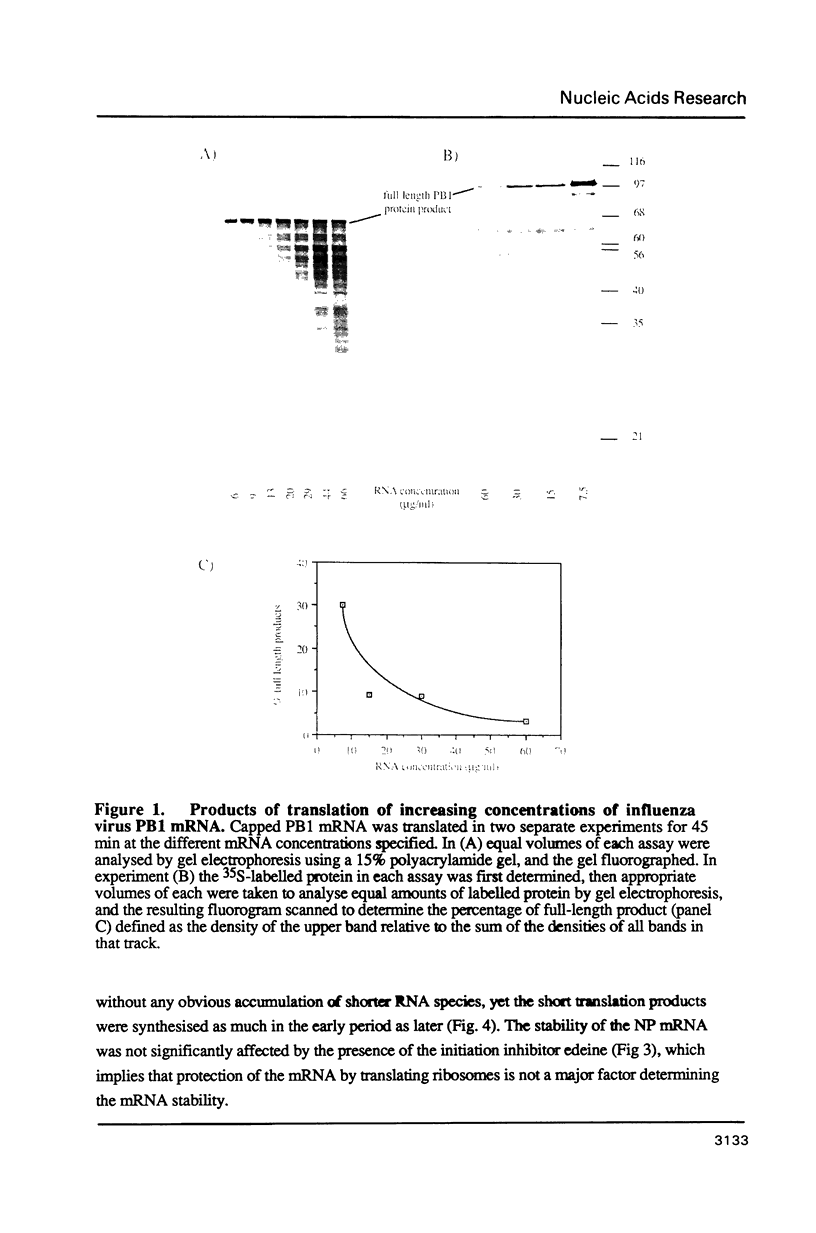
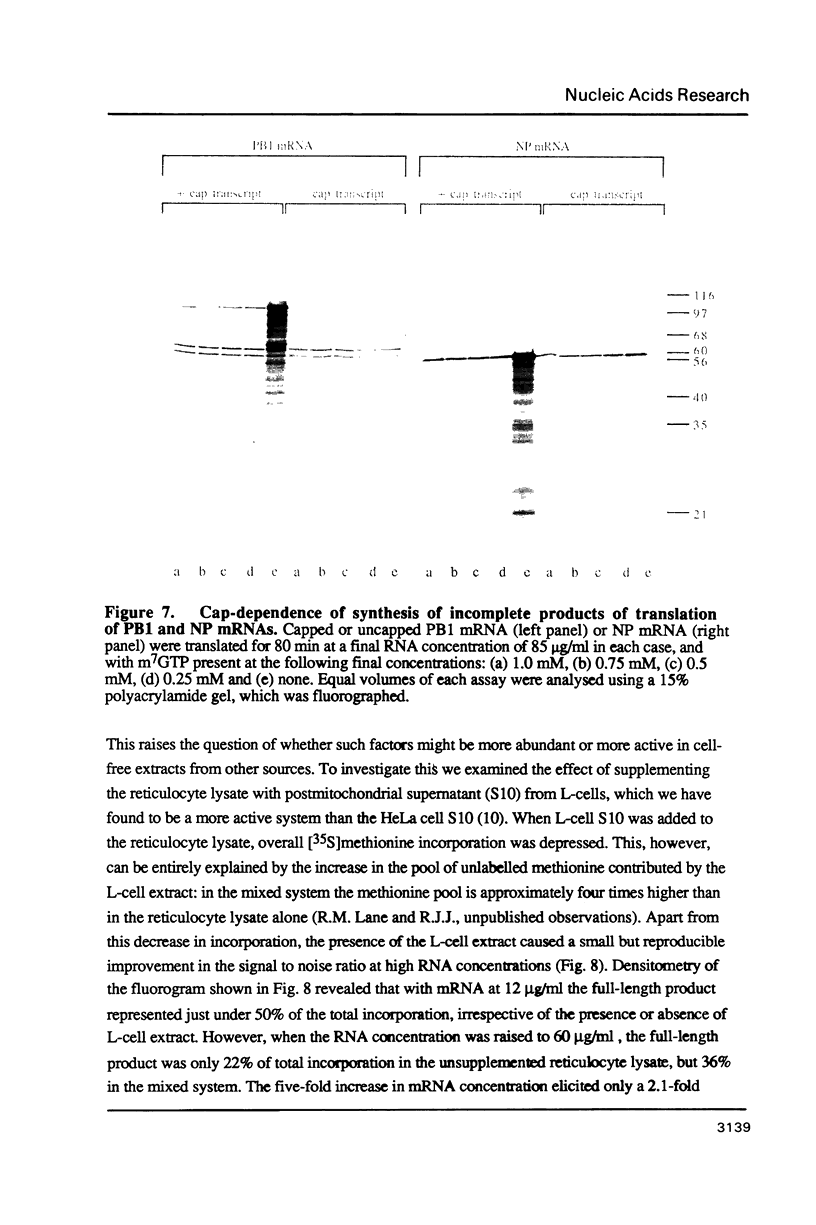
Abstract
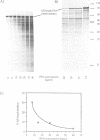
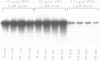
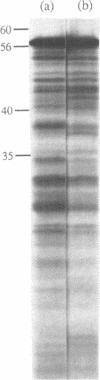
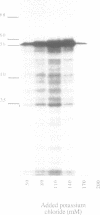
As a test of the fidelity of the rabbit reticulocyte lysate system, we have examined the products of translation of various different influenza virus mRNAs, produced by in vitro transcription. A common finding with all mRNA species was that the ratio of full-length translation product to incomplete products decreased with increasing mRNA concentration. These short products are a mixture of (i) polypeptides initiated at the authentic initiation site but terminated prematurely, and (ii) polypeptides initiated at internal sites and terminated at the correct site. Analysis of mRNA stability during the translation assay showed very little degradation, quite insufficient to be the principle cause of incomplete product synthesis. Investigation of the influence of various parameters on the ratio of full-length to incomplete products leads to the conclusion that a high fidelity of translation can be obtained provided certain precautions are followed: the use of capped, rather than uncapped, mRNAs at low concentrations, with KCl concentrations about 20 mM above the level that gives maximum incorporation.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brierley I., Boursnell M. E., Binns M. M., Bilimoria B., Blok V. C., Brown T. D., Inglis S. C. An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J. 1987 Dec 1;6(12):3779–3785. doi: 10.1002/j.1460-2075.1987.tb02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Semler B. L., Jackson R. J., Hanecak R., Duprey E., Wimmer E. In vitro translation of poliovirus RNA: utilization of internal initiation sites in reticulocyte lysate. J Virol. 1984 May;50(2):507–514. doi: 10.1128/jvi.50.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H., Turcotte B., Quirin-Stricker C., Bocquel M. T., Meyer M. E., Krozowski Z., Jeltsch J. M., Lerouge T., Garnier J. M., Chambon P. The chicken progesterone receptor: sequence, expression and functional analysis. EMBO J. 1987 Dec 20;6(13):3985–3994. doi: 10.1002/j.1460-2075.1987.tb02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Brownstein M. J. Use of a cDNA clone to identify a supposed precursor protein containing valosin. Nature. 1987 Feb 5;325(6104):542–545. doi: 10.1038/325542a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Matlashewski G. J., Tuck S., Pim D., Lamb P., Schneider J., Crawford L. V. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol. 1987 Feb;7(2):961–963. doi: 10.1128/mcb.7.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley J. W., Mahy B. W. Structure and function of the influenza virus genome. Biochem J. 1983 May 1;211(2):281–294. doi: 10.1042/bj2110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. J., Buhl W. J., Jackson R. J. A rabbit reticulocyte factor which stimulates protein synthesis in several mammalian cell-free systems. Biochim Biophys Acta. 1985 May 24;825(1):57–69. doi: 10.1016/0167-4781(85)90079-x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Sykes J. M., Hunt T. Characteristics of a coupled cell-free transcription and translation system directed by vaccinia cores. Eur J Biochem. 1978 Jan 2;82(1):199–209. doi: 10.1111/j.1432-1033.1978.tb12012.x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Emmert A. Modulation of the expression of poliovirus proteins in reticulocyte lysates. Virology. 1986 Jan 30;148(2):255–267. doi: 10.1016/0042-6822(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Priess H., Zillig W. Inhibitor für pankreatische Ribonuclease aus roten Blutzellen. Hoppe Seylers Z Physiol Chem. 1967 Jul;348(7):817–822. [PubMed] [Google Scholar]