Abstract
The skeletal muscle extracellular matrix (ECM) plays an important role in muscle fiber force transmission, maintenance, and repair. In both injured and diseased states, ECM adapts dramatically, a property thathas clinical manifestations and alters muscle function. Here, we review the structure, composition, and mechanical properties of skeletal muscle ECM, describe the cells that contribute to the maintenance of the ECM and, finally, overview changes that occur with pathology. New scanning electron micrographs of ECM structure are also presented with hypotheses about ECM structure-function relationships. Detailed structure-function relationships of the ECM have yet to be defined and, as a result, we propose areas for future studies.
Keywords: Skeletal muscle, extracellular matrix, collagen, biomechanics, fibrosis
Introduction
Skeletal muscles are composed primarily of contractile material. Thus, it is no surprise that the vast majority of skeletal muscle studies focus on its contractile properties. However, because muscle is a composite tissue of connective tissue, blood vessels, and nerves as well as contractile material, these “minor” tissues (in terms of relative mass) may strongly influence muscle function. In this review, we focus on the skeletal muscle extracellular matrix (ECM), because there is a growing body of evidence indicating that ECM strongly affects muscle’s normal function, its ability to adapt, and the biological reservoir of muscle stem cells that it provides. Specifically, recent biomechanical studies support the idea the ECM bears the majority of muscle passive load, which implies that clinical examination of patient range of motion and stiffness primarily reflect their ECM properties. In addition, while muscle pathology is typically described in terms of altered fiber type, fiber size distribution or centralized nuclei, nearly every pathological change reported in muscle is also associated to some degree with ECM fibrosis. Therefore, based on its important functional role, its consistent response to disease and injury, and its clinically significant manifestations, it is timely to review our current understanding of muscle ECM structure, function, and biology.
Skeletal Muscle ECM Structure
Unfortunately, there is a paucity of objective information about muscle ECM compared to the other connective tissues of mesenchymal origin, such as tendon, ligament, bone, and cartilage. It follows logically that much of the muscle ECM literature simply represents extrapolation of knowledge gleaned from other tissues. This is especially true in terms of understanding the functional properties of muscle ECM, primarily because the geometry of muscle ECM is tremendously complex compared to other connective tissues. Didactic presentations regarding muscle ECM often subdivide it into endomysial (around the muscle cell), perimysial (around groups of muscle cells), and epimysial (around the whole muscle) connective tissues (Fig. 1). However, direct inspection of actual skeletal muscle samples by light, transmission or scanning electron microscopy reveals that such definitive subdivisions are relatively arbitrary. Systematic studies of muscle ECM, in which the proper rules of three-dimensional sampling are followed, are lacking, resulting in a literature where sampling is typically biased according to what “appears” most prominent in tissue sections. Those studying ECM are encouraged to emulate the outstanding morphometric studies of muscle sarcoplasmic reticulum (SR) that led to our current understanding of the excitation-contraction coupling mechanism. These studies revealed that, while the SR is unimpressive in any single EM plane, reconstruction of the SR network reveals a highly ordered, extensive, and functionally critical component of muscle that would have been missed if it were simply viewed in any single traditional plane.1 It is therefore likely that a higher order organization of muscle ECM exists that is yet to be defined, and our understanding of the “three” subdivisions of ECMis of a rudimentary nature.
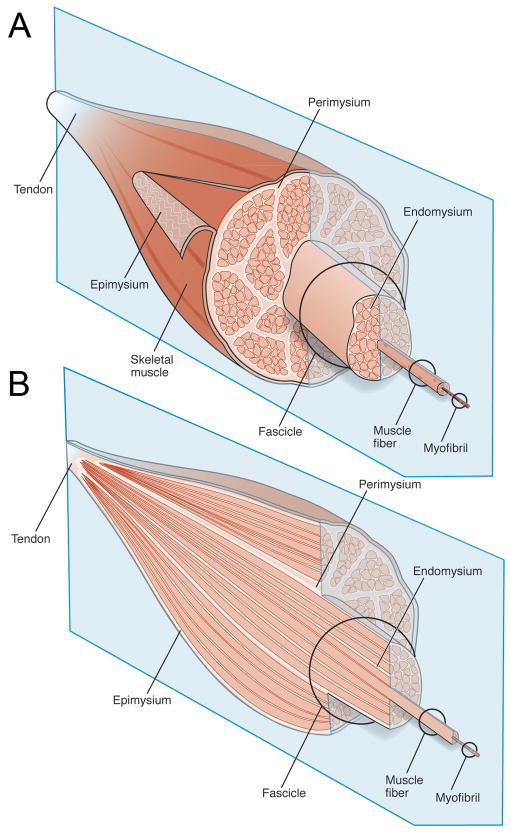
Figure 1.
Schematic diagram of the gross organization of muscle tissue and muscle ECM-tendon organization. (A) Muscle ECM can be categorized as epimysium (surrounding the muscle), perimysium (surrounding muscle fascicles), and endomysium (surrounding muscle fibers). (B) Cross-section of muscle tissue indicating that the perimysium may be continuous with the tendon, whereas endomysium is contained within muscle fascicles.
The Muscle Endomysium
An exception to the casual sampling performed in most ECM studies is the systematic and quantitative description of the muscle endomysial ECM reported for feline and bovine muscle by Purslow and Trotter.2, 3 They showed that a highly ordered network surrounds individual muscle fibers that deforms nonlinearly with increasing sarcomere length. The result is a load-bearing network whose mechanical properties reflect more the network geometry than the constitutive properties of the composite collagen fibers (except at very long lengths). The significance of this geometry is that force is most likely transmitted by shear through the endomysium. Their dramatic topographical photographs (Fig. 2) and quantitative models,3 although performed about 25 years ago, still form our basic understanding of muscle cell-endomysium structural interactions. Because other muscles have not been studied at the same level of detail, it is not clear whether this level of organizationis typical of all muscles across species or even different muscles within the same species.
Figure 2.

Scanning EM of the collagen us endomysial network around muscle fibers observed after digesting fibers with NaOH. (A) Low power overview of theendomysium reveals an array of “tubes” into which muscle fibers insert (arrows) as well as a thickened area surrounding the fibers that is presumably perimysium (arrowhead). (B) Higher power view of endomysial network revealing the fine structure of the endomysial surfaces (arrow) as well as some undigested muscle fiber (arrowhead). This picture suggests that muscle fibers are embedded in a complex connective tissue matrix and are intimately associated with ECM. Figure from reference2 used with permission.
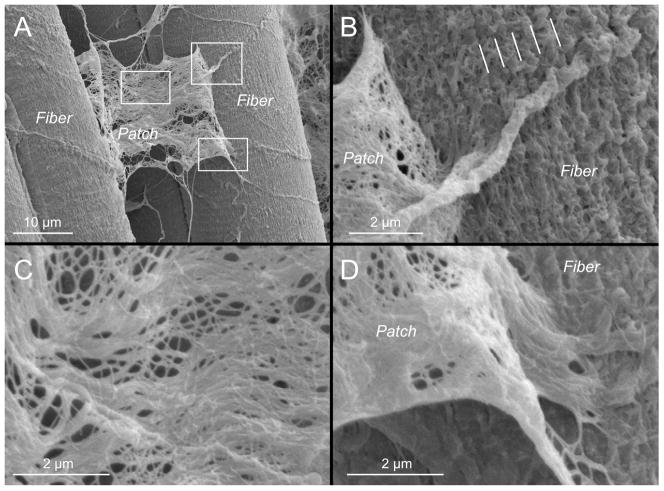
We have examined the endomysial structure of mouse extensor digitorum longus (EDL) muscles using scanning electron microscopy. When fixed at resting length, we observed discreet patches of ECM that could be lifted away from myofibers in regions where fibers were apparently pulled apart during processing (Fig. 3A). Based on the fact that the edges of this “patch” appeared to become contiguous with the muscle fiber (Figs. 3Band 3D), we suspect that this patch represents an elevated piece of muscle fiber ECM, presumably endomysium. Interestingly, on muscle fibers adjacent to this patch, endomysial ECM appeared to have a longitudinal periodicity on the myofiber surface (see lines in Fig. 3B), but no such longitudinal periodicity is obvious on the released patches (Fig. 3C). Often a large tubular extension passed over the surface of the fiber, which may represent a portion of the microcirculation or, perhaps an axon traversing the tissue (Fig. 3B).
Figure 3.
Mouse extensor digitorum longus muscles formalin-fixed at resting length, dehydrated in graded ethanol, lyophilized, and observed by SEM(same preparatory method used for Figs 4, 5, 6, and 7). (A) Patch of ECM pulled away from muscle fibers during sample preparation. Each white rectangle is enlarged in subsequent figure components. (B) A regular, longitudinal organization of ECM structures is observable on the fiber surface and its periodicity is noted with lines. (C) Central region of the patch of ECM pulled away from muscle fiber surface showing the wavy collagen fiber organization and collagen fibril network. (D) Apparent connection between the ECM patch pulled away and the muscle fiber surface. The patch appears to be continuous with the ECM on the fiber surface.
The Muscle Perimysium
Muscle perimysial studies are much more sparse, variable and less defined compared to the studies described above for the endomysium, primarily because there is no strict definition of perimysium. Light micrographic cross-sections reveal thickened ECM that “surrounds” bundles of muscle cells. Thus, this “thicker” ECM region is often considered a distinct entity simply because it presents a more visually obvious pattern; its higher order arrangement (if any) is not known. For example, it is not known whether perimysium surrounds a bundle of fibers from origin to insertion analogous to a telephone cable or whether it is interconnected across the muscle belly similar to the endomysial network. Addressing this issue has profound implications for understanding the normal and pathological properties of the muscle perimysium and has not previously been addressed systematically.
Surface topology of the perimysium suggests that, unlike the endomysium, perimysial collagen fibers are organized into more-or-less discrete populations that extend along and across muscle fibers.4–7 This can be observed on micrographs where longitudinally oriented fibers form a dense series of bands along fibers while transverse collagen fibers interconnect muscle fibers at discrete points (“Perimysial Plate,” Fig. 4).
Figure 4.
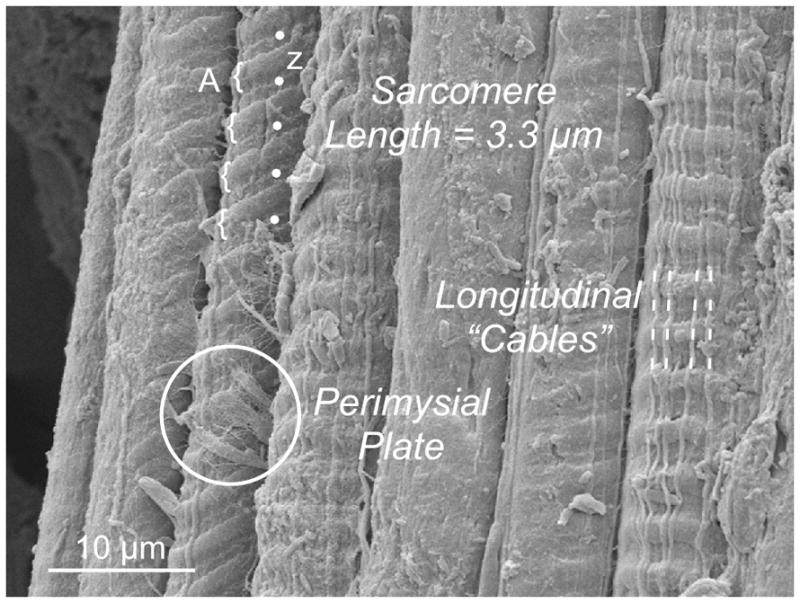
Scanning electron micrograph of 7 adjacent muscle fibers. Note that the surface topology varies among the fibers. In 5 cases, surface striations are visible, some of which clearly show A-band (curved brackets) and Z-band periodicity (dots). In 3 fibers, stout longitudinal “cables” extend a distance of at least 100 μm. In one case, a discrete connection is seen on the surface of a fiber that presumably represents the “perimysial plate” (circled) that connects adjacent fibers. Micrograph was obtained from a mouse EDL muscle stretched to a sarcomere length of 3.3 μm.
Intriguingly, it has been reported that these transverse connection points are co localized with focal adhesions and intracellular subdomains,7, 8 suggesting that the perimysium may be involved in cellular signaling. The degree of intimacy between the muscle cell and the perimysium can be appreciated based on the fact that cellular landmarks such as Z-bands and A-bands are visualized at the fiber surface (Fig. 4). However, it is not clear to what extent this arrangement is typical of all muscle fibers, and no data exist for human muscle. In the context of our understanding of normal muscle physiology, it would be interesting to determine whether perimysial arrangements vary among muscles of different functions, such as those that are chronically active, perform antigravity functions, operate at long lengths, or are involved in rapid movements. In the same way that muscle fiber type has come to be understood in the context of muscle’s physiological function,9 it is likely that some degree of ECM customization exists across muscles of different function. We frequently observed extended perimysial cables in the interstitium between muscle cells. Under conditions where the cells were extended (by about 30%), long cables were observed to be taut (Fig. 5A) and sometimes even frayed (Fig. 5B) as they passed between fibers.
Figure 5.
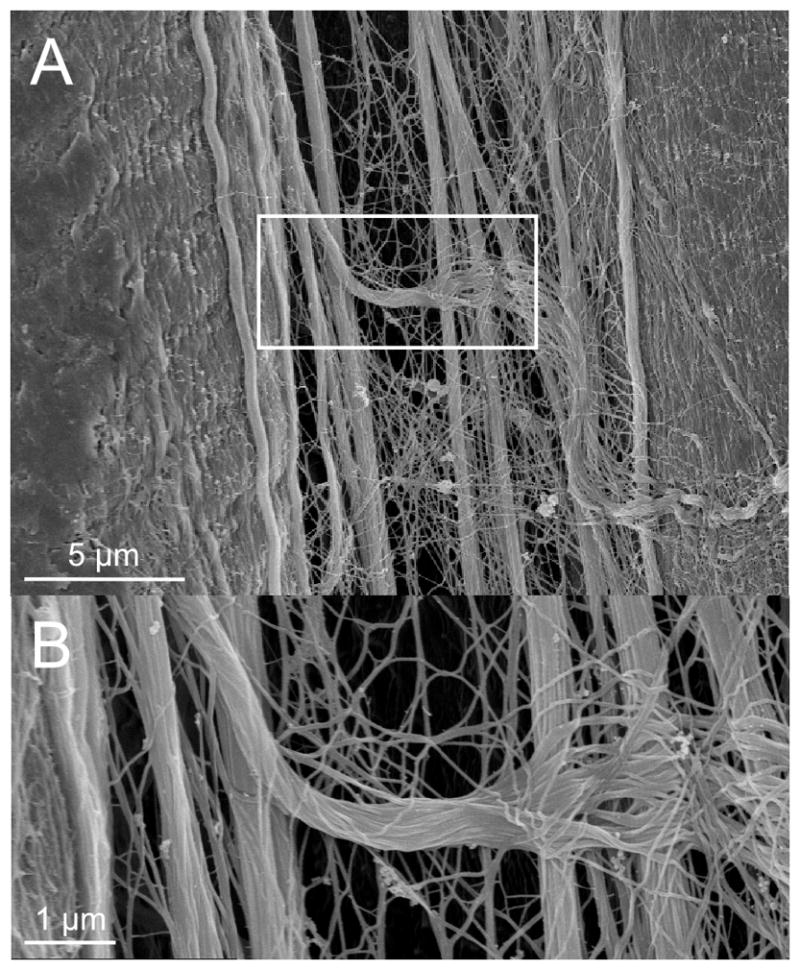
Scanning electron micrograph of mouse EDL muscle stretched about 30% beyond resting length showing longitudinally aligned perimysial collagen cables. (A) Two muscle fibers separated by stretched collagen cables. The collagen cables are distinct from the muscle fiber surface. (B) A collagen cable becomes frayed as it traverses across collagen cables.
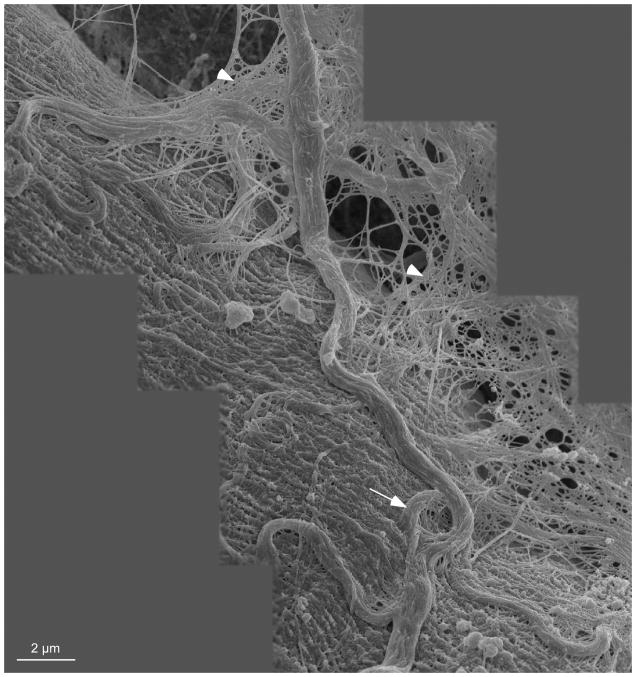
The cables themselves were organized as bundles of collagen fibers, but the actual collagen isoform or the presence/absence of proteoglycan in these cables is not known. These cables terminated on muscle cells as the perimysial plate (Fig. 4). Under conditions where the muscle was fixed while slack, the perimysial cables were tortuous and intimately associated with the fiber surface (Fig. 6). It is of interest that the cable reproduced in the montage of Figure 6 has a gross structure reminiscent of strain relief (arrow) that is commonly present in load-bearing structures that are subjected to length changes. Also visible in this micrograph is the close association between the so-called perimysial cables and the endomysial mesh (arrowheads). Clearly, “unraveling” the biological and biomechanical associations between these structures represents a formidable challenge.
Figure 6.
Montage of scanning electron micrographs from shortened mouse EDL muscle. Large collagen cables arranged in a slack configuration are visible on the surface of the muscle fiber and integrated with the fiber surface. Coils in the collagen cables may indicate that the cables have a strain relief function (arrow). These large cables are believed to be perimysial in nature while the mesh in the background of the montage (arrow heads) is believed to be endomysial. It is clear that these two levels of ECM are intimately associated.
To understand the organization of collagen fibrils in ECMmore completely, confocal microscopy can be used to examine ECM components.10, 11 This method offers the ability to measurereal-time deformation in ECM components in response to loading. Taken together, the above descriptive studies of skeletal muscle ECM strongly suggest that there is a well-organized collagen network that can be quantified in terms of fibril size distribution, interconnection, and orientation as a function of muscle length and activity. However, the exact nature of the network and its site(s) of mechanical integration with the surrounding muscle tissue, including the microcirculation and motor unit axons, remain to be determined.
The Muscle Epimysium
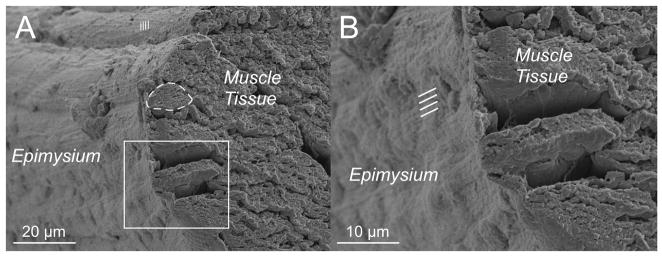
The epimysial layer of muscle ECM can be discretely isolated by dissection and, thus, lends itself to more discrete definition and mechanical testing. Gao and colleagues described the deep and superficial surfaces of epimysium as being primarily composed of very large collagen bundles with a familiar “crimp” pattern reminiscent of that seen in tendon.12, 13 In light of this structural specialization, a micromechanical model was developed that could describe the highly nonlinear behavior of this connective tissue.14 These investigators speculated that stiffness of the epimysium increased with age, which could have implications for understanding biomechanical properties of aged muscle both in terms of normal function and response to injury. In addition to the epimysium, connective tissue linkages may be involved in force transmission between muscles, but this will not be discussed here (see review15). Based on the morphological appearance of epimysium (Fig. 7), it is not clear whether epimysium has truly distinct structural properties compared to perimysium. Apparent sarcomere length periodicity (Fig. 7A, lines) as well as longitudinal ECM periodicity are both observable in the epimysium (Fig. 7B, lines). However, because these preparatory and visualization methods are so disruptive, it is not clear that these properties reflect native ECM structure.
Figure 7.
Epimysial layer of a mouse EDL muscle viewed in cross-section. A connective tissue sheath is clearly seen surrounding muscle fibers. (A) Survey view of the muscle where a region can be observed with sarcomere periodicity through the connective tissue layer. An individual muscle fiber is outlined (dashed line) (B) Closer view of the epimysium reveals longitudinal periodicities (lines) with approximately 1 μm of spacing.
Putative Structure Hierarchy of Skeletal Muscle ECM
The hierarchical organization of ECM into endo-, peri-, and epimysium is generally accepted, but this simplified organization does not explain how ECM transitions through these “zones” from muscle to tendon. Passerieux and colleagues digested muscle fibers in bovine flexor carpi radialis muscles and visualized the resulting connective tissue using scanning electron microscopy.16 The images they obtained suggest that sheets of perimysial collagen join and become continuous with tendon. It is interesting to note that tendon and perimysium both contain primarily type I collagen, and the primary proteoglycan (PG)for both structures is decorin. In contrast, epimysium and endomysium are made up of almost equal amounts of types I and III collagen and contain other PGs(see below). The structure of perimysium is also different from the mesh-like structure of endomysium. This evidence supports the hypothesis that perimysium is continuous with tendon (Fig. 1B). Using this logic, muscle fascicles are viewed as being surrounded by perimysium, and endomysiumis continuous within fascicles but does not cross the perimysial border. Collagen in tendon is much more organized than collagen in perimysium, but collagen organization may have initially been similar during development before differentiating as a result of loading conditions. High tensile loads on the tendon may organize collagen fibers to align with the muscle axis while complex stress and strain distributions within the muscle belly may allow perimysial collagen to maintain a less organized structure. Based on the fact that muscle fibers within a motor unit do not extend the length of afascicle17 and the observation of an intimate interaction between tendon and perimysium, a current structural model for muscle tissue is one in which muscle fibers are embedded within a matrix of ECM that forms discrete layers that are mechanically interconnected. Thus, muscle fiber force generated by actin-myosin interactions will be transmitted to the ECM at multiple focal adhesions along the muscle fiber itself.18 Once force is transmitted to the ECM, there would be nearly infinite paths of force transmission to the external tendon. In this way, focal ECM or muscle fiber injuries would have negligible functional significance due to the mechanical redundancy built into the ECM. It would be extremely helpful to pursue detailed studies of the transition zones between endomysium, perimysium, epimysium, and tendon.
Composition
Skeletal Muscle Collagens
Collagen is the major structural protein in skeletal muscle ECM; it accounts for 1–10% of muscle mass dry weight.19, 20 Collagen structure has been extensively studied and will not be described here (see review21). Types I, III, IV, V, VI, XI, XII, XIV, XV, and XVIII collagen are expressed during skeletal muscle development,22–26 although fibrillar types I and III predominate in adult endo-, peri-, and epimysium.27, 28 Several studies suggest that perimysial collagen is predominantly type I, whereas type III collagen appears to be more evenly distributed between endomysium and epimysium.28 However, these studies are largely qualitative duet difficulty isolating various “regions” of ECM; thus, it is not clear if collagen ratios vary in muscles with different functions. Type V collagen, another fibril-forming collagen, associates with types I and III and may form a core for type I collagen fibrils in perimysium and endomysium.29 No data exist regarding the effect of a type V collagen core on type I collagen fibril crimp pattern, tensile strength, or fibril diameter. Types XII and XIV collagen are fibril-associated collagens with interrupted triple helices (FACITs) localized primarily to perimysium.23 While they appear to link fibrillar collagen to other ECM components, their precise function is not known.
Muscle basement membrane consists primarily of a type IV collagen network, however types VI, XV, and XVIII are also present.22, 24–26, 30 Types XV and XVIII collagen are classified as multiplexins, which are heparan sulfate proteoglycans (HSPGs). The multiplexins can bind growth factors and also aid in linking the basement membrane to other basement membrane glycoproteins and endomysium.31 Although the basement membrane is considered to be distinct from endomysium, the two are intimately connected and probably involved in the transmission of force from the myofiber to the tendon.3, 32
Attempts to characterize skeletal muscle collagens fully have been hampered by inadequate biochemical techniques that do not accurately differentiate and/or quantify collagen types. This is partially due to the complex and conserved structure of collagen molecules. Current methods include quantification by the ratio of hydroxyproline to collagen, Western blots of digested collagen fractions, antibody binding assays, and high pressure liquid chromatography (HPLC). Identification by hydroxyproline and HPLC does not allow for differentiation of specific collagen types, whereas Western blotting may be used to successfully quantify collagen types. However, this method is laborious, fairly insensitive, difficult to quantify and not suitable for large-scale screening. Immunohistochemistry may be used to localize collagen in muscle tissue, however this approach suffers from the fact that antibody affinities vary tremendously and, as described above, collagen “visualization” is highly biased due to the complex geometry of ECM itself. Visualization is further complicated by the presence of heterotrimers of different collagen types, because antibody-binding epitopes may be masked by associated collagen fibrils. New techniques must be developed to identify and quantify different collagen types.
Proteoglycans and Glycosaminoglycans
Proteoglycans are ubiquitous in connective tissue, and muscle ECM is no exception. A number of PGsare present in muscle ECM, and many belong to the family of small leucine-rich proteoglycans (SLRPs). SLRPs consist of a core protein with attached GAG chains and include decor in, biglycan, fibromodulin, and lumican. The most abundant PGs in skeletal muscle ECM have chondroitin sulfate and derma tan sulfate GAG chains, including decor in and biglycan.33 The heparan sulfate PGs collagen XVIII, perlecan, and agrin comprise approximately 30% of the PGs in ECM33 and are known to bind growth factors.34, 35 Growth factors in skeletal muscle can be stored and released by negatively charged GAGs, particularly HSPGs. This is especially important in the basement membrane and endomysium, which surround muscle fibers where they can act in an autocrine fashion. Importantly, enzymes in the ECM can cleave GAG chains with their associated growth factors,36–38 allowing for their interaction in cell signaling and mechanotransduction. Again, this field is in its infancy.
Collagen-Proteoglycan Interactions
Because they comprise the major structural components of ECM, collagen and proteoglycans have unique interactions that maintain the structure and organization of the matrix. Proteoglycans bind to collagen in specific locations39–41 and are present in different ratios throughout ECM. Decorin, the major PG in the perimysium,42 is arch shaped,43, 44 and its leucine-rich repeatsbind to type I collagen45 near the d and e bands.40 Due to its binding interaction with type I collagen in vitro, decorin has been proposed to bea regulator of type I collagen fibrillogenesis in skeletal muscle. Decorin inhibits the lateral growth of collagen fibrils, both with and without its GAG chain, implying that the core protein is the source of inhibition.46 Biglycan is structurally similar to decorin and also binds to type I collagen, likely at the same position as decorin.47 The binding of proteoglycans to collagen and the location of collagen crosslinks mightbe key factors that determine the structural organization of skeletal muscle ECM. In the absence of decorin48 or biglycan,49 collagen fibril diameter in tendon is irregular. Additionally, biglycan-null mice display a mild muscular dystrophic phenotype,50 and skin from decorin-null mice has reduced tensile strength,48 indicating the importance of these PGs in maintaining normal tissue function and mechanical properties (see review51).
Glycoprotein sand Matricellular Proteins
Many glycoproteins function as linker molecules between type IV collagen in the basement membrane and sarcolemma (muscle fiber membrane). At the sarcolemma, laminins are bound by integrins and α-dystroglycan,52, 53 and fibronectin may also bind to integrins.54 Laminins can bind directly to type IV collagen,55, 56 but may also be indirectly linked by fibronectin55, 57 or nidogen.58, 59 Interactions between these glycoproteins provide potential mechanisms for lateral force transmission from the myofiber and have been reviewed.32 Together with the branched network structure of type IV collagen, these glycoproteins form the basis for basement membrane architecture(see reviews60, 61).
Matricellular proteins are secreted into the extracellular matrix but do not play a structural role. In skeletal muscle they include osteopontin, secreted protein acidic and rich in cysteine (SPARC), thrombospondin, and tenascin-C. Osteopontin has cytokine-like functions and is not normally observed in normal skeletal muscle, but it appears during muscle regeneration. Although typically secreted by inflammatory cells, myoblasts may also secrete osteopontin.62 SPARC has been shown to bind collagen and may chaperone collagen interactions in the extracellular matrix.63 Thrombospondin has been identified in skeletal muscle ECM, and thrombospondin knockout mice had higher skeletal muscle capillarity than wild type mice, indicating that its role may be to prevent excessive capillary formation.64 Tenascin-C is localized to the neuromuscular junction (NMJ)65 and binds perlecan and agrin,66, 67 which could indicate that it plays arole in maintaining NMJ architecture.68 Although these proteins do not provide structural support, they are vital in ECM signaling and maintaining ECM organization.
Matrix Remodeling Enzymes
In normal skeletal muscle, a delicate balance exists between enzymes responsible for ECM synthesis and their inhibitors. Turnover of the ECM is required for cell migration, myotube formation, and reorganization of the matrix during muscle adaptation. Matrix metalloproteinase(MMP)levels in uninjured muscle are generally low, but secreted MMPs that can be expressed in skeletal muscle include the gelatinases MMP-2 and MMP-969 that degrade type IV collagen, fibronectin, proteoglycans, and laminin, as well as the collagenases MMP-170 and MMP-1371 that degrade types I and III collagen. In addition to secreted MMPs, membrane type-1 MMP activates MMP-2 and has proteolytic functions near the cell surface.72 Their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMPs) 1–3,70, 73 either bind to active MMPs or stabilize inactive forms, thereby inhibiting their enzymatic activity. Given the wide range of components involved in ECM synthesis, degradation, and inhibition of this degradation, it is clear that skeletal muscle ECM composition and, therefore, its mechanical properties, are under tight regulation.
ECM Mechanics
Muscle ECM Mechanical Properties
Muscle develops passive tension as it is stretched beyond its slack length and, within fibers, passive tension has been attributed primarily to titin, a giant protein that spans from the Z-disc to the M-line within sarcomeres.74 Magid and Law demonstrated in frog muscle fibers that passive tension originated from within myofibrils, not extracellularly, which led to decades of defining titin’s structure and function.75 However, acorrelation between titin size and fiber or myofibrillar stiffness is only weak,76 suggesting that other aspects of titin’s structure may be more functionally important than size (e.g. protein phosphorylation) or that other structures are significantly involved. Biomechanical studies of muscle fiber bundles and even whole muscle provide more evidence that titin plays only a minor role in passive load-bearing at the tissue level. For example, when measuring the passive mechanical modulus of muscle from single fibers to fiber bundles, the modulus often increases two-fold (Table 1).77–80 Given that the ECM comprises only about 5% of the area fraction of these muscle samples, these studies suggest that the ECM is a tremendously stiff structure surrounding relatively compliant fibers.
Table 1.
Comparison of single fiber and fiber bundle modulus values from various muscles
| Muscle | Single Fiber Modulus (kPa) | Fiber Bundle Modulus (kPa) | Bundle:Fiber Modulus Ratio | Reference |
|---|---|---|---|---|
| Human Iliocostalis | 37.05 | 58.83 | 1.59 | 77 |
| Human Longissimus | 32.80 | 62.85 | 1.92 | 77 |
| Human Multifidus | 33.71 | 91.34 | 2.71 | 77 |
| Human Upper Extremity | 28.2 | 462.5 | 16.4 | 78 |
| Spastic Human Upper Extremity | 55 | 111.15 | 2.02 | 78 |
| Mouse Fifth Toe EDL | 6.26 | 40.53 | 6.48 | 79 |
| Rabbit Diaphragm | 30.14* | 57.01* | 1.89 | 76 |
| Rabbit EDL | 70.95* 81.31 |
158.47* 89.41 |
2.23 1.10 |
76 80 |
| Rabbit Gastrocnemius | 53.52* | 106.53* | 1.99 | 76 |
| Rabbit Psoas | 157.11* | 91.59* | 0.58 | 76 |
| Rabbit Tibialis Anterior | 112.08 | 127.69 | 1.14 | 80 |
| Rabbit EDII | 80.52 | 90.91 | 1.13 | 80 |
| Mean ± Standard Deviation | 60± 41 | 119± 108 | 3.2 ± 4.2 |
Abbreviations: No data (ND), extensor digitorum longus (EDL), extensor digitorum II (EDII)
Modulus values calculated as the tangent modulus corresponding to a strain of 44.9%.
As mentioned above, to date, measurement of ECM biomechanical properties has consisted of studying epimysial sheets that are easily dissected and tested or isolation studies that remove all components from muscle with the exception of endomysium, perimysium, and epimysium collagen fibrils. However, biaxial testing of ECM sheets or isolated collagen fibrils may not represent the in vivo loading or structural environment of ECM, and new methods are needed to accurately measure the in vivo mechanical properties of the composite ECM.
Direct and Indirect Measurement of Composite ECM Mechanical Properties
Two recent studies provide examples of novel methods thatindirectly79 or directly81 define muscle ECM mechanical properties. To indirectly define ECM mechanical properties, a single muscle fiber modulus can be measured and compared to the modulus of muscle bundles. ECM modulus can then be calculated using the rule of mixtures for composites after the load-bearing area of fiber and ECM are first defined.79 Using this approach, isolated muscle fiber stress-strain behavior was shown to be linear, and muscle bundle stress-strain behavior was nonlinear (Fig. 8A). However, it was not clear whether the inherent nonlinearities in the ECM material properties were due to the fact that muscle fiber bundles are composed of numerous individual muscle fibers with slightly different linear material properties or whether the ECM itself was nonlinear. It is possible that nonlinear stress-strain behavior of muscle bundles can be a manifestation offibers with linear stress-strain relationships that develop tension at different sarcomere lengths. To address this question, mechanical experiments were performed in which numerous single fibers were isolated to exclude ECM and then “grouped” together to be the size of a muscle fiber bundle. Then, a comparison between fiber “groups” and fiber bundles provided a comparison between multiple muscle fibers with and without ECM. The results clearly demonstrated that the modulus of a fiber group was nearly identical to that of single fibers; in contrast, the modulus of bundles was about five times higher (Fig. 8B). These experiments demonstrate that ECM is inherently stiffer than muscle fibers and that its material properties are highly nonlinear.
Figure 8.
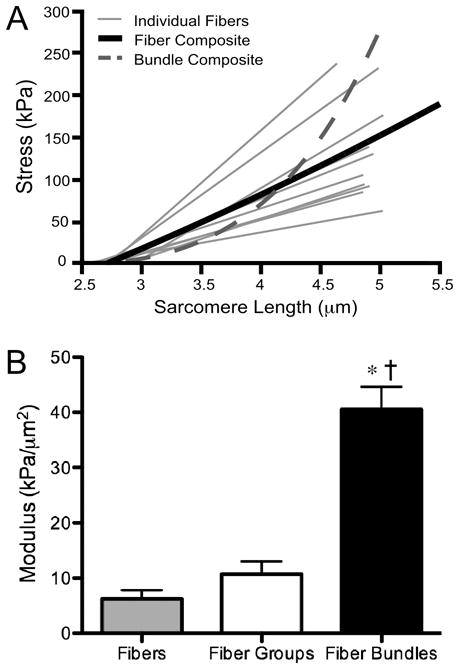
Mechanical contribution of the ECM to muscle bundle modulus. (A) Individual fibers display linear stress-strain behavior, whereas muscle bundles composed of these fibers and ECM display a nonlinear relationship. (B) Fiber groups do not contain ECM (see text), and the modulus value is comparable to that of single fibers. Fiber bundles do contain ECM and have a significantly higher modulus, suggesting that the addition of ECM is responsible for the increased modulus (data replotted from reference79). Asterisk indicates p<0.05 compared to fibers, dagger indicates p<0.05 compared to fiber groups.
In a different approach, instead of using a subtractive method to define ECM properties, ECM properties were directly measured by treating the muscle in such a way that removes every thing except the ECM from a muscle.81 Unlike previous maceration techniques used to observe collagen in the ECM82 or enzymatic and detergent digestion methods that remove glycosaminoglycans that may affect mechanical properties,83, 84 this so-called “decellularization” protocol did not remove glycosaminoglycans from the muscle. Importantly, biomechanical properties of the decellularized muscles were nearly identical to those of intact muscles. Creative experiments such as these will provide a first step toward elucidating the mechanical properties of skeletal muscle ECM.
ECM Mononuclear Cells
While most descriptions of “cells” within muscle tissue refer to the multinucleated, post-mitotic, highly differentiated muscle fibers, muscle tissue, in general, and Ermine particular, is resident to a wide variety of mononuclear cell types that are involved in the maintenance of ECM and the regeneration of muscle. Satellite cells within muscle have been described in detailin a number of recent reviews85, 86 and will not be discussed further in this review. Instead, we focus on the cell types in muscle that contribute to the production and maintenance of ECM. In terms of the “minor” cell types, nerve axons secrete agrin, a HSPG that aids in clustering acetylcholine receptors at the neuromuscular junction.87 Their supporting Schwann cells are capable of producing laminins both in vitro and in vivo,88 and, when cultured with nerve cells, they secrete types I and III collagen.89 The primary mononuclear cell in normal muscle ECM is the fibroblast, which isresponsible for producing the majority of ECM components, including collagen, fibronectin, MMPs, and PGs.90–92 In cases of muscle injury, however, other cell types may contribute to the production of ECM components. For example, satellite cells have been shown to secrete MMPs in culture,93 which may aid in their migration through the ECMto sites of muscle injury. It is even possible that multinucleated myofibers contribute to ECM production in normal muscle. Myogenic cells secrete collagen,94 MMP-2,69 and decorin95 in culture, and embryonic myoblasts secrete collagens prior to fusion,96 but fibroblasts appear to be necessary to organize these ECM components into a functional matrix.92, 97 Fibroblasts are sensitive to mechanical loading and convert mechanical signals into altered gene expression (see review98). Fibroblasts near the myotendinous junction likely experience a different mechanical environment compared to those located in the midbelly of the muscle, and this could be responsible for increased collagen production to aid in tendon development and maintenance as well as laminins and talins that link the ends of muscle fibers to the basement membrane at the myotendinous junction. Because the amount of collagen in muscle varies, it is reasonable to speculate that muscles may have phenotypically different fibroblasts for ECM production that correspond to the needs of that muscle type. There is precedent for such a differential function of generically identified “fibroblasts”; extraocular fibroblasts are phenotypically distinct from leg muscle fibroblasts even when isolated from the same species. These fibroblasts are derived from neural ectoderm and mesenchymal origin, respectively, so transcriptional differences would not be surprising.99 A recent study found that fibroblasts isolated from different muscle types have different in vitro growth potentials and express different levels of MMP-2.90 Fibroblasts cultured from bovine semitendinosus and sternomandibularis muscles had higher growth capacity than fibroblasts from longissimus dorsi muscle, and active MMP-2 expression was generally highest in semitendinosus fibroblast cultures and lowest in sternomandibularis fibroblast cultures. The mechanical properties and collagen content of these muscles were not analyzed in parallel, so these differences cannot be correlated with muscle function or ECM turnover. The location and number of fibroblasts in skeletal muscle have not been systematically addressed, but fluorescence-activated cell sorting (FACS) makes it possible to identify and separate fibroblasts from muscle tissue. Measuring changes in the fibroblast compartment of muscle after injury and pathology could explain the measured changes in ECM of these muscles.
The fact that a significant fraction of muscle-resident mononuclear cells have stem cell properties86 is especially provocative in light of recent studies that clearly demonstrate that the biomechanical properties of the cell substrate strongly affects gene expression patterns that pertain to fate determination.100 This raises the distinct possibilities that mechanical properties of ECM are not only modified in disease and injury, but that mechanical modifications or the ECM affect subsequent tissue properties. Again, this field is in its infancy.
ECM Pathological Changes
Fibrosis
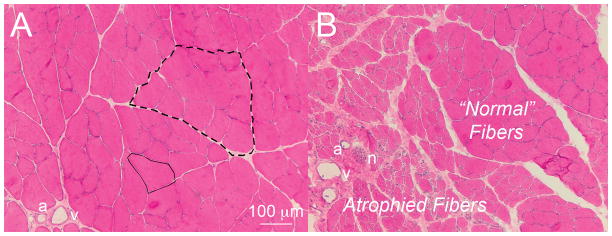
A common theme across almost all myopathies is the accumulation of excess ECM, which is generically termed “fibrosis.” This term actually has no objective definition butis typically used as a qualitative descriptor in muscle pathology. Fibrosis in skeletal muscle is clinically relevant especially in light of the very strong mechanical influence that the ECM plays in muscle function. It is unclear, however, whether fibrosis in muscle is characterized by excessive production of ECM components, altered activity or gene expression of ECM-degrading Mumps and their inhibitors, or a combination of these. Skeletal muscle fibrosis occurs in muscular dystrophies, diabetes, immobilization, and aging.101–105 Histological analysis of fibrosis in muscle (Fig. 9) is characterized by increased endomysium and perimysium. This type of descriptionis highly sensitive but entirely nonspecific. Fibroblasts are typically the collagen-producing cells in mature muscle, but other cell types, including inflammatory cells, myofibers, endothelial cells, and satellite cells, may contribute to collagen production in fibrotic muscle, as described above. To answer the question of which cell types produce collagen in fibrotic muscle, it would be interesting to study a Cre recombinase expressing mouse under the control of the type I collagen promoter106 in a fibrosis-inducing injury model. Cre/loxP methodology allows tissue-specific manipulation of a given gene of interest. Through molecular biology techniques, loxP sites are inserted into a specific sequence of DNA that is responsible for the expression of the target gene/protein. By crossing mice that express this “floxed” gene with mice expressing Cre recombinase (an enzyme that binds loxP sequences), the region of DNA that is flanked by loxP site is excised. Thus, in a mouse in which Cre-recombinase is driven by the type I collagen promoter, it is possible to determine which cells are producing the collagen that is presumably leading to fibrosis. Furthermore, multiple reaction monitoring may be used in the future to quantify proteins from labeled cell types in muscle.107 This method can detect and quantify peptides in multiplex format using a triple quadrupole instrument. An advantage to this method is that it might be used to identify protein signals in fibrotic muscle that cause cells to overexpress collagen.
Figure 9.
Cross-sectional view of normal and neurotoxin-injected rat tibia is anterior muscle stained with hematoxylin and eosin. (A) Normal muscle showing endomysium connective tissue (sample outlined in solid line) separating muscle fibers and perimysium (sample outlined in dashed line) separating bundles of muscle fibers (artery, a; vein, v). (B) Increased connective tissue around atrophied fibers in neurotoxin-injected muscle (nerve, n). Unaffected regions show normal fiber morphology (image courtesy of Drs. Sam Ward and Viviane Minamoto).
Transforming growth factor-beta (TGF-β) is a cytokine with many functions in skeletal muscle. The role of TGF-β in fibrosis has been the subject of many studies (see reviews108, 109). Activated TGF-β induces fibroblasts to produce type I collagen, fibronectin, and connective tissue growth factor (CTGF) and suppresses matrix metalloproteinases. When repeated muscle injury occurs, elevated TGF-β continues to signal ECM production and eventually leads to a fibrotic response. In the case of Duchenne muscular dystrophy (DMD), decorin and biglycan, which normally sequester TGF-β, are differentially expressed,110–112 which may lead to altered TGF-β signaling and eventual fibrosis. CTGF has only recently been discovered, and its role in muscle fibrosis is not fully understood, but the expression of TGF-β and CTGF seem to be related.113–116 Mechanical stress appears to stimulate CTGF expression,117, 118 and CTGF may be involved in the muscle fibrosis that occurs after stretch injury.
In addition to changes in composition, fibrosis has been associated with changes in muscle mechanics. Spastic muscle from patients with cerebral palsy is characterized by increased extracellular material119 and stiffer fibers.78, 120 Interestingly, the modulus of spastic muscle fibers is about two times greater than normal fibers, but the modulus of spastic muscle bundles is significantly lower than normal bundles.78 It is tempting to speculate that the reduced spastic bundle modulus occurs in response to the altered stiffness of the muscle fibers themselves. Collagen content is increased in spastic muscle119 and correlates with the clinical severity of muscle spasticity,121 again demonstrating the clinical importance of ECM in pathology. Analysis of muscle from children with wrist flexion contractures revealed significant changes in transcripts related to ECM in a way that differed from other altered use or disease models such as DMD, immobilization-induced atrophy or hereditary spastic paraplegia.122 Thus, it is possible that the “final” observable effect is fibrosis, but there are numerous cellular processes that can produce it.
An equally provocative muscle adaptation was recently observed in a desmin knockout model.123 Incremental stress-relaxation tests were performed on EDL muscle fibers and fiber bundles from desmin knockout and wildtype mice. The linear modulus was defined as the slope of the stress-strain curve—a measure of tissue stiffness. Loss of the intermediate filament network ultimately resulted in ECM hypertrophy and, mechanically, stiffer muscle tissue. Thus, data from children with CP and transgenic animal models both suggest the possibility that muscle fiber mechanical properties and ECM properties are dependent upon each other. The detailed mechanistic basis for such an interaction remains to be defined.
Profibrotic cytokines and ECM components that interact with them have been specific targets for antifibrotic therapies for skeletal muscle based on their potential to directly affect ECM production. Huard and colleagues have implemented this mechanistic approach by using IGF-1, decorin, and TGF-β inhibitors to prevent fibrosis in skeletal muscle.124–126 These treatments enhance regeneration and prevent fibrosis in injured muscle, but their efficacy in muscle that is already fibrotic has not yet been determined.
Intramuscular Fat
Another pathological response of skeletal muscle to disease or injury is the accumulation of intramuscular, extracellular fat. This is distinct from the positive adaptation of muscle to exercise that includes increased intracellular fat deposition.127 Pathological examples of intramuscular fat deposition include fatty deposits that occur in advanced cases of DMD,128 obesity,129 type 2 diabetes,129 and aged muscle,129, 130 as well as the “fatty atrophy” that accompanies rotator cuff tears131, 132 and low back pathology in humans.133
Only recently has it been possible to address, in a relatively mechanistic fashion, the biological basis for fatty deposition in muscle tissue. This is based on the discovery of several cells types within muscle that have clear adipogenic potential, including satellite cells, mesenchymal stem cells, and certain side population cells.134–136 However, until recently, it was not clear which of these cells are responsible for adipose tissue in muscle. Uezumi and colleagues identified a PDGFRα+ progenitor cell population distinct from satellite cells that can differentiate into adipose tissue in vivo under non-regenerating conditions.135 Interestingly, these cells were observed more in perimysium than endomysium, consistent with the idea that fat accumulation in disease is generally localized to the perimysium.137 A detailed mechanism for the activation of these cells and the factor(s) that regulate their proliferation is not yet known.
Conclusions
The role of ECM in muscle mechanics and pathology is not well understood, but it is becoming more evident that changes in ECM are clinically significant. Further studies are required to define the detailed structure and composition of ECM, characterize its mechanical properties, and determine the way in which these relationships change in diseased states. Several studies have been proposed in this review that could address these areas, and the key questions are summarized below:
What is the three-dimensional perimysial collagen structure in muscle? How does this differ amongst muscles with different function, e.g. those that are chronically active, perform antigravity functions, operate at long lengths, or are involved in rapid movements?
What is the nature of the transition between structures of the endomysium, perimysium, epimysium, and tendon? How do these structures interconnect to transmit force along and across muscle?
What is the nature of the biological and biomechanical communication between muscle cells and the ECM? Which structures are responsible for transmission of biological and mechanical signals between muscle cells and the ECM?
What are the changes that occur in the fibroblast and mononuclear cell compartment of muscle with normal development, pathology, and injury? What cell types contribute to the fibrosis or fatty accumulation that occurs in muscle disease?
Answers to these questions will vastly improve our basic understanding of the ECM and how it contributes to normal muscle function. Only then can we begin to understand how compositional and structural changes in the ECM affect muscle function in disease and injury. Finally, identifying mechanisms by which ECM hypertrophies in disease will provide potential targets for therapeutic interventions.
Abbreviations
- CTGF
Connective tissue growth factor
- DMD
Duchenne muscular dystrophy
- ECM
Extracellular matrix
- EDL
Extensor Digitorum Longus
- FACIT
Fibril associated collagen with interrupted triple helices
- FACS
Fluorescence-activated cell sorting
- GAG
Glycosaminoglycan
- HPLC
High pressure liquid chromatography
- HSPG
Heparan sulfate proteoglycan
- MMP
Matrix metalloproteinase
- MTJ
Myotendinous junction
- PG
Proteoglycan
- SEM
Scanning electron microscopy
- SLRP
Small leucine rich proteoglycan
- SPARC
Secreted protein acidic and rich in cysteine
- SR
Sarcoplasmic reticulum
- TGF
β-Transforming growth factor-beta
- TIMP
Tissue inhibitor of metalloproteinase
References
- 1.Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, Adrian RH, Geiger SR, editors. Skeletal Muscle. Baltimore, MD: American Physiological Society; 1983. pp. 73–112. [Google Scholar]
- 2.Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. J Morphol. 1992;212:109–122. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- 3.Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil. 1994;15:299–308. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- 4.Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Jarvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil. 2002;23:245–254. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- 5.Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue Cell. 1980;12:197–207. doi: 10.1016/0040-8166(80)90061-0. [DOI] [PubMed] [Google Scholar]
- 6.Rowe RW. Morphology of perimysial and endomysial connective tissue in skeletal muscle. Tissue Cell. 1981;13:681–690. doi: 10.1016/s0040-8166(81)80005-5. [DOI] [PubMed] [Google Scholar]
- 7.Passerieux E, Rossignol R, Chopard A, Carnino A, Marini JF, Letellier T, et al. Structural organization of the perimysium in bovine skeletal muscle: Junctional plates and associated intracellular subdomains. J Struct Biol. 2006;154:206–216. doi: 10.1016/j.jsb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Young M, Paul A, Rodda J, Duxson M, Sheard P. Examination of intrafascicular muscle fiber terminations: implications for tension delivery in series-fibered muscles. J Morphol. 2000;245:130–145. doi: 10.1002/1097-4687(200008)245:2<130::AID-JMOR4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- 10.Hanley PJ, Young AA, LeGrice IJ, Edgar SG, Loiselle DS. 3-Dimensional configuration of perimysial collagen fibres in rat cardiac muscle at resting and extended sarcomere lengths. J Physiol. 1999;517:831–837. doi: 10.1111/j.1469-7793.1999.0831s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura YN, Iwamoto H, Yamaguchi T, Ono Y, Nakanishi Y, Tabata S, et al. Three-dimensional reconstruction of intramuscular collagen networks of bovine muscle: A demonstration by an immunohistochemical/confocal laser-scanning microscopic method. Anim Sci J. 2007;78:445–447. [Google Scholar]
- 12.Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. Exerc Sport Sci Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- 13.Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech. 2008;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y, Waas AM, Faulkner JA, Kostrominova TY, Wineman AS. Micromechanical modeling of the epimysium of the skeletal muscles. J Biomech. 2008;41:1–10. doi: 10.1016/j.jbiomech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 16.Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J Struct Biol. 2007;159:19–28. doi: 10.1016/j.jsb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Ounjian M, Roy RR, Eldred E, Garfinkel A, Payne JR, Armstrong A, et al. Physiological and developmental implications of motor unit anatomy. J Neurobiol. 1991;22:547–559. doi: 10.1002/neu.480220510. [DOI] [PubMed] [Google Scholar]
- 18.Patel TJ, Lieber RL. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev. 1997;25:321–363. [PubMed] [Google Scholar]
- 19.Bendall JR. Elastin Content of Various Muscles of Beef Animals. J Sci Food Agr. 1967;18:553–558. [Google Scholar]
- 20.Dransfield E. Intramuscular Composition and Texture of Beef Muscles. J Sci Food Agr. 1977;28:833–842. [Google Scholar]
- 21.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273:25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- 23.Listrat A, Lethias C, Hocquette JF, Renand G, Menissier F, Geay Y, et al. Age-related changes and location of types I, III, XII and XIV collagen during development of skeletal muscles from genetically different animals. Histochem J. 2000;32:349–356. doi: 10.1023/a:1004013613793. [DOI] [PubMed] [Google Scholar]
- 24.Marvulli D, Volpin D, Bressan GM. Spatial and temporal changes of type VI collagen expression during mouse development. Dev Dyn. 1996;206:447–454. doi: 10.1002/(SICI)1097-0177(199608)206:4<447::AID-AJA10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Myers JC, Dion AS, Abraham V, Amenta PS. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T, Ojima K, Hattori A, Takahashi K. Developmental expression of extracellular matrix components in intramuscular connective tissue of bovine semitendinosus muscle. Histochem Cell Biol. 1997;107:215–221. doi: 10.1007/s004180050106. [DOI] [PubMed] [Google Scholar]
- 27.Bailey AJ, Restall DJ, Sims TJ, Duance VC. Meat Tenderness -Immunofluorescent Localization of the Isomorphic Forms of Collagen in Bovine Muscles of Varying Texture. J Sci Food Agr. 1979;30:203–210. [Google Scholar]
- 28.Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 1984;219:1017–1026. doi: 10.1042/bj2191017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitch JM, Gross J, Mayne R, Johnson-Wint B, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea: monoclonal antibody studies. Proc Natl Acad Sci U S A. 1984;81:2791–2795. doi: 10.1073/pnas.81.9.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanes JR. Laminin, fibronectin, and collagen in synaptic and extrasynaptic portions of muscle fiber basement membrane. J Cell Biol. 1982;93:442–451. doi: 10.1083/jcb.93.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eklund L, Piuhola J, Komulainen J, Sormunen R, Ongvarrasopone C, Fassler R, et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci U S A. 2001;98:1194–1199. doi: 10.1073/pnas.031444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grounds MD, Sorokin L, White J. Strength at the extracellular matrix-muscle interface. Scand J Med Sci Spor. 2005;15:381–391. doi: 10.1111/j.1600-0838.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Brandan E, Inestrosa NC. Isolation of the heparan sulfate proteoglycans from the extracellular matrix of rat skeletal muscle. J Neurobiol. 1987;18:271–282. doi: 10.1002/neu.480180303. [DOI] [PubMed] [Google Scholar]
- 34.Mundhenke C, Meyer K, Drew S, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 receptor binding in breast carcinomas. Am J Pathol. 2002;160:185–194. doi: 10.1016/S0002-9440(10)64362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai K, Harames A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-beta 1 release. Biochem J. 1997;322:809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishai-Michaeli R, Eldor A, Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990;1:833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 39.Hedbom E, Heinegard D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- 40.Pringle GA, Dodd CM. Immunoelectron Microscopic Localization of the Core Protein of Decorin near the D-Bands and E-Bands of Tendon Collagen Fibrils by Use of Monoclonal-Antibodies. J Histochem Cytochem. 1990;38:1405–1411. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- 41.Scott JE, Haigh M. ‘Small’-proteoglycan: collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the ‘a’ and ‘c’ bands. Biosci Rep. 1985;5:765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- 42.Nakano T, Li X, Sunwoo HH, Sim JS. Immunohistochemical localization of proteoglycans in bovine skeletal muscle and adipose connective tissues. Can J Anim Sci. 1997;77:169–172. [Google Scholar]
- 43.Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 1996;35:8795–8799. doi: 10.1021/bi960773t. [DOI] [PubMed] [Google Scholar]
- 44.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 1996;271:31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 45.Svensson L, Heinegard D, Oldberg A. Decorin-Binding Sites for Collagen Type-I Are Mainly Located in Leucine-Rich Repeats 4–5. J Biol Chem. 1995;270:20712–20716. doi: 10.1074/jbc.270.35.20712. [DOI] [PubMed] [Google Scholar]
- 46.Vogel KG, Koob TJ, Fisher LW. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem Biophys Res Commun. 1987;148:658–663. doi: 10.1016/0006-291x(87)90927-2. [DOI] [PubMed] [Google Scholar]
- 47.Schonherr E, Witschprehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of Biglycan with Type-I Collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- 48.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 50.Mercado ML, Amenta AR, Hagiwara H, Rafii MS, Lechner BE, Owens RT, et al. Biglycan regulates the expression and sarcolemmal localization of dystrobrevin, syntrophin, and nNOS. FASEB J. 2006;20:1724–1726. doi: 10.1096/fj.05-5124fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 52.Ervasti JM, Campbell KP. A Role for the Dystrophin-Glycoprotein Complex as a Transmembrane Linker between Laminin and Actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von der Mark H, Durr J, Sonnenberg A, von der Mark K, Deutzmann R, Goodman SL. Skeletal myoblasts utilize a novel beta 1-series integrin and not alpha 6 beta 1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991;266:23593–23601. [PubMed] [Google Scholar]
- 54.Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletalinteraction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 55.Rao CN, Margulies IM, Liotta LA. Binding domain for laminin on type IV collagen. Biochem Biophys Res Commun. 1985;128:45–52. doi: 10.1016/0006-291x(85)91642-0. [DOI] [PubMed] [Google Scholar]
- 56.Charonis AS, Tsilibary EC, Yurchenco PD, Furthmayr H. Binding of laminin to type IV collagen: a morphological study. J Cell Biol. 1985;100:1848–1853. doi: 10.1083/jcb.100.6.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurie GW, Bing JT, Kleinman HK, Hassell JR, Aumailley M, Martin GR, et al. Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J Mol Biol. 1986;189:205–216. doi: 10.1016/0022-2836(86)90391-8. [DOI] [PubMed] [Google Scholar]
- 58.Mayer U, Nischt R, Poschl E, Mann K, Fukuda K, Gerl M, et al. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 1993;12:1879–1885. doi: 10.1002/j.1460-2075.1993.tb05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 61.Ervasti JM. Costameres: the Achilles’ heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 62.Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int J Biochem Cell Biol. 2008;40:2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol. 2009;94:749–760. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- 65.Chiquet M, Fambrough DM. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol. 1984;98:1937–1946. doi: 10.1083/jcb.98.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cotman SL, Halfter W, Cole GJ. Identification of extracellular matrix ligands for the heparan sulfate proteoglycan agrin. Exp Cell Res. 1999;249:54–64. doi: 10.1006/excr.1999.4463. [DOI] [PubMed] [Google Scholar]
- 67.Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J Cell Sci. 1997;110 ( Pt 12):1413–1419. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- 68.Cifuentes-Diaz C, Faille L, Goudou D, Schachner M, Rieger F, Angaut-Petit D. Abnormal reinnervation of skeletal muscle in a tenascin-C-deficient mouse. J Neurosci Res. 2002;67:93–99. doi: 10.1002/jnr.10109. [DOI] [PubMed] [Google Scholar]
- 69.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- 70.Singh A, Nelson-Moon ZL, Thomas GJ, Hunt NP, Lewis MP. Identification of matrix metalloproteinases and their tissue inhibitorstype 1 and 2 in human masseter muscle. Arch Oral Biol. 2000;45:431–440. doi: 10.1016/s0003-9969(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 71.Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol. 2003;121:926–932. doi: 10.1046/j.1523-1747.2003.12497.x. [DOI] [PubMed] [Google Scholar]
- 72.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 73.Chin JR, Werb Z. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development. 1997;124:1519–1530. doi: 10.1242/dev.124.8.1519. [DOI] [PubMed] [Google Scholar]
- 74.Granzier H, Labeit S. Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve. 2007;36:740–755. doi: 10.1002/mus.20886. [DOI] [PubMed] [Google Scholar]
- 75.Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science. 1985;230:1280–1282. doi: 10.1126/science.4071053. [DOI] [PubMed] [Google Scholar]
- 76.Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol. 2005;126:461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward SR, Tomiya A, Regev GJ, Thacker BE, Benzl RC, Kim CW, et al. Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. J Biomech. 2009;42:1384–1389. doi: 10.1016/j.jbiomech.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extra cellular matrix material. Muscle Nerve. 2003;28:464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 79.Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech. 2011 doi: 10.1016/j.jbiomech.2010.10.044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winters TM, Takahashi M, Lieber RL, Ward SR. Nonlinear scaling of passive tension in skeletal muscle. Workshop on Multi-Scale Muscle Mechanics; 2009 Sept 18–21; Woods Hole, MA. p. 38. [Google Scholar]
- 81.Gillies A, Smith LR, Lieber RL, Varghese S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng Part C Methods. 2010 doi: 10.1089/ten.tec.2010.0438. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohtani O, Ushiki T, Taguchi T, Kikuta A. Collagen fibrillar networks as skeletal frameworks: a demonstration by cell-maceration/scanning electron microscope method. Arch Histol Cytol. 1988;51:249–261. doi: 10.1679/aohc.51.249. [DOI] [PubMed] [Google Scholar]
- 83.Schenke-Layland K, Vasilevski O, Opitz F, Konig K, Riemann I, Halbhuber KJ, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143:201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 84.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393–2399. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–194. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, et al. Stemand progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 87.Reist NE, Magill C, McMahan UJ. Agrin-like molecules at synaptic sites in normal, denervated, and damaged skeletal muscles. J Cell Biol. 1987;105:2457–2469. doi: 10.1083/jcb.105.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cornbrooks CJ, Carey DJ, McDonald JA, Timpl R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983;80:3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bunge MB, Williams AK, Wood PM, Uitto J, Jeffrey JJ. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980;84:184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Archile-Contreras AC, Mandell IB, Purslow PP. Phenotypic differences in matrix metalloproteinase2 activity between fibroblasts from three bovine muscles. J Anim Sci. 2010 doi: 10.2527/jas.2010-3060. [DOI] [PubMed] [Google Scholar]
- 91.Gatchalian CL, Schachner M, Sanes JR. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin (J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol. 1989;108:1873–1890. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuhl U, Ocalan M, Timpl R, Mayne R, Hay E, von der Mark K. Role of muscle fibroblasts in the deposition of type-IV collagen in the basal lamina of myotubes. Differentiation. 1984;28:164–172. doi: 10.1111/j.1432-0436.1984.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 93.Guerin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev Dyn. 1995;202:91–99. doi: 10.1002/aja.1002020109. [DOI] [PubMed] [Google Scholar]
- 94.Beach RL, Rao JS, Festoff BW. Extracellular-matrix synthesis by skeletal muscle in culture. Major secreted collagen us proteins of clonal myoblasts. Biochem J. 1985;225:619–627. doi: 10.1042/bj2250619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brandan E, Fuentes ME, Andrade W. The proteoglycan decorin is synthesized and secreted by differentiated myotubes. Eur J Cell Biol. 1991;55:209–216. [PubMed] [Google Scholar]
- 96.Sasse J, von der Mark H, Kuhl U, Dessau W, von der Mark K. Origin of collagen types I, III, and V in cultures of avian skeletal muscle. Dev Biol. 1981;83:79–89. doi: 10.1016/s0012-1606(81)80010-3. [DOI] [PubMed] [Google Scholar]
- 97.Kuhl U, Timpl R, von der Mark K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev Biol. 1982;93:344–354. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- 98.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 99.Kusner LL, Young A, Tjoe S, Leahy P, Kaminski HJ. Perimysial fibroblasts of extraocular muscle, as unique as the muscle fibers. Invest Ophthalmol Vis Sci. 2010;51:192–200. doi: 10.1167/iovs.08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 101.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 102.Alnaqeeb MA, Alzaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat. 1984;139:677–689. [PMC free article] [PubMed] [Google Scholar]
- 103.Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, et al. Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290:E560–565. doi: 10.1152/ajpendo.00202.2005. [DOI] [PubMed] [Google Scholar]
- 104.Duance VC, Stephens HR, Dunn M, Bailey AJ, Dubowitz V. A role for collagen in the pathogenesis of muscular dystrophy? Nature. 1980;284:470–472. doi: 10.1038/284470a0. [DOI] [PubMed] [Google Scholar]
- 105.Williams PE, Goldspink G. Connective tissue changes in immobilised muscle. J Anat. 1984;138 ( Pt 2):343–350. [PMC free article] [PubMed] [Google Scholar]
- 106.Florin L, Alter H, Grone HJ, Szabowski A, Schutz G, Angel P. Cre recombinase- mediated gene targeting of mesenchymal cells. Genesis. 2004;38:139–144. doi: 10.1002/gene.20004. [DOI] [PubMed] [Google Scholar]
- 107.Fu Q, Schoenhoff FS, Savage WJ, Zhang P, Van Eyk JE. Multiplex assays for biomarker research and clinical application: translational science coming of age. Proteomics Clin Appl. 2010;4:271–284. doi: 10.1002/prca.200900217. [DOI] [PubMed] [Google Scholar]
- 108.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 109.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 110.Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E. Increase in decorin and biglycan in Duchenne Muscular Dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med. 2006;10:758–769. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zanotti S, Negri T, Cappelletti C, Bernasconi P, Canioni E, Di Blasi C, et al. Decorin and biglycan expression is differentially altered in several muscular dystrophies. Brain. 2005;128:2546–2555. doi: 10.1093/brain/awh635. [DOI] [PubMed] [Google Scholar]
- 112.Zanotti S, Saredi S, Ruggieri A, Fabbri M, Blasevich F, Romaggi S, et al. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol. 2007;26:615–624. doi: 10.1016/j.matbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 113.Blalock TD, Duncan MR, Varela JC, Goldstein MH, Tuli SS, Grotendorst GR, et al. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- 114.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang XF, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: downregulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 115.Maeda N, Kanda F, Okuda S, Ishihara H, Chihara K. Transforming growth factor-beta enhances connective tissue growth factor expression in L6 rat skeletal myotubes. Neuromuscul Disord. 2005;15:790–793. doi: 10.1016/j.nmd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 116.Mezzano V, Cabrera D, Vial C, Brandan E. Constitutively activated dystrophic muscle fibroblasts show a paradoxical response to TGF-beta and CTGF/CCN2. J Cell Commun Signal. 2007;1:205–217. doi: 10.1007/s12079-008-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schild C, Trueb B. Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res. 2002;274:83–91. doi: 10.1006/excr.2001.5458. [DOI] [PubMed] [Google Scholar]
- 118.Kivela R, Kyrolainen H, Selanne H, Komi PV, Kainulainen H, Vihko V. A single bout of exercise with high mechanical loading induces the expression of Cyr61/CCN1 and CTGF/CCN2 in human skeletal muscle. J Appl Physiol. 2007;103:1395–1401. doi: 10.1152/japplphysiol.00531.2007. [DOI] [PubMed] [Google Scholar]
- 119.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer ECM and increased in vivo sarcomere length. J Physiol. 2011 doi: 10.1113/jphysiol.2010.203364. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 121.Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol. 2001;43:314–320. doi: 10.1017/s0012162201000597. [DOI] [PubMed] [Google Scholar]
- 122.Smith LR, Ponten E, Hedstrom Y, Ward SR, Chambers HG, Subramaniam S, et al. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med Genomics. 2009;2:44. doi: 10.1186/1755-8794-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meyer GA, McCulloch AD, Ward SR, Lieber RL. Intermediate filament and ECM mechanics deduced from desmin knockout muscles. Biophysical Society 54th Annual Meeting; 2010 Feb 20–24; San Francisco, CA. p. 545a. [Google Scholar]
- 124.Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- 125.Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 126.Sato K, Li Y, Foster W, Fukushima K, Badlani N, Adachi N, et al. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- 127.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leroy-Willig A, Willig TN, Henry-Feugeas MC, Frouin V, Marinier E, Boulier A, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging. 1997;15:737–744. doi: 10.1016/s0730-725x(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 129.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes. 2004;5:219–226. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 130.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 131.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre-and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- 132.Nakagaki K, Ozaki J, Tomita Y, Tamai S. Fatty degeneration in the supraspinatus muscle after rotator cuff tear. J Shoulder Elbow Surg. 1996;5:194–200. doi: 10.1016/s1058-2746(05)80005-9. [DOI] [PubMed] [Google Scholar]
- 133.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 135.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 136.Uezumi A, Ojima K, Fukada S, Ikemoto M, Masuda S, Miyagoe-Suzuki Y, et al. Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Commun. 2006;341:864–873. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 137.Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]