Abstract
The present study examined the effects of a 9-month randomized control physical activity intervention aimed at improving cardiorespiratory fitness on changes in working memory performance in preadolescent children relative to a waitlist control group. Participants performed a modified Sternberg task, which manipulated working memory demands based on encoding set sizes, while task performance and the contingent negative variation (CNV) event-related brain potential were measured. Analyses revealed that the physical activity intervention led to increases in cardiorespiratory fitness and improved Sternberg task performance. Further, the beneficial effects of the physical activity intervention were greater for a task condition requiring greater working memory demands. In addition, the intervention group exhibited larger initial CNV at the frontal electrode site, relative to the waitlist group at post-test; an effect not observed during the pre-test. These results indicate that increases in cardiorespiratory fitness are associated with improvements in the cognitive control of working memory in preadolescent children.
Introduction
There is growing concern that children are becoming more sedentary and less fit, leading to an increased risk for chronic diseases such as cardiovascular diseases and type-2 diabetes (Department of Health and Human Services & Department of Education, 2000). Recent studies have suggested that physical activity and cardiorespiratory fitness are associated with not only chronic diseases prevention, but also cognitive and brain health (see Hillman, Erikson & Kramer, 2008, for review). For example, Castelli, Hillman, Buck and Erwin (2007) indicated that greater cardiorespiratory capacity was related to better academic achievement (mathematics and reading) among preadolescent children, with several other reports corroborating such a pattern of findings (see Centers for Diseases Control and Prevention, 2010, for review). Further, recent neuroelectric studies using event-related brain potentials (ERPs) have supported the positive relation of cardiorespiratory fitness with neurocognitive function in preadolescent children (Hillman, Buck, Themanson, Pontifex & Castelli, 2009; Hillman, Castelli & Buck, 2005; Pontifex, Raine, Johnson, Chaddock, Voss, Cohen, Kramer & Hillman, in press).
ERPs assess aspects of human information processing, and have provided insight into the underlying mechanisms involved in cognitive function beyond that of overt behavioral task performance (i.e. response accuracy and reaction time [RT]). Due to their high temporal resolution, ERPs provide information regarding discrete cognitive processes occurring between stimulus encoding and response execution. Hillman et al. (2005) used the P3 component of the stimulus-locked ERP to investigate the relation of cardiorespiratory fitness to cognitive function in preadolescent children (M = 9.6 years). They indicated that more-fit children exhibited larger P3 amplitude and shorter P3 latency with better task performance compared to their less-fit counterparts during a discrimination task (i.e. oddball task). Such a pattern of results suggests that cardiorespiratory fitness has a positive relation with neuroelectric indices of attention and cognition (Hillman et al., 2005).
In a second study, Hillman et al. (2009) further examined this relationship using a cognitive control task, because it has been well established that improvements in cognitive function that result from enhanced cardiorespiratory fitness are disproportionately larger for tasks or task components requiring extensive amounts of cognitive control in adult populations (Colcombe & Kramer, 2003; Kramer, Hahn, Cohen, Banich, McAuley, Harrison, Chason, Vakil, Bardell, Boileau & Colcombe, 1999). Cognitive control is used to describe ‘the ability to orchestrate thought and action in accord with internal goals’ (Miller & Cohen, 2001, p. 167), with inhibition, cognitive flexibility, and working memory thought to comprise core processes underlying such abilities (Diamond, 2006). Hillman et al. (2009) used a modified flanker task (Eriksen & Eriksen, 1974) requiring variable amounts of inhibitory control and indicated that more-fit children again had larger P3 amplitude and better response accuracy compared to their less-fit counterparts (M = 9.4 years). The results supported the fitness–cognitive control relationship, which has been observed in adult populations, and extended it to preadolescent childhood. In addition, Pontifex et al. (in press) extended these findings by manipulating stimulus–response compatibility (i.e. cognitive flexibility) during a modified flanker task. They indicated that more-fit children (M = 10.0 years) increased P3 amplitude for the incompatible condition compared to the compatible condition, whereas no such difference based on compatibility was observed for less-fit children. The results suggest that more-fit children had greater capacity to flexibly allocate attentional resources based on stimulus–response compatibility. Accordingly, more-fit participants were able to maintain response accuracy regardless of the compatibility manipulation, which required the upregulation of cognitive control (Pontifex et al., in press). Collectively, these previous ERP studies (Hillman et al., 2009; Pontifex et al., in press) suggest that cardiorespiratory fitness is associated with better cognitive control in children and is consonant with the findings observed in adult populations (Colcombe & Kramer, 2003; Kramer et al., 1999).
It is noteworthy to mention that these ERP studies investigating the relationship between cardiorespiratory fitness and cognitive function in preadolescent children used cross-sectional designs (Hillman et al., 2005, 2009; Pontifex et al., in press). Although these studies minimized the potential bias by matching groups on several demographic characteristics (e.g. pubertal status, IQ, ADHD rating scale), which have been associated with fitness or cognition, the prior cross-sectional studies have left open the possibility that other factors (e.g. genetics, personality) might influence the relationship between fitness and cognitive function. Accordingly, further investigation using longitudinal randomized control interventions is warranted to better establish a causal link between cardiorespiratory fitness and changes in cognitive control. Thus, the present study was conducted to investigate the effects of a 9-month physical activity intervention designed to improve cardiorespiratory fitness on cognitive control in preadolescent children. To our knowledge, this is the first ERP study using a longitudinal randomized control design with preadolescent children.
Given that a relatively small literature has explored the relation of cardiorespiratory fitness to cognitive control during childhood, the extent to which fitness affects cognitive control more broadly, or only during the expression of select functions, remains unclear. Accordingly, the present study focused on a different aspect of cognitive control (i.e. working memory) to elucidate the effects of a physical activity intervention leading to changes in cardiorespiratory fitness on cognitive control in preadolescent children. Working memory is considered a key aspect of cognitive control (Diamond, 2006) because individuals need to transiently hold and manipulate information in their mind to orchestrate goal-directed behaviors in a variety of cognitive contexts. For school-aged children, it has been reported that working memory is associated with academic performance such as mathematics and reading (Alloway, Gathercole, Adams, Willis, Eaglen & Lamont, 2005; Gathercole, Pickering, Knight & Stegmann, 2004; Silva-Pereyra, Fernández, Harmony, Bernal, Galán, Díaz-Comas, Fernández-Bouzas, Yáñez, Rivera-Gaxiola, Rodríguez & Marosi, 2001). As such, the current study was undertaken to examine whether the beneficial effects of a physical activity program would enhance working memory performance, supporting the importance of physical activity for cognitive development and providing a basis for academic performance (Castelli et al., 2007; Coe, Pivarnik, Womack, Reeves & Malina, 2006).
In this study, we used a modified Sternberg task (Sternberg, 1966) to evaluate working memory function, though it should be mentioned that some debate in the literature exists as others believe that this task taps short-term memory (Klein, Rauh & Biscaldi, 2010; Schooler, Caplan, Revell, Salazar & Grafman, 2008). The task required participants to encode a memory set (S1) containing an array of one, three, or five uppercase letters and respond whether a subsequently presented single lowercase probe (S2) letter appeared in the encoded array. That is, participants needed to actively maintain letters from the memory set during the delay interval (i.e. S1–S2 interval) and manipulate relevant information when the probe letter was presented to respond accurately and quickly. Accordingly, increased cognitive demands are required for larger set sizes, resulting in decreased accuracy (Marshall, Molle, Siebner & Born, 2005) and response speed (Sternberg, 1966). Further, the Sternberg task allows for the measurement of task preparation processes between S1 and S2 through the measurement of the contingent negative variation (CNV; Kamijo, O’Leary, Pontifex, Themanson & Hillman, 2010). Previous ERP studies with preadolescent children have only investigated the cardiorespiratory fitness–cognitive function relationship to inform about stimulus evaluation and action monitoring processes (Hillman et al., 2005, 2009; Pontifex et al., in press). By contrast, the current study focused on task preparation processes to better understand the contribution of changes in cardiorespiratory fitness to processes occurring between stimulus encoding and response execution. Thus, the present study stands to provide new insights into the beneficial effects of physical activity training on working memory and task preparation processes.
Specifically, the CNV is a negative-going slow cortical potential elicited during the interval between S1 and S2, which has been related to cognitive operations subserving stimulus engagement and task preparation processes. It is well known that the CNV is composed of at least two different components, the initial CNV (iCNV; also called O-wave) and the terminal CNV (tCNV; also called E-wave; Loveless & Sanford, 1974; Weerts & Lang, 1973). It is believed that the iCNV is associated with stimulus orientation (S1), and the tCNV is related to stimulus anticipation or response preparation (S2; Loveless & Sanford, 1974; Weerts & Lang, 1973). More recently, researchers have suggested that frontal CNV is associated with cognitive preparation processes rather than response preparation processes in adult populations (Falkenstein, Hoormann, Hohnsbein & Kleinsorge, 2003; Leynes, Allen & Marsh, 1998; Lorist, Klein, Nieuwenhuis, De Jong, Mulder & Meijman, 2000; Wild-Wall, Hohnsbein & Falkenstein, 2007). Further, the CNV has been found to increase for task conditions placing greater demands upon working memory in adults (Goffaux, Phillips, Sinai & Pushkar, 2006; Kamijo et al., 2010), suggesting that the CNV is associated with active maintenance of goal-relevant information during the interval between S1 and S2. Kamijo et al. (2010) investigated the relation of cardiorespiratory fitness to working memory processes using the tCNV during a Sternberg task in young adults. The results indicated that frontal tCNV amplitude was significantly smaller for more-fit participants compared to their less-fit counterparts, suggesting that active maintenance processes of more-fit individuals may be more efficient than those of less-fit individuals (Kamijo et al., 2010).
Developmental studies have indicated that CNV amplitude increases with age (Bender, Weisbrod, Just, Pfuller, Parzer, Resch & Oelkers-Ax, 2002; Segalowitz & Davies, 2004). Perchet and Garcia-Larrea (2005) found using a cued target detection task (i.e. Posner task) that a CNV-like component (cue-to-target interval = 500 ms) was observed for all cue conditions (valid, invalid, and neutral) in adults, whereas children (M = 7.2 years) exhibited the CNV-like component only for the neutral cue condition but not for the valid and invalid cue conditions. This suggests that adults might use cue information to anticipate and prepare for the target stimulus, whereas children might not be able to utilize such information, reflecting immature cognitive control (Perchet & Garcia-Larrea, 2005). Additionally, Segalowitz, Unsal, and Dywan (1992) indicated that iCNV was smaller in children (M = 12.2 years) than adults, whereas tCNV did not differ between age groups during a S1–S2 task requiring minimal cognitive control, suggesting that age differences are specific to iCNV. However, it should be noted that CNV was only recorded from a central electrode site. Jonkman, Lansbergen and Stauder (2003) replicated the previous findings of Segalowitz et al. (1992) using a cued go/nogo task (i.e. AX-CPT task) requiring inhibitory control, and observed smaller iCNV amplitude at fronto-central locations for children (M = 9.4 years) relative to adults. Given that tCNV amplitude was comparable between the children and adults (Jonkman et al., 2003), the findings suggest ineffective cue-orientation in children, but mature response preparation processes. Accordingly, it would appear that smaller iCNV, rather than tCNV, at fronto-central locations reflects immature cognitive control in children.
Based on the previous findings, we hypothesized that greater improvement in task performance as a function of enhanced cardiorespiratory fitness would be observed during task conditions placing greater demands upon working memory (i.e. larger set sizes), indicating that fitness exerts a disproportionately stronger effect on tasks requiring the upregulation of cognitive control. It has been reported that adults slow down their response speed during task conditions requiring greater amounts of cognitive control to preserve response accuracy, whereas children maintain a more constant response speed irrespective of cognitive control demands due to their impulsive performance tendency (Christakou, Halari, Smith, Ifkovits, Brammer & Rubia, 2009; Davidson, Amso, Anderson & Diamond, 2006). That is, children cannot effectively withhold their response to take the time necessary to ensure accurate performance. Thus, it would seem that RTs during cognitive tasks do not adequately reflect cognitive control demands in children. Indeed, the previous ERP studies using cognitive control tasks (Hillman et al., 2009; Pontifex et al., in press) indicated a positive relation of cardiorespiratory fitness with response accuracy, but not with RT. Thus, we hypothesized that the effects of physical activity training on cognitive control would be reflected in response accuracy, with selectively greater accuracy for the treatment group following the physical activity intervention. With regard to CNV, smaller iCNV, rather than tCNV, at fronto-central locations is considered to reflect immature cognitive control in children (Jonkman et al., 2003). Accordingly, we hypothesized that CNV amplitudes would increase as a result of physical activity training, and that this effect would be larger for iCNV compared to tCNV, with disproportionately larger effects over fronto-central electrode sites, due to the working memory demands of the task.
Methods
Participants
Forty-three children between 7 and 9 years old were randomly assigned to participate in either an afterschool physical activity program (intervention group; n = 22) or a waitlist control group (waitlist group; n = 21). None of the children received special education services related to cognitive or attentional disorders. All participants and their legal guardians provided written informed consent in accordance with the Institutional Review Board at the University of Illinois at Urbana-Champaign. Legal guardians completed the ADHD Rating Scale IV (DuPaul, Power, Anastopoulos & Reid, 1998) and provided information regarding participants’ demographics and socioeconomic status (SES). SES was determined by creating a trichotomous index based on: (1) participation in free or reduced-price lunch program at school, (2) the highest level of education obtained by the mother and father, and (3) number of parents who worked full-time (Birnbaum, Lytle, Murray, Story, Perry & Boutelle, 2002). All participants reported that they were free of neurological diseases, or physical disabilities, and had (corrected-to-) normal vision. Participants, in collaboration with their legal guardian, completed the Tanner Staging System (Taylor, Whincup, Hindmarsh, Lampe, Odoki & Cook, 2001), indicating that their pubertal status was at or below a score of 2 (i.e. prepubescent) on a 5-point scale. Additionally, the Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) was administered by a trained experimenter to assess intelligence quotient.
Seven participants (intervention = 2, waitlist = 5) were excluded from all analyses because they did not complete the cognitive task. Thus, analyses for the behavioral task performance were conducted on 36 participants.1 Table 1 lists participants’ demographic and fitness information for this sample. Lastly, data from 10 participants were discarded for the CNV analyses due to excessive noise in the electroencephalogram (EEG) signal. Thus, the CNV analyses were conducted on 26 participants (intervention = 12, waitlist = 14). Demographic data did not differ between the participants included in the task performance analyses and the subset of participants included in the CNV analyses, p ≥ .67.
Table 1.
Mean (SD) values for participant demographics and fitness data
| Measured variable | Intervention | Waitlist |
|---|---|---|
| n | 20 (11 girls) | 16 (8 girls) |
| Age (years) | 8.9 (0.5) | 9.1 (0.6) |
| Tanner | 1.6 (0.6) | 1.6 (0.5) |
| BMI (kg/m2) | 20.5 (3.8) | 20.7 (5.2) |
| K-BIT composite score (IQ) | 110.6 (12.8) | 106.3 (8.5) |
| SES | 1.9 (0.9) | 1.5 (0.8) |
| ADHD | 7.5 (7.3) | 6.8 (6.9) |
| Pre-VO2max (ml/kg/min) | 33.5 (7.3)a | 36.5 (8.1) |
| Post-VO2max (ml/kg/min) | 37.8 (7.1)a | 36.0 (7.2) |
| VO2max (ml/kg/min) | 4.2 (4.1)b | -0.5 (2.3)b |
| Attendance Rate (%) | 82.2 (8.2) | – |
Notes: BMI = body mass index; K-BIT = Kaufman Brief Intelligence Test; SES = socioeconomic status; ADHD = scores on the Attention Deficit Hyperactivity Disorder Rating Scale IV; VO2max = maximal oxygen consumption. Significant difference, Bonferroni-corrected post-hoc comparisons between pre- and post-test within a group:
p < .025, and unpaired t-test between groups,
p < .05.
Physical activity intervention
The physical activity intervention occurred for a 2-hour period following each school day, and focused on improvement of cardiorespiratory fitness through engagement in a variety of age-appropriate physical activities. Although cardiorespiratory fitness was the focus, muscle fitness was addressed at least 2 days a week, with the participants using their own body, Thera-bands® (Akron, OH), medicine balls, and other equipment as resistance. Within a daily lesson, the children intermittently participated in at least 70 minutes of moderate to vigorous physical activity (recorded by E600 Polar heart rate [HR] monitors; Polar Electro, Finland). Specifically, children completed approximately 40 minutes of physical fitness stations (i.e. individually, with partners, or in small groups). Next, a healthy snack and educational component was provided as a rest period, and children then engaged in low organizational games centered around a skill theme (i.e. dribbling). The activities were aerobically demanding, but simultaneously provided opportunities to refine motor skills. Some small-area games were offered as part of the motor skill practice. On the weekends, the children were encouraged to continue their participation in physical activity with their family unit. Because the program was offered 150 days of the 170-day school year, physical activity focused worksheets were utilized during extended periods of time away from the program (e.g. school holidays, vacation) to log continued engagement.
Cardiorespiratory fitness assessment
Maximal oxygen consumption (VO2max) was measured using a motor-driven treadmill and a modified Balke protocol (American College of Sports Medicine, 2006), which involved walking/running on a treadmill at a constant speed with increasing grade increments of 2.5% every 2 min until volitional exhaustion occurred. Oxygen consumption was measured using a computerized indirect calorimetry system (ParvoMedics True Max 2400) with averages for VO2 and respiratory exchange ratio (RER) assessed every 20 s. A polar HR monitor (Polar WearLink+ 31; Polar Electro, Finland) was used to measure HR throughout the test, and ratings of perceived exertion (RPE) were assessed every 2 min using the children’s OMNI scale (Utter, Robertson, Nieman & Kang, 2002). VO2max was based upon maximal effort as evidenced by (1) a peak HR ≥ 185 bpm (American College of Sports Medicine, 2006) and an HR plateau (Freedson & Goodman, 1993); (2) RER ≥ 1.0 (Bar-Or, 1983); (3) a score on the children’s OMNI RPE scale ≥ 8 (Utter et al., 2002); and/or (4) a plateau in oxygen consumption corresponding to an increase of less than 2 ml/kg/min despite an increase in workload.
Cognitive task
A modified Sternberg task (Sternberg, 1966) asked participants to encode a memory set containing an array of one, three, or five letters and press one of two buttons with their thumbs corresponding to whether a single probe letter was present (right) or absent (left) in the encoded letter array. They were asked to respond as quickly and accurately as possible. The memory sets comprised all uppercase consonants (e.g. KRM) and contained no alphabetical consonant strings, whereas the probe letters were lowercase consonants. The probe letter following an array of three or five letters was bilaterally flanked by one or two ‘?’, respectively, to match the memory set in physical size and visual content (e.g. ?k?). The experimenter provided the task instructions and 10 practice trials were presented repeatedly until the participant understood the task and exhibited task performance above chance. Next, four experimental blocks of 45 trials were administered with a brief rest between blocks. Probe presence/absence and the three letter conditions appeared with equal probability in a random order. The viewing distance was approximately 1 m. All stimuli were 7 cm tall white letters presented on a black background. The stimulus durations were 2500 ms for encoded array (S1) and 250 ms for probe letter (S2), with a 2000 ms inter-stimulus interval (from S1 offset to S2 onset) and a 2500 ms response window (from S2 onset to S1 onset).
ERP recording
EEG activity was measured from 64 electrode sites arranged in an extended montage based on the International 10-10 system (Chatrian, Lettich & Nelson, 1985), referenced to a midline electrode placed at the midpoint between Cz and CPz, with AFz serving as the ground electrode, and interelectrode impedances kept below 10 kΩ. Additional electrodes were placed above and below the left orbit and the outer left and right canthi to monitor electrooculogram (EOG) activity with bipolar recording. Continuous data were digitized at a sampling rate of 500 Hz, amplified 500 times with a DC to 70 Hz filter, and a 60 Hz notch filter using a Neuroscan Synamps2 amplifier (Neuro, Inc., Charlotte, NC, USA).
Offline EEG processing included: EOG correction using a spatial filter (Compumedics Neuroscan, 2003), re-referencing to average mastoids, creation of S2-locked epochs (−4600 to 0 ms relative to S2 onset), baseline correction (100 ms time window prior to S1 onset), low-pass filtering (10 Hz, 24 dB/octave), and artifact rejection (epochs containing signals that exceeded ± 100 µV were rejected). Trials with a response error were excluded from the CNV analyses as well as the RT analyses. Because there were not enough trials to average separately for each letter condition due to the number of response errors produced by the study participants as well as the noise present in the EEG signal on certain trials, ERPs were averaged across the letter conditions. Across the intervention and waitlist groups, a mean of 67 and 76 trials were averaged at pre- and post-test, respectively. The number of trials for the CNV averages did not differ between groups (intervention = 70, waitlist = 73). The mean amplitudes were calculated in two different time windows, from 500 to 1000 ms (iCNV) and from 1500 to 2000 ms (tCNV) after S1 offset.
Laboratory procedure
During pre- and post-tests, the experimental protocol occurred over two separate days for each participant. On the first visit to the laboratory, informed assent/consent was obtained, participants completed the K-BIT (Kaufman & Kaufman, 1990) and had their height and weight measured. Concurrently, participants’ legal guardians completed a health history and demographics questionnaire, the ADHD Rating Scale IV (DuPaul et al., 1998), the modified Tanner Staging questionnaire (Taylor et al., 2001), and the Physical Activity Readiness Questionnaire (Thomas, Reading & Shephard, 1992) to screen for any previous health issues that might be exacerbated by exercise. After completing all questionnaires, a cardiorespiratory fitness test was conducted.
On the second visit, participants were fitted with a 64-channel Quik-Cap (Compumedics Neuroscan, El Paso, TX) and seated in a sound-attenuated room where the Sternberg task took place. The participant was given the instructions and practiced the task prior to the start of testing. Upon completion of the cognitive task, all electrodes were removed. At the pre-test, participants were randomized to groups following their laboratory visits. At the post-test, the participant and guardian were briefed on the purpose of the experiment and compensated for their time.
Statistical analysis
VO2max data were analyzed using a 2 (Group: intervention, waitlist) × 2 (Time: pre-test, post-test) mixed-model MANOVA with repeated measures. Task performance data (i.e. response accuracy and RT) were submitted to a 2 (Group) × 2 (Time) × 3 (Set Size: one, three, five letter condition) mixed-model MANOVA with repeated measures. Mean amplitude of each CNV (i.e. iCNV and tCNV) was analyzed using a 2 (Group) × 2 (Time) × 5 (Site: Fz, FCz, Cz, CPz, Pz) mixed-model MANOVA with repeated measures (Kamijo et al., 2010). All analyses with three or more within-participants levels used the Wilks’ lambda statistic. Post-hoc analyses were conducted using Bonferroni corrected t-tests. Additionally, based on our hypotheses, planned comparisons were conducted. Specifically, for response accuracy, 2 (Group) × 2 (Time) mixed-model MANOVAs were conducted for each letter condition. For the CNV components, Bonferroni-corrected planned comparisons were conducted to compare group differences in pre–post change score at each electrode site. The alpha level was set at p = .05.
Results
Participant characteristics
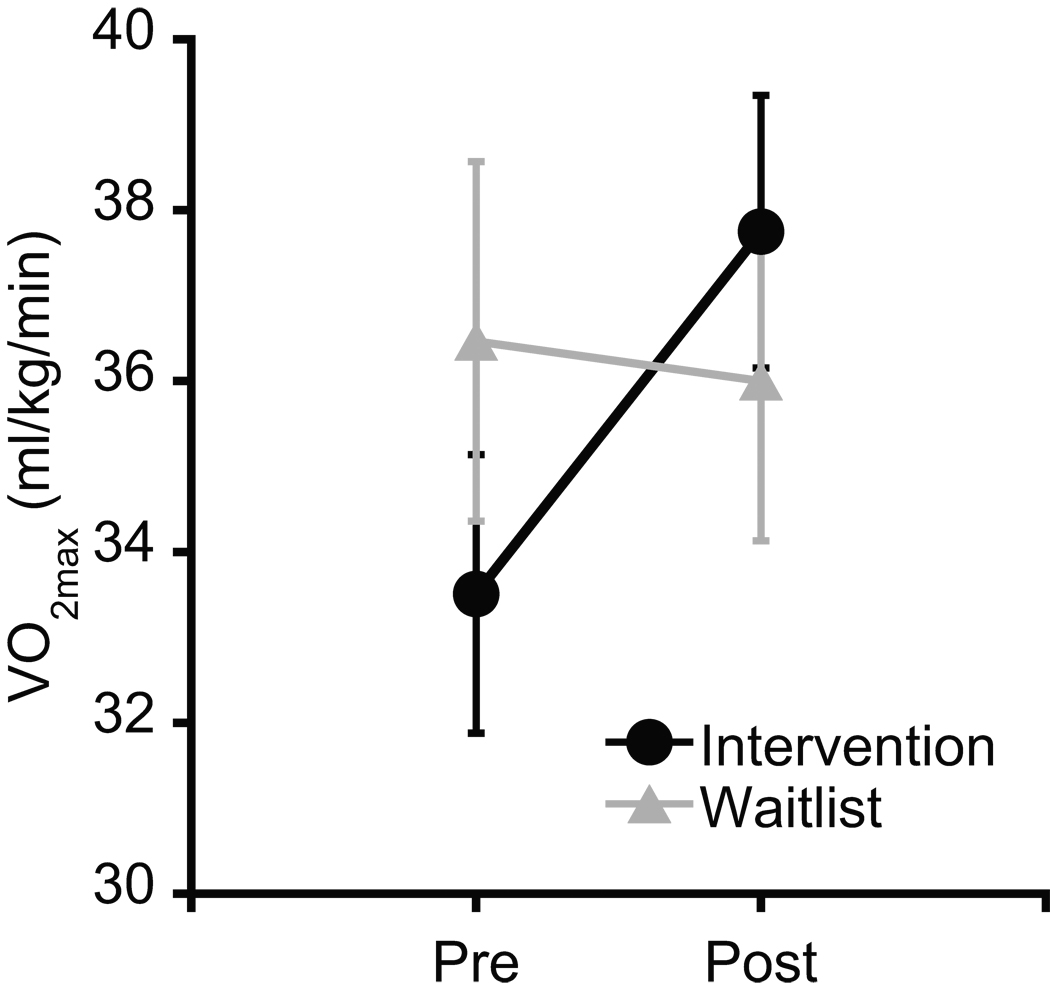
Participant demographic and fitness data are provided in Table 1. Demographic variables did not differ between the intervention and waitlist groups, ts(34) ≤ 1.4, ps ≥ .18. VO2max analysis revealed a Time main effect, F(1, 33) = 10.2, p = .003, η2p = .24, qualified by a Group × Time interaction, F(1, 33) = 15.8, p < .001, η2p = .32. Post-hoc analyses (p = .025) indicated that VO2max at post-test was significantly larger than at pre-test for the intervention group, t(19) = 4.6, p < .001, while no such difference was observed for the waitlist group, t(14) = 0.8, p = .45, confirming the efficacy of the physical activity intervention (see Table 1 and Figure 1). Additionally, pre–post change scores for VO2max were significantly larger for the intervention group compared to the waitlist group, t(33) = 4.0, p < .001 (see Table 1).
Figure 1.
Mean VO2max at pre- and post-test for the physical activity intervention and waitlist control groups. These data are sample values for participants including in the primary task performance analysis (n = 36). Error bars indicate SEs.
Task performance
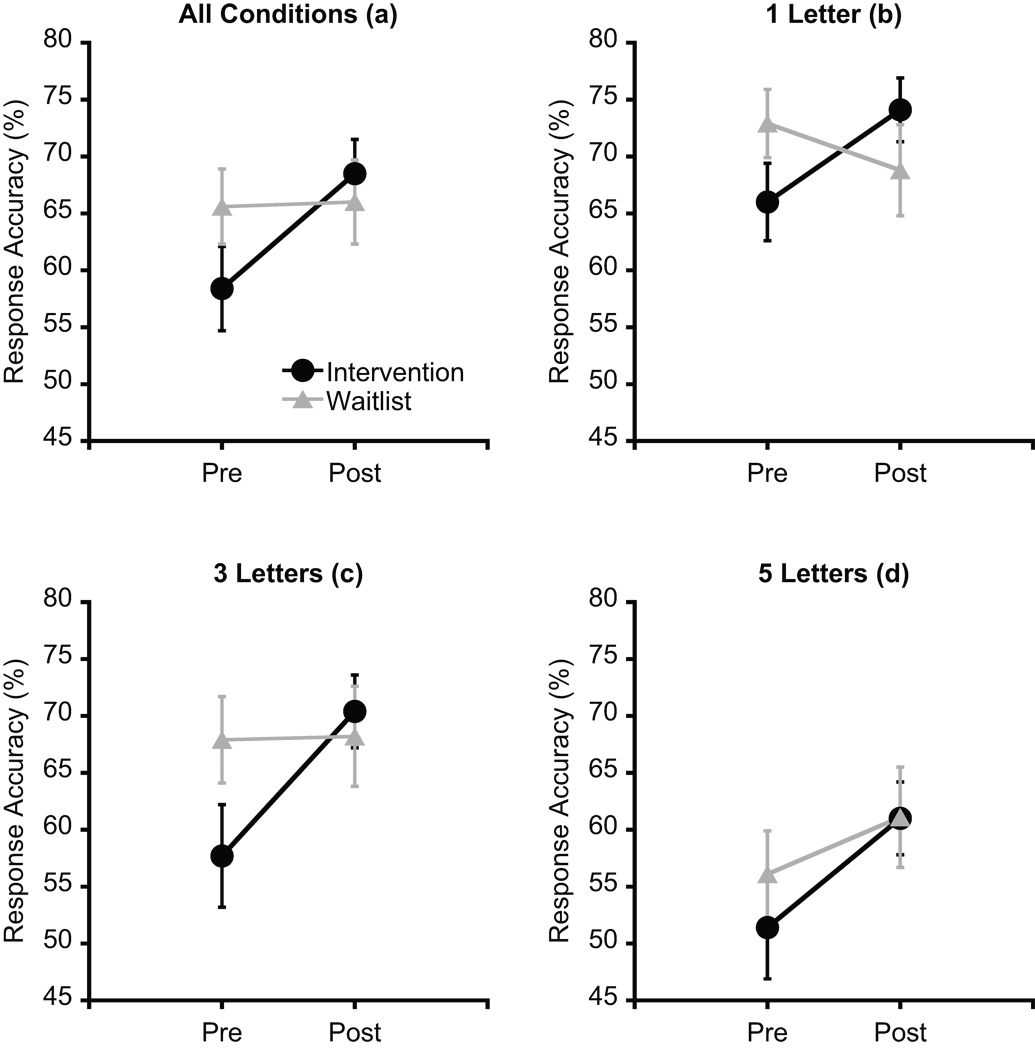
Response accuracy data are provided in Table 2 and Figure 2. Initially, preliminary t-tests were conducted to confirm that there were no significant differences in response accuracy between groups at pre-test for each letter condition. As expected, the preliminary analyses revealed no group differences for any of the letter conditions, ts(34) ≤ 1.7, ps ≥ .1. The omnibus analysis for response accuracy revealed a main effect for Time, F(1, 34) = 6.7, p = .01, η2p = .16, which was qualified by a Group × Time interaction, F(1, 34) = 5.8, p = .02, η2p = .15. Post-hoc analyses (p = .025) indicated that response accuracy at post-test was greater than at pre-test for the intervention group, t(19) = 3.6, p = .002, while no such difference was observed for the waitlist group, t(15) = 0.1, p = .9 (Figure 2a). A Set Size effect was also observed, F(2, 33) = 52.0, p < .001, η2p = .76, with follow-up Bonferroni-corrected t-tests (p = .017) indicating significant decreases in response accuracy from the one to five letter condition (one: M = 70.5%, SE = 2.0; three: M = 66.0%, SE = 2.6; five: M = 57.4%, SE = 2.4), ts(35) ≥ 4.0, ps < .001. Further, marginally significant Time × Set Size, F(2, 33) = 2.9, p = .07, η2p = .15, and Group × Time × Set Size, F(2, 33) = 2.9, p = .07, η2p = .15, interactions were observed.
Table 2.
Mean (SD) values for response accuracy data (%)
| Set size | Time | Intervention | Waitlist |
|---|---|---|---|
| All conditions | Pre | 58.4 (16.6)a | 65.6 (13.1) |
| Post | 68.5 (13.3)a | 66.0 (15.0) | |
| 1 Letter | Pre | 66.0 (15.4) | 72.9 (12.0) |
| Post | 74.1 (12.6) | 68.8 (15.8) | |
| 3 Letters | Pre | 57.7 (20.1)a | 67.9 (15.2) |
| Post | 70.4 (14.2)a | 68.2 (17.7) | |
| 5 Letters | Pre | 51.4 (18.3) | 56.1 (13.4) |
| Post | 61.0 (15.9) | 61.1 (14.1) |
Notes These data are sample values for participants including in the primary task performance analysis (n = 36). The set size factor was collapsed all conditions. Significant difference, Bonferroni-corrected post-hoc comparisons between pre- and post-test:
p < .025.
Figure 2.
Mean response accuracy at pre- and post-test across the physical activity intervention and waitlist control groups. The set size factor was collapsed all conditions (a). These data are sample values for participants including in the primary task performance analysis (n = 36). Error bars indicate SEs.
Based on our a priori hypothesis concerning selectively greater fitness effects for task components necessitating the upregulation of cognitive control, planned comparisons (Group × Time mixed-model MANOVAs for each letter condition) were conducted to examine the effect of cardiorespiratory fitness change on set size. For the one letter condition, the analysis revealed a Group × Time interaction, F(1, 34) = 6.7, p = .01, η2p = .17. However, Bonferroni-corrected post-hoc t-tests (p = .025) indicated no differences between pre- and post-test for both the intervention, t(19) = 2.4, p = .03, Cohen’s d = 0.57, and the waitlist group, t(15) = 1.3, p = .2, Cohen’s d = −0.29. For the three letter condition, the analysis revealed a main effect for Time, F(1, 34) = 6.6, p = .02, η2p = .16, which was qualified by a Group × Time interaction, F(1, 34) = 6.0, p = .02, η2p = .15. Bonferroni-corrected post-hoc t-tests (p = .025) indicated that response accuracy at post-test was greater than at pre-test for the intervention group, t(19) = 3.6, p = .002, Cohen’s d = 0.73, while no such difference was observed for the waitlist group, t(15) = 0.1, p = .9, Cohen’s d = 0.02 (Figure 2c). For the five letter condition, the analysis revealed a Time main effect, F(1, 34) = 10.8, p = .002, η2p = .24, with greater response accuracy at post-test compared to at pre-test (Figure 2d).
RT analysis revealed main effects for Time, F(1, 34) = 9.5, p = .004, η2p = .22, with shorter RT at post-test (M = 1257.3 ms, SE = 47.3) compared to at pre-test (M = 1376.9 ms, SE = 46.1), and Set Size, F(2, 33) = 87.6, p < .001, η2p = .84, with follow-up Bonferroni-corrected t-tests (p = .017) indicating significantly longer RTs for three and five letter conditions compared to the one letter condition (one: M = 1186.4 ms, SE = 45.0; three: M = 1387.7 ms, SE = 42.5; five: M = 1377.1 ms, SE = 42.7), t’s(35) ≥ 9.9, p’s < .001. There were no significant main effects or interactions involving the Group factor.
Task performance (subset of participants included in the CNV analyses)
Given that 10 participants were discarded from the CNV analyses due to poor signal quality, further task performance analyses were conducted on the participants who were included in the CNV analyses (intervention = 12, waitlist = 14) to confirm the results of the larger sample. Overall, the results for the subset of participants included in the CNV analysis were similar to those conducted for all participants.
Preliminary t-tests to compare response accuracy between groups at pre-test revealed no group differences for any of the letter conditions, ts(24) ≤ 1.1, ps ≥ .3. The omnibus analysis for response accuracy revealed a Time effect, F(1, 24) = 7.2, p = .01, η2p = .23, with greater response accuracy at post-test (M = 71.2%, SE = 2.4) compared to at pre-test (M = 65.9%, SE = 2.8). A Set Size effect was also significant, F(2, 23) = 37.0, p < .001, η2p = .76, with follow-up Bonferroni-corrected t-tests (p = .017) indicating significant decreases in response accuracy from the one to five letter condition (one: M = 73.9%, SE = 2.2; three: M = 69.4%, SE = 2.9; five: M = 62.2%, SE = 2.5), ts(25) ≥ 3.2, ps ≤ .003. Further, a marginal Group × Time interaction was observed, F(1, 24) = 3.9, p = .06, η2p = .14.
Similar to the analyses for all participants, planned comparisons were conducted. A Group × Time mixed-model MANOVA for the one letter condition revealed their interaction, F(1, 24) = 4.5, p = .04, η2p = .16. However, Bonferroni-corrected post-hoc t-tests (p = .025) indicated no difference between pre- and post-test for both the intervention, t(13) = 2.2, p = .05, Cohen’s d = 0.60, and the waitlist group, t(11) = 0.8, p = .4, Cohen’s d = −0.22. For the three letter condition, the analysis revealed a main effect for Time, F(1, 24) = 6.5, p = .02, η2p = .21, which was qualified by a Group × Time interaction, F(1, 24) = 4.2, p = .05, η2p = .15. Bonferroni-corrected post-hoc t-tests (p = .025) indicated that response accuracy at post-test was greater than at pre-test for the intervention group, t(11) = 3.2, p = .009, Cohen’s d = 0.65, while no such difference was observed for the waitlist group, t(13) = 0.4, p = .7, Cohen’s d = 0.09. For the five letter condition, the analysis revealed a Time main effect, F(1, 24) = 9.1, p = .006, η2p = .28, with greater response accuracy at post-test compared to at pre-test.
RT analysis revealed effects for Time, F(1, 24) = 4.7, p = .04, η2p = .16, with shorter RT at post-test (M = 1242.1 ms, SE = 54.1) compared to pre-test (M = 1343.9 ms, SE = 52.9), and Set Size, F(2, 23) = 56.0, p < .001, η2p = .83, with follow-up Bonferroni-corrected t-tests (p = .017) indicating significantly longer RTs for three and five letter conditions compared to the one letter condition (one: M = 1153.9 ms, SE = 50.7; three: M = 1363.4 ms, SE = 50.2; five: M = 1361.7 ms, SE = 47.0), ts(25) ≥ 8.9, ps < .001. There were no significant main effects or interactions involving the Group factor.
CNV
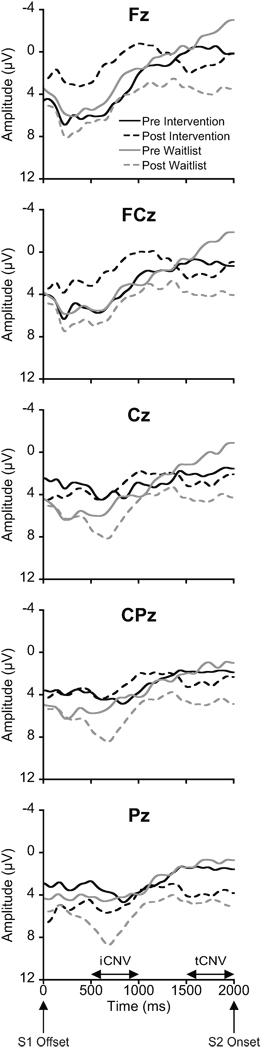
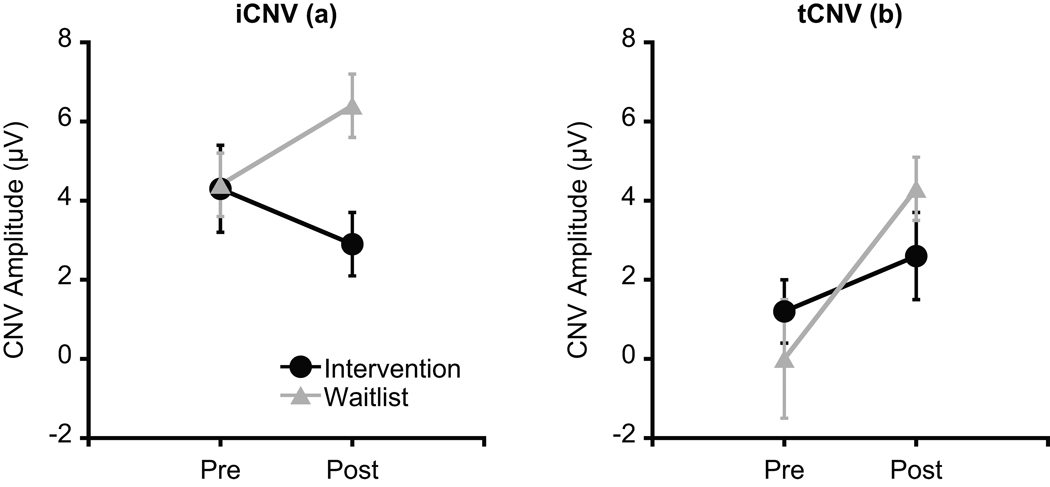
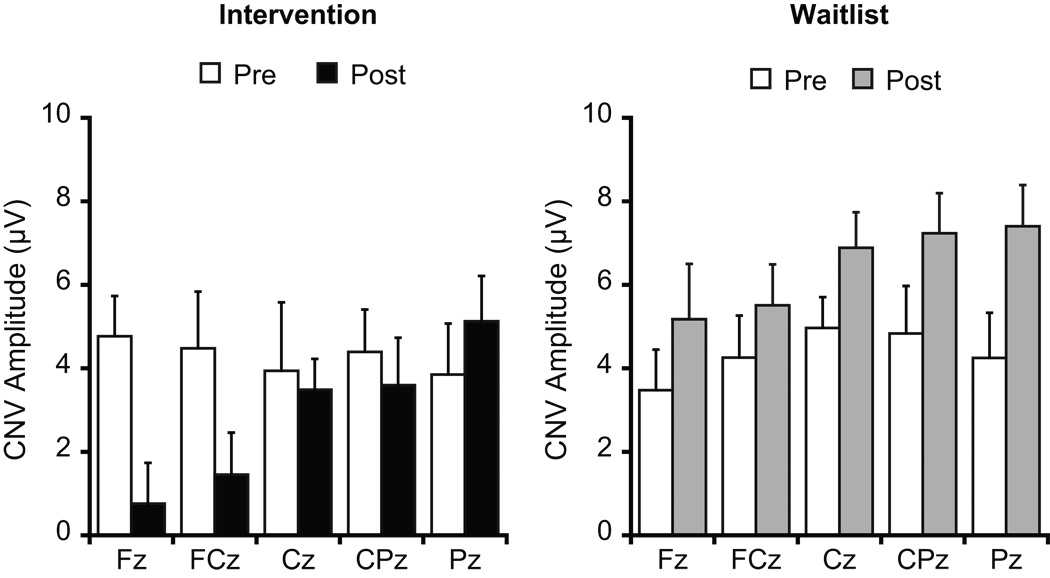
Figure 3 illustrates grand averaged ERP waveforms at pre- and post-test across the intervention and waitlist groups as a function of electrode site. Analysis of iCNV amplitude revealed a significant Group × Time interaction, F(1, 24) = 8.2, p = .01, η2p = .26. Post-hoc analyses (p = .025) indicated that iCNV amplitude did not differ between groups at pre-test, t(24) = 0.1, p = .96, whereas iCNV amplitude for the intervention group was significantly larger than the waitlist group at post-test, t(24) = 3.1, p = .01 (Figure 4a). A Time × Site interaction was also significant, F(4, 21) = 5.0, p = .01, η2p = .49. Post-hoc Bonferroni-corrected t-tests (p = .005) indicated that iCNV amplitudes did not differ among electrode sites at pre-test, ts(25) ≤ 1.1, ps ≥ .27, whereas iCNV amplitude at FCz was larger than at Cz, CPz, and Pz at post-test, ts(25) ≥ 3.4, ps ≤ .003. We hypothesized that the effects of the physical activity intervention on CNV would be disproportionately larger over fronto-central electrode sites. Based on our a priori hypothesis, planned comparisons were conducted to examine whether physical activity training effects (i.e. group differences in pre–post change score) differ by electrode sites. The Bonferroni-corrected planned comparisons (p = .01) revealed that the pre–post change score for iCNV amplitude for the intervention group was significantly larger than the waitlist group at Fz, t(24) = 3.3, p = .003, whereas no such group differences were observed at the other electrode sites, ts(24) ≤ 2.5, ps ≥ .02 (Figure 5).
Figure 3.
Grand averaged ERP waveforms at pre- and post-test across the physical activity intervention and waitlist control groups as a function of electrode site.
Figure 4.
Mean (a) iCNV and (b) tCNV amplitudes at pre- and post-test across the physical activity intervention and waitlist control groups. Error bars indicate SEs.
Figure 5.
Mean iCNV amplitudes at pre- and post-test for each group as a function of electrode site. Error bars indicate SEs.
Analysis of tCNV amplitude revealed a main effect for Time, F(1, 24) = 10.2, p = .004, η2p = .30, with smaller tCNV amplitude at post-test compared to at pre-test (Figure 4b). No other significant main effects or interactions were observed. Similar to iCNV, a priori planned comparisons (p = .01) were performed, and indicated that pre–post change scores of tCNV amplitude did not differ between the intervention and waitlist groups at any electrode site, ts(24) ≤ 1.8, ps ≥ .09.
Discussion
Task performance
We examined whether a physical activity program designed to promote increases in cardiorespiratory fitness improved cognitive control in preadolescent children using a randomized control design. A modified Sternberg task (Sternberg, 1966) was used to manipulate working memory demands, with variable set sizes requiring the upregulation of cognitive control. The main findings indicated that increases in cardiorespiratory fitness resulting from the physical activity intervention led to improvements in overall response accuracy, with no such effect observed for the waitlist group. More importantly, analyses revealed that the beneficial effects of fitness on task performance differed based on working memory demands. Specifically, planned comparisons indicated that the physical activity intervention improved response accuracy for the one and three letter conditions, with greater effects for the three letter condition. However, no such effect was observed for the five letter condition. These results, on the surface, appear to contradict our hypothesis suggesting greater improvement for task conditions placing greater demands upon working memory.
Specifically, the planned comparisons did not reveal an improvement in response accuracy for the largest set size (i.e. five letter condition) as a function of enhanced cardiorespiratory fitness. One possible explanation for this lack of improvement is that the largest set size was too difficult for preadolescent children. RTs did not differ between three and five letter conditions, while response accuracy decreased from the one to five letter conditions in the expected fashion. These results imply that preadolescent participants might not be able to effectively withhold their response to take the time necessary to ensure accurate performance for the largest set size due to their propensity to respond impulsively (Davidson et al., 2006). Thus, the task difficulty associated with the largest set size, paired with their impulsive response tendency, might account for the lack of improvement in task performance following the physical activity intervention. In other words, response accuracy for the five letter condition may not accurately reflect an increase in cognitive control demands for children. In addition, a marginal interaction of set size and time suggests that practice effects and/or developmental effects were greater for the larger set sizes. Indeed, this interaction for the waitlist group, which allows for comparison of the practice effects and/or developmental effects based on the set size without interference from a change in fitness status, revealed a significant relationship with the largest pre–post change score for the five letter condition (see Figure 2). Accordingly, it is speculated that greater practice and/or developmental effects for the five letter condition may have blurred fitness-related improvement in response accuracy.
In the one versus the three letter condition, it would appear that a beneficial effect of the physical activity intervention was disproportionately greater for the larger set size. Although the planned comparison indicated that group interacted with time for the one letter condition, post-hoc analyses indicated no difference between pre- and post-test for either group. By contrast, for the three letter condition, post-hoc analyses revealed significant improvements in response accuracy for the intervention group, and the effect size for the three letter condition was larger than for the one letter condition. No such relationship was observed for the waitlist group. Thus, this pattern of results might support our a priori hypothesis that greater improvement would be observed during task conditions requiring the upregulation of cognitive control.
If this interpretation is correct, the disproportionate effect of physical activity training on response accuracy as a function of the differential set sizes may be explained from a neural developmental perspective. A recent functional magnetic resonance imaging (fMRI) study demonstrated that adults recruited right dorsolateral prefrontal cortex (DLPFC) and superior parietal cortex for task conditions requiring greater amounts of cognitive control during the delay period in a working memory task, whereas children (8–12 years) failed to recruit these regions (Crone, Wendelken, Donohue, Leijenhorst & Bunge, 2006). Such differences in recruitment were associated with poorer behavioral adaptation to the task manipulation in children (Crone et al., 2006), and suggest that immaturity of the fronto-parietal network including DLPFC and superior parietal cortex may be associated with inferior cognitive control performance during working memory demands in children. These findings are well founded given that the DLPFC matures later in the development of the frontal cortex (Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent, Herman, Clasen, Toga, Rapoport & Thompson, 2004), implying that poorer adaptation to cognitive control requirements in children may be related to immaturity of the neural network underlying working memory. The current findings suggest that physical activity training may improve the effectiveness of the fronto-parietal network in support of working memory, as evidenced by selectively better performance at post-test only for the intervention group. Such a finding is especially interesting given the protracted maturation of this neural network during preadolescent development.
CNV
The current CNV findings support the observed improvements in response accuracy, as the intervention group exhibited larger iCNV amplitude at post-test compared to the waitlist group, with the greatest effect observed at the frontal site. It has been suggested that the frontal CNV reflects cognitive preparation processes rather than response preparation processes (Falkenstein et al., 2003; Leynes et al., 1998; Lorist et al., 2000; Wild-Wall et al., 2007). Although the CNV has multiple generators located in frontal, premotor, and sensory areas, iCNV might be primarily generated in the frontal areas (Cui, Egkher, Huter, Lang, Lindinger & Deecke, 2000; Hamano, Luders, Ikeda, Collura, Comair & Shibasaki, 1997). Developmental studies have indicated that children have smaller iCNV relative to adults at fronto-central locations (Jonkman et al., 2003; Segalowitz et al., 1992). Jonkman et al. (2003) suggested that, using an inhibitory control task, the smaller iCNV at fronto-central locations in children might reflect incomplete development of the frontal lobes, resulting in ineffective cognitive control. From these findings, it is plausible that the larger frontal iCNV amplitude at post-test for the intervention group may indicate more effective cognitive control, which in turn may underlie the production of more accurate task performance.
Interestingly, the effect of the physical activity intervention was observed for the iCNV, but not for the tCNV. This selective effect suggests that changes in cognitive strategy might underlie more effective cognitive control for the intervention group. Braver, Gray and Burgess (2007) proposed the dual mechanisms of control (DMC) theory, in which cognitive control operates along two distinct strategies referred to as proactive control and reactive control. Proactive control is characterized by future-oriented early selection, which is associated with sustained or preparatory lateral PFC activation prior to the imperative stimulus to actively maintain goal-relevant information (Braver et al., 2007). By contrast, reactive control is characterized by past-oriented late correction, which is associated with transient activation of lateral PFC and other brain regions such as anterior cingulated cortex (ACC) to reactivate task goals only as needed (Braver et al., 2007). Speer, Jacoby and Braver (2003) manipulated participants’ strategy (i.e. proactive or reactive) during the Sternberg task, indicating that the proactive control strategy resulted in greater response accuracy and sustained activation of DLPFC during the encoding and delay periods compared to the reactive control strategy. By contrast, activation of anterior PFC, which is associated with episodic retrieval processes (Lepage, Ghaffar, Nyberg & Tulving, 2000), was transiently greater for the reactive, relative to proactive, control strategy only during the period of prove judgment. From these findings, proactive control, which results in sustained active maintenance of task goals, is considered to be a more effective strategy compared to reactive control, at least in the context of a Sternberg task. Thus, according to the DMC theory, it is plausible that the selective increases in iCNV at post-test for the intervention group may indicate a strategic change from reactive to proactive control, which may be associated with a more effective working memory network.
This speculation regarding shifts in cognitive strategy is consistent with previous studies examining the relation of fitness to action monitoring processes using the error-related negativity (ERN) in preadolescent children (Hillman et al., 2009; Pontifex et al., in press). These previous studies indicated that more-fit children exhibited smaller ERN amplitude with greater post-error response accuracy compared to their less-fit counterparts during a modified flanker task (Hillman et al., 2009; Pontifex et al., in press). It has been well established that the ERN is an indicator of action monitoring, with a neural source located in the dorsal ACC (Carter, Braver, Barch, Botvinick, Noll & Cohen, 1998; Dehaene, Posner & Tucker, 1994; Miltner, Lemke, Weiss, Holroyd, Scheffers & Coles, 2003; van Veen & Carter, 2002). According to the DMC theory, when reactive control is dominant, relatively strong transient activation of the ACC should be reflected in an increase in ERN. By contrast, smaller ERN amplitudes in more-fit children may indicate a relative decrease in ACC activation due to a proactive control strategy, which would likely included decreased reliance upon action monitoring (Hillman et al., 2009; Pontifex et al., in press). Taken together, it is speculated that more fitness may be associated with a shift away from reactive control toward the adoption of a proactive strategy, resulting in more effective task preparation (i.e. larger iCNV) and more efficient action monitoring (i.e. smaller ERN) during the cognitive control task.
In contrast to the iCNV, tCNV amplitude decreased at post-test relative to pre-test across both groups, suggesting that smaller tCNV amplitudes may reflect more efficient task preparation (Casini & Macar, 1996; Hillman Weiss, Hagberg & Hatfield, 2002; Kamijo et al., 2010). Further, smaller tCNV at post-test was observed across all midline electrode sites. From these findings, it is plausible that the smaller tCNV at post-test reflects more efficient response preparation due to practice or learning as a result of repeated exposure to the task. Indeed, RT also decreased at post-test across both groups. Although previous studies indicated a positive relation of cardiorespiratory fitness to response preparation processes in adult populations (Hillman et al., 2002; Kamijo et al., 2010; Kamijo & Takeda, 2009), the relationship is more robust for cognitive control processes (Kamijo et al., 2010). Thus, the present CNV findings provide support for Kamijo et al. (2010), as cardiorespiratory fitness is more closely related to cognitive control processes relative to response preparation processes.
Limitations
Although the present study provides causal evidence for a relationship between fitness and cognitive control using a randomized control intervention, several limitations should be acknowledged. First, the relatively small sample size in the present study might incidentally cause numerical, but non-significant, group differences in the collected measures. The numerically lower response accuracy for the intervention group at pre-test holds out the possibility that improvement in response accuracy may reflect ‘regression toward the mean’ at post-test due to practice effects and/or developmental effects. However, because VO2max and response accuracy showed similar changes from pre- to post-test (see Figures 1, 2), it is plausible that the disproportionately larger improvements in response accuracy for the intervention group may be related to the disproportionate enhancement in cardiorespiratory fitness rather than regression toward the mean.2 Second, CNV could not be averaged separately based on the encoded set size. Although the selective effects of the physical activity intervention on the iCNV may indicate more effective cognitive control as discussed above, the current CNV findings cannot support the disproportionate effect of physical activity training on response accuracy based on set size. Thus, further studies with larger sample sizes and a greater number of trials are needed to elucidate the disproportionate effects based on working memory demands. Third, some have argued that the Sternberg task requires short-term memory rather than working memory (Klein et al., 2010; Schooler et al., 2008). During the Sternberg task, error rate and RT increase during conditions in which a probe letter matches a letter from the previous trial’s memory set (D’Esposito, Postle, Jonides & Smith, 1999). That is, it is plausible that this task requires manipulation of inhibitory control to respond effectively, indicating that cognitive control is necessary for successful task completion. Regardless, a theoretical account of the cognitive processes elicited by the Sternberg task is beyond the scope of the present study. Less controversial is the contention that larger set sizes require greater amounts of cognitive control to orchestrate information for more accurate and quicker responses, and that physical activity training is beneficial to these processes.
In addition, limitations exist with the use of a waitlist control group. The effects of physical activity on cognitive function can interact with other lifestyle factors such as social interaction and diet. In the present study, children in the intervention group had social interaction with exercise personnel and each other during the physical activity training. However, because children in both groups engaged in general school activities (i.e. were recruited from within the same schools), the effects of social interaction might be relatively small compared to similar studies with older adults. Next, children in the intervention group received a healthy snack. Although nutritional supplementation can influence cognitive development, the findings remain controversial (Bryan, Osendarp, Hughes, Calvaresi, Baghurst & van Klinken, 2004), and the snack provided to participants in the intervention group did not comprise the majority of their daily caloric intake. Further, SES, which impacts nutritional status (Bradley & Corwyn, 2002), was not different between groups (see Table 1). Thus, the possibility of the effects of supplementation for the intervention group on cognitive function cannot be denied, but are likely negligible. Lastly, although the physical activity program was designed to enhance cardiorespiratory fitness, several kinds of activity (e.g. small-area games, motor skill practices, etc.) were needed to keep children engaged during the 9-month duration. Although unknown, other factors (i.e. rule-based exercises, strength training, etc.) might influence children’s cognitive function as well.
Summary
In summary, physical activity training improved working memory in preadolescent children, as reflected by greater response accuracy and larger iCNV. Working memory ability is a crucial function of classroom activities that form the basis for learning, and thereby has been related to scholastic performance (Alloway et al., 2005; Gathercole et al., 2004; Silva-Pereyra et al., 2001). Thus, the present results support previous findings indicating a positive relation of cardiorespiratory fitness to academic achievement (Castelli et al., 2007; Coe et al., 2006). The present study indicates that regular physical activity leading to increases in cardiorespiratory fitness is closely associated with cognitive and brain health and may shape cognitive development in preadolescent children.
Acknowledgements
Support for our research and the preparation of this manuscript were provided by the Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowships for Research Abroad to Keita Kamijo, and grants from the National Institute of Child Health and Human Development (NICHD R01 HD055352) to Charles Hillman.
Footnotes
Although a few participants had missing data for VO2max (n = 1) and the Tanner Staging System (n = 2), they were included in the analyses. To confirm our results, we further conducted all analyses without these three participants. The results remained unchanged from those including the three participants.
To support our contention that greater response accuracy for the intervention group at post-test indicates physical activity-based improvements in task performance rather than regression toward the mean, additional analyses were conducted. We systematically removed one, two, three, four, and five participants from the intervention group beginning with the worst performer and reran the analysis after each participant was removed. Overall, statistical analyses for the subset discarding the one and two worst performing participants indicate similar findings to those conducted for all participants, which is reported in the text. Beyond that point, the effects of the physical activity intervention disappeared gradually due to an increasingly smaller sample size. However, even when the mean response accuracy at pre-test was equivalent between groups after removing the five worst performing participants (intervention: M = 65.0%, SE = 3.5; waitlist: M = 65.6%, SE = 3.3), response accuracy for the intervention group was still greater at post-test than at pre-test for the three letter condition, t(14) = 2.3, p = .04 (i.e. uncorrected t-test). Accordingly, greater response accuracy for the intervention group at post-test does not appear to indicate regression toward the mean, but rather physical activity-related improvements in task performance.
References
- Alloway TP, Gathercole SE, Adams AM, Willis C, Eaglen R, Lamont E. Working memory and phonological awareness as predictors of progress towards early learning goals at school entry. British Journal of Developmental Psychology. 2005;23:417–426. [Google Scholar]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7th edn. New York: Lippincott Williams & Wilkins; 2006. [DOI] [PubMed] [Google Scholar]
- Bar-Or O. Pediatric sports medicine for the practitioner: From physiologic principles to clinical applications. New York: Springer-Verlag; 1983. [Google Scholar]
- Bender S, Weisbrod M, Just U, Pfuller U, Parzer P, Resch F, Oelkers-Ax R. Lack of age-dependent development of the contingent negative variation (CNV) in migraine children? Cephalalgia. 2002;22:132–136. doi: 10.1046/j.1468-2982.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum AS, Lytle LA, Murray DM, Story M, Perry CL, Boutelle KN. Survey development for assessing correlates of young adolescents’ eating. American Journal of Health Behavior. 2002;26:284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken JW. Nutrients for cognitive development in school-aged children. Nutrition Reviews. 2004;62:295–306. doi: 10.1111/j.1753-4887.2004.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulated cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casini L, Macar F. Can the level of prefrontal activity provide an index of performance in humans? Neuroscience Letters. 1996;219:71–74. doi: 10.1016/s0304-3940(96)13188-8. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. Journal of Sport and Exercise Psychology. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The association between school-based physical activity, including physical education, and academic performance. Atlanta, GA: US Department of Health and Human Services; 2010. [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. American Journal of EEG Technology. 1985;25:83–92. [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Coe DP, Pivarnik JM, Womack CJ, Reeves MJ, Malina RM. Effect of physical education and activity levels on academic achievement in children. Medicine and Science in Sports and Exercise. 2006;38:1515–1519. doi: 10.1249/01.mss.0000227537.13175.1b. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui RQ, Egkher A, Huter D, Lang W, Lindinger G, Deecke L. High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clinical Neurophysiology. 2000;111:1847–1859. doi: 10.1016/s1388-2457(00)00388-6. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Department of Health and Human Services, & Department of Education. Promoting better health for young people through physical activity and sports. A Report to the President from the Secretary of Health and Human Services and the Secretary of Education. Silver Spring, MD: Centers for Disease Control; 2000. [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. New York: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York: The Guilford Press; 1998. [Google Scholar]
- Eriksen CW, Eriksen BA. Effects of noise letters upon the identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J, Kleinsorge T. Short-term mobilization of processing resources is revealed in the event-related potential. Psychophysiology. 2003;40:914–923. doi: 10.1111/1469-8986.00109. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Goodman TL. Measurement of oxygen consumption. In: Rowland TW, editor. Pediatric laboratory exercise testing: Clinical guidelines. Champaign, IL: Human Kinetics; 1993. pp. 91–113. [Google Scholar]
- Gathercole SE, Pickering SJ, Knight C, Stegmann Z. Working memory skills and educational attainment: evidence from national curriculum assessments at 7 and 14 years of age. Applied Cognitive Psychology. 2004;18:1–16. [Google Scholar]
- Goffaux P, Phillips NA, Sinai M, Pushkar D. Behavioural and electrophysiological measures of task switching during single and mixed-task conditions. Biological Psychology. 2006;72:278–290. doi: 10.1016/j.biopsycho.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T, Luders HO, Ikeda A, Collura TF, Comair YG, Shibasaki H. The cortical generators of the contingent negative variation in humans: a study with subdural electrodes. Electroencephalography and Clinical Neurophysiology. 1997;104:257–268. doi: 10.1016/s0168-5597(97)96107-4. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: event-related brain potential and task performance indices of executive control in preadolescent children. Developmental Psychology. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Medicine and Science in Sports and Exercise. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Weiss EP, Hagberg JM, Hatfield BD. The relationship of age and cardiovascular fitness to cognitive and motor processes. Psychophysiology. 2002;39:303–312. doi: 10.1017/s0048577201393058. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Lansbergen M, Stauder JE. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Kamijo K, O’Leary KC, Pontifex MB, Themanson JR, Hillman CH. The relation of aerobic fitness to neuroelectric indices of cognitive and motor task preparation. Psychophysiology. 2010;47:814–821. doi: 10.1111/j.1469-8986.2010.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Takeda Y. General physical activity levels influence positive and negative priming effects in young adults. Clinical Neurophysiology. 2009;120:511–519. doi: 10.1016/j.clinph.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Klein C, Rauh R, Biscaldi M. Cognitive correlates of anti-saccade task performance. Experimental Brain Research. 2010;203:759–764. doi: 10.1007/s00221-010-2276-5. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leynes PA, Allen JD, Marsh RL. Topographic differences in CNV amplitude reflect different preparatory processes. International Journal of Psychophysiology. 1998;31:33–44. doi: 10.1016/s0167-8760(98)00032-4. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Klein M, Nieuwenhuis S, De Jong R, Mulder G, Meijman TF. Mental fatigue and task control: planning and preparation. Psychophysiology. 2000;37:614–625. [PubMed] [Google Scholar]
- Loveless NE, Sanford AJ. Slow potential correlates of preparatory set. Biological Psychology. 1974;1:303–314. doi: 10.1016/0301-0511(74)90005-2. [DOI] [PubMed] [Google Scholar]
- Marshall L, Molle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neuroscience. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH. Implementation of error-processing in the human anterior cingulated cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biological Psychology. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Perchet C, Garcia-Larrea L. Learning to react: anticipatory mechanisms in children and adults during a visuospatial attention task. Clinical Neurophysiology. 2005;116:1906–1917. doi: 10.1016/j.clinph.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, Hillman CH. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21528. (in press) [DOI] [PubMed] [Google Scholar]
- Schooler C, Caplan LJ, Revell AJ, Salazar AM, Grafman J. Brain lesion and memory functioning: short-term memory deficit is independent of lesion location. Psychonomic Bulletin and Review. 2008;15:521–527. doi: 10.3758/pbr.15.3.521. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain and Cognition. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Unsal A, Dywan J. Cleverness and wisdom in 12-year-olds: electrophysiological evidence for late maturation of the frontal lobe. Developmental Neuropsychology. 1992;8:279–298. [Google Scholar]
- Silva-Pereyra J, Fernández T, Harmony T, Bernal J, Galán L, Díaz-Comas L, Fernández-Bouzas A, Yáñez G, Rivera-Gaxiola M, Rodríguez M, Marosi E. Delayed P300 during Sternberg and color discrimination tasks in poor readers. International Journal of Psychophysiology. 2001;40:17–32. doi: 10.1016/s0167-8760(00)00123-9. [DOI] [PubMed] [Google Scholar]
- Speer NK, Jacoby LL, Braver TS. Strategy-dependent changes in memory: effects on behavior and brain activity. Cognitive, Affective and Behavioral Neuroscience. 2003;3:155–167. doi: 10.3758/cabn.3.3.155. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Pediatric and Perinatal Epidemiology. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Canadian Journal of Sport Sciences. 1992;17:338–345. [PubMed] [Google Scholar]
- Utter AC, Robertson RJ, Nieman DC, Kang J. Children’s OMNI Scale of Perceived Exertion: walking/running evaluation. Medicine and Science in Sports and Exercise. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulated cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Weerts TC, Lang PJ. The effects of eye fixation and stimulus and response location on the contingent negative variation (CNV) Biological Psychology. 1973;1:1–19. doi: 10.1016/0301-0511(73)90010-0. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Hohnsbein J, Falkenstein M. Effects of ageing on cognitive task preparation as reflected by event-related potentials. Clinical Neurophysiology. 2007;118:558–569. doi: 10.1016/j.clinph.2006.09.005. [DOI] [PubMed] [Google Scholar]