Abstract
Background
The incidence of chronic renal disease in women increases with aging especially after menopause suggesting that the loss of sex hormones contributes to the development and progression of renal disease. However, the mechanisms by which sex hormones, estrogens in particular, contribute to the disease process are unclear.
Objective
The present study examined the effects of ovariectomy (OVX) with or without 17β-estadiol (E2) supplementation (OVX+E2) on the expression of inducible (iNOS) and endothelial (eNOS) nitric oxide synthase in the kidney.
Methods
The study was performed in young (4 months, 4M) and aged (12 months, 12M) Dahl salt sensitive (DSS) rats fed a low salt (0.1% NaCl) diet.
Results
OVX in the aged rats was associated with 35% and 25% decreases, respectively, in medullary iNOS (4M OVX, 1.81±0.14 vs. 12M OVX, 1.17±0.16, P<0.05) and eNOS (4M OVX, 1.91±0.09 vs. 12M OVX, 1.43±0.15, P<0.05) protein expression and a 25-fold increase in the abundance of CD68-positive cells indicating macrophage infiltration (4M OVX, 1.18±0.09 vs. 12M OVX, 30.0±0.74, P<0.001). E2 supplementation either partially or completely attenuated these changes in iNOS (4M OVX+E2, 2.26±0.08 vs. 12M OVX+E2, 1.70±0.09, P<0.05), eNOS (4M OVX+E2, 2.03±0.07 vs 12M OVX+E2, 1.77±0.11) and CD68 (4M OVX+E2, 1.46±0.07 vs. 12M OVX+E2, 6.87±1.6, P<0.01) associated with OVX in the aging kidney.
Conclusions
These data suggest that ovarian E2 loss with aging may contribute to the development of age-related renal disease through downregulation of iNOS and eNOS protein abundance and increased renal inflammation. Furthermore, E2 supplementation may be protective in the aging kidney by attenuating these changes.
Keywords: kidney, aging, estradiol, hypertension, nitric oxide, nitric oxide synthase
INTRODUCTION
The incidence of chronic renal disease and hypertension is far lower in pre-menopausal women compared with age-matched men 1–4. With menopause and aging, however, the risk for development and progression of renal disease is increased in women and becomes comparable to that seen in men by the sixth decade of life 5,6. These observations suggest that females are protected against the development and progression of age-associated non-diabetic renal disease and hypertension, but that this protection may not be as apparent after menopause. Consequently, these observations underscore the importance of ovarian sex hormones in regulating renal function and blood pressure. However, the mechanisms by which ovarian sex hormones exert their protective effects in the kidney and on blood pressure control remain poorly understood.
Our previous studies have shown that the aging female Dahl salt sensitive (DSS) rat maintained on a low sodium diet develops hypertension and renal damage including glomerulosclerosis and interstitial fibrosis and that ovariectomy at a young age exacerbates the rate of progression of the hypertension and renal damage while 17β-estardiol (E2) replacement attenuates these effects of OVX 7–9. Furthermore, our studies suggest that one of the mechanisms by which E2 attenuates age-related renal disease and hypertension is through decreasing the expression and activity of the type 1 angiotensin II (Ang II) receptor (AT1R) 8. Others have also shown that aging kidney exhibits an increased responsiveness to Ang II and decreased production of nitric oxide (NO) 10–12,13. Furthermore, E2 has been suggested to play a role in the regulation of NO synthase (NOS) expression in the kidneys in young DSS 14 and mRen2.Lewis congenic rats 15. However, the role of E2 in the regulation of NOS expression in the development of age-related renal disease in the DSS rat has not been previously addressed. Thus, the aim of our study was to determine the age-related changes in the expression of renal NOS isoforms, namely iNOS and eNOS and the effect of E2 loss and replacement on their expression in the aging DSS rat.
METHODS
Animal Model
DSS rats (Harlan Sprague Dawley, Indianapolis, IN) were purchased at 6 weeks of age and were maintained on a low sodium (0.1% NaCl) diet for the duration of the study. At 10 weeks of age, the rats were implanted with radiotelemetry transmitters (Data Sciences, St. Paul, MN) for monitoring arterial pressure, the data of which was published previously 8. At 3 months of age, the rats were randomly divided into the following treatment groups: sham surgery (Intact), ovariectomy (OVX) and OVX with implantation of an E2 silastic pellet (OVX+E2). Treatments were carried out for 1 or 9 months, rendering the animals 4 months (4M) and 12 months (12M) of age at the time of sacrifice. The number of animals in each treatment grouped ranged between 4–6. The rats were sacrificed by decapitation and plasma samples were collected for measurement of estradiol levels. The right kidney was removed and immersion fixed in 4% paraformaldehyde (for immunohistochemistry) and the left kidney snap frozen in liquid nitrogen (for Western blot analysis). All experiments were performed according to the guidelines recommended by the National Institutes of Health and approved by the University of Texas Health Science Center and the Georgetown University Animal Care and Use Committees.
Ovariectomy and E2 supplementation
OVX was carried out under gas anesthesia (2% isoflurane) as previously described 8. Sham operations were carried out in the Intact group of animals in which only skin incisions were made without excising the ovaries. The animals receiving E2 supplementation were implanted with E2 (5 mg)-filled silastic tubes, which were replaced every 12 weeks for the duration of the study. This dose and method of administration of E2 resulted in circulating E2 levels that were in the peak physiological range 7.
Immunohistochemistry
Immunohistochemistry was performed as previously described 7. Briefly, 4 µm paraffin sections were incubated with antibodies against iNOS (mouse monoclonal, Transduction laboratories, San Jose, CA; cat. no. 610308), eNOS (mouse monoclonal IgG, Transduction laboratories; cat. no. 610296), CD68 (mouse monoclonal IgG, Serotec, Oxford, UK; cat. no. MCA341R) or Phospho-AKt (pAKt, mouse monoclonal IgG, Cell Signalling, Danvers, MA; cat. no. 4051) at 4°C overnight. The sections were then incubated with biotinylated goat-anti-mouse IgG followed by the avidin-biotin complex (Vectastain, Vector labs, Burlingame, CA). Positive reactions were identified following incubation with 3,3'-diaminobenzidine and counterstaining with Mayer’s hematoxylin. Sections incubated with 10% non-immune goat serum instead of the primary antibody were used as negative controls.
Western blotting
Western blotting was performed as previously described 7. Briefly, the renal cortex and medulla were separated, homogenized and the protein concentration determined using a colorimetric assay (BioRad, Hercules, CA). The samples (containing 15–40 µg of protein) were denatured, separated on 7% (iNOS and eNOS) or 12% (p-AKt) acrylamide gels then immunoblotted to nitrocellulose membranes. Following incubation with the primary antibodies (iNOS, eNOS and p-AKt) at 4°C overnight, membranes were incubated with anti-mouse IgG conjugated to horseradish peroxidase and proteins visualized by enhanced chemiluminescence (KPL, Gaithersburg, MD). The densities of specific bands were normalized to the total amount of protein loaded in each well following densitometric analysis of gels stained with Coomassie blue. The densities of specific bands were quantitated by densitometry using the Scion Image beta (version 4.02) software.
Quantitative analysis of CD68-positive cell abundance
The number of CD68-positive cells, suggesting the presence of macrophages was counted in twenty random fields (×400 magnification) per section in 4 sections per kidney from each treatment group.
Statistical analysis
Data are expressed as means±SEM and were analyzed using a two-way ANOVA followed by a Newman-Keuls multiple comparison test. Differences were considered statistically significant at P<0.05.
RESULTS
We previously described the effects of aging, ovariectomy and E2 replacement on body weight, E2 levels, and the development and progression of hypertension and the magnitude of glomerulosclerosis in these same animals 7,8. Here we examined iNOS and eNOS immunoreactivity and protein expression as well as macrophage infiltration in the kidneys from these animals.
iNOS protein expression
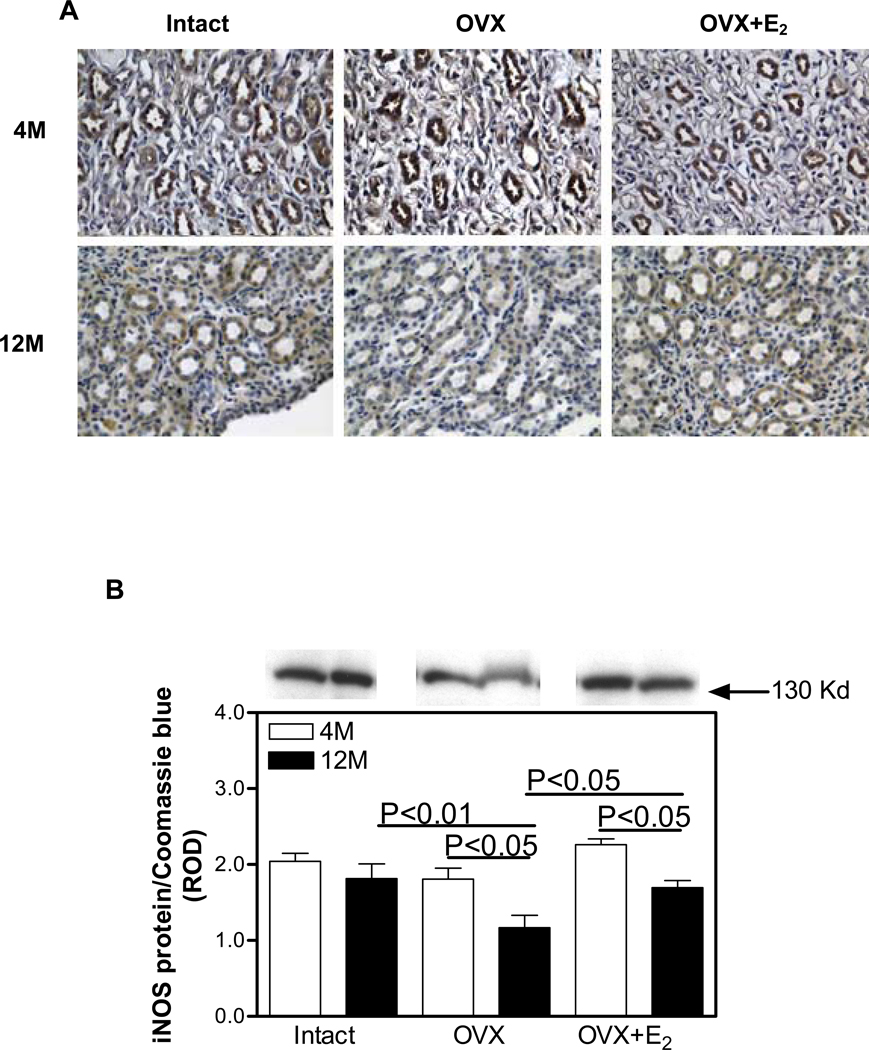
In the 4M Intact kidneys, iNOS was immunolocalized to proximal and distal tubules and both cortical (Figure 1A) and medullary collecting ducts (Figure 2A), while no immunostaining was observed in the glomerulus. The pattern of immunostaining was similar in all the other treatment groups; however, the overall intensity of immunostaining in both the cortex and medulla was reduced in the 12M animals compared with 4M. Western blotting confirmed the immunohistochemical observations in that no differences in the level of expression of iNOS protein in the renal cortex were observed in any of the treatment groups. While there was a trend towards a reduction in iNOS protein expression in the renal cortex with aging, this did not reach statistical significance (Figure 1B). No differences in the level of expression of iNOS protein in the renal medulla was observed among the Intact, OVX and OVX+E2 treatment groups at 4M (Figure 2B). In contrast, at 12M, OVX was associated with a 55% decrease in medullary iNOS expression compared with Intact, while iNOS protein levels in the OVX+E2 group were indistinguishable from the Intact group (Figure 2B).
Figure 1.
Immunohistochemical localization and expression of iNOS protein in the renal cortex. A. iNOS (brown staining) immunolocalization. Abbreviations: proximal tubule (pt); distal tubule (dt); collecting duct (cd); glomerulus (g); ovariectomy (OVX); ovariectomy with E2 supplementation (OVX+E2). Original magnification ×400. B. iNOS protein expression by Western blotting. Top panel, representative gels of iNOS protein expression. Bottom panel, densitometric scans of iNOS protein levels in relative optical density (ROD) expressed as a ratio of iNOS/Coomassie blue. Data are expressed as mean±SEM.
Figure 2.
Immunohistochemical localization and expression of iNOS protein in the renal medulla. A. iNOS (brown staining) immunolocalization. Original magnification ×400. B. iNOS protein expression by Western blotting. Top panel, representative gels of iNOS protein expression. Bottom panel, densitometric scans of iNOS protein levels in relative optical density (ROD) expressed as a ratio of iNOS/Coomassie blue. Data are expressed as mean±SEM.
eNOS protein expression
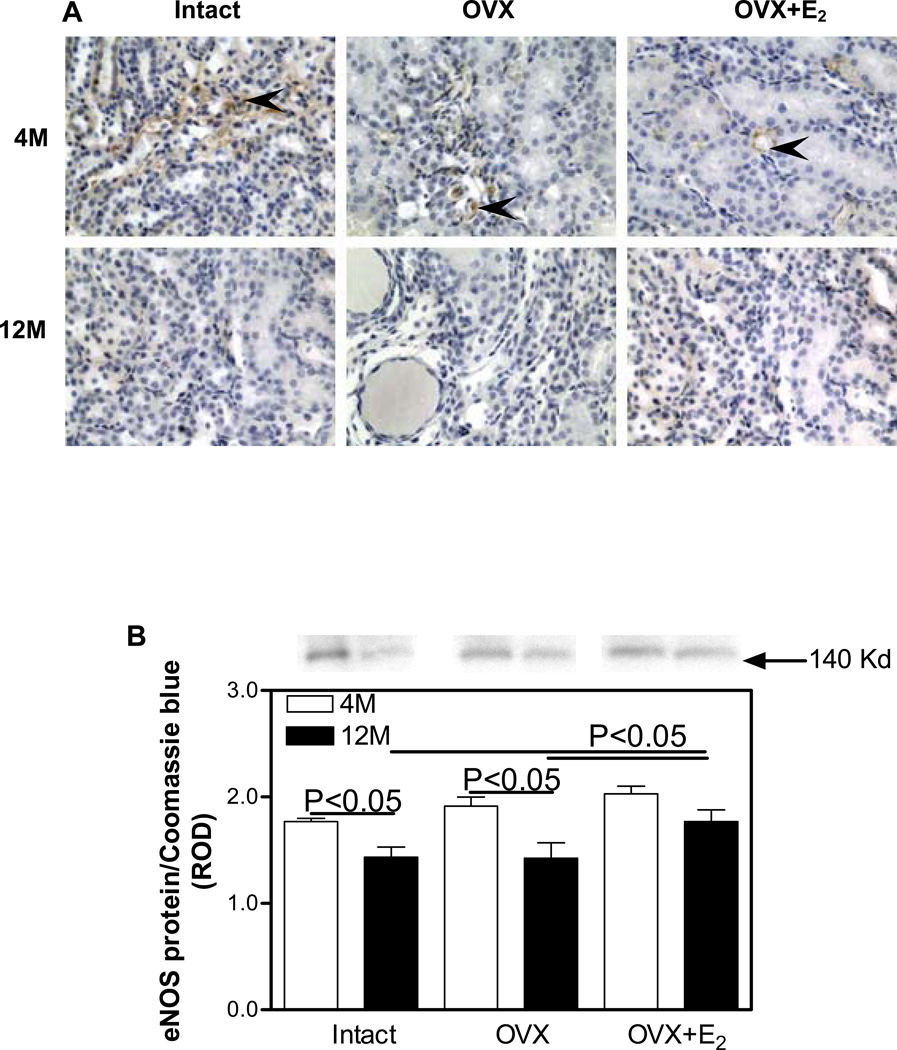
In the 4M Intact kidneys, eNOS was predominantly immunolocalized to glomerular (Fig. 3A) and peritubular capillaries in the outer medulla (Figure 4A). 12M kidneys showed an overall reduction in the intensity of eNOS immunostaining compared with 4M kidneys in the medulla (Figure 4A), especially in the OVX group; however, no apparent differences in eNOS immunostaining in the renal cortex were observed between the 4M and 12M animal groups (Figure 3A). Western blotting confirmed the immunohistochemical observations in that no differences in the levels of expression of cortical eNOS protein expression were detected in any of the treatment groups (Figure 3B). No differences in medullary eNOS were detected between any of the treatment groups at 4M. In contrast, eNOS levels were 23% lower in the Intact group and 34% lower in the OVX group in the 12M animals compared to medullary eNOS protein levels in the 4M animals (Figure 4B). The OVX+E2 12M group expressed 20% higher levels of eNOS protein compared to either the Intact or OVX groups at 12M.
Figure 3.
Immunohistochemical localization and expression of eNOS protein in the renal cortex. A. eNOS (brown staining) immunolocalization. Insets, higher magnification of the area outlined in the black square; arrow heads, endothelial cells. Original magnification ×400. B. eNOS protein expression by Western blotting. Top panel, representative gels of eNOS protein expression. Bottom panel, densitometric scans of eNOS protein levels in relative optical density (ROD) expressed as a ratio of eNOS/Coomassie blue. Data are expressed as mean±SEM.
Figure 4.
Immunohistochemical localization and expression of eNOS protein in the renal medulla. A. eNOS (brown staining) immunolocalization. Arrow heads, endothelial cells. Original magnification ×400. B. eNOS protein expression by Western blotting. Top panel, representative gels of eNOS protein expression. Bottom panel, densitometric scans of eNOS protein levels in relative optical density (ROD) expressed as a ratio of eNOS/Coomassie blue. Data are expressed as mean±SEM.
No changes in nNOS expression in either cortex or medulla were observed (data not shown).
pAKt protein expression
In the 4M Intact kidneys, pAKt was immunolocalized to the proximal and distal tubules (Figure 5A); no significant differences in the overall intensity of immunostaining were observed in any of the treatment groups at 4M. In the 12M animals, there were no apparent differences in the intensity of immunostaining compared with 4M; however, in addition to staining in the proximal and distal tubules, staining was also apparent in the glomerulus (Figure 5A). Western blotting confirmed the immunohistochemical observations in that that no significant differences in the level of expression of pAKt protein in the renal cortex were observed in any of the treatment groups at either 4M or 12M (Figure 5B).
Figure 5.
Immunohistochemical localization and expression of phospho-AKt (pAKt) protein in the renal cortex. A. pAKt (brown staining) immunolocalization. Original magnification ×400. B. pAKt protein expression by Western blotting. Top panel, representative gels of pAKt protein expression. Bottom panel, densitometric scans of pAKt protein levels in relative optical density (ROD) expressed as a ratio of pAKt/Coomassie blue. Data are expressed as mean±SEM.
CD68-positive cell abundance
In the 4M Intact kidneys, very few CD68-positive cells were observed (Figure 6A). In contrast, CD68-positive cells were highly abundant in cortical tubulointerstitial areas of 12M kidneys, especially in the OVX group (Figure 6A). CD68-positive cells were also highly abundant in the renal medulla of 12M kidneys, especially in the OVX group (data not shown). Quantitative analysis showed that OVX was associated with a 59% increase in CD68-positive cell abundance compared with 12M Intact while E2 supplementation (OVX+E2) markedly attenuated this age-related increase in CD68-positive abundance in the renal cortex (Figure 6B) and medulla (data not shown).
Figure 6.
Immunohistochemical localization of CD68. A. CD68 (arrow heads) immunolocalization. Original magnification ×400. B. Quantitation of CD68-positive cell abundance.
DISCUSSION
The major finding of our study is that the age-related hypertension and renal damage in female DSS rats, which we previously reported 7,8 is also associated with a reduction in renal medullary expression of iNOS and eNOS protein. These changes in renal medullary iNOS and eNOS protein expression are especially evident after ovariectomy while E2 supplementation attenuates these changes.
Our previous studies have shown that DSS rats, maintained on a low salt (0.1% NaCl) diet develop hypertension with aging, which is accelerated following ovariectomy 8,9. In addition, others have shown that the extent of hypertension after ovariectomy either in younger DSS rats 14 or in mRen2.Lewis congenic rats 15 is highly dependent on the underlying sodium status. Both of these studies suggest that the ovariectomy - induced hypertension is exacerbated with high salt and alleviated with low salt intake. Furthermore, these studies have shown that E2 supplementation reduces, while ovariectomy increases angiotensin converting enzyme activity, plasma Ang II levels and renal AT1 receptor expression 8, 15, 16. These observations suggest that one of the mechanisms by which E2 exerts its antihypertensive effect is through modulating the expression and activity of the renin-angiotensin system.
In addition to developing hypertension, our previous study showed that DSS rats develop age-related glomerulosclerosis and tubulointerstitial fibrosis that is most severe after ovariectomy and attenuated with E2 supplementation 7. Some of the mechanisms by which E2 exerts its renoprotective effect in this model are through attenuating extracellular matrix deposition and reducing transforming growth factor beta protein expression 7. Our present study shows that the aging kidney is characterized by high abundance of CD68-positive cells, suggesting the presence of macrophages. CD68-positive cells have been shown to be present in a number of inflammatory renal diseases 17 and diabetic nephropathy 18; however, thus far, the presence of CD68-positive cells has no been reported in the aging kidney. Our study shows that OVX increases while E2 supplementation reduces the abundance of CD68-positive cells in the aging kidney. These studies clearly indicate a renoprotective and anti-inflammatory effect of E2 in age-related renal disease.
Our study shows that aging is associated with a decrease in both iNOS and eNOS protein expression, but only in the renal medulla. Several studies have demonstrated that the renal medulla has a greater NO generating capacity than the renal cortex 19, 20. The significance of the medullary changes in NOS expression in the aging kidney of the DSS rat may be related to changes in the regulation of renal medullary blood flow as well as development of medullary tubulointerstitial fibrosis. Our previous studies have shown severe medullary interstitial fibrosis in the aging DSS rat 7. In addition, we have shown antipoliferative and extracellular matrix degrading properties of NO in the renal medulla 21. Thus, decreases in medullary NOS expression with aging may result in decreased medullary NO bioavailability. This loss in NO bioavailability may in turn contribute to the development of age-related renal medullary disease and hypertension. It is also conceivable that age-related renal injury and hypertension themselves lead to decreased NO bioavailability, contributing to a viscous cycle of disease progression. Interestingly, no changes in renal cortical iNOS or eNOS expression were observed in aging rats, despite a similar degree of renal injury in the cortex and medulla. These observations suggest that the underlying mechanisms of age-related renal disease are different in the two compartments. Further studies are needed to examine the specific mechanisms underlying renal cortical injury in aging.
The decrease in renal medullary iNOS protein expression was especially evident with OVX, while eNOS protein expression did not decrease with OVX beyond that of the age-dependent decrease. Most importantly, decreases in both iNOS and eNOS protein expression were attenuated with E2 supplementation indicating an important role for E2 in regulating renal medullary NOS expression. Interestingly however, no NOS expression was evident in macrophages that were abundant in kidneys of aging animals. Renal iNOS expression has been reported to be increased in infiltrating macrophages in response to pro-inflammatory cytokines, thus contributing to disease progression 22. It is conceivable that the lack of iNOS expression in macrophages of aging kidneys may be a protective mechanism against severe tissue injury induced by inflammation and aging. More importantly, these observations suggest that NOS expression is regulated in a cell-specific manner.
Although we did not measure NOS activity in the current study (due to inadequate sample availability), we sought to examine some of the other potential mechanisms involved in regulating NO production. Phospho-AKt has been reported to increase eNOS activity by enhancing its phosphorylation 23. We found no significant difference in the expression of p-AKt protein with aging, ovariectomy or E2 supplementation. A lack of change in p-AKt associated with a decrease in eNOS protein expression was also observed in the aging SHR rat 24. These observations indicate that NO production in the DSS rat may be largely due to decrease in NOS protein expression rather than NOS activity.
One of the caveats of the present study is that ovariectomy was performed at 3 months of age. Thus, the changes in estradiol levels do not truly mimic the age-related loss of ovarian hormones, as in menopause, but rather show effects of prolonged absence of estradiol. One the other hand, one of the advantages of the study design is that E2 supplementation began at the time of ovariectomy. Thus, the protective effects of E2 supplementation observed in this model underscores the importance of the timing of E2 administration.
CONCLUSIONS
The present study demonstrates that aging in the DSS rat, especially after ovariectomy is associated with downregulation of medullary iNOS and eNOS protein expression. Furthermore, our previous study demonstrates that aged, but not young DSS rats, develop hypertension following ovariectomy, which is attenuated with E2 supplementation. These findings suggest that the augmented expression of NOS isoforms may contribute to the development of hypertension and age-associated renal medullary disease in the DSS rat. Most importantly, this study provides evidence for a antihypertensive, anti-inflammatory and renoprotective role of E2, suggesting that hormone therapy, despite the controversy of its effectiveness, albeit mainly related to diseases other than renal, may be beneficial in preventing the progression of hypertension and age-related renal disease.
Table 1.
Body and kidney weight, plasma estradiol and blood pressure
| Intact | OVX | OVX+E2 | ||||
|---|---|---|---|---|---|---|
| 4M | 12M | 4M | 12M | 4M | 12M | |
| Body weight (g) | 262.5±9.4 | 377.7±17.9** | 317.3±5.2# | 421.0±18.0**,## | 228.3±2.3#,§§§ | 285.8±9.3*,###,§ |
| Kidney weight (g) | 0.88±0.02 | 1.11±0.04 | 0.83±0.02 | 1.03±0.04 | 0.93±0.03 | 1.4±0.11*,#,§§ |
| Kidney/body weight (mg/g) | 3.4±0.09 | 3.0±0.16 | 2.6±0.06# | 2.5±0.15 | 4.1±0.12#,§§ | 3.6±0.15#,§§ |
| Plasma estradiol (pg/ml) | 38.1±9.4 | 5.5±1.3* | 12.7±1.5# | 6.6±1.5## | 32.5±2.4§ | 19.4±2.6*,#,§ |
| MAP (mmHg) | 120.6±7.6 | 150.8±8.6* | 127.7±4.6 | 167.3±5.5**,# | 115.1±2.0 | 122.5±6.2*,#,§ |
Data are expressed as mean ± SEM.
P<0.05,
P<0.01 vs. 4M in the same treatment group
P<0.05,
P<0.01,
P<0.001 vs. Intact in the same age group
P<0.05,
P<0.01,
P<0.001 vs. OVX in the same age group
ACKNOWLEDGEMENTS
This work was supported by a Nathan Schock Minority Supplement Award and an RO1 grant (AG/HL20256) to C. Hinojosa-Laborde and by an RO3 grant (AG22233) to C. Maric from the National Institutes of Health.
REFERENCES
- 1.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10:219–225. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Neugarten J. Gender and the progression of renal disease. J Am Soc Nephrol. 2002;13:2807–2809. doi: 10.1097/01.asn.0000035846.89753.d4. [DOI] [PubMed] [Google Scholar]
- 3.Wassertheil-Smoller S, Anderson G, Psaty BM, et al. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension. 2000;36:780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 4.Tozawa M, Iseki K, Iseki C, et al. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 5.Schulman IH, Aranda P, Raij L, et al. Surgical menopause increases salt sensitivity of blood pressure. Hypertension. 2006;47:1168–1174. doi: 10.1161/01.HYP.0000218857.67880.75. [DOI] [PubMed] [Google Scholar]
- 6.Reckelhoff JF, Zhang H, Srivastava K, et al. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 7.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and Tubulointerstitial Fibrosis are Attenuated with 17beta-Estradiol in the Aging Dahl Salt Sensitive Rat. J Am Soc Nephrol. 2004;15:1546–1556. doi: 10.1097/01.asn.0000128219.65330.ea. [DOI] [PubMed] [Google Scholar]
- 8.Hinojosa-Laborde C, Craig T, Zheng W, et al. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 9.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 10.Baylis C, Schmidt R. The aging glomerulus. Semin Nephrol. 1996;16:265–276. [PubMed] [Google Scholar]
- 11.Erdely A, Greenfeld Z, Wagner L, et al. Sexual dimorphism in the aging kidney: Effects on injury and nitric oxide system. Kidney Int. 2003;63:1021–1026. doi: 10.1046/j.1523-1755.2003.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. Am J Pathol. 1995;146:742–752. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XZ, Qiu C, Baylis C. Sensitivity of the segmental renal arterioles to angiotensin II in the aging rat. Mech Ageing Dev. 1997;97:183–192. doi: 10.1016/s0047-6374(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 14.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;42:1157–1163. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- 15.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2).Lewis rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1557–R1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 16.Thompson MM, Oyama TT, Kelly FJ, et al. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1787–R1794. doi: 10.1152/ajpregu.2000.279.5.R1787. [DOI] [PubMed] [Google Scholar]
- 17.Rossini M, Cheunsuchon B, Donnert E, et al. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 2005;68:2621–2628. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 18.Chow F, Ozols E, Nikolic-Paterson DJ, et al. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL, Lu S, Nakanishi K, et al. Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–H1926. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 20.Taylor NE, Maier KG, Roman RJ, et al. NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension. 2006;48:1066–1071. doi: 10.1161/01.HYP.0000248751.11383.7c. [DOI] [PubMed] [Google Scholar]
- 21.Maric C, Aldred GP, Antoine AM, et al. Actions of endothelin-1 on cultured rat renomedullary interstitial cells are modulated by nitric oxide. Clin Exp Pharmacol Physiol./ 1999;26:392–398. doi: 10.1046/j.1440-1681.1999.03060.x. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Guerrero C, Lopez-Franco O, Suzuki Y, et al. Nitric oxide production in renal cells by immune complexes: Role of kinases and nuclear factor-kappaB. Kidney Int. 20002;62:2022–2034. doi: 10.1046/j.1523-1755.2002.00653.x. [DOI] [PubMed] [Google Scholar]
- 23.Giles ME, Fernley RT, Nakamura Y, et al. Characterization of a Specific Antibody to the Rat Angiotensin II AT1 Receptor. J Histochem Cytochem. 1999;47:507–515. doi: 10.1177/002215549904700409. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND, Wang XQ, Ni ZN, et al. Effects of aging and AT-1 receptor blockade on NO synthase expression and renal function in SHR. Biochim Biophys Acta. 2002;1592:153–161. doi: 10.1016/s0167-4889(02)00309-9. [DOI] [PubMed] [Google Scholar]