Abstract
Purpose
To present the use of a quality control ice-water phantom for DW-MRI. DW-MRI has emerged as an important cancer imaging biomarker candidate for diagnosis and early treatment response assessment. Validating imaging biomarkers through multi-center trials requires calibration and performance testing across sites.
Materials and Methods
The phantom consisted of a center tube filled with distilled water surrounded by ice-water. Following preparation of the phantom approximately 30 minutes was allowed to reach thermal equilibrium. DW-MRI data was collected at 7 institutions, 20 MRI scanners from three vendors and 2 field strengths (1.5 and 3T). The phantom was also scanned on a single system on 16 different days over a 25 day period. All data was transferred to a central processing site at the University of Michigan for analysis.
Results
Results revealed that the variation of measured ADC values between all systems tested was ±5% indicating excellent agreement between systems. Reproducibility of a single system over a 25 day period was also found to be within ±5% ADC values. Overall, the use of an ice water phantom for assessment of ADC was found to be a reasonable candidate for use in multi-center trials.
Conclusion
The ice water phantom described here is a practical and universal approach to validate the accuracy of ADC measurements with ever changing MRI sequence and hardware design and can be readily implemented in multicenter clinical trial designs.
Keywords: diffusion, MRI, phantom, ice water, quality control
Introduction
Development of new quantitative imaging methods for cancer diagnostic and treatment response assessment is under active investigation. The apparent diffusion coefficient (ADC), sensitive to tumor cellularity and response to cytotoxic therapy, is an imaging biomarker under active evaluation. While the basic biophysical premise and technical feasibility of these quantitative imaging approaches are well established, several important practical issues must be addressed prior to routine use in clinical trials. Many of these issues were enumerated in a recent consensus article on use of diffusion-weighted MRI (DW-MRI) as a cancer biomarker(1). Particularly relevant to the present article are recognized needs to: (a) standardize DW-MRI acquisition schemes across multiple vendors, software and hardware platforms; (b) develop phantoms to confirm quantitative agreement across platforms; and (c) certify proper calibration and performance of systems at participating multicenter trial sites. Phantoms are often developed for one of two distinct purposes: to reasonably mimic specific tissue properties or serve as a device to test performance of the imaging system. Diffusion in biological systems is complex as it involves water movement within and among cellular and subcellular elements consisting of macromolecular structures, tortuosity of the extracellular space and anisotropy of cytoarchitecture. Non diffusion-related motions from blood flow and bulk tissue motion further complicate in vivo ADC measurement. As a result, it is difficult to develop a stable phantom that truly mimics all tissue properties, thus some phantoms have been designed to emulate tissue anisotropy (2,3) or multi-exponential decay properties (4,5). In terms of phantoms to confirm accurate quantification of diffusion values by MRI systems, simple fluid-based test objects are preferred (1,6). Tofts et al. characterized relaxation and diffusion properties of 15 liquids (cyclic alkanes, n-alkanes and n-alcohols) as a function of temperature (15 to 30°C)(7). These materials were shown to have diffusion coefficients in the relevant tissue range (0.36-2.2×10-3mm2/s); are stable, readily available and safe when proper handling precautions are taken. For optimal accuracy in diffusion determination, the temperature of a fluid needs to be determined and controlled as diffusion coefficients are known to vary by 1.7-3.2%/°C near room temperature (8,9).
The objective of this article is to demonstrate use of ice water as a universal standard in an ADC phantom developed to address these needs. Once thermal equilibrium is achieved, the ice water-based alternative proposed herein offers inherent thermal control over an extended interval (several hours) to test/calibrate one or multiple MR systems. Additional favorable properties include safe handling, non toxic fluid disposal and low cost. While ice water presents only a single diffusion coefficient, its value is comparable to edematous and tumor tissues thus it offers a reasonable tissue-like b-value dependent DW-MRI signal decay property.
Methods
Phantom Design
The phantom was constructed from standard labware components: a 50ml polypropylene conical tube (30mm × 115mm) and 1000ml polypropylene wide-mouth jar. The 50ml tube was filled with distilled water, capped and cemented to the underside of the 1000ml jar top. Prior to diffusion measurements, cubed or crushed ice and water were added such that ice filled the full extent of the jar. By screwing on the jar top, the 50ml tube of water was held in the center ice water mixture. The phantom was wrapped in a hospital “blue pad” for insulation and to absorb surface condensate. Figure 1 illustrates the phantom (Figure 1A), its suggested positioning for scanning and a representative T1-weighted image through the center of the phantom (Figure 1B).
Figure 1.
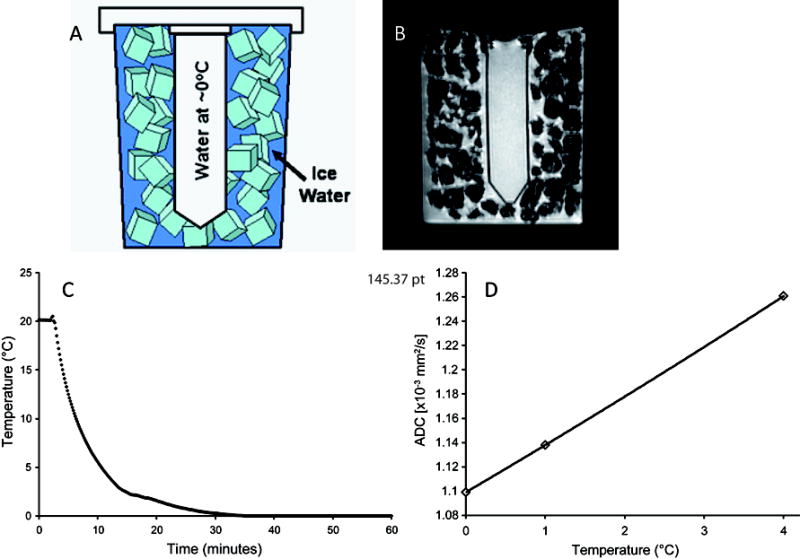
(A) Schematic and (B) axial MR image of the ice water phantom. (C) Water in the conical tube was found to reach thermal equilibrium within 30 minutes of placement in the ice water jacket. (D) The dependence of the self-diffusion of water to temperature based on Holtz et. al.(11), with a reported value of ~1.1×10-3 mm2/s at 0°C.
Separate from the MRI experiment, a thermocouple was inserted into the conical tube for determination of the time required to reach thermal equilibration between the water in the conical tube and the surrounding ice bath starting from room temperature..
MRI Systems Tested
A total of 8 phantoms were constructed with the 50ml tube filled and cemented in place prior to shipment. Two phantoms were retained for use on multiple systems at University of Michigan (Ann Arbor, MI) and the remaining were shipped to five additional sites including National Institutes of Health (Bethesda, MD), University of Massachusetts (Worcester, MA) Mount Vernon Hospital (Northwood, UK), Royal Marsden Hospital (Sutton, UK), William Beaumont Hospital (Royal Oak, MI). These 6 institutions provided data from 20 different human MRI scanners from three vendors (General Electric; Philips; and Siemens) and two field strengths (1.5T and 3T). In this study, vendors were denoted as Vendor A, B and C to maintain de-identification of data thus attribution of specific data to a specific Vendor is not provided. Key attributes for each scanner are provided in Table 1. In addition, to assess single-system repeatability, the ice water phantom was scanned on one system on 16 separate days over a 25 day interval.
Table 1.
Variability in sequence parameters
| Sequence Parameters | Mean | SD |
|---|---|---|
| FOV (cm) | 23.9 | 2.4 |
| Number of Slices | 16.9 | 6.1 |
| TR (ms) | 4349 | 2000 |
| TE (ms) | 87.1 | 32.6 |
Diffusion-weighted Image Acquisition
The primary objective was to demonstrate suitability of the ADC ice water phantom for multi-institutional trials, with a secondary aim to demonstrate its transportability for obtaining data of agreement in ADC values across different MRI Vendors (varying software and hardware platforms), field strengths and institutions. Rather than detail the specification of acquisition parameters which can be difficult across vendors and platforms, each center was requested to perform their “local-standard adult brain three-axis DWI protocol”, as well as DWI scans at b = 0, 500, 800, 1000 and 2000 s/mm2. Slice thickness, quantity of slices, acquisition and reconstruction matrix, receiver bandwidth, number of averages, use of parallel imaging, TR and TE were not specified. The simple nature of this diffusion phantom suggests the measured ADC should be independent of these acquisition parameters (including b-value) as long as there is adequate signal-to-noise and low artifact to avoid bias. All acquisition parameters were held constant for the single-system repeatability study.
Diffusion-weighted Image Analysis
Image data from each system were transferred in DICOM format to the University of Michigan for generation of ADC maps using customized software routines developed in Matlab (The MathWorks, Inc. Natick, MA). ADC maps were calculated from low b=0 images (S0) and high b (Sb) image pairs according to:
where high b = 500, 800, 1000 and 2000s/mm2. That is, four sets of ADC maps were generated for each scanner: ADC500, ADC800, ADC1000 and ADC2000. A rectangular region-of-interest (ROI) of area 360±30mm2 was manually defined on ADC maps on the central slice through the 50ml tube and ROI mean and standard deviation were recorded.
Statistical Analysis
Of the 20 scanners, 12 were 1.5T and 8 were 3T. Due to the small number of 3T systems from Vendors A and C, comparison of ADC measurements by field strength or vendor was performed on pooled data. An analysis of variance (ANOVA) was used to assess the differences in ADC values measured at each b-value between magnetic field strengths, vendors pooled, and vendors, field strengths pooled. Differences in ADC measurements obtained using b-values of 500, 800, 1000 and 2000 s/mm2 were assessed separately for each vendor, pooling data from both field strengths, by repeated measures (rm)-ANOVA. Multiple comparisons of the main effect were adjusted using a Bonferroni posthoc test. For the single-system repeatability study normality of ADC measurements at each b-value was determined using a one-sample Kolmogorov-Smirnov test. A one-sample t-test was performed to determine differences of ADC values at each b-value from the literature value of ~1.1×10-3 mm2/s (8-10). Finally, differences in ADC values acquired at different b-values were assessed as described for vendor comparisons. Statistical significance was assessed at p<0.05. Data are presented as the mean±SD.
Results
As depicted in Figure 1C, upon inserting the tube of room temperature water into ice water jacket, approximately 30 minutes was required for the central fluid to reach an equilibrium temperature approximately 0°C. Water maintained at 0°C has a literature ADC value of ~1.1×10-3 mm2/s (Figure 1D) (8-11). Water within the tube was easily delineated from the ice bath by MRI as a result of the lack of signal intensity from the tube wall (wall thickness ~1mm). This provided ease of prescribing a region of interest (ROI) within the tube. The high sensitivity of water diffusion with temperature makes it important to have a stable temperature over long periods of time. As reported from various sources in the literature and shown in Figure 1D, water ADC increases at a rate of 2.4%/°C (8,9). We have found that our ice water phantom was capable of maintaining a temperature ~0°C for up to 3-4 hours (data not shown). This provides the thermal stability necessary for acquiring ADC measurements on multiple systems using the same preparation over this period.
The objective of this study was not to propose a set of sequence parameters for acquiring an ADC measurement of ice water, but to determine the accuracy of these measurements using an ice water phantom over a variety of vendor instruments using site-specific protocols for acquiring ADC measurements of the human brain. As presented in Table 1, a wide variety of basic sequence parameter settings were used by different sites to acquire the DW-MRI data. Repetition time (TR) and echo time (TE) were found to be highly variable between sites, ranging from 1000-8000ms and 51-167ms, respectively.
Irrespective of the variability in sequence parameters between sites, we found that ADC measurements of the ice water phantom were consistent with the literature value for water at ~0°C (8-10). Presented in Figure 2 are the grouped ADC values, acquired at b-values of 500, 800, 1000 and 2000 s/mm2, from the ice water phantom for each individual system (n=20). A total of 80 measurements were obtained and 86% of the values were within 5% (blue field in Figure 2) of the literature value of 1.1×10-3 mm2/s (10). All measurements were within 10% (denoted by red fields in Figure 2) of this value. ADC values acquired using Vendor C systems at 1.5T were noticeably higher than the sole 3T system from the same manufacturer. Nevertheless, magnet field strength, when pooled over all systems, was not found to have an impact on ADC measurements. In contrast, differences in ADC were observed between system manufactures. Vendor C, driven heavily by the 1.5T systems, had a significantly higher ADC measurement than Vendor’s A and B, when acquired using b-values of 800 (p=0.02 and p<0.0001), 1000 (p=0.01 and p<0.0001) and 2000 (p=0.002 and p=0.001) s/mm2. At a b-value of 500 s/mm2 Vendor B was found to have a significantly lower ADC value than both Vendor A (p=0.02) and C (p<0.0001). In general, all systems manufactured by Vendor B produced ADCs that increased with increasing b-values, with differences in ADC values observed between all b-value combinations (p<0.01).. Vendor A produced ADC values that differed when acquired using b-values of 1000 and 2000s/mm2 (p=0.02), whereas no statistical difference in ADC with b-value was observed for Vendor C.
Figure 2.
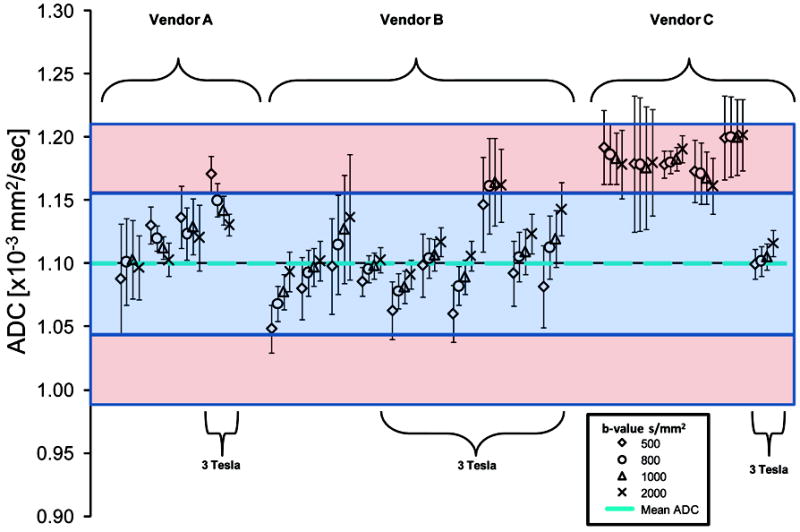
Measurements of the apparent diffusion coefficient (ADC) of the ice water phantom separated by site, vendor, magnetic field strength and b-values.
The single-system repeatability study using a Vendor B manufactured system showed a steady increase in ADC values for increasing b-values at each examination (Figure 3). Nevertheless, a mean ADC of 1.09×10-3mm2/s was obtained using all measurements. At individual b-values, ADCs (mean±SD) were 1.07±0.02, 1.09±0.02, 1.09±0.02 and 1.1±0.02 ×10-3mm2/s acquired at 500, 800, 1000 and 2000 s/mm2, respectively. These measurements were not found to deviate from normality. When comparing these ADC values to a test value of 1.1×10-3mm2/s, only measurements obtained with b-values of 500 and 800 s/mm2 deviated significantly from the test value (p<0.0001 and p=0.02, respectively). All combinations of b-values resulted in significantly different ADC values.
Figure 3.
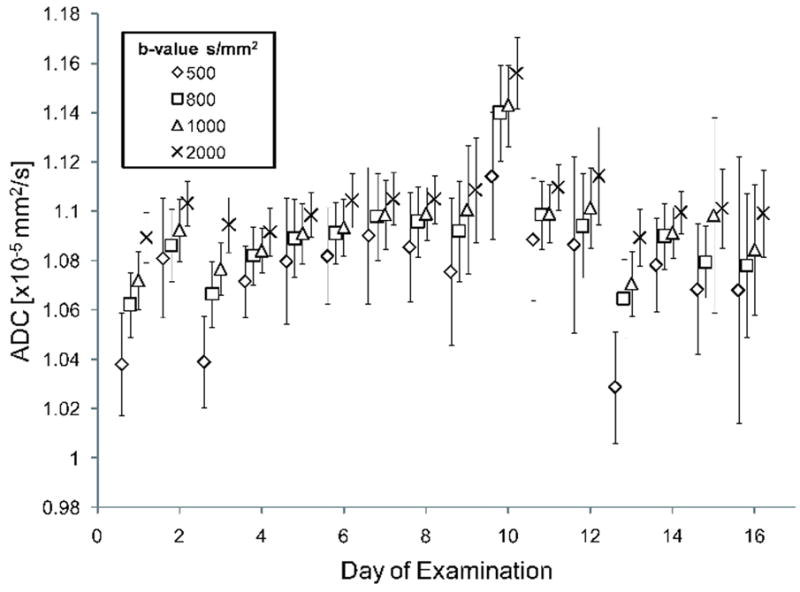
Repeatability in the measurements of the apparent diffusion coefficient (ADC) of the ice water phantom acquired at different b-values was performed at a single-site on 16 different occasions over a 25 day period.
Discussion
DW-MRI, a standard sequence on most MRI systems, has found widespread use for diagnostic and prognostic applications in the medical community (1,12-15). The attractiveness of this technique is its high sensitivity to microenvironmental changes in living tissue that commonly occurs upon the onset or treatment of disease. In addition, this MRI technique is inherently noninvasive; requiring no contrast administration.. Although widely used, there remains little conformity in the “preferred” diffusion MRI hardware, sequence and parameters, with scan protocols defined differently between vendors and sites. We sought to determine the reproducibility of the diffusion measurements across clinical platforms and sites. by employing a universal, temperature-controlled fluid. The diffusion sequences were locally-defined for brain imaging using parameters and hardware specific to each individual site. No request was made to modify the standard operating procedure, only that they follow the setup procedure for measuring ADC using the ice water phantom.
The physical properties of water are well characterized in the literature making it a good candidate for diffusion measurements by MRI(8-10,16). Unlike some other proposed fluids, water is inert making it free of special handling requirements and is readily available(7). A limitation in using any fluid where viscosity and molecular mobility vary with temperature is the need for temperature determination and control. To circumvent this limitation liquid water was jacketed within ice water and allowed to equilibrate. This not only sets the test fluid to a known temperature, but also maintains that temperature as long as sufficient ice remains in phase transition to water. We found that approximately 30 minutes was required for the liquid water to reach a temperature ~0°C (Figure 1C) and that this temperature was maintained for several hours.
As expected, there were site-specific variations in MR hardware and sequence parameters (Table 1) with repetition and echo times varying quite substantially. Despite these variables, the large majority of ADC values were within 5% of literature values, with all 80 measurements within 10%. These variations were in agreement with the work performed by Sasaki and colleagues, who, using healthy subjects, demonstrated the variability in absolute ADC of white and gray matter in brain tissue across different platforms(17). For their study, sequence parameters were attempted to be as consistent as possible. Sasaki and colleagues report inter-vendor variability up to 7% and intra-variability up to 8%. ADC values fluctuated by up to 5% when the same volunteers were imaged on the same scanner. Use of our ice water phantom across vendors and multiple examinations on the same scanner are consistent with their results despite not setting controls on site sequence parameters. In addition, we observed ADC variability at different b-values. Based on first principles, ADC of water should not have a b-value dependence. Nevertheless Vendor B systems were found to steadily increase ADC with increasing b-value. This effect was small (<5%) but significant and may be a result of eddy-current effects resulting in the true b-value being slightly higher than the assumed b-value. Detection of such small effects could not be easily performed on living tissue (i.e. brain), due to non-monoexponential trends in diffusion-weighted signal attenuation typically observed in biological systems (2,15,18,19).
In Conclusion, the extensive use of the absolute ADC as a predictive surrogate biomarker of disease and treatment-induced response has made validation and calibration of individual systems a prerequisite for quality control (1,12-15). With advances in rapid imaging techniques, DW sequences and radio-frequency coil design, conformity to a pre-described DWI protocol is difficult to achieve across multiple sites and system platforms. Requiring only inexpensive material that is readily available and 30 minutes to reach thermal equilibrium, the proposed ice water phantom is a practical and universal approach to validating the accuracy of ADC measurements with ever changing MRI sequence and hardware design.
Acknowledgments
Contract grant sponsor: National Institutes of Health (NIH); Contract grant number: P01-CA85878 and P50-CA93990. We would like to acknowledge assistance with data collection by Anil Shetty, David Thomasson, Yanping Sun, David Collins, Anwar Padhani.
References
- 1.Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark CA, Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med. 2000;44:852–859. doi: 10.1002/1522-2594(200012)44:6<852::aid-mrm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Komlosh ME, Horkay F, Freidlin RZ, Nevo U, Assaf Y, Basser PJ. Detection of microscopic anisotropy in gray matter and in a novel tissue phantom using double Pulsed Gradient Spin Echo MR. J Magn Reson. 2007;189:38–45. doi: 10.1016/j.jmr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Delakis I, Moore EM, Leach MO, De Wilde JP. Developing a quality control protocol for diffusion imaging on a clinical MRI system. Phys Med Biol. 2004;49:1409–1422. doi: 10.1088/0031-9155/49/8/003. [DOI] [PubMed] [Google Scholar]
- 5.Laubach HJ, Jakob PM, Loevblad KO, et al. A phantom for diffusion-weighted imaging of acute stroke. J Magn Reson Imaging. 1998;8:1349–1354. doi: 10.1002/jmri.1880080627. [DOI] [PubMed] [Google Scholar]
- 6.Pierpaoli C, Sarlls J, Nevo U, Basser PJ, et al. Polyvinylpyrrolidone (PVP) water solutions as isotropic phantoms for diffusion MRI studies. Proceedings of the 8th Anual Meeting of ISMRM; Honolulu. 2009. abstract 1414. [Google Scholar]
- 7.Tofts PS, Lloyd D, Clark CA, et al. Test liquids for quantitative MRI measurements of self-diffusion coefficient in vivo. Magn Reson Med. 2000;43:368–374. doi: 10.1002/(sici)1522-2594(200003)43:3<368::aid-mrm8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Krynicki K, Green CD, Sawyer DW. Pressure and temperature dependence of self-diffusion in water. Faraday Discuss Chem soc. 1978;66:199–208. [Google Scholar]
- 9.Simpson JH, Carr HY. Diffusion and nuclear spin relaxation in water. Phys Rev. 1958;111:1201–1202. [Google Scholar]
- 10.Mills R. Self-diffusion in normal and heavy water in the range 1-45.deg. J Phys Chem. 1973;77:685–688. [Google Scholar]
- 11.Holz M, Heil SR, Sacco A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys Chem Chem Phys. 2000;2:4740–4742. [Google Scholar]
- 12.Chenevert TL, Ross BD. Diffusion imaging for therapy response assessment of brain tumor. Neuroimaging Clin N Am. 2009;19:559–571. doi: 10.1016/j.nic.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galban CJ, Mukherji SK, Chenevert TL, et al. A feasibility study of parametric response map analysis of diffusion-weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl Oncol. 2009;2:184–190. doi: 10.1593/tlo.09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamstra DA, Lee KC, Moffat BA, Chenevert TL, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an imaging treatment response biomarker to chemoradiotherapy in a mouse model of squamous cell cancer of the head and neck. Transl Oncol. 2008;1:187–194. doi: 10.1593/tlo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier SE, Bogner P, Bajzik G, et al. Normal brain and brain tumor: multicomponent apparent diffusion coefficient line scan imaging. Radiology. 2001;219:842–849. doi: 10.1148/radiology.219.3.r01jn02842. [DOI] [PubMed] [Google Scholar]
- 16.Cooke R, Kuntz ID. The properties of water in biological systems. Annu Rev Biophys Bioeng. 1974;3:95–126. doi: 10.1146/annurev.bb.03.060174.000523. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki M, Yamada K, Watanabe Y, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008;249:624–630. doi: 10.1148/radiol.2492071681. [DOI] [PubMed] [Google Scholar]
- 18.Mulkern RV, Gudbjartsson H, Westin CF, et al. Multi-component apparent diffusion coefficients in human brain. NMR Biomed. 1999;12:51–62. doi: 10.1002/(sici)1099-1492(199902)12:1<51::aid-nbm546>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Riches SF, Hawtin K, Charles-Edwards EM, de Souza NM. Diffusion-weighted imaging of the prostate and rectal wall: comparison of biexponential and monoexponential modelled diffusion and associated perfusion coefficients. NMR Biomed. 2009;22:318–325. doi: 10.1002/nbm.1328. [DOI] [PubMed] [Google Scholar]


