani W., Chiarotti F., Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Anim. Behav. 2002;64:541–546. [Google Scholar]
- McAlonan K., Brown V.J. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Newman L.A., McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev. Psychobiol. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.M., Hayden B.Y., Raghavachari S., Platt M.L. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr. Biol. 2009;19:1532–1537. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M.E., Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behav. Neurosci. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Rhodes M.G. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol. Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Roberts A.C., Robbins T.W., Everitt B.J. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q. J. Exp. Psychol. B. 1988;40:321–341. [PubMed] [Google Scholar]
- Romine C.B., Lee D., Wolfe M.E., Homack S., George C., Riccio C.A. Wisconsin Card Sorting Test with children: a meta-analytic study of sensitivity and specificity. Arch. Clin. Neuropsychol. 2004;19:1027–1041. doi: 10.1016/j.acn.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Somsen R.J.M. The development of attention regulation in the Wisconsin Card Sorting Task. Dev. Sci. 2007;10:664–680. doi: 10.1111/j.1467-7687.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Belsky J. A sociobiological perspective on psychopathology in adolescence. In: Cicchetti D., Toch S., editors. Rochester Symposium on Developmental Psychopathology. University of Rochester Press; Rochester, NY: 1996. pp. 93–124. [Google Scholar]
- Sturman D.A., Mandell D.R., Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav. Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth C.E., Spear L.P. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol. Biochem. Behav. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]