Abstract
Background
The majority of studies relating amyloid pathology with brain volumes have been cross-sectional. Apolipoprotein E4 (APOE4), a genetic risk factor for Alzheimer’s disease (AD), is also associated with hippocampal volume loss. No studies have considered the effects of amyloid pathology and APOE4 together on longitudinal volume loss.
Methods
We evaluated whether an abnormal level of cerebrospinal fluid beta-amyloid (CSF Aβ) and APOE4 carrier status were independently associated with greater hippocampal volume loss over 1 year. We then assessed whether APOE4 status and CSF Aβ acted synergistically, testing the significance of an interaction term in the regression analysis. We included 297 participants: 77 cognitively normal (NC), 144 with mild cognitive impairment (MCI), and 76 with AD.
Results
An abnormal CSF Aβ level was found to be associated with greater hippocampal volume loss over 1 year in each group. APOE4 was associated with hippocampal volume loss only in the NC and MCI groups. APOE4 carriers with abnormal CSF Aβ in the MCI group acted synergistically to produce disproportionately greater volume loss than noncarriers.
Conclusion
Baseline CSF Aβ predicts progression of hippocampal volume loss. APOE4 carrier status amplifies the degree of neurodegeneration in MCI. Understanding the effect of interactions between genetic risk and amyloid pathology will be important in clinical trials and our understanding of the disease process.
Keywords: apolipoprotein E4, hippocampal atrophy, beta-amyloid, biomarker, MRI
1. Introduction
Fibrillar beta-amyloid (Aβ) plaques, one of the hallmarks of Alzheimer’s disease (AD), have been shown to be associated with hippocampal atrophy in multiple cross-sectional positron emission tomography (PET) studies using the amyloid ligand, Pittsburgh Compound B (PiB) [1-5]. A few studies have found similar correlations between cerebrospinal fluid (CSF) Aβ, an indirect measure of cerebral amyloid deposition [6,7], and hippocampal atrophy [8,9]. However, studies relating Aβ pathology with longitudinal volume loss have been mixed. One PiB-PET study found a strong association between brain Aβ and change in regional MRI volumes in normals, but only a trend in AD [3]. One study reported an association between CSF Aβ and the rate of hippocampal atrophy [10], although CSF p-tau was found to be a better predictor, and two other studies found no correlation between Aβ and the rate of whole brain atrophy [11,12].
The primary goal of our study was to determine whether baseline CSF Aβ level is associated with longitudinal hippocampal volume loss, incorporating data from the multicenter Alzheimer’s Disease Neuroimaging Initiative (ADNI) (www.loni.ucla.edu\ADNI). Since apolipoprotein E4 (APOE4), a well-documented genetic risk factor for developing AD [13,14], is associated with increased brain Aβ [15-18] and hippocampal atrophy [19-21], we further explored whether APOE4 modifies the relationship between abnormally low CSF Aβ and hippocampal volume loss.
2. Methods
2.1. Participants
The participants in this study were recruited through the ADNI between 2005 and 2008, a longitudinal study including 56 centers in the U.S. and Canada with the purpose of identifying biomarkers of early Alzheimer’s disease (AD) for clinical trials (www.adni-info.org). The ADNI was funded by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies, and non-profit organizations, as a 5-year public-private partnership.
2.2. APOE genotyping and clinical assessment
All participants underwent APOE genotyping at the baseline visit. Approximately 6 ml of blood were obtained from each participant in an EDTA tube, gently mixed by inversion, and shipped at ambient temperature to a single designated laboratory within 24 hours of collection for analysis.
Participants ranged in age from 55 to 90, did not have major depression or severe systemic illnesses that would interfere with participation, and did not take investigational or psychometric medications. The normal controls (NC) subjects had no memory complaint, had preserved activities of daily living, scored between 26 and 30 on a baseline Mini-Mental Status Examination (MMSE) [23], scored a 0 on the Clinical Dementia Rating scale (CDR) [24], and scored within the normal range on the Logical Memory II subscale (delayed paragraph recall) from the Wechsler Memory Scale – Revised (WMS-R Log Mem) [25]. The subjects with mild cognitive impairment (MCI) had a memory complaint that was verified by a study partner, had preserved activities of daily living, and scored between 24 and 30 on the MMSE, 0.5 on the CDR, and below the normal range on the WMS-R Log Mem. The AD subjects scored between 20 and 26 on the MMSE, between 0.5 or 1 on the CDR, and met NINCDS/ADRDA criteria for probable AD [26]. Written consent was obtained from all subjects participating in the study, and the study was approved by the institutional review board at each participating site.
2.3. CSF analysis
As described in the ADNI protocol (www.adni-info.org), all 56 participating centers were asked to perform lumbar punctures on at least 20% of their participants. Approximately half of the participants recruited at each center underwent lumbar puncture for cerebrospinal fluid analysis. CSF samples were banked and batch-processed at a single laboratory, as described previously [27]. Briefly, lumbar puncture was performed with a 20- or 24-gauge spinal needle at the baseline visit after an overnight fast. The CSF samples were then transferred into polypropylene transfer tubes, frozen on dry ice within an hour after collection, and shipped on dry ice overnight to a single designated laboratory. After thawing for 1 hour at room temperature and gentle mixing, 0.5 ml aliquots were prepared from these samples. The aliquots were then stored in bar code-labeled polypropylene vials at −80°C and measured using the xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics (INNOBIA AlzBio3, Ghent, Belgium) immunoassay kit-based reagents, which included the monoclonal antibody specific for Aβ1-42 (4D7A3).
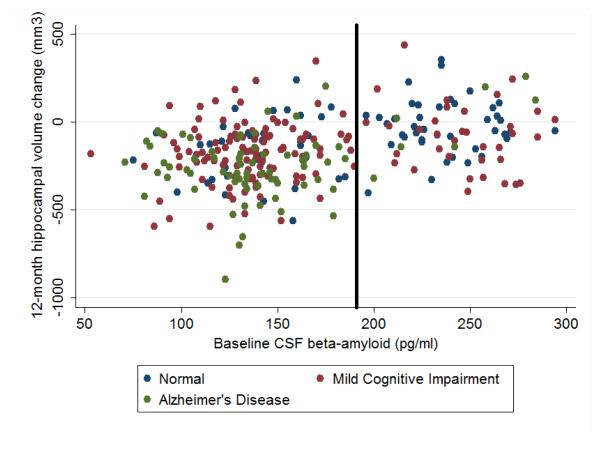
In our analysis, the baseline CSF Aβ level was dichotomized as either abnormal, i.e. reflective of underlying Alzheimer’s pathology, or normal (Figure 1). It was previously published that using a threshold CSF Aβ value of 192 pg/ml yielded a sensitivity of 96% for detecting Alzheimer’s disease, based on a sample of non-ADNI normal controls and subjects with Alzheimer’s disease using the same CSF assay [28]. Furthermore, this cutoff value showed 91% agreement with evidence of brain amyloid using Pittsburgh Compound B in positron emission tomography imaging [29].
Figure 1. Association between baseline CSF Aβ level and 1-year change in hippocampal volumes.
The CSF Aβ level of less than 192 pg/ml (as delineated by the solid line) is considered abnormal in this study, i.e. reflective of underlying Alzheimer’s pathology. Greater than 192 pg/ml is considered normal.
2.4. MRI acquisition
Participants underwent the following standardized 1.5 T MRI protocol (http://www.loni.ucla.edu/ADNI/Research/Cores/index.shtml): two T1-weighted MRI scans, using a sagittal volumetric magnetization prepared rapid gradient echo (MP-RAGE) sequence, with an echo time (TE) of 4 ms, repetition time (TR) of 9 ms, flip angle of 8°, and acquisition matrix size of 256 × 256 × 166 in the x-, y- and z-dimensions with a nominal voxel size of 0.94 × 0.94 × 1.2 mm [30].
2.5. MRI post-processing
The raw Digital Imaging and Communications in Medicine MRI data were downloaded from the Laboratory of Neuro Imaging (LONI) Image Database Archive (http://www.loni.ucla.edu/ADNI/Data/index.shtml). The images were aligned, skull-stripped, and segmented using FreeSurfer software, version 4.3 (http://surfer.nmr.mgh.harvard.edu/) [31]. Bilateral hippocampal volumes, obtained from this segmentation, were averaged in the analyses. The change in hippocampal volumes over 1 year was calculated by subtracting the baseline hippocampal volume from the volume at follow-up and normalized by the time difference.
2.6. Statistical analyses
We excluded 33 subjects who carried at least one APOE2 allele to avoid confounding the analysis, since APOE2 is believed to be protective against development of AD and slow rates of hippocampal atrophy [32,33]. Our final cohort thus includes 297 subjects who had a lumbar puncture and at least 2 MRI scans, spaced 1 year apart – 77 NC, 144 MCI, and 76 with probable AD (Table 1).
Table 1.
Group characteristics
| NC | p- value |
MCI | p- value |
AD | p- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| APOE 3/3 |
APOE 3/4 or 4/4** |
APOE 3/3 |
APOE 3/4 or 4/4** |
APOE 3/3 |
APOE 3/4 or 4/4** |
||||
| N | 55 | 22 | 65 | 79 | 24 | 52 | |||
| Age (years) | 76 (5.0) |
76 (6.3) |
0.37 | 76 (8.5) |
73 (6.5) |
0.03* | 77 (9.1) |
74 (7.1) |
0.19 |
| Female (%) | 55 | 36 | 0.21 | 26 | 44 | 0.04* | 42 | 42 | 0.91 |
| Education (years) |
16 (2.4) |
16 (3.4) |
0.84 | 16 (2.9) |
16 (2.9) |
0.82 | 15 (5.3) |
14 (4.0) |
0.14 |
| CSF Aβ (pg/ml) |
209 (48.8) |
147 (43.5) |
<0.001* | 188 (59.8) |
141 (38.8) |
<0.001* | 169 (53.6) |
130 (29.3) |
0.001* |
| Baseline hippocampal volume (mm3) |
6752 (731.5) |
6691 (724.2) |
0.70 | 5904 (1072.9) |
5599 (923.8) |
0.06 | 5433 (1466.4) |
5073 (792.0) |
0.50 |
| MMSE | 29 (1.0) |
29 (0.9) |
0.45 | 27 (1.8) |
27 (1.8) |
0.49 | 23 (2.0) |
24 (1.8) |
0.68 |
|
| |||||||||
| Unadjusted 1-year change in hippocampal volume (mm3) |
−57.8 (179.2) |
−160.5 (150.9) |
0.01* | −103.5 (176.0) |
−199.9 (166.2) |
0.003** | −231.0 (227.3) |
−252.1 (174.1) |
0.80 |
Data shown are means (SD).
Significant at the α = 0.05 level.
Number of (APOE3/4, APOE4/4) carriers in each group: NC (20, 2), MCI (63, 16), AD (34, 18).
All statistical analyses were programmed in R, version 2.9.2 (www.r-project.org). Model assumptions were assessed with plots of residuals. APOE genotype was dichotomized into APOE4 carriers (E3/4 or E4/4) and noncarriers (APOE3/3). Age, baseline hippocampal volume, gender, and years of education were included as covariates in every model.
We first determined whether an abnormal baseline CSF Aβ level and APOE4 carrier status were independently associated with 1-year change in hippocampal volumes in all stages, after adjusting for covariates, using ordinary least squares regression. If both risk factors were significantly associated with volume loss, we then tested for interaction between APOE4 and CSF Aβ. To do so, we centered CSF Aβ on its mean to reduce collinearity and included an interaction term between APOE4 and CSF Aβ, which was considered significant at the α = 0.05 level.
3. Results
3.1. Group characteristics
The group characteristics are summarized in Table 1. Mean CSF Aβ was significantly lower in APOE4 carriers compared to noncarriers at each clinical stage, consistent with previous literature [15-18]. The APOE4 MCI group was slightly younger and included more women. No significant differences in MMSE were seen between carriers and noncarriers. Without adjusting for covariates, the change in raw hippocampal volumes over 1 year was significantly different by APOE4 status in the NC and MCI groups, but not in AD.
3.2. Association between CSF Aβ and 1-year change in hippocampal volumes
Participants with an abnormally low CSF Aβ level had greater volume loss in all groups. In the NC group, participants with a low CSF Aβ level had a 138 mm3 greater 1-year volume loss than those with a normal CSF Aβ level (p < 0.001). In the MCI group, participants with a low CSF Aβ level had a 71 mm3 greater volume loss than those with a normal CSF Aβ level (p = 0.03). In the AD group, participants with a low CSF Aβ level had a 300 mm3 greater 1-year volume loss than those with a normal CSF Aβ level (p < 0.001).
3.3. Association between APOE4 and 1-year change in hippocampal volumes
Participants who carried at least one APOE4 allele had greater volume loss in the NC and MCI groups. In the NC group, APOE4 participants had a 121 mm3 greater 1-year volume loss than those without an APOE4 allele (p < 0.007). In the MCI group, APOE4 participants had a 76 mm3 greater volume loss than those without an APOE4 allele (p = 0.01). In the AD group, APOE4-positive and APOE4-negative participants demonstrated no difference in 1-year volume loss (p = 0.66).
3.4. Testing for APOE4-CSF Aβ interaction in NC and MCI
Since both APOE4 and a low CSF Aβ level were associated with greater volume loss in the NC and MCI groups, we then tested for an APOE4-CSF Aβ interaction in these groups. No significant APOE4-CSF Aβ interaction was seen in the NC group (β = 138, p = 0.19). There was however a significant interaction between APOE4 and CSF Aβ in the MCI group (β = −181, p = 0.02). Compared to APOE4-noncarriers with normal CSF Aβ, APOE4-carriers with abnormal CSF Aβ had 88 mm3 greater volume loss over 1 year (p = 0.02). Compared to APOE4-noncarriers with abnormal CSF Aβ, APOE4-carriers with abnormal CSF Aβ had 97 mm3 greater volume loss over 1 year (p = 0.004).
4. Discussion
The major findings of this study are: 1) an abnormally low baseline CSF Aβ level, suggestive of underlying Alzheimer’s pathology, predicted 1-year change in hippocampal volumes in all groups; 2) APOE4 carriers demonstrate greater hippocampal volume loss only in the NC and MCI groups; 3) APOE4 and low CSF Aβ are synergistic risk factors, such that APOE4 carrier status amplifies the predicted 1-year volume loss beyond that predicted by a low CSF Aβ alone.
The finding that an abnormally low CSF Aβ predicted 1-year hippocampal volume loss is consistent with the predominantly cross-sectional literature, which describes an association between amyloid pathology and hippocampal atrophy [1-5, 8-10]. Some have postulated that the large extracellular amyloid plaques disrupt cortico-hippocampal pathways, leading to neurodegeneration [2]. Another hypothesis is that insoluble plaques detected in cerebrospinal fluid are an indirect marker of soluble Aβ oligomers, which may be the inciting agent in Alzheimer’s disease, by disrupting hippocampal synapses and promoting volume loss [34, 35].
The second finding that APOE4 is associated with greater longitudinal hippocampal volume loss in the NC and MCI groups is also compatible with prior literature. Numerous studies suggest that APOE4 carriers demonstrate increased vulnerability to developing AD, which is manifested through neurodegeneration [36-39]. A reason for the lack of increase volume loss among APOE4 carriers in the AD group may be that, although APOE4 carriers develop AD at an earlier age [13], once the disease is clinically apparent in an individual, APOE4 no longer alters the course of the disease. The lack of a significant difference in hippocampal volumes among APOE4 carriers and noncarriers with AD has also been reported in prior studies [40,41].
Finally, the finding that the presence of a genetic risk factor, APOE4, amplifies the association between CSF Aβ and progressive hippocampal volume loss in MCI is novel. One possible explanation for this is that the APOE4 carriers with low CSF Aβ are more likely to have Alzheimer’s pathology. Although an abnormally low CSF Aβ is highly sensitive for detecting brain amyloid associated with AD, it is not an entirely specific for AD [28]. Some of the participants with low CSF Aβ may have frontotemporal dementia and would not demonstrate the same degree of hippocampus-specific volume loss as prodromal Alzheimer’s patients [42]. However, this argument would also be true among NC subjects, in which no interaction was demonstrated.
A second explanation for the APOE4-CSF Aβ interaction in MCI could be explained by a temporal progression of pathological mechanisms resulting from the APOE4 genotype. Early on when subjects demonstrate normal cognition, the predominant effect of APOE4 appears to be to increase brain amyloid deposition, as reported by numerous prior studies [15-18]. Since, both APOE4 carrier status and a low CSF Aβ, as defined by our cutoff value, reflect greater brain amyloid, no interaction is seen in our NC group. However, once cognitive impairment is evident clinically, as in the MCI group, the effects of Aβ and APOE4 on pathogenesis of Alzheimer’s disease may diverge, thus resulting in disproportionately greater volume loss in those with both risk factors. Indeed, APOE4 has been found to be associated with an inability to repair synaptic damage, more rapid promotion of other neurotoxic species, such as tau, susceptibility to oxidative stress, and promotion of inflammatory cascades [17], beyond simply increasing levels of brain amyloid. Further work examining this interaction is warranted.
A third possible explanation is that both APOE4 and a low CSF Aβ are markers of disease progression. According to the literature, only 10-15% of individuals with MCI will convert to AD each year [43]. The other 85-95% of stable MCI individuals may be more likely have higher levels of CSF Aβ and be APOE4-negative, thus resulting in slower hippocampal volume loss.
Several study limitations deserve mention. First, the ADNI was designed to mimic a trial population, so participants were more educated, more Caucasian, and had fewer comorbidities than a community-based cohort [22]. The generalizability of our conclusions is thus controversial, and the length of follow-up was short. Second, this was a secondary analysis of the cohort, so there were different proportions of APOE4 carriers individuals at each clinical stage. Overall, the NC and AD groups had about half the number of participants as the MCI group, resulting in reduced power to detect differences. Rather than take a sample with balanced proportions, we wanted to include all available data. Furthermore, an allelic dose-dependent effect of APOE4 could not be explored, since only two NC were homozygous for APOE4, and the MCI and AD had imbalanced proportions of heterozygotes and homozygotes. Third, we only included hippocampal volumes as a marker of structural change to limit the number of comparisons. Inclusion of other limbic or whole brain markers would potentially detect more APOE4 effects not described in our analysis. Further prospective studies are needed to validate our findings.
In summary, we demonstrated that baseline CSF levels of Aβ are predictive of near-term hippocampal volume loss. The strengths of this study include the recruitment of participant from multiple centers, longitudinal follow-up, and consideration of all 3 clinical stages. We further raised the possibility of an APOE4-CSF Aβ interaction on longitudinal hippocampal atrophy among MCI participants. As interest grows in using hippocampal atrophy as an outcome in clinical trials, it will be important to consider how varying risk factors and biomarkers interact and influence the progression of neurodegeneration.
Acknowledgements
This work is funded by the National Institutes of Health (NIH), National Institute of Biomedical Imaging and Bioengineering (NIBIB) [T32 EB001631-05].
Dr. Fox also receives funding from the Medical Research Council (MRC), the Alzheimer’s Research Trust (ART), and the National Institute for Health Research (NIHR).
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Footnotes
Conflicts of interest The authors report no conflicts of interest with regard to this work.
Disclosures Dr. Reiman serves on the scientific advisory boards of Accera, AstraZeneca, Elan Pharmaceuticals, Eli Lilly, GlaxoSmithKline, and Siemens; serves as a consultant to Amnestix/Sygnis; holds US Patent Number 6,374,130, issued April 16, 2002; and receives research support from Kronos Life Sciences, GlaxoSmithKline, AstraZeneca, Avid, the NIA [9R01 AG031581-10 (PI)], and the State of Arizona.
Dr. Jack is an investigator in clinical trials sponsored by Pfizer; serves as a consultant for Elan Pharmaceuticals; and receives research support from the NIH [R01-AG11378] and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation.
Dr. Fox has served on the scientific advisory boards of Alzheimer’s Research Forum, Alzheimer’s Society and Alzheimer’s Research Trust and editorial boards of Alzheimer’s Disease and Associated Disorders; Neurodegenerative Diseases and BioMed Central - Alzheimer’s Research and Therapy. He holds a patent for QA Box that may accrue revenue. In the last five years his research group has received payment for consultancy or for conducting studies from Abbott Laboratories, Elan Pharmaceuticals, Eisai, Eli Lilly, GE Healthcare, IXICO, Lundbeck, Pfizer Inc, Sanofi-Aventis and Wyeth Pharmaceuticals. He receives research support from MRC [G0801306 (PI), G0601846 (PI)] NIH [U01 AG024904 (Co-investigator(sub contract)], Alzheimer Research Trust [ART/RF/2007/1 (PI)] and the NIHR (as a Senior Investigator).
Dr. Jagust serves on a scientific advisory board of Genentech; has served as a consultant to Synarc, Elan Pharmaceuticals, Genentech, Ceregene, Schering-Plough, and Merck & Co.; and receives research support from the NIH [AG027859 (PI), AG027984 (PI), and AG 024904 (Coinvestigator)] and the Alzheimer’s Association [ZEN-08-87090 (PI)].
Dr. Aisen serves on a scientific advisory board for NeuroPhage; serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, and Medivation, Inc.; receives research support from Pfizer Inc, and Baxter International Inc., and the NIH [NIA U01-AG10483 (PI), NIA U01-AG024904 (Coordinating Center Director), NIA R01-AG030048 (PI), and R01-AG16381 (Co-I)]; and has received stock options from Medivation, Inc. and NeuroPhage.
Dr. Petersen serves as a consultant to Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIA [AG 06786 (PI) and AG 16574 (PI)].
Dr. Weiner serves on scientific advisory boards for Bayer Schering Pharma, Eli Lilly, Nestle, CoMentis, Neurochem, Eisai, Avid, Aegis, Genentech, Allergan, Lippincott, Bristol Meyers Squibb, Forest, Pfizer, McKinsey, Mitsubishi, and Novartis. He has received non–industry-supported funding for travel; serves on the editorial board of Alzheimer’s & Dementia; received honoraria from the Rotman Research Institute and BOLT International; receives research support from Merck & Co, Avid, NIH [U01AG024904 (PI), P41 RR023953 (PI), R01 AG10897 (PI), P01AG19724 (Coinvestigator), P50AG23501(Coinvestigator), R24 RR021992 (Coinvestigator), R01 NS031966 (Coinvestigator), and P01AG012435 (Coinvestigator)], the Department of Defense [DAMD17-01-1-0764 (PI)], and the Veterans Administration [MIRECC VISN 21 (Core PI)]; and holds stock in Synarc and Elan Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinner JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1511. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- [2].Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scheinen NM, Aalto S, Koikkalainen J, Lotjonen J, Karrasch M, et al. Follow-up of [11C]PIB uptake and brain volume in patients with Alzheimer disease and controls. Neurology. 2009;73:1186–1192. doi: 10.1212/WNL.0b013e3181bacf1b. [DOI] [PubMed] [Google Scholar]
- [4].Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bourgeat P, Chetelat G, Villemagne VL, Fripp J, Raniga P, Pike K, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- [6].Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- [7].Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- [8].Herukka SK, Pennanen C, Soininen H, Pirttila T. CSF Abeta42, tau and phosphorylated tau correlate with medial temporal lobe atrophy. J Alzheimers Dis. 2008;14:51–57. doi: 10.3233/jad-2008-14105. [DOI] [PubMed] [Google Scholar]
- [9].Fjell AM, Walhovd KB, Amlein I, Bjornerud A, Reinvang I, Gjerstad L, et al. Morphometric changes in the episodic memory network and tau pathologic features correlate with memory performance in patients with mild cognitive impairment. Am J Neuroradiol. 2008;29:1183–1189. doi: 10.3174/ajnr.A1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Henneman WJ, Vrenken H, Barnes J, Sluimer IC, Verwey NA, Blankenstein MA, et al. Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology. 2009;73:935–940. doi: 10.1212/WNL.0b013e3181b879ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sluimer JD, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, et al. Whole brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.016. (in press) [DOI] [PubMed] [Google Scholar]
- [13].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- [14].Seshadri S, Drachman DA, Lippa CF. Apolipoprotein E epsilon 4 allele and the lifetime risk of Alzheimer’s disease: what physicians know, and what they should know. Arch Neurol. 1995;52:1074–1079. doi: 10.1001/archneur.1995.00540350068018. [DOI] [PubMed] [Google Scholar]
- [15].Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Small GW, Siddarth P, Burggren AC, Kepe V, Ercoli LM, et al. Influence of Cognitive Status, Age, and ApoE-4 Genetic Risk on Brain FDDNP-PET Binding in Non-Demented Persons. Arch Gen Psychiatry. 2009;66:81–87. doi: 10.1001/archgenpsychiatry.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].van de Pol LA, van der Flier WM, Korf ES, Fox NC, Barkhof F, Scheltens P. Baseline predictors of rates of hippocampal atrophy in mild cognitive impairment. Neurology. 2007;69:1491–1497. doi: 10.1212/01.wnl.0000277458.26846.96. [DOI] [PubMed] [Google Scholar]
- [20].Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, et al. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45:S3–15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [24].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- [25].Wechsler D. Wms-R Weschsler Memory Scale – Revised Manual. The Psychological Corporation, Harcourt Brace Jovanovich, Inc.; New York, NY: 1987. [Google Scholar]
- [26].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: a report of the NINCDS Alzheimer’s disease RDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [27].Shaw LM. PENN Biomarker Core of the Alzheimer’s Disease Neuroimaging Initiative. Neurosignals. 2008;16:19–23. doi: 10.1159/000109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- [32].Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. Protective effect of apolipoprotein E type 2 allele for late onset zheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- [33].Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang S, et al. Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normals. Neurology. 2011 doi: 10.1212/WNL.0b013e3181ffe4d1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- [35].Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- [36].Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55:134–136. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- [37].Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- [38].Fleisher A, Grundman M, Jack CR, Petersen RC, Taylor C, Kim HT, et al. Sex, apolipoprotein E epsilon4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- [39].den Heijer T, Oudkerk M, Launder LJ, van Duijin CM, Hofman A, Breteler MMB. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- [40].Jack CR, Petersen RC, Xu YC, O’Brien PC, Waring SC, Tangalos EG, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- [42].Riemenschneider M, Wagenpfeil S, Diehl J, Lautenschlager N, Theml T, Heldmann B, et al. Tau and Aβ42 protein in CSF of patients with frontotemporal degeneration. Neurology. 2002;58:1622–1628. doi: 10.1212/wnl.58.11.1622. [DOI] [PubMed] [Google Scholar]
- [43].Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]