Abstract
We have demonstrated that neonatal exposure to selective serotonin reuptake inhibitors has lasting effects on behavior and serotonergic neurons in Long Evans rats. Hyperserotoninemia and altered sensory processing are reported in autistic spectrum disorders (ASD). We hypothesized that early life exposure to SSRIs alters sensory processing, disrupts responses to novelty and impairs social interactions in a manner similar to that observed in ASD. Male and female Long-Evans rat pups were administered citalopram, buproprion, fluoxetine, or saline from postnatal day (P) 8 to 21. Rats were tested for response to a novel tone before weaning (P25). Later, rats were tested 2× for response to a novel object (P39), and to a novel conspecific (P78, P101). In addition, rats were assessed for juvenile play behaviors (P32–P34) and later, we assessed sexual response to an estrus female in male rats (P153–184). Antidepressant exposure increased freezing after tone, diminished novel object exploration and reduced conspecific interaction up to 3× compared to saline exposed rats. Juvenile play was profoundly reduced in antidepressant-exposed males when compared to saline exposed groups. Exposure to the SSRIs, but not bupropion disrupted male sexual behaviors. Moreover, specific male responses to female proceptive behaviors were disrupted in SSRI, but not bupropion exposed rats. We conclude that neonatal exposure to antidepressants in rats results in sensory and social abnormalities that parallel many of those reported in ASD.
INTRODUCTION
Autism Spectrum Disorders (ASD) are severe neurodevelopmental disorders with early childhood onset that continue to evolve during development. They are characterized by qualitative alterations in three behavioral areas: social reciprocity, communication and breadth of interests manifest by repetitive behaviors or restricted interests (The Task Force on DSM-IV, 1994). More than four times as many males as females are affected(Nicholas et al., 2009). Within these core diagnostic areas, great variability in clinical presentation and characteristics may appear in individuals with ASD. Behavioral problems such as impulsivity, anxiety, hyperactivity, irritability and aggression, may be also be present(Harkin et al., 2000; Sikich, 2001). Likewise, sensory alterations such as auditory hyper-reactivity to novel targets, together with processing alterations have been reported in autistic children. In fact, autistic children have been described as displaying tactile defensiveness, which includes escaping from the stimulation (Baranek and Berkson, 1994; Khalfa et al., 2001; Gomot et al., 2008). The relationship between the sensory and behavioral alterations is not fully understood.
Among various attempts to design an animal model for autism, those that alter serotonin metabolism during neurodevelopment replicate some of the neurobiological features of autistic individuals. Further, recognition of the morphogenetic role of serotonin in central nervous system development (Whitaker-Azmitia, 2005; McNamara et al., 2008)has encouraged the development of animal models based on early life alterations in serotonin availability. Many of these models replicate anatomical and behavioral abnormalities observed in ASD. For example, early exposure to the serotonin agonist, 5-methoxytryptamine, results in structural alterations in cortical column development and behaviors that include decreased social bonding, sensory-hyperesponsiveness, seizures and motor changes (Winslow and Insel, 1990; Kahne et al., 2002; Whitaker-Azmitia, 2005).
The PN8–21 antidepressant exposure paradigm was originally introduced by Mirmiran et al. as a chemical means of suppressing rapid eye movement sleep during development (Mirmiran et al., 1981). These and subsequent investigators reported a number of behavioral changes in these rats as adults including alterations in locomotor activity, anxiety-like behavior, increased immobility in forced swim test, reduced exploratory behaviors in open field testing, reduced male sexual activity and competence, increased ethanol consumption, dysregulation of the hypothalamic-pituitary-adrenal axis and alterations in REM sleep. (Mirmiran et al., 1981; De Boer et al., 1989; Maciag et al., 2006). Although some of the reported behavioral deficits have been difficult to replicate across laboratories, impairment of male sexual behavior and disruption of aggressive behaviors have been remarkably consistent sequelae of early life antidepressant exposure (reviewed in (Vogel et al., 2000)).
Our laboratory has utilized early life exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants to elevate extracellular serotonin levels during specific periods of development. Using this paradigm, we have demonstrated that male rodents chronically exposed to SSRIs or other antidepressants such as clomipramine from postnatal day 8 (P8) to P21 present severe disruptions in both sexual and aggressive behaviors as well as lasting effects on serotonergic synthetic and synaptic clearance proteins(Maciag et al., 2006). Likewise, the cytoarchitectual features of serotonergic axons projecting to the frontal cortex and hippocampus are disrupted by this treatment with the appearance of “beaded” and “thick” fibers in place of the fine branching arbors typically observed in control animals (Weaver et al., 2010).
The effects of early life SSRI exposure on serotonergic neurons and projections parallel the neurobiological features ASD (Maciag et al., 2006; Weaver et al., 2010). These studies have contributed to the understanding of the mechanisms underlying the neurobiological characteristics of autism, however it is obvious that there is a gap between the anatomy and the complex behavior of ASD that remains to be explained. Moreover, the adult behaviors in rodents previously reported to be disrupted by early life SSRI exposure are complex and not clearly relatable to the behaviors observed in humans with ASD. In order to begin to behaviorally validate the hypothesis that early life SSRI exposure results in neurobiological features of ASD, it is necessary to determine whether this paradigm disrupts behaviors that can be closely linked with ASD.
To do this, we approached this model using simple behaviors that are components of sexual (Beyer et al., 1981; Hull and Dominguez, 2007)or aggressive behavior(Albert and Walsh, 1982; Blanchard et al., 2003)that also model behavioral elements of ASD (Winslow and Insel, 1990; Kahne et al., 2002; Whitaker-Azmitia, 2005). Specifically, we chose models to examine the effect of early life antidepressant exposure on response to auditory, visual and conspecific novelty. In addition, several behaviors previously observed to be disrupted after neonatal antidepressant exposure are closely related to social or affiliative behaviors such as those disrupted in ASD. Thus, we examined the effects of neonatal antidepressant exposure on social recognition and juvenile play behavior as well as response to novel sensory input. As described above, alterations in serotonin metabolism during brain development translate into structural alterations in regions involved in sensory processing and in abnormal behaviors that parallel some of the autistic behaviors and might be also related to the response to novel stimulus. Thus, we hypothesized that early exposure to SSRIs, but not a selective dopamine/norepinephrine reuptake inhibitor (bupropion), alters the development of sensory processing regions which might translate in an abnormal response to novel stimulus. This disruption in sensory processing might affect simple sensory-response behaviors but also complex social interactions that depend on specific sensory cues.
MATERIALS AND METHODS
Design
Both female and male offspring of timed-pregnant Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN) were used in these experiments. All procedures were approved by the UMMC Institutional Animal Care and Use Committee and complied with AAALAC and NIH standards. On the first day after birth, pups were cross-fostered as needed within the adult females to achieve groups of 12 to 14 per litter. All animals thrived and grew normally and none were lost during development. On PN6–7, pups were tattooed for identification. Beginning at PN8, rat pups were injected subcutaneously with citalopram-HBr at a dose of 10 mg/kg, fluoxetine-HCl at a dose 5 mg/kg, bupropion-HCl at a dose 15 mg/kg or saline in a volume of 0.1 ml twice daily (0600 and 1200 hours) from PN8–PN21. This dosing schedule was chosen to allow a more dilute concentration of drug per injection and minimize the risk of irritation/inflammation due to subcutaneous injection. All drugs were obtained from Toronto Research Chemicals (Toronto, Canada). Each litter included at least one pup in each treatment group. At PN28 pups were weaned and housed in groups of 2–3/cage under standard laboratory conditions with ad lib access to food and water.
Behavioral Testing
Behavioral testing was conducted on rats during the dark phase of the light : dark cycle. Rats were brought in covered cages to a sound-attenuated (ambient sound level in testing area ≤30 dB), dim light (~50 lux) testing room to acclimate for 1 h before each test.
Locomotor tone
On P25 rats were placed individually into locomotor activity-monitoring units (transparent Plexiglas, 43 cm2 floor, 20 cm walls Opto-Varimex, Columbus Instruments) for 6 minutes. Four monitoring units were arranged in parallel so that at least one rat from each exposure group was recorded in each observation period. After 120 sec acclimation, a tone (radio tuned to static–sound level in testing area = 70 dB) was turned on for 120 sec. Locomotor activity was recorded for the 6 min test. A computer acquisition system recorded horizontal (ambulation time, distance traveled) and vertical activity(rearing)in 1 min epochs. In addition, time spent immobile (resting) was recorded. At the conclusion of each squad’s test, animals were removed from the units and returned to their home cages. Fecal boli were removed and the units were swabbed with a 10%alcohol/water solution to remove scents. Data were analyzed using two factor (neonatal treatment, gender) repeated measures (pretone, tone, post-tone) analysis of variance for ambulation time, distance traveled, immobility, zone of activity, stereotypies, and rearing. Individual between-group analyses were conducted with a′ priori contrast analysis using univariate F-tests. The primary measure presented is the freezing (immobility) response to the novel tone.
Spontaneous locomotor activity
At intervals during development after weaning (P36, P89 and P120) were brought to the testing room 1 hr prior to testing. Rats were placed individually into the locomotor activity monitoring units described above in a sound-attenuated, lowlight room for 20 minutes. Data were analyzed for time ambulating, zone of activity, distance traveled, stereotypies, and rearing. At the conclusion of each squad’s test, animals were removed from the units and returned to their home cages. Fecal boli were removed and the units were swabbed with a 10%alcohol/water solution to remove scents. Data were analyzed using two factor (neonatal treatment, gender) repeated measures (4 epochs of 5 min. each) analysis of variance for time ambulating, zone of activity, distance traveled, stereotypies, immobility and rearing. Individual between-group analyses were conducted with a′ priori contrast analysis using univariate F-tests.
Novel object approach
On P39, rats were brought to the testing room 1 hr prior to testing. Rats were placed individually into the locomotor activity-monitoring units described above in a sound-attenuated, moderate light room for 20 minutes. After10 minutes of adaptation in the chamber a novel object (lead block, 2 cm high, 5 cm by 5 cm each side, covered in white Plexiglas, weight ~ 700 g) was placed in one corner of the locomotor arena. A computer acquisition unit recorded horizontal and vertical activity in 5 min epochs. At the conclusion of each squad’s test, animals were removed from the units and returned to their home cages. Fecal boli were removed and the units were swabbed with a 10% alcohol/water solution to remove scents. Data were analyzed for time spent in each corner and entries into each corner using two factor (neonatal treatment, gender) repeated measures (target corner zone, opposite corner zone) analysis of variance for time in zone. Individual between-group analyses were conducted with a′ priori contrast analysis using univariate F-tests.
Juvenile play behavior
Between P32 and P34, pups were tested for the appearance and sequence of juvenile play behavior. Pups were isolated for 3.5 hr prior to testing to facilitate the appearance of play behavior. Pups were then paired within gender and treatment groups with conspecifics of comparable weight and placed in an observation chamber (60 × 30 cm) under low light conditions (~50 lux) in a sound-attenuated testing room. After allowing 10 min to acclimate to the test chamber, pairs were video recorded for 15 min. The pups were then returned to their home cage. Using a video scoring program (Noldus Observer), we recorded behaviors (pinning, pouncing, boxing/wrestling, following/chasing, social grooming and social exploration) associated with social play in peri-adolescent rats(Kim et al., 2006; Venkatesha et al., 2006). Data were analyzed for frequency of each behavior analyzed (see Table 1)using two factor (neonatal treatment, gender) analysis of variance. Individual between-group analyses were conducted with a posteriori analysis using Fisher’s Least Significant Difference test.
Table 1.
Juvenile Play behaviors by gender and exposure. Data represent frequency of behavior over 15 min. observation.
| BEHAVIOR | Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTM | FLX | BUP | SAL | |||||||||
| M | SEM | N | M | SEM | N | M | SEM | N | M | SEM | N | |
| Pinning | 1.16a | 1.02 | 18 | 0.13a | 0.13 | 22 | 0.70a | 0.54 | 20 | 6.46 | 2.32 | 16 |
| Boxing | 1.61a | 0.72 | 18 | 2.36a | 0.50 | 22 | 1.95a | 0.49 | 20 | 8.00 | 3.00 | 16 |
| Immobility | 2.11a | 0.48 | 18 | 1.36 | 0.35 | 22 | 1.65 | 0.42 | 20 | 0.81 | 0.36 | 16 |
| Crawling | 1.39 | 0.42 | 18 | 1.27 | 0.34 | 22 | 1.40 | 0.45 | 20 | 0.50 | 0.23 | 16 |
| Other Behavior | 10.0 | 2.03 | 18 | 13.5 | 1.95 | 22 | 12.8 | 1.49 | 20 | 10.2 | 2.31 | 16 |
| Social Grooming | 5.78a | 0.65 | 18 | 9.50 | 1.38 | 22 | 9.30 | 1.65 | 20 | 9.44 | 1.61 | 16 |
| Personal Grooming | 8.89 | 1.17 | 18 | 7.23 | 0.76 | 22 | 8.90 | 0.67 | 20 | 9.75 | 1.24 | 16 |
| Social Exploration | 9.44 | 1.43 | 18 | 4.14 | 1.10 | 22 | 4.65 | 0.96 | 20 | 5.94 | 0.83 | 16 |
| Following/ Chasing | 2.72 | 1.00 | 18 | 2.50 | 0.68 | 22 | 1.75 | 0.67 | 20 | 4.00 | 1.47 | 16 |
| Ambulation | 16.9a | 2.18 | 18 | 15.7a | 1.45 | 22 | 19.5a | 2.94 | 20 | 26.1 | 3.13 | 16 |
| Stereotypic behavior | 4.89a | 0.73 | 18 | 2.41 | 0.70 | 22 | 2.15 | 0.62 | 20 | 0.81 | 0.30 | 16 |
| Rearing | 23.6a | 2.92 | 18 | 28.8 | 2.67 | 22 | 34.7 | 4.57 | 20 | 38.2 | 4.79 | 16 |
| BEHAVIOR | Female | |||||||||||
| CTM | FLX | BUP | SAL | |||||||||
| M | SEM | N | M | SEM | N | M | SEM | N | M | SEM | N | |
| Pinning | 0.12 | 0.13 | 16 | 1.95 | 0.87 | 12 | 8.45a,b | 3.60 | 16 | 3.12 | 1.30 | 18 |
| Boxing | 2.25a | 0.72 | 16 | 4.67 | 1.90 | 12 | 10.8b | 1.96 | 16 | 7.67 | 2.20 | 18 |
| Immobility | 0.75b | 0.32 | 16 | 0.08b | 0.09 | 12 | 0.44b | 0.33 | 16 | 0.00 | 0.00 | 18 |
| Crawling | 1.56 | 0.60 | 16 | 1.33 | 0.59 | 12 | 0.94 | 0.55 | 16 | 1.28 | 0.38 | 18 |
| Other Behavior | 3.38a,b | 0.92 | 16 | 8.33b | 1.00 | 12 | 3.44a,b | 0.55 | 16 | 8.33 | 2.27 | 18 |
| Social Grooming | 3.56 | 0.81 | 16 | 5.17b | 1.44 | 12 | 4.00b | 0.97 | 16 | 6.39 | 1.33 | 18 |
| Personal Grooming | 6.19a | 1.39 | 16 | 9.75 | 0.95 | 12 | 6.81a | 0.83 | 16 | 10.6 | 1.39 | 18 |
| Social Exploration | 3.56a,b | 0.81 | 16 | 5.17b | 1.44 | 12 | 4.00a | 0.97 | 16 | 6.39b | 1.33 | 18 |
| Following/ Chasing | 5.25 | 1.71 | 16 | 5.17 | 1.88 | 12 | 9.81b | 2.09 | 16 | 9.06 | 1.54 | 18 |
| Ambulation | 19.8a | 3.75 | 16 | 27.7b | 3.80 | 12 | 32.2b | 4.31 | 16 | 32.1 | 2.94 | 18 |
| Stereotypic behavior | 3.25 | 1.42 | 16 | 1.00 | 0.59 | 12 | 1.06 | 0.46 | 16 | 2.00 | 0.52 | 18 |
| Rearing | 35.9a | 7.24 | 16 | 55.0b | 6.30 | 12 | 58.7b | 4.83 | 16 | 50.3 | 4.68 | 18 |
p < 0.05 vs. Saline (SAL),
p<0.05 vs. Male–Two Factor (Drug Exposure × Gender) ANOVA followed by Fisher’s Least Significant Difference Test.
Object-Conspecific preference
At P78 and P101, rats were tested for conspecific preference. Rats were brought to the testing room 60 min prior to testing. Animals were placed in the test chamber (20 × 120 cm) on three successive days for 10 min acclimation. The chamber contained a 15 × 15 × 20 cm perforated plexiglas enclosure at each end which the rat could see, touch and smell, but could not enter. On the fourth day, a novel conspecific rat was placed in one of the perforated Plexiglas chambers a comparable size and colored Plexiglas block was placed in the other. The placement was randomized across testing to avoid side-biases. The rat to be tested was placed in the test chamber for 10 min and video recorded for time spent in proximity and contacts with perforated wall of the chamber containing either the conspecific rat or the inanimate object. Data were analyzed for the ratio of contact with the conspecific rat enclosure vs. contact with the inanimate object enclosure using two factor (neonatal treatment, gender) analysis of variance. The interaction ratio (IR) derived is the ratio of contacts with the conspecific wall to contacts with the object wall. Individual between-group analyses were conducted with a posteriori analysis using Fisher’s Least Significant Difference test.
Male sexual behavior
Between P153 and P184 male rats were brought to the sound-attenuated testing room 1 hr prior to testing. A group of ovariectomized females (the original dams and additional retired breeders) were brought into estrous with estrogen benzoate (5 μg SC, 48 and 24 hr prior to testing) and progesterone (500 μg SC 4–6 hr prior to testing) to be used as stimulus females. Males were placed in the observation chamber (30 × 60 cm) to acclimate for 10 min. The test was initiated by placing a female into the arena with the male. Each test lasted 30 min and was conducted under dim red light (7–10 lux) during the dark phase of the light: dark cycle. Each encounter was videotaped and the tape later rated by a technician blind to treatment. Using a video scoring program (Noldus Observer), we recorded sexual and social behaviors (incomplete mount, mount, intromission, ejaculation, social grooming and social exploration) associated with social male sexual behavior rats. Two trials were conducted for acclimation and a third one for analysis. The third trial was analyzed using Kaplan-Meier survival analysis and log-rank statistics. Lag-sequential analysis of the relationship between female behaviors and first order male responses were conducted using log-linear models over all three behavioral trials.
RESULTS
Locomotor Response to Tone
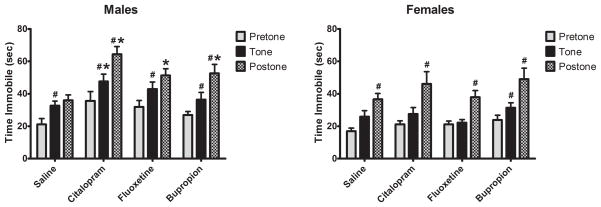
Response to a novel auditory stimulus was examined using the freezing response to a novel tone. Exposure to a novel tone reduces locomotor activity and increases immobility (freezing)which recovers slowly after sound termination in normal juvenile rats. In control male rats, exposure to a novel tone resulted in a 54% increase in immobility that remained stable for 2 min. after the tone ended. In contrast, the immobility of antidepressant-exposed males continued to increase, even after the end of the tone period. Moreover, the increase in immobility was exaggerated, reaching 180% of pretone baseline (Figure 1a).
Figure 1.
Effect on neonatal antidepressant exposure on locomotor activity in response to a novel tone. Data represent the mean ± SEM of 15–21 males and 13–17 females. Two factor (Drug Exposure × Gender) repeated measures (Testing Condition) ANOVA revealed main effects of Testing Condition (F2,246 = 75.857, p < 0.001), Drug Exposure (F3,123 = 5.256, p = 0.002) and, Gender (F1,123 = 20.617, p < 0.001) as well as an Exposure × Gender interaction (F3,123 = 2.815, p = 0.042). *P<0.05 vs SAL, #P<0.05 vs previous Testing Condition, Fisher’s Least Significant Difference Test.
The response of control female rats resembled that of males with presentation of a novel tone eliciting a rapid increase in immobility. However, unlike control males, immobility in control females continued to increase to 116% of pretone levels. While novel tone elicited an increase in immobility in antidepressant-treated females, the effect differed somewhat from males and from control females in that a significant increase in immobility was not observed until after the end of the tone period. The magnitude of the post-tone response was similar across treatment groups in the females (Figure 1b).
Novel Object Exploration
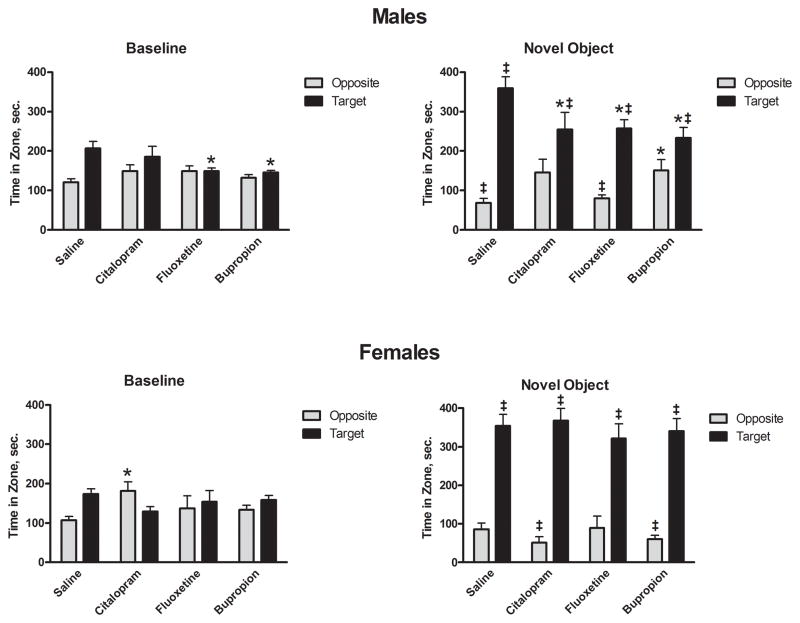
Response to a novel visual/tactile stimulus was examined using the investigation of a novel object in a familiar setting. When presented a novel object, both male and female saline-exposed rats exhibit a pronounced preference for the area with the target over any other area in the chamber. Conversely, saline-exposed rats spent less time in the area opposite the target much less than any other area in the chamber (Figure 2). In contrast, male antidepressant exposed groups spent less time in the novel object area and more time in the area furthest from the novel object when compared to control group. Although pronounced reductions in target preference were evident in males exposed to antidepressants, particularly citalopram and bupropion, no such reduction was evident in antidepressant-exposed females (Figure 2)
Figure 2.
Effect of neonatal antidepressant exposure on time spent in field relativeto novel object. Data presented are time spent in the target grid square and the grid square in the corner opposite the target. Data represent the mean ± SEM of 15–21 males and 13–17 females. Two factor (Drug Exposure × Gender), nested repeated measures (Corner, Object Presence) ANOVA revealed main effects of Drug Exposure (F3,123 = 4.835, p = 0.003), Corner (F3,369 = 76.553, p < 0.001) and, Object Presence (F1,123 = 34.907, p < 0.001). In addition, ANOVA revealed Corner × Treatment (F9, 369 = 2.117, p = 0.027), Corner × Object Presence (F3,369 = 97.550, p < 0.001), Corner × Object Presence × Gender (F3,369 = 8.443, p < 0.001) and Corner × Object Presence × Treatment × Gender (F9,369 = 2.160, p = 0.024). *p<0.05 compared to saline,‡ p<0.05 compared to baseline, Fisher’s Least Significant Difference Test.
Object-Conspecific Interaction
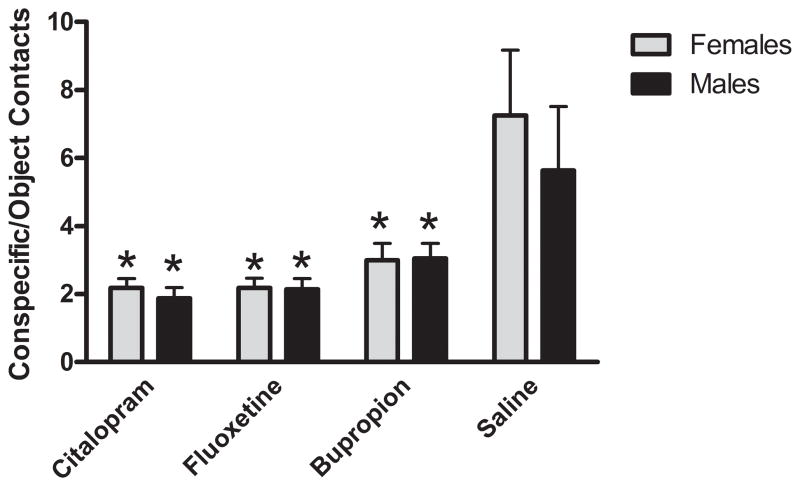
Response to a novel conspecific animal and preference for investigating a novel animal versus a novel object was tested by examining object-conspecific interaction at P78. The ratio of conspecific contacts/object contacts (i.e. contacts with the Plexiglas wall separating the test rat from the object or conspecific) was defined as the interaction ratio (IR). Non-exposed rats interacted with conspecifics up to 3 times more than the exposed group (Figure 3). Citalopram-exposed rats displayed the lowest values, although fluoxetine-exposed rats were very similar. Bupropion-exposed rats values were somewhat higher, but still only represented 50% of the control values. There were no differences between males and females. In order to determine whether interaction was stable, rats were tested again at P101. The results were comparable with those from P78 (data not shown).
Figure 3.
Effect of neonatal antidepressant exposure on the number of interactions with conspecific in relation with interaction with object. Data represents the mean ± SEM of the ratio of contacts with the conspecifics enclosure to that of the novel object for 8–20 males and 11–16 females. Two factor (Drug Exposure × Gender), repeated measures (Interaction Zone) ANOVA revealed a main effect of Interaction Zone (F1,104 = 238.48, p < 0.001) and a Drug Exposure × Interaction Zone (F3,104 = 10.495, p < 0.001) interaction. *p<0.05 compared to saline, Fisher’s Least Significant Difference Test.
Juvenile play
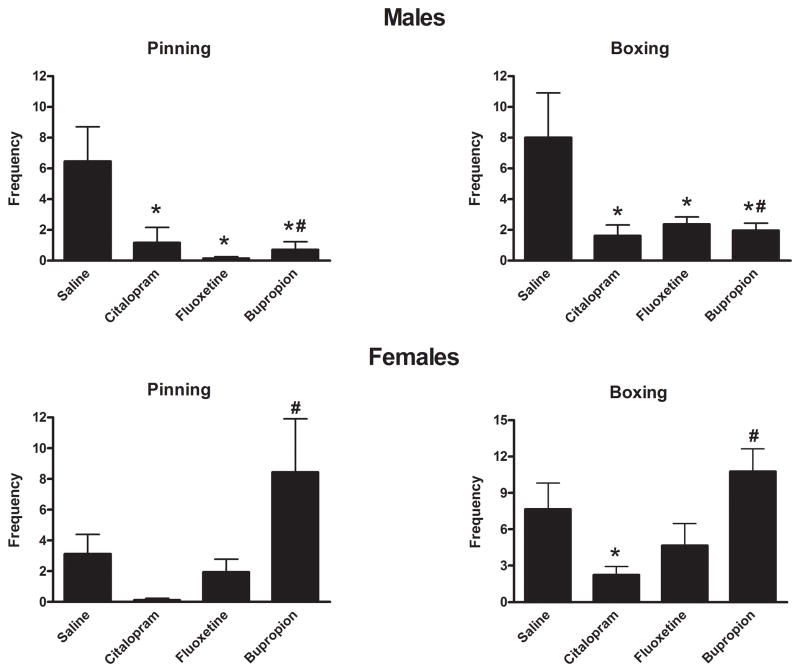
Interest/ability to interact in a normal social setting during development was tested by examining juvenile play behaviors. Juvenile play was profoundly reduced in antidepressant-exposed males when compared to control groups. The frequency of playing activities such as pinning or boxing where reduced up to 98% of control values.(Figure 4) Corresponding increases in stereotypic behaviors and immobility were also observed in antidepressant-exposed males. Finally, all antidepressant exposures resulted in reduced ambulatory activity in this social situation which corresponded to the reduction in play behaviors (pinning, boxing). Female SSRI exposed rats displayed less marked effects than those observed in males. However, control play behavior in females was lower than males. In contrast, the bupropion exposed group showed a 170% increase of these juvenile play activities.(Table 1)
Figure 4.
Effect on neonatal antidepressant exposure on pinning and boxing during juvenile play. Data represent the mean ± SEM of 16–22 males and 12–18 females. Two factor (Drug Exposure × Gender) ANOVA of Boxing/Wrestling behavior revealed main effects of Gender (F1,130 = 6.833, p = 0.010) and Drug Exposure (F3,130 = 6.050, p = 0.001) as well as a Gender by Drug Exposure interaction (F3,130 = 3.709, p = 0.013). Similarly two factor ANOVA of Pinning behavior revealed a main effect Drug Exposure (F3,130 = 4.233, p = 0.007) as well as a Gender by Drug Exposure interaction (F3,130 = 5.066, p = 0.002).*P<0.05 vs saline, #P<0.05 vs opposite gender, Fisher’s Least Significant Difference Test.
The frequency of stereotypical behavior episodes was markedly increased in the male group that was exposed to antidepressants, with the citalopram-exposed group displaying a 6-fold increase in stereotypies compared with control group. In females there was no evident difference between exposed and control groups.(Table 1)
In male rats, the SSRI exposed groups displayed a significant reduction of ambulatory activity with an accompanying increase in immobility. However, bupropion-exposed rats displayed less suppression of motor activity. However in females, ambulatory activity was higher in bupropion and fluoxetine exposed groups compared to control, whereas it was25% lower in citalopram exposed females.(Table 1)
Male sexual behavior
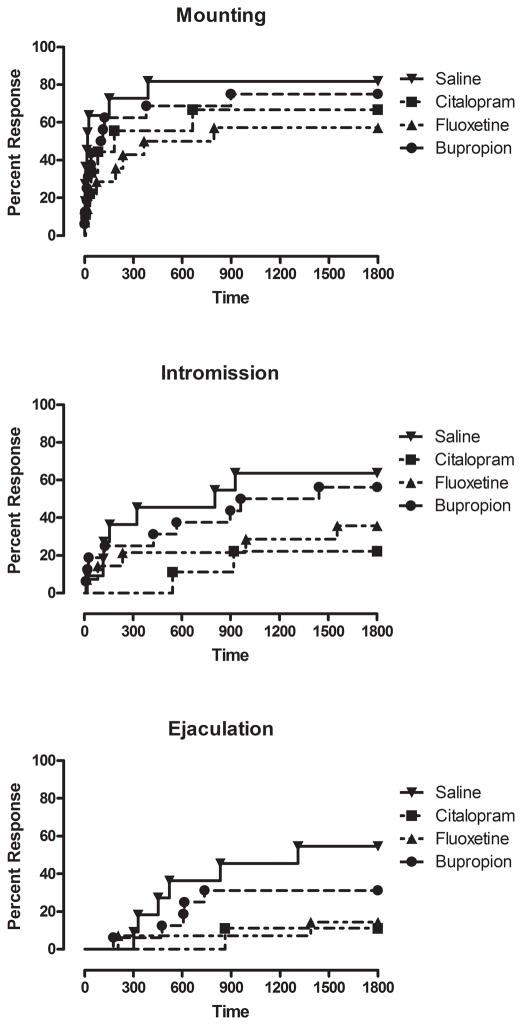
In order to relate these studies to both our own previous studies and to previous studies of early life antidepressant exposure in other laboratories, we examined adult male sexual behavior. Moreover, using lag-sequential analysis, we were able to determine whether the previously observed deficits in male sexual behavior could be accounted for by a deficit in response to normal social cues from the female rat. As noted previously, early life exposure to citalopram significantly impaired male sexual behavior with reductions observed in mounting, intromission and ejaculation (Figure 5). Similar results were obtained in animals exposed to the other SSRI, fluoxetine. In contrast, the non-SSRI antidepressant, bupropion, was without effect on male sexual behaviors. During the observation of male sexual behavior SSRI exposed males were less prone to engage in sexual activities such as mounting, intromission and ejaculation and these effects were more pronounced in citalopram- than in fluoxetine-exposed rats.
Figure 5.
Effect of neonatal antidepressant exposure on male sexual behaviors. (A) Kaplan-Meier survival analysis of mounting behavior using Gehan-Breslow-Wilcoxon Test, saline vs. citalopram χ2 = 2.684, df 1, P = 0.10; saline vs. fluoxetine χ2 = 4.602, df 1, P = 0.0319 and saline vs. bupropion = χ2 = 1.127, df 1, P > 0.10. (B) Kaplan-Meier survival analysis of intromission using Gehan-Breslow-Wilcoxon Test, saline vs. citalopram χ2 =4.125, df 1, P= 0.0422; saline vs. fluoxetine χ2 =1.894, df1, P > 0.10 and saline vs. bupropion χ2 =0.2626, df 1, P > 0.10. (C) Kaplan-Meier survival analysis of ejaculation using Gehan-Breslow-Wilcoxon Test, saline vs. citalopram χ2 =4.357, df 1, P = 0.0369; saline vs. fluoxetine χ2 =4.358, df 1, P = 0.0368 and saline vs. bupropion χ2 =1.319, df 1, P > 0.10.
There was considerable lack of independence within each group between the male and female behaviors (all 4 groups p<0.0001) and when combined across all 4 groups (p<0.0001). That is, male behaviors for lag-1 period seem to depend, to some extent, on female behaviors at lag-0. To compare the dependence relationships across groups, a model that included main effects for group, female behavior, male behavior and male-female interaction was fit to the data. Residuals from the log-linear fit provide “Z” statistics that can be compared to the standard normal. These residuals will give some indication about where the proposed model does not fit the data. That is, large residuals imply that the observed frequency is higher/lower than expected if the dependence among male and female behaviors is similar across the 4 groups.
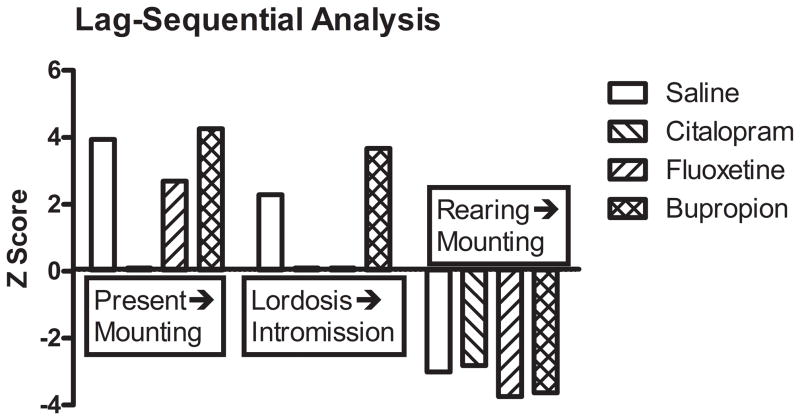
Lag-sequential analysis of the behavioral response of male rats to the behavior of estrus females revealed that, among controls, female proceptive behaviors such as presentation or lordosis were normally followed by appropriate male sexual behaviors, such as mounting or intromission (Figure 6). Similarly, certain nonsexual female behaviors, such as rearing suppress male sexual behavior such as mounting. Neonatal exposure to citalopram disrupted male responses to both proceptive behaviors and neonatal fluoxetine exposure disrupted the male response (intromission) to lordosis. In contrast, neonatal bupropion exposure had no effect on male response to female proceptive behaviors. Notably, none of the antidepressants to which neonates were exposed affected male response to nonsexual female behavior
Figure 6.
Lag-sequential analysis of the effect of neonatal antidepressant exposure on male response to female behaviors. Data are presented as Z-scores from derived from data accumulated over three trials (see Results).
DISCUSSION
The data presented here demonstrate that chronic early life exposure to SSRIs leads to persistent alterations in the response to novel stimuli presented in several sensory fields. SSRI-exposed rats displayed hyper-reactivity towards a novel auditory stimulus when compared to the control group. Exploration of a novel object was also altered with the SSRI-exposed groups exhibiting reduced investigation and a higher rate of avoidance. When given a choice between a novel object and a novel conspecific animal, drug-exposed groups chose to investigate the novel object while controls chose to investigate the novel conspecific. The data presented here also demonstrate that social interactions are affected throughout life. In juvenile play, exposed rats showed less prosocial behavior than controls, while in adult animals this lack of normal social interaction was expressed in disrupted male sexual behavior and lack of appropriate response to female partner (proceptive) cues. Within the antidepressant-exposed groups, more marked differences were observed in the citalopram-exposed group than in the fluoxetine-exposed group, and in both SSRI exposed groups than in the bupropion group, suggesting an important role of serotonin in these behavioral features. Overall, the effects of antidepressant exposure were more robust in males than in females.
A recent metanalysis of sensory processing in autism, has described sensory processing alterations as universally present in autistic individuals (Genn et al., 2003). Both primary (auditory, visual, tactile and olfactory) and multimodal sensory processing have been reported as abnormal in individuals with ASD (Cohen et al., 2004). This reaction to stimuli has been described as sensory over-responsivity’, ‘sensory under-responsivity’ and ‘sensory seeking’ (Cohen et al., 2004). Here we show that when SSRI-exposed rats were presented with a novel auditory stimulus, they tend to react as the non exposed rats by stopping whatever movement they were doing at that time. However the SSRI-exposed rats take significantly longer to restore their movement when compared the control group, which can be understood as over-responsivity to a sensory stimulus.
Impaired social interaction is a hallmark of ASD. In fact, a lack of social reciprocity, which is gross and sustained, is one of the features of this impairment. Younger individuals may not empathize with other peers, or may not even notice them. There is generally a severe reduction or even a lack of interest in social interaction during early stages. There is a preference in solitary activities in preference to those that involve interaction, like social playing (Task Force on DSM-IV, 1994). Interest in social interaction may appear in later stages of development, but the lack of understanding of social cues, conventions or the needs of others, seriously impairs these attempts at interaction. The sensory stimuli primarily tested in these studies focused on visual, auditory and/or tactile cues. Inasmuch as rats are macroosmotic, receiving primary sensory input via olfactory cues, further studies will be needed to determine whether the disruption of sensory processing produced by early life SSRI exposure extends to this modality.
Our data indicate that SSRIs such as citalopram and fluoxetine, can produce lasting disruption of both social and sensorimotor behaviors. In order to determine whether the effects of early life SSRI exposure on behavior were specific to the SSRI class of drugs or a more general feature of antidepressants as a class, we compared the effects of two SSRIs, citalopram and fluoxetine, to bupropion, an antidepressant with high affinity for the dopamine-and norepinephrine transporters. On several behaviors, notably the locomotor response to a novel tone, exploration of a novel object and object-conspecific preference, the effects of bupropion were indistinguishable from those of the SSRIs. This would suggest that these effects resulted from a feature common to many antidepressants rather than a specific monoamine reuptake site. Since the acute actions of almost all antidepressants involve monoaminergic targets, this suggests that a target downstream from the synapse, such as the regulation of glutamatergic signaling or synaptic plasticity that is produced by the antidepressant class of drugs (Pittenger and Duman, 2008)might better account for their effects on these behaviors.
In contrast, bupropion exposure did not disrupt male sexual behaviors or female juvenile play behavior, suggesting that the effects of antidepressants at the serotonin transporter may play a more critical role in mediating the development of these behaviors. Nonetheless, it is notable that bupropion, as with the SSRIs, disrupted male juvenile play behaviors. Thus, although these data suggest that inhibition of serotonin reuptake may be necessary to disrupt these behaviors, it would appear to do so in a gender-specific fashion. Clearly, these data indicate the need for additional studies to resolve this issue. In particular, it will be important in future studies to compare the dose-dependency of the effects of bupropion to those of citalopramin both male and female subjects.
Diverse epidemiological reports have described a higher prevalence of male individuals diagnosed with ASD than females (typically 3 or 4 to 1) (Haroutunian et al., 1986). Our study has demonstrated for the first time that the early exposure to a given dose of SSRIs affects male rats more severely than females. Males showed a greater restriction in their behaviors and were less prone to interact with a novel object. The action of both androgens and estrogens play an important role, not only in serotonin metabolism during brain development, but also during adulthood(Maki and Dumas, 2009). The difference in estrogen modulation of some of the components of the serotonin metabolism, may result in a sexually dimorphic response to exposure to SSRIs. For example, it has been demonstrated that estrogen but not testosterone increases the density of 5HT2A receptor in caudate nucleus(Severino and Yonkers, 1993; Fink et al., 1999; Zhang et al., 1999). This matter should also be of special interest for the understanding of the pathophysiological processes underlying ASD.
The mechanism by which early life exposure to SSRIs produces lasting disruption of response to novelty and social behaviors is unknown. However, serotonin is well known to play a major trophic role, particularly in the development of sensory cortical projections. Although it is beyond the scope of this manuscript to discuss the myriad effects of serotonin on sensory development, two sensory structures deserve particular attention in the context of our data. It is worth noting that, with the possible exception of exploration of a novel inanimate object, almost all of the behaviors disrupted by early SSRI exposure in the present studies are heavily dependent on intact olfactory and auditory function. Moreover, the development of both auditory as well as olfactory circuits is closely tied to both transient expression of SERT and serotonin uptake as well as increased expression of serotonin and its receptors in auditory cortex (Egaas et al., 1995; Lebrand et al., 1996; Hansson et al., 1998; Lebrand et al., 1998) and olfactory bulb (McLean and Shipley, 1987b, 1987a). It is tempting to suggest that disruption of normal serotonin reuptake during this developmental period could result in lasting disruption of normal sensory processing.
These series of observations indicate the need to better understand the effects of early exposure to increased extrasynaptic concentrations of serotonin during brain development. The increase in extrasynaptic concentrations of serotonin suggests that abnormal stimulation of serotonin receptors during development plays an important role in the production of these lifelong changes in prosocial behavior. An important first step will be a precise description of the role that each serotonin receptor plays in this process, as well as the investigation of other potential functions of the SERT during brain development.
Acknowledgments
We thank Emily C. Nichols, Gabrielle Matthews and Mariel Parman for their excellent technical assistance.
Grant sponsor(s): NIMH, NCRR
Grant number(s) MH084194 to RCSL and RR017701 to IAP
References
- Albert DJ, Walsh ML. The inhibitory modulation of agonistic behavior in the rat brain: a review. Neurosci Biobehav Rev. 1982;6:125–143. doi: 10.1016/0149-7634(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Berkson G. Tactile defensiveness in children with developmental disabilities: responsiveness and habituation. J Autism Dev Disord. 1994;24:457–471. doi: 10.1007/BF02172128. [DOI] [PubMed] [Google Scholar]
- Beyer C, Contreras JL, Morali G, Larsson K. Effects of castration and sex steroid treatment on the motor copulatory pattern of the rat. Physiol Behav. 1981;27:727–730. doi: 10.1016/0031-9384(81)90247-x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Yonkers KA, Bellew KM, Bridges IM, Steiner M. Paroxetine controlled release for premenstrual dysphoric disorder: a double-blind, placebo-controlled trial. Psychosom Med. 2004;66:707–713. doi: 10.1097/01.psy.0000140005.94790.9c. [DOI] [PubMed] [Google Scholar]
- De Boer S, Mirmiran M, Van Haaren F, Louwerse A, van de Poll NE. Neurobehavioral teratogenic effects of clomipramine and alpha-methyldopa. Neurotoxicol Teratol. 1989;11:77–84. doi: 10.1016/0892-0362(89)90089-5. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci S, Edwards JE, File SE. Dietary restriction and nicotine can reduce anxiety in female rats. Neuropsychopharmacology. 2003;28:1257–1263. doi: 10.1038/sj.npp.1300168. [DOI] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131:2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Harkin A, Nowak G, Paul IA. Corrigendum to: "Noradrenergic lesion antagonizes desipramine-induced adaptation of NMDA receptors". Eur J Pharmacol. 2000;397:399. doi: 10.1016/s0014-2999(00)00327-7. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Kanof PD, Tsuboyama GK, Campbell GA, Davis KL. Animal models of Alzheimer’s disease: behavior, pharmacology, transplants. Can J Neurol Sci. 1986;13:385–393. doi: 10.1017/s0317167100036957. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahne D, Tudorica A, Borella A, Shapiro L, Johnstone F, Huang W, Whitaker-Azmitia PM. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav. 2002;75:403–410. doi: 10.1016/s0031-9384(01)00673-4. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bruneau N, Roge B, Georgieff N, Veuillet E, Adrien JL, Barthelemy C, Collet L. Peripheral auditory asymmetry in infantile autism. Eur J Neurosci. 2001;13:628–632. doi: 10.1046/j.1460-9568.2001.01423.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee SY, Lee C, Kim I, An HJ, Kim JY, Baek KH, Kim EJ, Kim JM, Lee JB, Lee JW, Jung WW, Chun T, Oh YK. Differential expression profiling of genes in a complete hydatidiform mole using cDNA microarray analysis. Gynecol Oncol. 2006;103:654–660. doi: 10.1016/j.ygyno.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med. 2009;27:250–259. doi: 10.1055/s-0029-1216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci. 1987a;7:3016–3028. doi: 10.1523/JNEUROSCI.07-10-03016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: II. Changes in fiber distribution during development. J Neurosci. 1987b;7:3029–3039. doi: 10.1523/JNEUROSCI.07-10-03029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008;1189:203–214. doi: 10.1016/j.brainres.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, van de Poll NE, Corner MA, van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Nicholas JS, Carpenter LA, King LB, Jenner W, Charles JM. Autism spectrum disorders in preschool-aged children: prevalence and comparison to a school-aged population. Ann Epidemiol. 2009;19:808–814. doi: 10.1016/j.annepidem.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Severino SK, Yonkers KA. A literature review of psychotic symptoms associated with the premenstruum. Psychosomatics. 1993;34:299–306. doi: 10.1016/S0033-3182(93)71863-0. [DOI] [PubMed] [Google Scholar]
- Sikich L. Psychopharmacologic treatment studies in autism. In: Schopler E, Yirmiya N, Schulman C, Marcus L, editors. The research basis for autism intervention. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 199–218. [Google Scholar]
- Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders, Fourth edition (DSM-IV) 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- The Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav. 2000;68:453–461. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- Weaver KJ, Paul IA, Lin RC, Simpson KL. Neonatal exposure to citalopram selectively alters the expression of the serotonin transporter in the hippocampus: dose-dependent effects. Anat Rec (Hoboken) 2010;293:1920–1932. doi: 10.1002/ar.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1990;254:212–220. [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]