Abstract
Studies of brain development suggest that the increase in risk taking observed during adolescence may be due to insufficient prefrontal executive function compared to a more rapidly developing subcortical motivation system. We examined executive function as assessed by working memory ability in a community sample of youth (n = 387, ages 10 to 12 at baseline) in three annual assessments to determine its relation to two forms of impulsivity (sensation seeking and acting without thinking) and a wide range of risk and externalizing behavior. Using structural equation modeling, we tested a model in which differential activation of the dorsal and ventral striatum produces imbalance in the function of these brain regions. For youth high in sensation seeking, both regions were predicted to develop with age. However, for youth high in the tendency to act without thinking, the ventral striatum was expected to dominate. The model predicted that working memory ability would exhibit (1) early weakness in youth high in acting without thinking but (2) growing strength in those high in sensation seeking. In addition, it predicted that (3) acting without thinking would be more strongly related to risk and externalizing behavior than sensation seeking. Finally, it predicted that (4) controlling for acting without thinking, sensation seeking would predict later increases in risky and externalizing behavior. All four of these predictions were confirmed. The results indicate that the rise in sensation seeking that occurs during adolescence is not accompanied by a deficit in executive function and therefore requires different intervention strategies from those for youth whose impulsivity is characterized by early signs of acting without thinking.
Adolescence is a period of major change in brain development and behavior. Although adolescents gain important skills and maturity, many also engage in considerable risk taking, putting them at risk for drug dependence (Grant & Dawson, 1998), driving accidents (Shope & Bingham, 2006), and other injuries (Pickett, Garner, Boyce, & King, 2002). One explanation for this developmental pattern attributes it to insufficient cortical control over a rapidly developing subcortical motivation system (Casey, Getz, & Galvan, 2008; Chambers, Taylor, & Potenza, 2003; Galvan, A., Hare, T. A., Parra, C. E., Penn, J., Voss, H., Glover, G., et al. (2006); Steinberg, 2008). Support for this explanation comes from structural brain imaging that finds slower cortical thinning and white matter growth in dorsal and frontal brain areas than in ventral and occipital areas during and beyond the adolescent period (Gogtay, Giedd, Lusk, Hayashi, Greenstein, et al., 2004; Sowell, Peterson, & Thompson, 2003). Because frontal brain regions are critically involved in executive function and behavior control, the findings have been interpreted to indicate that higher order cortical functions are insufficiently developed to counter the strong increase in subcortical-limbic activation that is implicated in the rise in impulsivity and risk taking during adolescence.
Despite the plausibility of this hypothesis, there is little direct evidence linking rates of prefrontal cortical maturation with risky adolescent behavior (Lu & Sowell, 2009). Indeed, a recent study found that the tendency to engage in risky activity was positively related to white matter growth in the cerebral cortex (Berns, Moore, & Capra, 2009). That is, after controlling for age, youth ages 12 to 18 who had more advanced white matter development engaged in more risky activity. Furthermore, research regarding the relation between cortical thinning and cognitive ability (i.e., IQ) indicates that more intelligent youth exhibit delayed cortical thinning (Shaw, Greenstein, Lerch, Classen, Lenroot, Gogtay, et al., 2006). Thus, the evidence regarding maturation in cortical thinning and white matter growth in the prefrontal and association areas is not particularly supportive of an inverse relation with either risk taking or executive function.
A perhaps more serious problem for the hypothesis is the recognition that adolescent risk taking is subject to considerable individual variation. High proportions of adolescents do not use drugs, drive unsafely, or engage in delinquent behavior (Jessor, 1992; Romer, 2010). Indeed, a small proportion of adolescents account for a majority of hazardous risk behavior during adolescence. For example, Biglan and Cody (2003) found that about 18% of youth ages 12 to 20 accounted for the majority of the occurrences of drunk driving and criminal arrests in that age group. If adolescents experienced a universal deficit in cognitive control, one would expect its effects to be more widespread than it actually is (Romer, 2010).
In this study, we examine individual differences in prefrontal executive function as well as subcortical motivation systems as predictors of risk taking and associated externalizing behaviors. We focus on two dopamine systems that are heavily involved in executive cognitive function and motivational drives underlying impulsivity, and we propose a model in which imbalance between these systems leads to maladaptive risk taking and externalizing behavior problems. Unlike the hypotheses drawn from brain maturation studies, the model predicts that not all risk taking is associated with weakness in executive cognitive function and that some individuals experience sufficient balance between the two systems that their risk taking is associated with stronger not weaker executive cognitive function. Hence, individual differences in the balance between cognitive executive function and impulsive tendencies are critical in characterizing adolescent risk taking tendencies.
Increase in Dopamine During Adolescence
Research in both animals and humans indicates that levels of the neurotransmitter dopamine increase in the brain during adolescence (Chambers et al., 2003; Spear, 2000). One important dopamine pathway ascends from the ventral tegmental area to the ventral striatum and ventral prefrontal cortex (PFC). This pathway passes through the nucleus accumbens, an important center for controlling motivation and reward seeking (Berridge & Robinson, 2003; Ikemoto, 2007). Individual differences in the strength of this pathway have been linked with individual variation in sensation seeking and other impulsive tendencies (Chambers et al., 2003; Cloninger, 1988; Zuckerman, 1994).
A second dopamine pathway also originates in the midbrain but courses through the dorsal striatum before linking with dorsolateral PFC. Although the two circuits are activated by dopamine, they have very different effects on behavior (Previc, 2009). The dorsal striatal pathway subserves more thoughtful and controlled processes (Cools & Robbins, 2004; Ikemoto, 2007), while the ventral striatal motivates search for novel experience and facilitates learning of new relationships in the environment (Panksepp, 1998; Schultz et al., 1997).
There is growing evidence for the role of the dorsal striatum in cognitive executive function. For example, synthesis of dopamine in the dorsal striatum coincides with working memory performance, indicating that the pathway plays an important role in cognitive control over behavior (Cools, Gibbs, Miyakawa, Jagust, & D’Esposito, 2008; Landau, Lal, O’Neil, Baker, & Jagust, 2009). Indeed, extreme dopamine deficits in the pathway are associated with Parkinson’s disease, which severely interferes with working memory and other executive functions (Cools & Robbins, 2004; Previc, 1999). Furthermore, low doses of the drug amphetamine have been linked to increases of dopamine in the pathway thereby improving attention in persons with attention deficits (Arnsten & Li, 2005; Previc, 2009). This effect is attributed to greater ability to filter out distracting influences in working memory (Cools & Robbins, 2004). It is not surprising therefore that intensive training to improve working memory in children with attention deficits reduces subsequent impulsive behavior (Klingberg, Fernell, Olesen, Johnson, Gustafsson, Dahlstrom, et al., 2005).
Although both pathways undergo development during adolescence, there may be an imbalance between them that is responsible for individual differences in impulsivity and risk taking during adolescence. Indeed, research examining the development of impulsivity during adolescence suggests that this construct is multidimensional encompassing several different forms of risk taking (Smith, Fischer, Cyders, Annus, Spillane, & McCarthy, 2007; Whiteside & Lynam, 2001). For example, considerable research indicates that sensation seeking, which refers to the tendency to pursue exciting and novel experience, predicts a variety of potentially harmful risky behaviors, especially in adolescents (Arnett, 1992; Roberti, 2004; Zuckerman, 1994). However, sensation seeking can be distinguished from a tendency to act without thinking and to experience harmful consequences of impulsive decisions, what Whiteside and Lynam (2001) termed urgency. Consistent with this distinction, researchers have found that sensation seeking is primarily linked to the frequency of risk behaviors, such as alcohol consumption and gambling, while urgency is much more strongly linked to symptoms of addiction, such as problem drinking and gambling, and binge eating (Magid, MacLean, & Colder, 2007; Smith et al., 2007). Hence, although sensation seeking is clearly a marker of impulsive behavior, it is not as predictive of adverse experience as acting without thinking.
Despite evidence for divergence between sensation seeking and acting without thinking, these two forms of impulsivity are moderately correlated in college students (Magid et al., 2007; Whiteside & Lynam, 2001). Indeed, research using brain imaging techniques has verified that both forms of impulsivity are related to activation in the ventral striatum (Zald, Cowan, Riccardi, Baldwin, Ansari, Li, et al., 2008; Buckholtz, Teadway, Cowan, Woodward, Li, Ansari, et al., 2010). In addition, in a recent study of preadolescents, ages 10 to 12, the two forms of impulsivity were moderately correlated and linked to early risk taking (Romer, Betancourt, Giannetta, Brodsky, Farah, & Hurt, 2009). Furthermore, both forms of impulsivity loaded on the same factor that was positively related to age and inversely related to working memory performance and other measures of executive function. These findings suggest that despite their divergence in older youth, sensation seeking and acting without thinking exhibit similar age trajectories and associations with risk taking during early adolescence.
Despite the rise in activation of the ventral striatum during adolescence, there is also considerable evidence that working memory and other indicators of executive function either increase or plateau during the same period (Bunge & Crone, 2009). This raises the question as to whether all increases in risk taking during adolescence are associated with weakness in cognitive control. Indeed, many forms of adolescent risk taking have been linked with adaptive patterns of behavior that appear to serve useful developmental tasks, such as peer socialization and transition to adulthood (Jessor, 1992; Schedler & Block, 1990; Spear, 2007). However, it may be the youth who engage in risk taking without sufficient cognitive control that are at greater risk for the deleterious forms of risk behavior during adolescence. Thus, we propose that imbalance between the two striatal systems may explain individual differences in risk taking as exhibited by differences between sensation seeking and acting without thinking.
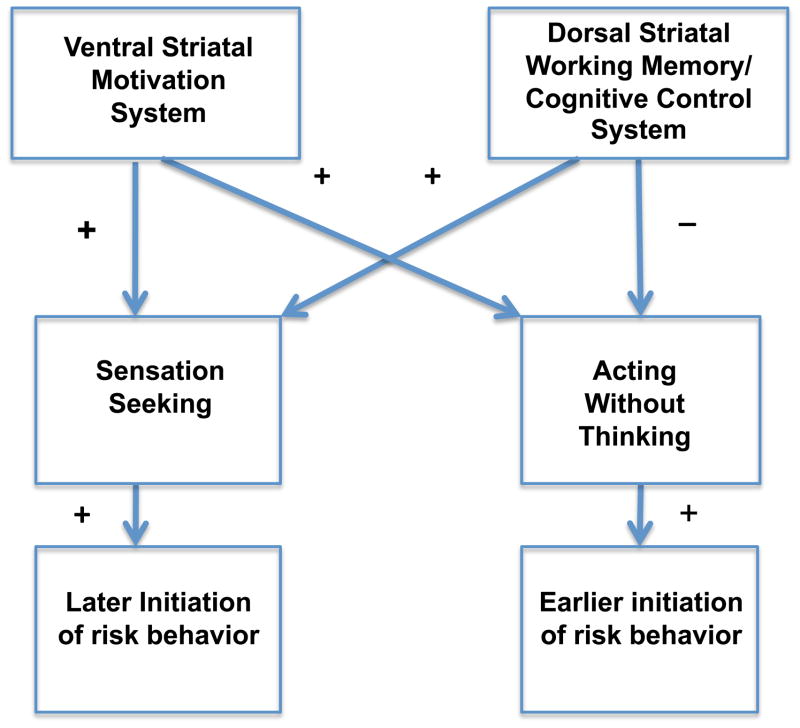
According to this striatal imbalance model (see Figure 1), sensation seeking and acting without thinking share the same predisposition to risk taking identified in neurobiological theories of adolescent risk behavior (Casey et al., 2008; Chambers, et al., 2003; Steinberg, 2008), but differ in regard to executive control processes, in particular working memory ability. As a result, we expect acting without thinking to be more strongly related to early risk taking and externalizing behavior than sensation seeking, a prediction that is also consistent with findings regarding these two forms of impulsivity (Magid, et al., 2007; Smith, et al., 2007).
Figure 1.
Hypothesized relations (positive and negative) between ventral and dorsal striatal systems, two forms of impulsivity, and risk behavior in adolescents.
Research by Tarter and colleagues (2003; 2004) provides additional evidence that risk for drug dependence in late adolescence is predicted by neurodevelopmental deficits in executive function and associated forms of externalizing behavior in early adolescence. Longitudinal studies suggest that weak executive function in combination with impulsive traits are an even earlier marker of subsequent externalizing behavior (Moffitt & Caspi, 2001), a pattern Moffitt (1993) calls a life-course persistent developmental trajectory. Indeed, this pattern of behavior may be evident in preadolescence if not sooner (Romer, et al., 2009).
In contrast to the early risk taking that characterizes acting without thinking, research suggests that sensation seeking may be more reflective of preferences for novel and exciting experiences than of deficits in executive function. Indeed, sensation seeking tends to be positively related to IQ (Zuckerman, 1994), while acting without thinking is linked to lower IQ and weaker executive function (Horn, Dolan, Elliott, Deakin, & Woodruff, 2003). Sensation seeking is therefore likely to be a marker of a more controlled form of risk taking that is normative for adolescents as they age but that declines as activation of the ventral striatum declines. Thus, the model predicts that sensation seeking is increasingly associated with the emergence of risk behavior as adolescents age but that the relation is accompanied by growing rather than declining executive function.
It is important to determine the distinctive characteristics of these forms of impulsivity because they have important implications for interventions aimed at reducing harmful risk taking during adolescence. If sensation seeking does not represent a deficit in executive function, then it may be more appropriate to target interventions that channel sensation seeking toward less hazardous activities, such as sports. For those who are more prone to acting without thinking, it may be more effective to increase executive function or other skills that enable better control over behavior. Such targeting will benefit from knowing the distinguishing characteristics of different forms of impulsivity.
The Present Study
In this study we tested the striatal imbalance model by examining the inter-relations between sensation seeking and acting without thinking in a community sample of youth ages 10 to 12 at study inception. The study examined the relations of each form of impulsivity with risk taking and executive function as assessed by working memory ability in a longitudinal design covering three years of annual assessments. We examine working memory because it is an important component of cognitive executive function and depends on activation of the dorsolateral prefrontal cortex and associated parietal regions (Fuster, 1997; Miller & Cohen, 2001). It has also been linked to activation of dorsal striatal dopamine circuit in adults (Cools, et al., 2008; Landau, et al., 2009). In addition, it has been found to be inversely related to a general impulsivity factor comprised primarily of acting without thinking in early adolescence (the first assessment in this study) (Romer et al., 2009).
We examined a wide range of risk behaviors, including early use of drugs (i.e., tobacco and alcohol), gambling for money, and fighting. We also examined a range of related externalizing behaviors, such as rule breaking and conduct problems. We tested our predictions using structural equation modeling (SEM). This methodology allowed us to examine relations between each of our individual differences contemporaneously as well as prospectively. SEM also allows rigorous analysis of relations between underlying factors rather than observed scores, which are subject to measurement error that can be correlated across repeated assessments. In addition, it allows one to assess whether relations between constructs, such as working memory and risk taking, are unique to particular measures of these variables or reflect more general underlying abilities and dispositions (see Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000, for a similar approach).
Method
Participants
The sample for this longitudinal study has been previously described (Romer et al., 2009). The sample, comprised of youth ages 10 to 12 at baseline (49% male), was drawn from various neighborhoods of Philadelphia and included youth of non-Hispanic white (63%) and black (27%) backgrounds. The families were in the lower range of middle class with parents having a median education of 14 years. Parental consent and youth assent were obtained in accordance with the protocol that was approved by the IRB of the Children’s Hospital of Philadelphia. Youth were reimbursed for their time and travel.
Assessments
Annual assessments lasting about 2 hours per session were conducted in local schools, community libraries, or in research center testing rooms. Examiners, who were carefully trained to administer all tasks in a standardized manner, tested participants one at a time. Tasks were administered on touch-screen laptops with e-Prime (Schneider, Eschman, & Zuccolotto, 2002) and Medialab (Jarvis, 2004) with the audio-computer assisted self-interviewing (ACASI) method of both visual and aural presentation. Use of ACASI served to maximize subjects’ comfort in answering truthfully about their behaviors (Metzger, Koblin, Turner, Navaline, Valenti, Holte, S., et al., 2000) while also reducing differences that might result from reading a self-administered survey. Although a break was scheduled during the session to reduce fatigue and maintain interest in the tasks, the use of computerized testing and variation in tasks worked to sustain attention.
Working Memory Performance
Working memory plays an essential role in many activities that are not tests of memory per se, such as the ability to hold the context or goals of a complex task in mind while operating on the contents of memory (Cohen, Cohen, & Ayache, 1992; Kimberg & Farah, 1993). In particular, working memory is weak in children at risk for drug use (Romer et al., 2009; Tarter, et al., 2004). Working memory is most reliably associated with activation in dorsolateral PFC (Mehta, Owen, Shahakian, Mavaddat, Pickard, Robbins, 2000; Paus, 2005). However, it has also been linked to activation in dorsal striatum (Cools et al., 2008; Landau et al., 2009). Four tasks were used to assess working memory performance with.
Digit Span Backwards
This is a well-known task that tests auditory-verbal working memory by having participants immediately repeat back orally presented sequences of digits to the experimenter. In functional imaging studies, this task reliably activates lateral PFC (Owen, 2000). Following the protocol given in the WISC-IV (Wechsler, 2003), both forward and backward digit span was administered. We used the digit-backward score as the measure of this ability with scores ranging from 0 to 15.
Visual Spatial Working Memory
This self-directed computerized task, a subtest of the CANTAB battery (Cambridge Cognition, 1999), requires the participant to search for hidden tokens one at a time within sets of four to eight randomly positioned boxes. Tokens are hidden only once in each box. Working memory skills are tapped as the participant, while searching, must hold in working memory the locations already checked and, as tokens are found, must remember and update the information about the locations of the found tokens (Elliott et al., 1997). In functional imaging studies, this task reliably activates dorsolateral PFC (Smith & Jonides, 1999). We used the between-search error score to index this ability with scores ranging from 0 to 110.
Corsi Block Tapping
This task is a nonverbal variant of the Digit Span task (Milner, 1971). The participant views a set of identical blocks that are spatially dispersed on the screen. The blocks are individually lit up in a random sequence. The participant is required to respond by the tapping each box in the reverse order of the sequence of lit boxes. This task is considered a task of spatial working memory as the sequence must be maintained and reversed in working memory in order to guide the response. Imaging studies show that performance on this task is dependent on right prefrontal brain regions (Goldman-Rakic, 1987). We used the total correct score to index this ability with scores ranging from 0 to 13.
Letter Two-Back
This task, adapted for children by Casey et al (1995), requires the participant to continually update working memory in order to compare the current letter to the one presented two trials back. The total number of correct responses (correct rejections + correct hits) served as the index of this ability. The score ranged from 38 to 122. Imaging studies find dorsolateral PFC activation with the task (Casey et al., 1995)
Because working memory tasks were readministered each year, we conducted analyses adapted from the method outlined by Slade, et al. (2008) to determine whether practice effects might obscure changes in working memory performance across testing sessions. Using whole-year-age specific, assessment-independent z-scores for each task score, we found age related increases in performance but no age-by-session interaction, p > 0.32; partial eta squared = 0.002 – 0.010. These results indicated that increasing age was related to performance; however, children who performed the tasks for the second or third time did not score significantly better than same age children performing the tasks for the first time.
Risk behaviors
Specific risk behaviors were assessed using questions derived from the CDC’s State and Local Youth Risk Behavior Survey (YRBS) (Centers for Disease Control and Prevention, 2003) and the Monitoring the Future Study (MTF) (Johnston, Bachman, & O’Malley, 2006). Risk behaviors were coded on four point scales (0 to 3) as follows: gambling for money: never to more than 9 times in the past 30 days; use of alcohol and cigarettes: never to all 30 of the past 30 days; and fighting: not in the past year to 10 or more times in the past year. We reduced these scales to a single score based on the first principal component at each time of assessment. This component accounted for 39% to 47% of the variation in these scores with no additional component larger than 1.0 at any time of assessment.
Externalizing Problems
Raw scores for externalizing behavior were obtained from the Youth Self-Report (YSR) of the Achenbach System of Empirically Based Assessment (Achenbach & Rescorla, 2001). The YSR provides an overall score for externalizing problems, indexed by rule breaking and aggressive behavior. Youth are asked to identify the degree to which 15 rule-breaking and 17 aggressive behaviors are presently true of themselves using a three-point scale: 0 = never; 1 = somewhat/sometimes; 2 = very/often (2). Thus scores could range from 0 to 64. The YSR has strong reliability and validity and has been used in many studies to assess problem behavior (Hofstra, Van der Ende, & Verhulst, 2002; Visser, Van der Ende, Koot, & Verhulst, 2000; Weinstein, Noam, Grimes, Stone, & Schwab-Stone, 1990).
Impulsivity
Acting Without Thinking
This tendency was measured using 13 self-report items from the Impulsivity Subscales of the Eysenck Personality Inventory (Acton, 2003; Eysenck & Eysenck, 1977; Horn, et al., 2003). The items were coded in binary format (0,1) with1 indicating greater impulsivity. Preliminary factor analyses at each assessment revealed two correlated factors as was observed at time 1 (Romer et al., 2009). One factor consisting of 6 items represented the tendency to act quickly without much thought (e.g., “I do and say things without thinking”) while the other consisting of 4 items represented the tendency to experience bad outcomes as a result (“I need lots of self control to stay out of trouble”). Three items that did not consistently load on either of these factors across assessments (e.g., “I change interests often”) were dropped from scores created by averaging the item values for each factor. Coefficients alphas (α) for the entire ten items ranged from .753 to .765 across assessments.
Sensation Seeking
Sensation seeking was measured using 4 items (“I would like to explore strange places;” “I like to do frightening things;” “I like new and exciting experiences even if I have to break the rules;” and “I prefer friends who are exciting and unpredictable;” Items 1 to 4 in Table 2) from the Brief Sensation Seeking Scale (Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, 2002), which was derived from the longer battery developed by Zuckerman (M. Zuckerman, 1994;M. Zuckerman & Kuhlman, 2000). Questions were answered on a 4-point Likert scale (strongly agree to strongly disagree) and coded from 1 to 4, with 4 indicating higher sensation seeking. Mean scores for these items had α’s that ranged from .728 to .767 across assessments.
Table 2.
Standardized factor loadings for observed scores at each assessment.
| Observed Score by Factor | Assessment Time | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Risk Behavior | |||
| Risk Component Score | .603 | .587 | .640 |
| Externalizing Behaviors | .809 | .816 | .904 |
| Working Memory | |||
| Corsi Block Tapping | .520 | .548 | NA |
| Digit Span | .397 | .424 | NA |
| Spatial Working Memory (Errors) | −.690 | −.676 | NA |
| Letter 2-Back | .428 | .351 | NA |
| Sensation Seeking | |||
| Explore strange places | .629 | .612 | .671 |
| Frightening | .695 | .701 | .738 |
| New & exciting | .674 | .718 | .746 |
| Exciting friends | .581 | .541 | .541 |
| Acting without Thinking | |||
| Factor 1 | .708 | .761 | .725 |
| Factor 2 | .641 | .605 | .649 |
Note: All loadings were significant, p < .01. These loadings do not include covariation between measures of the same factors that are captured by correlated measurement error.
Analysis
Preliminary analyses were conducted to identify trends in mean values of all relevant indicators assessed at the three time points in the study. Paired t tests were conducted between times 1 and 3 to determine changes in scores from the first to third assessment.
We used the program EQS to test the overall measurement model, representing the proposed factor structure of the indicators as well as relations between factors across assessments (Bentler, 2004). This analysis attempts to fit the covariance matrix of observed scores using only relations between the factors hypothesized to underlie those scores and correlations between the same indicators across times of assessment (i.e., correlated errors of measurement). The program uses maximum likelihood estimation procedures to impute missing values, which in this dataset were primarily observed for one measure of working memory using the digit-span test at time 1. Due to an administration error, scores for this test were not available for approximately 16% of the sample. In addition, small percentages of participants (3.6% at time 2 and 5.4% at time 3) were not available to complete follow-up assessments. EQS also provides robust estimates that adjust for the effects of departures from multivariate normality of which kurtosis is a particular concern (Yuan & Bentler, 1998). All coefficients and model tests in the results have probabilities evaluated with robust standard errors using the Yuan-Bentler scaled chi-square test.
We also used the program to assess prospective relations between factors across time as indicated by the model in Figure 1. This analysis controlled for autoregressive paths between the same factors over time (e.g., risk behavior assessed at times 1 and 2) while assessing the ability of earlier factors to predict later but different factors (“cross-lagged” paths, such as working memory at time 1 to sensation seeking at time 2). To assess the prediction that acting without thinking is more strongly related to early risk behavior than sensation seeking, we first tested this prediction using only time 1 data. In addition, we retested the relation that was observed in Romer et al. (2009) that working memory is inversely related to the underlying impulsivity component that is registered in acting without thinking. Because our sample varied in age, we also controlled for age in the analysis. Including age in the model not only held constant a source of positive relation between working memory and acting without thinking, but also controlled for possible effects of maturation apart from our measures of impulsivity and working memory.1
We assessed goodness of fit using two criteria. Although EQS provides a Chi-square test (χ2) to compare the predicted covariance matrix with the observed matrix (Bentler, 2004), such tests are very sensitive to sample size, and significant values do not necessarily indicate a poor fit with large samples. For this reason, we used indices that are not as sensitive to sample size and represent a graded index of fit (Hu& Bentler, 1995): the Comparative Fit Index (CFI) and Root Mean Square Error of Approximation (RMSEA). CFI is a comparison of two fit functions: one from the covariance matrix estimated from the fitted model and one from a model that assumes no association between the observed variables. Higher values reflect the relative advantage of the proposed model over a model with no association. Values greater than .95are considered to reflect a good fit (Hu & Bentler, 1995). The RMSEA measures the mean residuals between the observed and predicted covariance matrix. Departures from zero represent poorer fit. RMSEA values less than or equal to .06are considered acceptable (Kaplan, 2000). We also used χ2 difference tests to assess change in goodness of fit for parameters of theoretical interest.
Results
Table 1 shows the number of youth tested and the means and standard deviations of their ages at each of the three years of assessment. Table 1 also shows means and standard deviations of the measures included in the analysis. Risk behaviors (except fighting) and externalizing problems increased over the three waves (p < .02). Working memory measures improved as well (p < .001). Sensation seeking items tended to increase, with one doing so strongly (Item 3: “I like new and exciting experiences…”); the direct measure of acting without thinking also increased with age (p < .01). These trends are consistent with the expectation that risk taking and externalizing problems increase with age along with measures of impulsivity and executive function.
Table 1.
Descriptive statistics for measures of risk behavior, externalizing problems, working memory, sensation seeking, and acting without thinking at each time of assessment and tests of differences over time.
| Time of Assessment | t-test, Time 1 vs. 3 | Probability | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||||||
| Number tested | 387 | 373 | 366 | |||||||||||
| Mean | Std. Dev. | Skewness | Kurtosis | Mean | Std. Dev. | Skewness | Kurtosis | Mean | Std. Dev. | Skewness | Kurtosis | |||
| Age at testing, yrs Risk Behavior | 11.4 | 0.87 | 0.26 | −1.06 | 12.6 | 0.89 | 0.28 | −0.96 | 13.5 | 0.95 | .33 | −0.88 | NA | |
| Gambling | 0.36 | 0.63 | 1.62 | 1.65 | 0.32 | 0.74 | 2.18 | 3.53 | 0.75 | 0.88 | 0.77 | −0.63 | 8.84 | < .001 |
| Alcohol | 0.21 | 0.51 | 2.87 | 10.8 | 0.33 | 0.64 | 2.00 | 3.90 | 0.43 | 0.69 | 1.34 | 0.59 | 5.86 | < .001 |
| Fighting | 0.35 | 0.63 | 2.17 | 5.54 | 0.33 | 0.56 | 1.83 | 4.38 | 0.32 | 0.58 | 2.17 | 5.83 | 0.22 | 0.83 |
| Cigarettes | 0.03 | 0.17 | 5.67 | 30.4 | 0.07 | 0.34 | 5.73 | 37.5 | 0.10 | 0.43 | 4.28 | 18.0 | 4.13 | < .001 |
| Externalizing Problems | 9.01 | 6.99 | 1.59 | 3.71 | 9.39 | 6.77 | 1.16 | 1.70 | 10.1 | 7.53 | 1.01 | 0.58 | 2.66 | 0.008 |
| Working Memory | ||||||||||||||
| Corsi Block Tapping, Total Correct | 5.20 | 2.96 | 0.05 | −0.50 | 6.12 | 3.04 | −0.14 | −0.57 | 6.44 | 3.11 | 0.15 | −0.28 | 7.29 | < .001 |
| Digit Span Backward, Correct score | 7.28 | 1.84 | 0.91 | 1.81 | 7.29 | 1.79 | 0.64 | 0.25 | 7.67 | 1.87 | 0.37 | −0.05 | 3.74 | < .001 |
| Spatial WM, Between-Search Errors | 3.07 | 1.93 | 0.69 | −0.05 | 2.78 | 1.90 | 1.07 | 1.74 | 2.45 | 1.82 | 1.02 | 0.88 | 6.05 | < .001 |
| Letter 2-Back, Total correct | 111.0 | 13.4 | −3.18 | 11.1 | 113.4 | 9.10 | −4.25 | 24.9 | 114.9 | 7.6 | −3.10 | 14.9 | 4.30 | < .001 |
| Sensation Seeking | ||||||||||||||
| 1) Explore places | 2.39 | 1.07 | 0.03 | −1.31 | 2.38 | 0.99 | 0.27 | −1.10 | 2.41 | 1.04 | 0.09 | −1.23 | 0.48 | 0.63 |
| 2) Frightening | 1.78 | 0.97 | −0.92 | −0.39 | 1.78 | 0.92 | −0.85 | −0.47 | 1.90 | 1.00 | −0.63 | −0.96 | 1.74 | 0.08 |
| 3) New & exciting | 1.80 | 0.99 | −0.97 | −0.29 | 1.93 | 0.98 | −0.63 | −0.79 | 2.10 | 1.04 | −0.43 | −1.07 | 4.34 | < .001 |
| 4) Exciting friends | 2.60 | 1.05 | 0.19 | −1.15 | 2.72 | 1.00 | 0.35 | −0.93 | 2.65 | 0.96 | 0.34 | −0.80 | 0.76 | 0.45 |
| Acting Without Thinking | ||||||||||||||
| Factor 1 | 0.40 | 0.32 | 0.37 | −1.06 | 0.45 | 0.33 | 0.17 | −1.24 | 0.45 | 0.34 | 0.23 | −1.24 | 2.70 | 0.007 |
| Factor 2 | 0.32 | 0.30 | 0.61 | −0.56 | 0.36 | 0.28 | 0.33 | −0.82 | 0.34 | 0.28 | 0.54 | −0.48 | 0.90 | 0.37 |
Measurement Model
To assess the measurement model, a SEM was fit to the indicators in Table 1 allowing the factors to correlate with each other within and across time. This model had high loadings from all of the indicators at each time point, indicating that the measures reflected the factors they were intended to assess (see Table 2). We also plotted observed summary scores for each factor against other scores to identify outliers that might produce spurious correlations. However, all relations were linear in form. In addition, some of the variables exhibited high levels of kurtosis (i.e., risk behaviors and some of the working memory indicators). Although EQS provides robust estimates that are corrected for such departures from normality (Bentler, 2004), the program also permits one to identify the most extreme contributors to multivariate kurtosis in the dataset. Using this procedure, we deleted 5 extreme cases in the data and reran the model. The model fit and parameters were virtually identical, providing confirming evidence that the solution was stable and not an artifact of outliers. Indeed, the overall model provided a good fit to the data, CFI = .97; RMSEA = .023 (90% confidence interval (CI) = .013, .030) despite the significant overall chi-square test, χ2(408) = 557, p < .001.
Table 3 shows the inter-correlations between age and factors underlying risk taking, working memory, sensation seeking, and acting without thinking. It is noteworthy that correlations between factors across assessments revealed considerable stability. Risk behavior correlations ranged from .622 to .729. Working memory was very stable across times 1 and 2 (.922) and produced an illegitimate solution when all three assessments were included. Even though working memory at time 2 was essentially indistinguishable from the same score at time 1, we included it in the model to demonstrate that it did not add any prediction beyond its assessment at time 1. Sensation seeking correlations ranged from .547 to .742, while acting without thinking ranged from .660 to .788. Finally, as expected, age tended to be positively related to all of the measures, and its relations tended to increase over time.
Table 3.
Correlations between factors and age in confirmatory measurement model at three time points.
| Factor | Age | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||||
| 1) RB1 | .113 | ||||||||||
| 2) RB2 | .181 | .729 | |||||||||
| 3) RB3 | .193 | .622 | .709 | ||||||||
| 4) WM1 | .268 | −.041 | −.046 | −.142 | |||||||
| 5) WM2 | .116 | −.213 | −.209 | −.194 | .922 | ||||||
| 6) SS1 | .082 | .528 | .465 | .337 | .047 | .047 | |||||
| 7) SS2 | .128 | .551 | .735 | .532 | .203 | .061 | .642 | ||||
| 8) SS3 | .119 | .454 | .570 | .653 | .232 | .169 | .547 | .742 | |||
| 9) AWT1 | .144 | .856 | .672 | .659 | −.143 | −.219 | .587 | .439 | .400 | ||
| 10) AWT2 | .120 | .610 | .842 | .637 | −.182 | −.268 | .349 | .530 | .435 | .660 | |
| 11) AWT3 | .242 | .639 | .741 | .875 | −.140 | −.206 | .235 | .364 | .527 | .788 | .764 |
Note: All bold correlations significant at p < .05. Abbreviations: RB = risk behavior; WM = working memory performance; SS = sensation seeking; and AWT = acting without thinking.
Several patterns in the correlations were relevant to our predictions. First, sensation seeking and acting without thinking were positively related at each assessment (at about .5). This confirms the expectation that both of these measures are sensitive to activation in an underlying impulsivity dimension. Second, risk behavior was related to both sensation seeking and acting without thinking at each assessment, but the relation was stronger for acting without thinking. This confirms the expectation that acting without thinking represents a more serious form of impulsivity with a stronger relationship to risk behavior and externalizing problems. Third, working memory was positively related to sensation seeking in a prospective fashion but negatively related to acting without thinking at time 1, confirming the expectation that executive function has different relations with each form of impulsivity.
Time 1 Model Tests
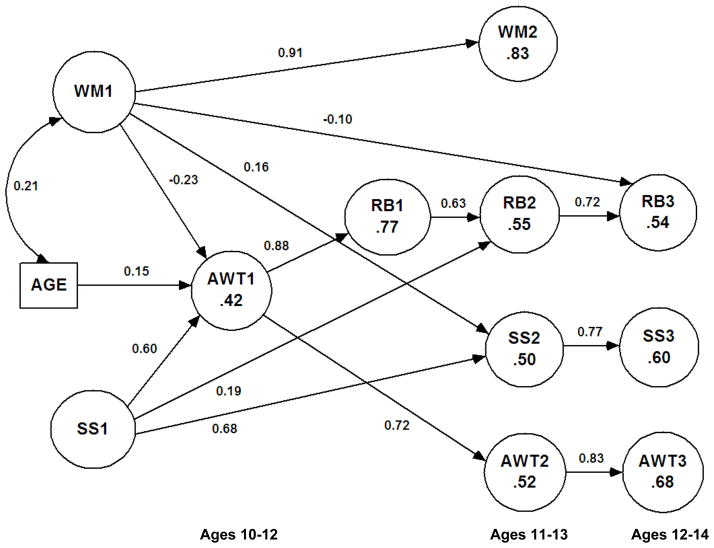
We first tested the prediction from the striatal imbalance model that early risk behavior would be largely dependent on acting without thinking with little or no contribution from sensation seeking. This prediction was tested in a model that allowed time 1 measures of age, working memory, and sensation seeking to predict acting without thinking at time 1. In addition, acting without thinking was defined as the sole predictor of time 1 risk behavior. As seen in the predictors of time 1 factors in Figure 2 and the parameter estimates in Table 4, this model contained significant parameters for each predictor of acting without thinking. In addition, acting without thinking was strongly related to risk behavior. The model provided an excellent fit to the data, χ2(61) = 82, p = .03, CFI = .97, RMSEA = .029 (90% CI = .005, .044), and explained 77% of the variation in risk behavior. Allowing sensation seeking also to directly predict risk behavior produced no improvement in fit, χ2(1) = 0.08, p > .25. In addition, a model in which the roles of acting without thinking and sensation seeking were reversed provided a poorer fit to the data, χ2(60) = 145, p < .01, CFI = .89, RMSEA = .060 (95% CI = .047, .072). Alternative models were also tested in which risk behavior was the cause of working memory, but these models also provided much poorer fits to the data. Hence, these tests confirmed the predictions that acting without thinking is inversely related to working memory performance but that it is able to account for early risk taking without any additional contribution from sensation seeking.
Figure 2.
Final model for relations between factors and age at each time point. RB = risk behavior; WM = working memory performance; SS = sensation seeking; and AWT = acting without thinking. Predicted variation in dependent factors (R2) shown within circles. All paths significant at p < .05.
Table 4.
Parameters in final model for assessments at times 1, 2 and 3.
| Path | Standardized Coefficient | Robust z value | Robust (two-tailed) Probability |
|---|---|---|---|
| Time 1 | |||
| WM1 → AWT1 | −.232 | −3.42 | <.001 |
| Age → AWT1 | .149 | 2.49 | .012 |
| SS → AWT1 | .597 | 7.28 | <.001 |
| AWT1 → RB1 | .878 | 7.96 | <.001 |
| Time 1 → Time 2 | |||
| WM1 → WM2 | .908 | 7.64 | <.001 |
| WM1 → SS2 | .156 | 2.34 | .020 |
| SS1 → RB2 | .186 | 2.47 | .012 |
| RB1 → RB2 | .626 | 6.76 | <.001 |
| AWT1 → AWT2 | .724 | 10.79 | <.001 |
| SS1 → SS2 | .684 | 7.43 | <.001 |
| Time 1 → Time 3 | |||
| WM1 → RB3 | −.096 | −2.023 | .042 |
| Time 2 → Time 3 | |||
| RB2 → RB3 | .720 | 7.61 | <.001 |
| SS2 → SS3 | .772 | 10.46 | <.001 |
| AWT2 → AWT3 | .826 | 11.15 | <.001 |
| Residual Correlations | |||
| SS2, RB2 | .703 | 5.25 | <.001 |
| SS2, AWT2 | .533 | 4.33 | <.001 |
| SS3, RB3 | .545 | 5.16 | <.001 |
| SS3, AWT3 | .585 | 3.56 | <.001 |
| AWT2, RB2 | .869 | 6.34 | <.001 |
| AWT3, RB3 | .815 | 5.76 | <.001 |
Abbreviations: RB = risk behavior; WM = working memory performance; SS = sensation seeking; and AWT = acting without thinking.
Model Tests over Time
To examine the relations between the factors over time, we tested a longitudinal model that predicted assessments at times 2 and 3 from the model that was identified at time 1 (as shown in Figure 2). We allowed the residuals in assessments to be inter-correlated at each time point but also tested paths from prior assessments to subsequent time points, controlling for prior status in the factors. Included in these analyses were controls for correlated errors in observed scores. After these initial tests, we dropped paths that were not significant, producing the final model in Figure 2 with estimates in Table 4. This model provided a good fit to the data, χ2(451) = 731, p < .01. CFI = .96, RMSEA = .022 (90% CI = .014, .029). In addition, the model accounted for at least half of the variation in each factor at times 2 and 3. We also reran the model excluding the cases that contributed the most to kurtosis in the data, but again found the same solution.
Our analyses indicated that stronger working memory at time 1 predicted greater sensation seeking at time 2 (β = .16), χ2(1) = 8.3, p < .001. There was no additional effect of working memory on sensation seeking at time 3. However, the effect at time 2 carried over to time 3 because of stability in sensation seeking. Thus, about 80% of the effect at time 2 was absorbed into time 3. In addition, stronger working memory at time 1 predicted lower levels of risk behavior at time 3 (β = −.10), χ2(1) = 5.00, p = .025. Finally, stronger sensation seeking at time 1 predicted greater risk behavior at time 2 (β = .19), χ2(1) = 8.2, p < .001. Acting without thinking did not predict risk behavior at time 2, p > .20. Age was not directly related to changes in any factors at times 2 or 3 apart from the other factors in the model.
It is noteworthy that acting without thinking was not prospectively related at either times 1 or 2 to change in subsequent risk behavior (paths not shown). This indicates that its strong relation to risk behavior at time 1 carried over to subsequent assessments. However, there were also strong contemporaneous relations between residuals in this measure and risk behavior at times 2 (r = .87) and 3 (r = .82). These correlations indicate that changes in each factor between assessments operated in a manner similar to what was observed at time 1 when the correlation between acting without thinking and risk behavior was also high (r = .86). This pattern supports the hypothesis that high levels of this form of impulsivity are evident early in development and remain so during early adolescence.
Contemporaneous relations in the residuals at times 2 and 3 between sensation seeking, risk taking, and acting without thinking also tended to mirror what was observed at time 1. Sensation seeking and acting without thinking were moderately correlated (r’s = .53 and .59); and sensation seeking and risk behavior were correlated (r’s = .70 and .55) but not as strongly as acting without thinking.
We left working memory at time 2 in the model even though it was highly collinear with its status at time 1. Indeed, unlike any of the other factors in the model, the residual for the factor at time 2 was not significantly different from zero. Nevertheless, the excellent fit of the model without any other paths from or to working memory at time 2 supports the conclusion that its status was fully captured at time 1.
Discussion
Our analysis of three annual assessments in a community sample of early adolescents supports the striatal imbalance model for the relation between two forms of impulsivity (sensation seeking and acting without thinking) and their relations to risk behavior and working memory performance. We found that although risk behavior and impulsivity increased across assessments, working memory performance also increased. This pattern suggests that not all risk taking need be accompanied by deficits in executive function. Indeed, we expected that the two forms of impulsivity would be positively related to each other at each assessment and positively related to risk behavior. However, we expected them to be differentially related to working memory performance with sensation seeking related positively and acting without thinking negatively related. In addition, because acting without thinking is linked to a deficit in executive function relative to subcortical impulse motivation, we expected it to be more strongly related to risk behavior than sensation seeking. Confirmation of all these predictions supported the proposed role of relative deficits in executive function as a precursor to risk behavior and associated externalizing problems.
Our findings supported a unique prediction of the striatal imbalance model, namely that adolescent risk taking is a product of both a dysfunctional form of impulsivity, associated with deficits in executive function and the tendency to act without thinking, and of a more controlled form of impulsivity associated with sensation seeking and relatively greater executive function. Indeed, at the same time that sensation seeking predicted increases in risk behavior (from time 1 to 2), it was accompanied by stronger working memory performance, as indicated by the prospective relation between working memory at time 1 and sensation seeking at time 2.
We also found that working memory performance was inversely related to subsequent risk behavior. Although this relation was not predicted by the striatal imbalance model, it is consistent with models that posit early weakness in executive cognitive function as increasing susceptibility to later problem behavior (Nigg, et al., 2006; Tarter et al., 2003; 2004). Furthermore, we could not rule out the effects of other forms of impulsivity that may mediate effects of working memory deficits on subsequent emergence of risk behavior. For example, a third form of impulsivity, temporal discounting, has also been found to be independent of sensation seeking (Wilson & Daly, 2006) and acting without thinking (Reynolds, Penfold, & Patak, 2008) and to predict drug use and other risky behavior (Reynolds, 2006). Although we did not measure this form of impulsivity, it has also been found to be inversely related to working memory performance (Shamosh et al., 2008). It is possible therefore that after controlling for prior influence of sensation seeking and acting without thinking, this or other forms of impulsivity could also influence risk taking in mid-adolescence.
Although acting without thinking was not associated with later emergence of risk behavior, this form of impulsivity was highly related to risk behavior at each assessment, suggesting that its effects were already evident at the first assessment in the study. This pattern is consistent with Tarter and colleagues (2003; 2004) model of neurobehavioral disinhibition, which predicts that early forms of externalizing problems and executive function deficits are associated with a pattern of increased risk for later problem behaviors. We suspect that this tendency is well under way prior to adolescence given evidence from studies that observe children as young as age 3 who exhibit impulsive traits that predict later problems in behavior (Caspi, Henry, McGee, Moffitt, & Silva, 1995; Caspi, Moffitt, Newman, & Silva, 1996; White, Moffitt, Caspi, Bartusch, Needles, & Stouthamer-Loeber 1994).
Despite the strong relation between risk taking and acting without thinking that was evident at all three assessments, the direct path from sensation seeking at time 1 to risk behavior at time 2 indicates that atleast some of the risk taking that occurs during adolescence is the unique result of sensation seeking. While the size of this relation was not large after controlling for acting without thinking, it is likely to continue to grow as adolescents age. Indeed, many studies find a robust relation between sensation seeking and risk taking during later adolescence (e.g., Crawford, Pentz, Chou, Li, & Dwyer, 2003; Romer & Hennessy, 2007). The finding that working memory was positively related to sensation seeking is also consistent with research that finds a positive relation between sensation seeking and IQ. Indeed, IQ tends to have a small but persistent negative relation with maladaptive forms of risk taking (Henry & Moffitt, 1997), Although the size of the relation between working memory and sensation seeking was not large, it is also likely to grow as adolescents age. As seen in the correlations in Table 3, the relation between working memory at time 1 and sensation seeking increased from .05 to .23 over the course of three years.
The separate effect of sensation seeking on risk taking suggests that later emergence of risk behavior associated with sensation seeking is actually marked by greater executive function. In this form of risk taking, attraction to risky activities is promoted by a combination of executive function and interest in novel and exciting stimuli of all kinds. Such a tendency would have clear adaptive benefits to adolescents who are expected to leave home, find mates, and start on a path toward self-sufficiency (Spear, 2007). It would also encourage engaging in new experiences that could permit the adolescent to identify unique strengths and abilities as an independent actor (Jessor, 1992). Unfortunately in a modern society with many hazards associated with risk taking during adolescence, such behavior is not without its perils, including risk for substance abuse and injury. However, it appears to be of a different sort from the risk taking that is implicated in the early emergence of weak executive function that may lead to more serious forms of disorder. Strategies designed to prevent those outcomes will need to distinguish between the two forms of impulsivity.
An alternative explanation for early weakness in executive function among high risk takers concerns the possibility that engagement in problem behavior disrupts the normal development of executive cognitive function. For example, heavy alcohol use during adolescence might affect the development of the hippocampus and thereby disrupt executive functioning (Medina, Schweinburg, Cohen-Zion, Nagel, & Tapert, 2007). This explanation seems unlikely given that our early risk takers were not engaging in high levels of alcohol use. In addition, we found no effect of early risk taking on working memory. Furthermore, the finding that early weakness in working memory predicted later increases in risk behavior suggests that this deficit predates the heavier uptake of drug use that occurs later in the adolescence. Hence, the data are more consistent with the hypothesis that early deficits in working memory relative to motivational circuits is partly responsible for high levels of early risk taking.
Our results are also interesting in the context of theories that attribute increased risk and externalizing behavior during adolescence to a structural imbalance between dorsolateral PFC and mesolimbic control over behavior. These theories tend to focus on increasing dopamine transmission in the ventral mesolimbic system and to overlook the other major (dorsal striatal) dopamine pathway that subserves more controlled cognitive processes related to working memory ability. Our results suggest that this pathway also exhibits increasing activation during adolescence. As a result, rather than reflecting universally slower adolescent maturation of dorsal versus ventral control systems, such differences may primarily affect a subset of adolescents who tend to exhibit greater ventral than dorsal striatal maturation early in development. This imbalance appears to continue into mid-adolescence placing youth with these characteristics at increased risk for drug dependence and other externalizing problems. At the same time, youth who also experience increasing ventral striatal activation but who do not suffer from relative weakness in executive function appear to be less susceptible to impulsive outcomes. Indeed, the striatal imbalance model predicts that some risk taking is accompanied by stronger executive function, a prediction that is clearly at odds with models that attribute all adolescent risk taking to weak executive function. Nevertheless, it will be important to continue to follow these youth as they enter later adolescence to determine whether the two types of impulsivity lead to different risks to healthy development. It would be expected that youth who are high in acting without thinking would experience more serious risks to healthy development than those who are high in sensation seeking.
Limitations
Limitations in this study should also be noted. We did not have direct measures of dopamine or striatal function in either of the pathways that were proposed to be critical to risk taking in our model. We assumed that variation in dopamine synthesis in the ventral striatum played a role in both forms of impulsivity we measured. However, the precise role of dopamine function in the dorsal pathway is less clear. In particular, the effects of drugs that increase dopamine function in this system appear to depend on the resting levels of dopamine activity, in keeping with the Yerkes-Dodson principle (Cools & Robbins, 2004). Hence, it could be that dopamine function in this pathway is weak leading to poor executive function, or it could be that it is overly active, also leading to the same result. Future research should be directed to observing such activation more directly in youth who exhibit high versus low levels of the two forms of impulsivity.
We primarily base our conclusions regarding risk behavior on the self-report of our participants. However, in regard to many forms of risk behavior in adolescents, youth reports are more sensitive than those of parents (or teachers), who often do not know what drugs their children use, or what they do with their peers (Crouter, Bumpus, Davis, & McHale, 2005; Herjanic & Reich, 1997). Finally, an analysis that identifies unique trajectories over time and tests relations between them should also be conducted as this cohort ages to determine whether an early risk-taking trajectory has different relations with trajectories in impulsivity and executive function. It would be expected that such an analysis would show that an early risk-taking trajectory is more highly related to an early trajectory in acting without thinking and weak executive function than to later emerging trajectories in risk behavior.
Implications for Intervention
Our results suggest that interventions to prevent adverse consequences for either of the pathways we have identified will need to consider the differences between the two types of impulsivity. Interventions to prevent adverse effects of acting without thinking will need to focus on weaknesses in executive function that appear to emerge early in development. Our results indicate that if untreated such deficits will lead to increasing levels of risk and externalizing behavior as youth age. Interventions to reduce weaknesses in executive function include training in working memory (Klingberg, et al., 2005) as well as in cognitive strategies of self-control and problem solving (Botvin & Schinke, 1997). Interventions for those high in sensation seeking might more fruitfully focus on diverting sensation seeking drives to less harmful activities, such as sports that can also satisfy needs for novelty and excitement. However, cognitive control strategies may also be appropriate for such youth as these abilities can help to channel impulsive drives into more constructive activity.
Acknowledgments
This research was supported by a grant from the U. S. National Institute on Drug Abuse (R01 DA 18913). We thank Chris Jarrold, the anonymous reviewers, and Martha J. Farah for helpful comments regarding earlier versions of this paper.
Footnotes
We also tested for differences in gender, but it was only directly related to acting without thinking at time 1 and its inclusion did not alter the significance of the paths in the model. Thus, it was not included in this or the larger model in Figure 2.
Contributor Information
Daniel Romer, University of Pennsylvania.
Laura M. Betancourt, Children’s Hospital of Philadelphia
Nancy L. Brodsky, Children’s Hospital of Philadelphia
Joan M. Giannetta, Children’s Hospital of Philadelphia
Wei Yang, University of Pennsylvania.
Hallam Hurt, Children’s Hospital of Philadelphia.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Acton GS. Measurement of impulsivity in a hierarchical model of personality traits: implications for substance use. Substance Use & Misuse. 2003;38(1):67–83. doi: 10.1081/ja-120016566. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Arnsten AFT, Li BM. Neurobiology of executive functions: Catecholomine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bentler PM. EQS 6 structural equation manual. Encino, CA: Multivariate Software, Inc; 2004. [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. Public Library of Science One. 2009;4(8):1–12. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Biglan A, Cody C. Preventing multiple problem behaviors in adolescence. In: Romer D, editor. Reducing adolescent risk: Toward an integrated approach. Thousand Oaks, CA: Sage Publications; 2003. pp. 125–131. [Google Scholar]
- Block J, Block J, Keyes S. Longitudinally foretelling drug usage in adolescence: Early childhood personality and environmental precursors. Child Development. 1988;59:336–355. doi: 10.1111/j.1467-8624.1988.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Botvin G, Schinke S. The etiology and prevention of drug abuse among minority youth. Binghamton, New York: Hayworth Press; 1997. [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Crone EA. Neural correlates of the development of cognitive control. In: Ramsey JM, Ernst M, editors. Neuroimaging in developmental clinical neuroscience. New York: Cambridge University Press; 2009. pp. 22–37. [Google Scholar]
- Cambridge Cognition Ltd. Cambridge Neuropsychological Test Automated Battery (CANTAB) Cambridge, UK: 1999. [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental origins of child and adolescent behavior problems: From age three to age fifteen. Child Development. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Archives of General Psychiatry. 1996;53(v):1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2003 State and Local Youth Behavior Survey. 2003 http://www.cdc.gov.
- Chambers RA, Taylor JR, Potenza M. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Journal of American Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcholism: Clinical and Experimental Research. 1988;121(4):494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. The Journal of Neuroscience. 2008;28(5):1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philosophical Transactions of the Royal Society London. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Crawford AM, Pentz MA, Chou CP, Li C, Dwyer JH. Parallel developmental trajectories of sensation seeking and regular substance use in adolescents. Psychology of Addictive Behaviors. 2003;17(3):179–192. doi: 10.1037/0893-164X.17.3.179. [DOI] [PubMed] [Google Scholar]
- Crouter AC, Bumpus MF, Davis KD, McHale SM. How do parents learn about adolescents’ experiences? Implications for parental knowledge and adolescent risky behavior. Child Development. 2005;76(4):869–882. doi: 10.1111/j.1467-8624.2005.00883.x. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, Ehlers KM, Whitmore EA, MacDonald MJ. Validity of structured clinical evaluations in adolescents with conduct and substance problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(3):265–273. doi: 10.1097/00004583-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131(2):196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- E-Prime [computer program, Version 1.1.4.1] Pittsburgh PA: Psychology Software Tools, Inc; 2002. p. 2002. [Google Scholar]
- Eysenck SBG, Eysenck HJ. The place of impulsiveness in a dimensional system of personality description. British Journal of Social and Clinical Psychology. 1977;16:57–68. doi: 10.1111/j.2044-8260.1977.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk I, Hayashi KM, Greenstein D, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of Physiology: Nervous System Vol V: Higher Functions of the Brain. Bethesda, MD: American Psychological Society; 1987. [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1998;10(2):163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Henry B, Moffitt TE. Neuropsychological and neuroimaging studies of juvenile delinquency and adult criminal behavior. In: Stoff DM, Breiling J, editors. Handbook of antisocial behavior. New York: Wiley; 1997. pp. 280–288. [Google Scholar]
- Herjanic B, Reich W. Development of a structured psychiatric interview for children: Agreement between child and parent on individual symptoms. Journal of Abnormal Child Psychology. 1997;25(1):21–31. doi: 10.1023/a:1025703323438. [DOI] [PubMed] [Google Scholar]
- Hofstra MB, Van der Ende J, Verhulst FC. Pathways of self-reported problem behaviors from adolescence into adulthood. American Journal of Psychiatry. 2002;159(3):401–407. doi: 10.1176/appi.ajp.159.3.401. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41(14):1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences. 2002;32:401–414. [Google Scholar]
- Hu L, Bentler PM. Evaluating model fit. In: Hoyle RH, editor. Structural equation modeling: Concepts, issues and applications. Newbury Park, CA: Sage Publications; 1995. pp. 76–99. [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopmaine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R. Reply: Risk Behavior in Adolescence: A Psychosocial Framework for Understanding and Action. Developmental Review. 1992;12:374–390. doi: 10.1016/1054-139x(91)90007-k. [DOI] [PubMed] [Google Scholar]
- Johnston LD, Bachman JG, O’Malley PM. Monitoring the Future: Questionnaire responses from the nation’s high school seniors. Ann Arbor: Institute for Social Research; 2006. [Google Scholar]
- Kaplan D. Structural equation modeling. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Cognitive functions in prefrontal cortex -working memory and executive control. Current Directions in Psychological Science. 1998:185–192. [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD: A randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O’Neil JP, Baker S, Jagust W. Striatal dopamine and working memory. Cerebral Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Sowell ER. Morphological development of the brain: What has imaging told us? In: Rumsey JM, Ernst M, editors. Neuroimaging in developmental clinical neurscience. New York: Cambridge University Press; 2009. pp. 5–21. [Google Scholar]
- Magid V, MacLean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addictive Behaviors. 2007;32:2046–2061. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MediaLab [computer program, Version 2004.2.87] New York: Empirisoft; 2004. [Google Scholar]
- Medina KL, Schweinburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. Journal of Neuroscience. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DS, Koblin B, Turner C, Navaline H, Valenti F, Holte S, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. American Journal of Epidemiology. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Development and Psychopathology. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive planning in humans: neuropsychological, neuroanatomical and neuropharmacological perspectives. Progress in Neurobiology. 1997a;53(4):431–450. doi: 10.1016/s0301-0082(97)00042-7. [DOI] [PubMed] [Google Scholar]
- Owen AM. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Experimental Brain Research. 2000;133(1):33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pickett W, Garner MJ, Boyce WF, King MA. Gradients of risk for youth injury associated with multiple-risk behaviours: A study of 11,329 Canadian adolescents. Social Science and Medicine. 2002;55(6):1055–1068. doi: 10.1016/s0277-9536(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Previc FH. Dopamine and the origins of human intelligence. Brain and Cognition. 1999;41:299–350. doi: 10.1006/brcg.1999.1129. [DOI] [PubMed] [Google Scholar]
- Previc FH. The dopaminergic mind in human evolution and history. New York: Cambridge University Press; 2009. [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behavioural Pharmacology. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: Laboratory behavioral assessments. Experimental and Clinical Psychopharmacology. 2008;16(2):124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Roberti JW. A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality. 2004;38:256–279. [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology. 2010;52(3):263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: the role of affect evaluation and peer-group influence in adolescent drug use. Prevention Science. 2007;8(2):89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague R. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: Relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Classen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shedler J, Block J. Adolescent Drug Use and Psychological Health: A Longitudinal Inquiry. American Psychologist. 1990;45:612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Shope JT, Bingham CR. Teen driving: Motor vehicle crashes and factors that contribute. American Journal of Preventive Medicine. 2006;35:S261–271. doi: 10.1016/j.amepre.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Slade PD, Townes BD, Rosenbaum G, Martins IP, Luis H, Bernardo M, et al. The serial use of child neurocognitive tests: development versus practice effects. Psychological Assessment. 2008;20(4):361–369. doi: 10.1037/a0012950. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14(2):155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM. Mapping cortical change across the lifespan. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The developing brain and adolescent-typical behavior patterns: An evolutionary approach. In: Romer D, Walker EF, editors. Adolescent psychopathology and the developing brain: Integrating brain and prevention science. New York: Oxford University Press; 2007. pp. 9–30. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavioral disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73(2):121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Visser JH, Van der Ende J, Koot HM, Verhulst FC. Predictors of psychopathology in young adults referred to mental health services in childhood or adolescence. British Journal of Psychiatry. 2000;177:59–65. doi: 10.1192/bjp.177.1.59. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Weinstein SR, Noam GG, Grimes K, Stone K, Schwab-Stone M. Convergence of DSM-III diagnoses and self-reported symptoms in child and adolescent inpatients. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29(4):627–634. doi: 10.1097/00004583-199007000-00018. [DOI] [PubMed] [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch DJ, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. Journal of Abnormal Psychology. 1994;103(2):192–205. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five-factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Wilson M, Daly M. Are juvenile offenders extreme future discounters? Psychological Science. 2006;17(11):989–994. doi: 10.1111/j.1467-9280.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- Winters KC, Stinchfield RD, Henly GA, Schwartz RH. Validity of adolescent self-report of alcohol and other drug involvement. International Journal of Addiction. 1990;25(11A):1379–1395. doi: 10.3109/10826089009068469. [DOI] [PubMed] [Google Scholar]
- Yuan KH, Bentler PM. Normal theory based statistics in structural equation modeling. British Journal of Mathematical and Statistical Psychology. 1998;51:289–309. doi: 10.1111/j.2044-8317.1998.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, et al. Midbrain dopamine receptor availability is inversely associated with novelty seeking traits in humans. Journal of Neuroscience. 2008;28(53):14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York: Cambridge University Press; 1994. [Google Scholar]
- Zuckerman M, Kuhlman D. Personality and risk-taking: common biosocial factors. Journal of Personality. 2000;68(6):999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]