Abstract
In animals, microRNAs (miRNAs) generally repress gene expression by binding to sites in the 3′-untranslated region (UTR) of target mRNAs. miRNAs have also been reported to repress or activate gene expression by binding to 5′-UTR sites, but the extent of such regulation and the factors that govern these different responses are unknown. Liver-specific miR-122 binds to sites in the 5′-UTR of hepatitis C virus (HCV) RNA and positively regulates the viral life cycle, in part by stimulating HCV translation. Here, we characterize the features that allow miR-122 to activate translation via the HCV 5′-UTR. We find that this regulation is a highly specialized process that requires uncapped RNA, the HCV internal ribosome entry site (IRES) and the 3′ region of miR-122. Translation activation does not involve a previously proposed structural transition in the HCV IRES and is mediated by Argonaute proteins. This study provides an important insight into the requirements for the miR-122–HCV interaction, and the broader consequences of miRNAs binding to 5′-UTR sites.
INTRODUCTION
MicroRNAs (miRNAs) are 21–23 nt non-coding RNA molecules that are expressed by a broad range of eukaryotic species and are important regulators of many cellular processes (1,2). Animal miRNAs generally repress gene expression by binding to imperfectly complementary sites in the 3′-untranslated regions (UTRs) of target mRNAs. The mechanism of repression is not fully understood, although both translation inhibition and mRNA degradation are implicated (1,2). miRNAs function in association with a complex of proteins, including an Argonaute (Ago) protein (3), known as the miRNA-induced silencing complex (miRISC). Immunoprecipitation of RNA regions bound by Ago and bioinformatic analysis indicate that miRNA target sites may also be located in the open reading frame (ORF) and to a lesser extent in the 5′-UTR (4–6). Although miRNA repression via sites in actively translated ORFs may be inhibited by translating ribosomes that displace the miRISC (7), there are several mammalian examples of miRNAs that mediate repression by binding to sites in the ORF of target mRNAs (8–11).
A few experimental studies have shown miRNAs to regulate gene expression by binding to 5′-UTRs. Both positive and negative effects were observed and it is not clear what drives these different responses. Repression of protein synthesis is directed by let-7 binding to multiple sites located upstream of the hepatitis C virus (HCV) internal ribosome entry site (IRES) in the 5′-UTR of a reporter mRNA (12). Repression via six 5′-UTR sites in a cap-dependent reporter mRNA is mediated by Drosophila miR-2 (13). Such repression can also occur in cellular mRNAs, as a human cytomegalovirus miRNA down-regulates expression of a number of cellular proteins by binding to sites in the 5′-UTR of mRNAs (14). Positive regulation via 5′-UTR sites was observed for miR-10a, which interacts directly with the 5′ TOP motif of ribosomal protein mRNAs and is involved in the serum-dependent translational activation of these messages (15), while miR-346 binds to a single site in the receptor-interacting protein 140 (RIP140) 5′-UTR and activates translation independently of Ago proteins (16). A detailed analysis of the mechanisms mediated by miRNAs binding to 5′-UTR sites is necessary to resolve the different outcomes observed in these studies.
An important example of a miRNA that targets a 5′-UTR is the liver-specific miR-122, which binds to two adjacent sites upstream of the IRES in HCV genomic RNA (Figure 1A) and is essential for HCV replication in cultured cells (17). HCV is a positive sense RNA virus with a 9.6 kb genome that establishes persistent infections in the liver, eventually leading to cirrhosis and hepatocellular carcinoma (18). Following entry into cells, HCV RNA first serves as a template for translation of viral proteins, which then mediate replication of the viral RNA via a negative strand intermediate. Sequestration of miR-122 by a locked nucleic acid (LNA)/DNA antisense oligonucleotide reduced HCV titre in chronically infected chimpanzees (19). The mechanism of regulation is not fully understood; miR-122 stimulates translation via the HCV 5′-UTR (20), but this is not sufficient to explain in full the effects of miR-122 on HCV replication, implying that a second regulatory process also occurs (21).
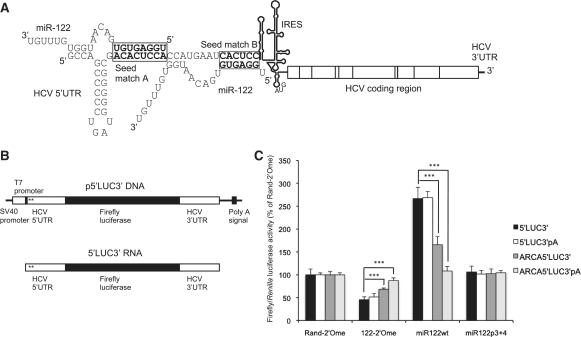
Figure 1.
miR-122-dependent activation of HCV 5′-UTR reporter RNA translation is substantially reduced by a cap and poly (A) tail. (A) Schematic of HCV RNA with the structure of the IRES and the sequence of nucleotides 1–45, containing the two miR-122 seed matches, shown in detail (genotype 1a). A model for two molecules of miR-122 binding via the seed and nucleotides 14–16, as proposed by Machlin et al. (33), is shown. (B) The structure of the p5′LUC3′ plasmid and the 5′LUC3′ reporter RNA synthesized from this plasmid using the T7 promoter. The locations of the two miR-122 seed matches are marked with asterisks. (C) 5′LUC3′ RNA with or without an ARCA cap or a poly (A) tail was introduced into Huh7 cells by transfection with a capped, polyadenylated Renilla luciferase RNA transfection control, in combination with a randomized control 2′-O-methylated oligonucleotide (Rand-2′Ome), an antisense 2′-O-methylated oligonucleotide to sequester miR-122 (122-2′Ome), a wild-type miR-122 duplex (miR122wt), or a miR-122 duplex with mutations at p3 + 4 of the seed (miR122p3 + 4). Repression by 122-2′Ome or activation by miR122wt was unaffected by a poly (A) tail alone, but significantly inhibited by an ARCA cap (***P < 0.0001 compared to uncapped 5′LUC3′ RNA). The ARCA cap and poly (A) tail in combination abolished activation of translation by miR122wt, although repression by 122-2′Ome was still significant (P < 0.0001 compared to Rand-2′Ome). Data are shown as firefly/Renilla luciferase activity at 6 h post-transfection and expressed as a percentage of the Rand-2′Ome control for each RNA. All values are averages of three independent triplicate experiments, with error bars representing standard deviation.
In this study, we have analyzed the requirements for miR-122 to stimulate translation via the HCV 5′-UTR in order to understand this important host–virus interaction and to gain insight into the broader consequences of miRNAs binding to 5′-UTR sites. We used a luciferase reporter RNA flanked by the HCV 5′- and 3′-UTRs to dissect individual components of this regulation (Figure 1B), as miR-122 effectively stimulates translation of such an RNA (20). This allowed us to determine the requirements for a functional miR-122-HCV interaction without complication from RNA sequence elements involved in other aspects of the HCV life cycle. We observe specific requirements for uncapped RNA, the HCV IRES and the 3′ region of miR-122 for this regulation to occur. These features suggest that miRNA-mediated translational stimulation via 5′-UTR sites is a highly specialized mechanism that is unlikely to be widely used in cellular mRNAs, but could be applicable to other viral systems. Finally, it has been shown in vitro that miR-122 binding mediates a structural transition in the viral IRES that was proposed to govern translational stimulation by the miRNA (22). We see no evidence for this mechanism in cultured cells. Our data suggest instead that this activation is promoted directly by an Ago-containing miRISC.
MATERIALS AND METHODS
Plasmid constructs
To generate p5′LUC3′, the T7 promoter and the HCV 5′-UTR, including 11 amino acids of coding sequence, were amplified from the plasmid pH77ΔE1/p7 (a gift from Stan Lemon) (23) using the primers 5′-UTR F and 5′-UTR R and the HCV 3′-UTR was amplified using 3′-UTR F and 3′-UTR R. These PCR products were inserted flanking the coding region of the firefly luciferase expression vector pGL3-MCS (24) on HindIII-NcoI and SpeI-EcoRI respectively. The HCV 5′-UTR was replaced with a longer variant including coding sequence to nucleotide 538, amplified with 5′-UTR F and 5′core R, to generate p5′coreLUC3′. Similarly, 1–45 R was used as a reverse primer to amplify only nucleotides 1–45 of the HCV 5′-UTR and create p1–45LUC3′. The CSFV IRES (Alfort Tübingen strain) from domain II to amino acid 19 of coding sequence and the minimal FMDV IRES (01 k strain) were amplified from dicistronic plasmids (gifts from Richard Jackson) using the primers CSFV F and CSFV R, or FMDV F and FMDV R, respectively. These IRESs were inserted at the NcoI site in p1–45LUC3′ to create p1–45CSFVLUC3′ and p1–45FMDVLUC3′.
Mutagenesis of the miR-122 binding region was carried out by overlap PCR and ligation of the mutant region in place of the wild-type sequence between the KpnI and NarI sites in p5′LUC3′. The outer primers used for the generation of all mutants were Kpn F and LUC R and the internal primers have the appropriate name for the mutation incorporated. Mutagenesis of pH77ΔE1/p7 was carried out by the same method, except that the external primers were H77Xmn F and H77Kpn R and the mutant PCR product was ligated into pH77ΔE1/p7 between the XmnI and KpnI sites. To generate pHCV/CSFVLUC3′, p5′LUC3′ was mutated to incorporate a BssHII site at the 3′-end of domain II of the HCV IRES. CSFV IRES domain III was then amplified from p1–45CSFVLUC3′ using CSFVIII F and LUC R and inserted between the BssHII and NarI sites in place of the equivalent HCV sequence. p5′coreLUC3′ was used as a template to create p5′mutLRALUC3′.
To generate the luciferase sensor plasmids pLUC21si, pLUC122si, pLUC21/122si and pLUC21/26asi, the forward primer LUC F and mutant reverse primers containing an exactly complementary target for each miRNA were used with pGL3-MCS as a template. The mutant sequences were inserted in pGL3-MCS between the NarI and EcoRI sites, such that the complementary miRNA target sites were in the 3′-UTR.
All primers were purchased from Invitrogen and the sequences are shown in Supplementary Table S1. pSV40-RL (Promega) was used as a transfection control.
In vitro transcription
The plasmid p5′LUC3′ and its derivatives were linearized with EcoRI and all other plasmids with XbaI. RNA was synthesized using the T7 Megascript kit (Applied Biosystems). To generate transcripts with poly (A) tails, the poly (A) tailing kit (Applied Biosystems) was then used. To synthesize m7G- or A-capped transcripts, anti-reverse cap analogue (ARCA) (Applied Biosystems) or A(5′)ppp(5′)G (NEB) was included in the reaction mix at 3.75 mM and the GTP concentration was reduced to 3.75 mM. 5′-monophosphorylated RNA was generated by treating 5′-triphosphophorylated RNA with calf intestinal alkaline phosphatase (NEB). RNA integrity was confirmed by denaturing agarose gel electrophoresis.
RNA and 2′-O-methylated oligonucleotides
Synthetic miRNAs were introduced into cells as duplexes based on the structure of the Dicer cleavage product of the pre-miRNA. These were generated by annealing the miRNA to the appropriate miRNA* sequence. Where miRNA mutations resulted in disruption of the duplex structure, appropriate mutant miRNA* molecules were synthesized to restore base-pairing. The sequences of 2′-O-methylated and RNA oligonucleotides (synthesized by Dharmacon) are shown in Supplementary Tables S2 and S3.
Cell culture and transfection
Huh7 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 1% non-essential amino acids and l-glutamine (Invitrogen). Lipofectamine 2000 (Invitrogen) was used to transfect cells in 24-well plates. Plating volumes of 1 ml containing 0.2 µg firefly luciferase reporter RNA or plasmid DNA, 0.01 µg capped, polyadenylated Renilla luciferase control RNA or pSV40-RL DNA and 20 nM 2′-O-methylated oligonucleotide or miRNA duplex were assembled and 300 µl applied to each of three wells. Samples were harvested after 6 h (RNA transfections), 24 h (luciferase sensor DNA transfections) or 48 h (p5′LUC3′ DNA transfections). Luciferase activity was determined using the Dual Luciferase Assay system (Promega) in a Glomax 96 microplate luminometer (Promega).
For electroporation, 5 × 106 Huh7 cells were trypsinized, washed once in phosphate-buffered saline and once in electroporation medium and resuspended in 1 ml electroporation medium [75% cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4, 25 mM HEPES, 2 mM EDTA, 5 mM MgCl2, pH 7.6), 25% phosphate-buffered sucrose (277 mM sucrose, 7 mM KH2PO4, 1 mM MgCl2, pH 7.4)], containing 2 µg luciferase RNA, 0.1 µg capped, polyadenylated Renilla luciferase control RNA and 200 nM 2′-O-methylated oligonucleotide or 5 µg H77ΔE1/p7 RNA. Cells were pulsed twice in a 0.4 cm gap width cuvette at 900 V, 25 µF, and ∞ resistance and incubated at room temperature for 10 min before plating. Cells electroporated with luciferase RNA were harvested at 6 h. Cells containing H77ΔE1/p7 RNA were transfected with miRNA duplexes using lipofectamine 2000 at Days −2 and +1 and harvested immediately after electroporation at Day 0, and at Day +4, for m21A + B experiments. Oligonucleotide and duplex transfections were carried out at Day +3 and the cells harvested at Day +5 for mutLRA experiments.
RNA isolation and northern blotting
RNA was harvested using TRI Reagent (Sigma). Northern blotting was carried out as described (24), using random-primed 32P-labeled DNA probes corresponding to nucleotides 84–374 of the HCV IRES, the entire coding region of firefly luciferase, or nucleotides 685–1171 of γ-actin.
RNA interference
siRNAs were introduced into Huh7 cells in 6 cm plates at 20 nM total siRNA concentration using lipofectamine RNAiMax (Invitrogen). Following a second siRNA hit at 48 h, cells were split into 24-well plates for transfection and 6-well plates to harvest RNA. Transfections with 5′LUC3′ RNA were carried out at 72 h post RNAi. siRNAs with published sequences were used to target the Ago mRNAs (25) and TNRC6B (26), and were synthesized by Dharmacon. SMARTpool siRNAs were used to target TNRC6A and Dicer and the control siRNA was ON-TARGETplus Non-targeting siRNA #3 (Dharmacon).
Quantitative RT–PCR
Total RNA extracted from cells following RNAi was subjected to reverse transcription with Superscript III (Invitrogen). The resulting cDNA was analyzed by quantitative PCR using GoTaq (Promega) in a Stratagene Mx3005P machine (Agilent Technologies). Primer sequences are shown in Supplementary Table S1.
Statistical analysis
All statistical analysis was carried out using two-tailed Student's t-test for unpaired samples of equal variance. P-values of <0.01, <0.001 and <0.0001 were considered to represent degrees of significance.
RESULTS
miR-122-dependent stimulation of HCV IRES-driven translation is impeded by an m7G cap
To test the requirements for miR-122 to activate translation via the HCV 5′-UTR, we constructed a reporter plasmid, p5′LUC3′, in which the firefly luciferase coding region is flanked by the complete HCV 5′- and 3′-UTRs (genotype 1a) (Figure 1B). This plasmid was linearized downstream of the HCV 3′-UTR and in vitro transcription carried out using the T7 promoter upstream of the HCV 5′-UTR. The resulting 5′LUC3′ RNA has the exact 5′-end of HCV (Figure 1B). HCV RNA does not have a 5′ cap (27), so in vitro transcribed uncapped 5′LUC3′ RNA mimics the viral 5′-UTR.
This reporter RNA was introduced into Huh7 human liver cells by liposome-mediated transfection in combination with an antisense 2′-O-methylated oligonucleotide to sequester miR-122 (122-2′Ome), a randomized control 2′-O-methylated oligomer (Rand-2′Ome), a synthetic wild-type miR-122 duplex (miR122wt) or a control miR-122 duplex with mutations at positions 3 and 4 to abolish target interaction (miR122p3 + 4). These molecules have previously been used to effectively regulate miR-122 activity (17,24). An in vitro transcribed Renilla luciferase mRNA, with a cap and poly (A) tail introduced in vitro to allow efficient translation, was included in all transfection experiments as a control, as Renilla luciferase activity was unaffected by miR-122 (Supplementary Figure S1A). Firefly/Renilla luciferase activity was determined as an average of three independent experiments, each performed in triplicate and shown as a percentage of Rand-2′Ome values for this and subsequent experiments. miR-122 inhibition led to a decrease in firefly luciferase expression and miR122wt overexpression to an increase, similar to the observations of Henke et al. (20) (Supplementary Figure S1B). miR-122 stimulates luciferase expression at the level of translation, as RNA levels and integrity were unaffected, as determined by either transfection and subsequent electrophoresis of radiolabeled 5′LUC3′ RNA, or qPCR with primers directed against different regions of the reporter RNA (Supplementary Figure S2). Quantification of RNA following liposome-mediated translation may give inaccurate results (28), so the 5′LUC3′ reporter RNA was also introduced into Huh7 cells by electroporation. miR-122 sequestration inhibited luciferase expression in electroporated cells without affecting the RNA level, confirming that miR-122 activates translation (Supplementary Figure S3). The intracellular RNA level was not important for miR-122 regulation, as electroporated and lipofected RNA showed the same regulation despite different transfection efficiencies. Subsequent experiments were carried out using liposomal transfection.
When p5′LUC3′ DNA was introduced into Huh7 cells by transfection, luciferase expression was unaffected by miR-122 (Supplementary Figure S4) (20). This suggested that features of the mRNA produced in transfected cells, such as the 5′ 7-methylguanosine (m7G) cap and the poly (A) tail, might impede miR-122-dependent translation stimulation. This hypothesis was investigated by synthesis of 5′LUC3′ reporter RNA bearing a 5′ m7G cap, incorporated using ARCA to ensure the correct orientation of the cap structure, and/or a poly (A) tail. These RNAs were introduced into Huh7 cells with or without sequestration or overexpression of miR-122. We observed a significant reduction in the response to miR-122 in ARCA-capped RNA compared to uncapped RNA, whereas a poly (A) tail alone did not affect regulation by miR-122 (Figure 1C). The combination of an ARCA cap and a poly (A) tail reduced the response to miR-122 more than an ARCA cap alone, such that the RNA was completely resistant to translational stimulation by miR122wt and only showed 9% inhibition on transfection of 122-2′Ome (Figure 1C). The inhibitory effect of a 5′ m7G cap on miR-122 regulation is not due to a specific requirement for the 5′-triphosphate generated by T7 RNA polymerase, as 5′LUC3′ RNA bearing an inactive 5′ ApppG cap or a 5′-monophosphate showed similar miR-122-dependent translation activation to 5′-triphosphorylated 5′LUC3′ RNA (Supplementary Figure S5). This implies that inhibition of miR-122 regulation is due to specific functions of the m7G cap, such as recruitment of the eIF4F complex, and suggests it is enhanced by the RNA circularization that occurs when an mRNA is both capped and polyadenylated.
A specific role for the HCV IRES in supporting activation of translation by miR-122
Stimulation of translation by miR-122 only requires the 5′-UTR of HCV RNA, as it was unaffected when the HCV 3′-UTR in our reporter RNA was replaced by a poly (A) tail (data not shown), so we analyzed the role of 5′-UTR elements in miR-122 regulation. HCV translation is driven by an IRES, a structured region of the 5′-UTR that mediates cap-independent translation initiation by directly recruiting 40S ribosomal subunits and eIF3 and does not require most of the canonical eukaryotic translation initiation factors (29). We tested the possibility that the mode of translation initiation influences miR-122 regulation by replacing the HCV IRES in the p5′LUC3′ plasmid with related or unrelated viral IRES sequences. The classic swine fever virus (CSFV) or foot and mouth disease virus (FMDV) IRES was inserted downstream of nucleotides 1–45 of the HCV 5′-UTR, which contain the miR-122 binding sites (Figure 2A). The FMDV IRES has a distinct sequence, structure and mechanism to the HCV IRES, requiring all the canonical translation initiation factors except eIF4E and some additional trans-acting factors (30). By contrast, the secondary structure and mechanism of the CSFV IRES are very similar to those of the HCV IRES (29). Reporter RNA synthesized from these plasmids was introduced into Huh7 cells with sequestration or overexpression of miR-122 (Figure 2C). RNA molecules containing either the CSFV or FMDV IRES showed a considerable reduction in the response to miR-122 compared to the wild-type HCV IRES-driven 5′LUC3′ RNA. The responses of the CSFV and FMDV IRESs did not differ significantly. This indicates that there is a specific role for the HCV IRES in effective miR-122-mediated translation activation that is not due to its mechanism of translation initiation.
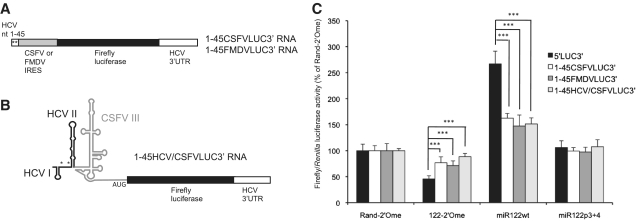
Figure 2.
The HCV IRES is necessary for full regulation of translation by miR-122. (A) The HCV IRES was excised from the plasmid encoding 5′LUC3′ RNA and replaced by either the CSFV or FMDV IRES downstream of HCV nucleotides 1–45, which contain the miR-122 binding sites. (B) The HCV IRES downstream of HCV nucleotides 1–45 was replaced by a chimeric IRES in which HCV IRES domain II was fused to CSFV IRES domain III. (C) RNA synthesized from these plasmids was introduced into Huh7 cells by transfection with sequestration or overexpression of miR-122, with a capped, polyadenylated Renilla luciferase RNA transfection control. Average firefly/Renilla luciferase activity from three independent triplicate experiments is shown as a percentage of the Rand-2′Ome values for each RNA. Error bars represent standard deviation. The CSFV, FMDV and chimeric IRES RNAs all showed a significantly reduced response to sequestration and overexpression of miR-122 compared to 5′LUC3′ RNA (***P < 0.0001).
We took advantage of the similar structures of the HCV and CSFV IRESs to construct a reporter RNA with a chimeric IRES, in which domains I (including the miR-122 binding sites) and II of the HCV 5′-UTR are fused to domain III of the CSFV IRES (Figure 2B). This RNA showed a reduction in miR-122-dependent translation stimulation compared to 5′LUC3′ RNA, similar to that observed with the full CSFV IRES (Figure 2C). Full translation stimulation by miR-122 therefore shows a specific requirement for HCV IRES sequence or structural features downstream of domain II.
The 3′ region of miR-122 is necessary for activation of translation
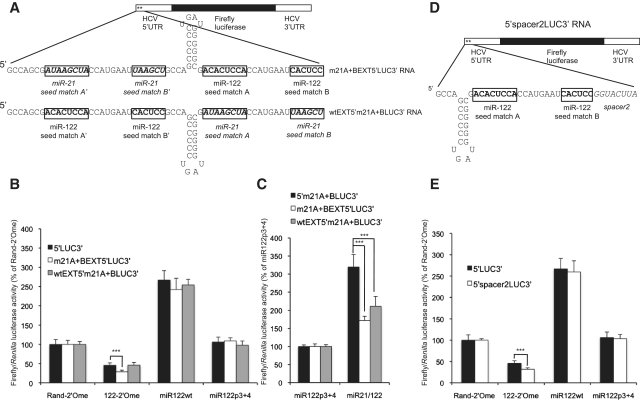
As miRNAs binding to 5′-UTRs mediate different responses in different studies, we considered the possibility that the identity of the miRNA might influence the mechanism used. The major determinant of a functional miRNA interaction is exact Watson–Crick base-pairing of nucleotides 2–7 or 2–8 of the miRNA, known as the seed, to the target site (31). To test whether a different miRNA can replace miR-122 in translational stimulation, we mutated both miR-122 seed matches in the p5′LUC3′ reporter plasmid to miR-21 seed matches (Figure 3A). In vitro transcribed 5′m21A + BLUC3′ mutant RNA was introduced into Huh7 cells, which express miR-21, with 2′-O-methylated complementary oligonucleotides to sequester either miR-122 or miR-21 (Figure 3B). Sensor plasmids with exactly complementary sites for miR-21 or miR-122 in the luciferase 3′-UTR confirmed that both inhibitors were effective (Supplementary Figure S6B). Luciferase activity from 5′m21A + BLUC3′ RNA was not affected by sequestration of miR-21 (Figure 3B), indicating that endogenous miR-21 cannot activate translation via miR-21 seed matches in the HCV 5′-UTR.
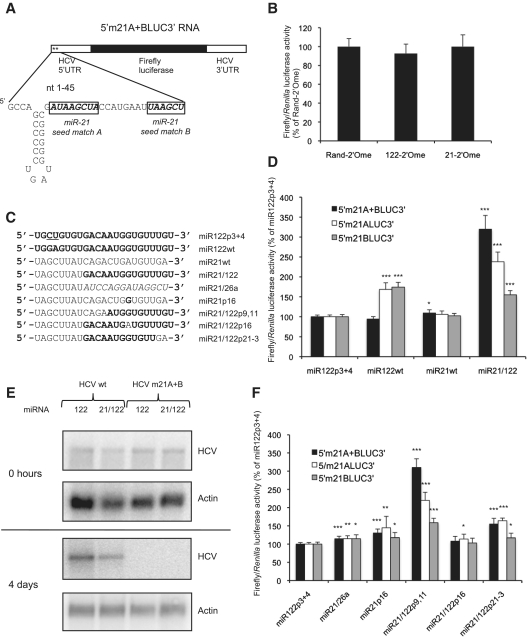
Figure 3.
The 3′ region of miR-122 is necessary for stimulation of translation. (A) 5′m21A + BLUC3′ RNA was generated from a p5′LUC3′ plasmid in which both miR-122 seed matches were mutated to miR-21 seed matches. (B) 5′m21A + BLUC3′ RNA and the Renilla luciferase control RNA were introduced into Huh7 cells with 2′-O-methylated antisense oligonucleotides to sequester either miR-122 or miR-21 (122-2′Ome or 21-2′Ome), or a randomized control (Rand-2′Ome), and showed no response to endogenous miR-21. Average firefly/Renilla luciferase activity across three independent triplicate experiments is shown as a percentage of the Rand-2′Ome values. Error bars represent standard deviation. (C) The sequences of transfected miRNAs. (D) RNA molecules with miR-21 seed matches at site A, site B, or A + B were introduced into Huh7 cells with overexpression of miR122p3 + 4, miR122wt, miR21wt or a miR21/122 chimera in which nucleotides 1–8 of miR-21 are fused to nucleotides 9–23 of miR-122. (E) Both miR-122 seed matches were mutated to miR-21 seed matches in a plasmid encoding a replication-competent H77ΔE1/p7 HCV RNA. Wild-type and mutant RNAs were introduced into Huh7 cells by electroporation, with transfection of miR122wt or miR21/122. HCV RNA was detected by northern blotting, with γ-actin as a loading control. At 4 days post electroporation, m21A + B mutant HCV did not replicate to a detectable level even when miR21/122 was present in the cells. (F) Similar to (D), except that individual nucleotides were mutated to test more precisely the requirements for translation activation. For both (D) and (F), luciferase activity is shown as in (B) but as a percentage of miR122p3 + 4 values for each RNA (*P < 0.01; **P < 0.001; ***P < 0.0001 compared to miR122p3 + 4). miR21/122 effectively stimulated translation via the miR-21 seed matches and nucleotide 16 and 21–23 of miR-122 were important for this regulation.
To test whether miR-21 is unable to substitute for miR-122 because miRNA features other than seed complementarity are required, we synthesized a chimeric miRNA in which the first 8 nt of miR-21 (the seed) are fused to nucleotides 9–23 of miR-122 (Figure 3C). This was annealed to a chimeric passenger strand to generate a chimeric miR21/122 duplex, which was effectively incorporated into miRISC following transfection as it targeted a luciferase sensor mRNA (Supplementary Figure S6C). This miR21/122 chimera strongly stimulated translation of 5′m21A + BLUC3′ RNA (Figure 3D), whereas overexpression of wild-type miR-21 only resulted in a very small increase in translation that is unlikely to be biologically relevant. This indicates that elements within nucleotides 9–23 of miR-122 are required for activation of translation, but that the only important feature of the miRNA seed is its complementarity to the target site. Examination of reporter RNAs with a single miR-21 seed match indicated that, whereas miR-122 regulated translation to a similar extent via both seed matches, activation of translation by miR21/122 was more efficient via miR-21 seed match A than seed match B (Figure 3D).
We have previously shown that a full length HCV RNA in which seed match A is mutated to a miR-21 seed match does not replicate in cells containing endogenous miR-21 (24). This fits with the requirement we now observe for the 3′ region of miR-122. To test this further, we mutated both miR-122 seed matches to miR-21 seed matches in a plasmid encoding a replication-competent HCV RNA that has the E1-p7 coding region deleted to prevent formation of infectious virus (genotype 1a, strain H77) (23). This mutant RNA was introduced into Huh7 cells with overexpression of miR122wt or miR21/122, but did not replicate to a detectable level even in the presence of miR21/122 (Figure 3E). Replication of wild-type H77ΔE1/p7 RNA was stimulated by transfection with wild-type miR-122, indicating that miRNA overexpression was effective (Figure 3E). This suggests that, in the context of replicating HCV, the sequence of the miR-122 binding region is critical for other aspects of the HCV life cycle in addition to miR-122 interaction.
The role of the 3′ region of miR-122 in translation activation was further probed by the cotransfection of additional miRNA mutants with 5′m21A + BLUC3′, 5′m21ALUC3′ and 5′m21BLUC3′ RNA. To exclude the possibility that the 3′ region of miR-21 specifically prevents translation activation, a second chimeric miRNA, in which the seed of miR-21 is fused to the 3′ region of miR-26a, was tested and only slightly enhanced translation (miR21/26a, Figure 3F). Contiguous 4 nt pairing starting at nucleotides 12, 13 or 14 of a miRNA is a feature of some miRNA target sites (32) and an interaction between GCCA at nucleotides 1–4 of the HCV 5′-UTR and UGGU at nucleotides 14–17 of miR-122 binding to site A was recently shown to be essential for miR-122 to regulate HCV replication (Figure 1A) (33). The requirement for the 3′ region of miR-122 to activate translation might be due to a need for base-pairing between these nucleotides. miR-21 mutated to give the miR-122 UGGU sequence at nucleotides 14–17 in place of the miR-21 UGAU sequence mediated a slight enhancement in translation of all three reporter RNAs (miR21p16, Figure 3F). This suggests that interaction with nucleotides 2–4 of HCV may make a contribution to translation stimulation, but there is an additional role for the 3′ region of miR-122 in this regulation other than interaction with these nucleotides. The slight stimulation mediated by miR21/26a could be due to base-pairing between the GG at nucleotide 14–15 of miR21/26a (Figure 3C) and nucleotides 2 and 3 of HCV.
Individual nucleotides that differ between miR-21 and miR-122 were mutated in the miR21/122 chimera to determine the features of the 3′ region of miR-122 that allow translation stimulation. A chimera with nucleotides 9 and 11 of miR-122 mutated to the miR-21 sequence was as effective as miR21/122 in stimulating translation (miR21/122p9,11, Figure 3F). Nucleotide 16 of miR-122 was essential for activation of translation, which is likely to be due to an interaction with nucleotide 2 of HCV RNA as discussed above (miR21/122p16, Figure 3F). Interestingly, we found that nucleotides 21–23 of miR-122 also make a strong contribution to translation stimulation (miR21/122p21-3, Figure 3F). This is an important observation, as these nucleotides are not predicted to interact with the RNA target.
Target site sequence requirements for miR-122 to activate translation
The importance of nucleotide 16 of miR-122 for translation stimulation suggested that the interactions between nucleotides 14–17 of miR-122 and the HCV 5′-UTR that are required for miR-122 to regulate HCV replication (33) (Figure 1A) are also necessary for regulation of translation. This possibility was tested by mutagenesis of various sequence elements in 5′LUC3′ RNA.
Mutation of nucleotides 2–4 in the 5′LUC3′ reporter RNA (Figure 4A) resulted in a slight decrease in the response to miR-122 in Huh7 cells, suggesting that pairing to this sequence might also be important for miR-122 to activate translation (Figure 4B). We could not mutate the G at nucleotide 1 of 5′LUC3′ RNA as it is required by the T7 promoter. The nucleotides 2–4 mutation was combined with a second mutation at positions 3 and 4 of seed match B in order to distinguish between translation activation via seed matches A (mediated by miR122wt) and B (mediated by miR122p3 + 4) (Figure 4A). Translation of this mutant reporter RNA was activated by miR122p3 + 4 but not by endogenous or overexpressed wild-type miR-122 (Figure 4B), indicating that nucleotides 2–4 of the HCV 5′-UTR are required for translational activation via seed match A.
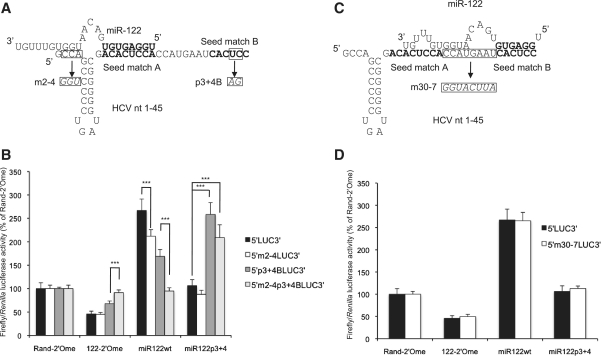
Figure 4.
The sequence of nucleotides 2–4 of HCV RNA is important for translational regulation by miR-122. (A) Model for a miR-122 molecule binding to seed match A in HCV nucleotides 1–45 and making additional contacts with nucleotides 1–4. This was tested by mutating nucleotides 2–4 in 5′LUC3′ RNA with or without mutations at p3 + 4 of seed match B. (B) The mutant RNAs in (A) were introduced into Huh7 cells by transfection, with sequestration or overexpression of miR-122. Mutation of nucleotides 2–4 abolished regulation by miR122 via seed match A in the context of a p3 + 4 mutant seed match B (***P < 0.0001). (C) Model for miR-122 binding to seed match B in HCV nucleotides 1–45 and making additional contacts with the spacer and an unoccupied seed match A. The spacer was mutated as shown. (D) 5′m30-7LUC3′ RNA was introduced into Huh7 cells with sequestration or overexpression of miR-122 and did not show a significant difference in the response to miR-122 compared to wild-type 5′LUC3′ RNA. All values are firefly luciferase activity relative to Renilla luciferase activity from the transfection control, as a percentage of Rand-2′Ome values for each RNA. The average of three independent triplicate experiments is shown, with error bars representing standard deviation.
A miR-122 molecule binding to seed match B could potentially pair with several nucleotides in the 8 nt spacer separating the two sites and also with nucleotides within an unoccupied seed match A (Figure 4C). We have shown previously that mutation or deletion of the two Cs in the spacer strongly inhibits replication of full length HCV RNA (24) and it was recently demonstrated that this requirement is due to an interaction with the GG motif at nucleotides 15 and 16 of miR-122 (33). Each nucleotide in the spacer was mutated to its complement in the 5′LUC3′ reporter RNA to disrupt any pairing to this region (Figure 4C). Following transfection into Huh7 cells, this mutant reporter (5′m30-7LUC3′) responded to miR-122 sequestration or overexpression to the same extent as the wild-type 5′LUC3′ reporter RNA (Figure 4D). Additionally, effective translation stimulation by miR21/122 binding to the 5′m21A + BLUC3′ reporter suggests that interaction between a miRNA bound to seed match B and sequence elements in seed match A is unlikely to be necessary for function.
Taken together, these mutagenesis experiments demonstrate that, although some base-pairing outside the miR-122 seed matches is important for translation stimulation, the 3′ region of miR-122 has a specific role in this regulation that is independent of target binding.
The role of target site location in miR-122-mediated activation of translation
As the location and spacing of the miR-122 seed matches in HCV is highly conserved (24), we tested whether the location of these sites relative to the RNA 5′-end and the HCV IRES is important. 5′LUC3′ RNA was extended at the 5′-end by a 31 nt sequence derived from the first 45 nt of the HCV 5′-UTR. Stem-loop I (SLI) is not required for miR-122 to activate translation (Supplementary Figure S7) so was not included in the extension. To distinguish between regulation via upstream and downstream sites, we tested versions of this reporter with miR-122 seed matches in either the extension or the original 5′-UTR replaced by miR-21 seed matches (Figure 5A). The presence of a 5′ extension slightly enhanced the response to 122-2′Ome via miR-122 sites in the original 5′-UTR, but this was the only difference in miR-122 regulation of these reporters and 5′LUC3′ RNA (Figure 5B), suggesting that site location is not critical for function. Interestingly, miR21/122 did not stimulate translation as effectively via seed matches in either of the extended RNA reporters as it did in 5′m21A + BLUC3′ RNA (Figure 5C). The reason for this is not clear, but the similar response of both extended reporters supports the conclusion that the location of the seed matches relative to either the 5′-end or the HCV IRES is not important, at least within the nucleotide lengths we have tested.
Figure 5.
The location of the miR-122 binding sites relative to the HCV IRES and 5′-end is not important for translation stimulation by miR-122. (A) 5′LUC3′ RNA was extended by 31 nucleotides at the 5′-end. The extension comprised nucleotides 1–45 of 5′LUC3′ RNA with SLI deleted. Variants were generated with either the miR-122 seed matches in the extension or in the original 5′-UTR replaced by miR-21 seed matches, so that regulation via upstream or downstream sites could be distinguished. (B) Both extended RNA reporters were introduced into Huh7 cells with sequestration of miR-122 or overexpression of miR122wt or miR21/122. Regulation by miR-122 via upstream or downstream seed matches was similar to that observed in 5′LUC3′ RNA. (C) Activation of translation by miR21/122 binding to miR-21 seed matches was significantly reduced in both extended RNAs compared to 5′m21A + BLUC3′ RNA. (D) A mutant 8 nt spacer sequence was introduced downstream of seed match B in 5′LUC3′ reporter RNA. (E) 5′spacer2LUC3′ RNA was introduced into Huh7 cells with sequestration or overexpression of wild-type miR-122. Repression by 122-2′Ome was slightly enhanced, but activation by miR122wt was unaffected. All data are shown as average firefly luciferase activity relative to Renilla luciferase activity from the transfection control for three independent triplicate experiments, as a percentage of Rand-2′Ome or miR122p3 + 4 values. Error bars represent standard deviation. (***P < 0.0001).
The influence of site location relative to the HCV IRES was further tested by insertion of an 8 nt spacer between seed match B and SLII of the IRES in 5′LUC3′ reporter RNA to generate 5′spacer2LUC3′ RNA (Figure 5D). This additional spacer sequence led to a slight increase in repression by sequestration of endogenous miR-122, but did not affect activation by overexpressed miR-122 (Figure 5E). Analysis of reporters with p3 + 4 mutant seed match A or seed match B indicated that the spacer did not consistently affect activation of translation via either seed match (Supplementary Figure S8). This supports our conclusion that a small increase in the distance between the miR-122 seed matches and the HCV IRES does not affect translation regulation.
miR-122 does not regulate HCV translation by disruption of an inhibitory interaction between the miR-122 binding sites and the HCV ORF
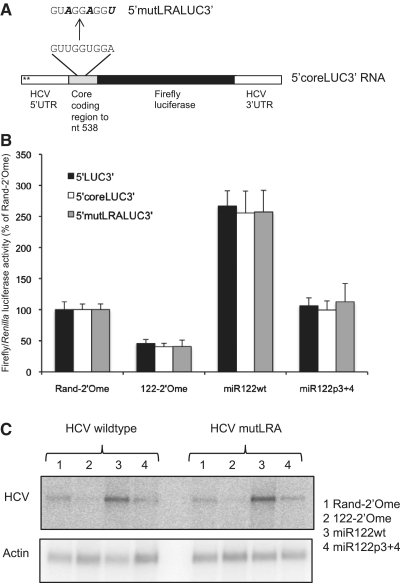
An in vitro structure probing study of HCV RNA suggested that miR-122 acts by disruption of an inhibitory long-range annealing (LRA) motif that forms between the miR-122 binding region and part of the core protein coding sequence (22). However, miR-122 stimulates translation in our 5′LUC3′ RNA, which does not include the ORF sequence predicted to form the LRA, suggesting that miR-122 may activate translation by an alternative mechanism. To test whether this interaction is involved in the response to miR-122 in Huh7 cells, part of the core coding sequence, including the region predicted to form the LRA, was fused to the HCV IRES in the plasmid encoding 5′LUC3′ RNA (Figure 6A). The 5′coreLUC3′ RNA synthesized from this plasmid responded to miR-122 in the same manner as 5′LUC3′ RNA (Figure 6B). The mutations that had previously been shown to enhance HCV IRES activity in a dicistronic luciferase reporter plasmid by disrupting the LRA, while maintaining the amino acid sequence (34), were introduced into the core coding sequence in 5′coreLUC3′ RNA (Figure 6A). These mutations had no effect on the response to miR-122, so translational regulation in this RNA reporter is not affected by this tertiary interaction (Figure 6B). We also introduced the same mutations into the core coding sequence in the plasmid encoding H77ΔE1/p7 RNA. We observed no difference in HCV replication in Huh7 cells or in the response to miR-122 between the wild-type and mutant RNAs (Figure 6C). We conclude that this particular LRA is not involved in activation of translation by miR-122. Our observation that translational regulation can still occur even when the binding site is extensively mutated implies that miR-122 is unlikely to function by disruption of alternative inhibitory interactions.
Figure 6.
miR-122 does not activate translation by disruption of a proposed long-range annealing (LRA) motif. (A) Part of the HCV core protein coding region, encompassing the sequence predicted to form an LRA with the miR-122 binding site, was fused in frame to the HCV IRES in p5′LUC3′ to generate a plasmid encoding 5′coreLUC3′ RNA. The 5′mutLRALUC3′ RNA has three point mutations that disrupt the LRA while maintaining the amino acid sequence. (B) 5′coreLUC3′ and 5′mutLRALUC3′ RNA were introduced into Huh7 cells with sequestration or overexpression of miR-122. Firefly luciferase activity is shown relative to Renilla luciferase activity of the transfection control as a percentage of the Rand-2′Ome values for each RNA. Data are an average of three independent triplicate experiments and error bars represent standard deviation. The responses of 5′coreLUC3′ and 5′mutLRALUC3′ to 122-2′Ome or miR122wt did not differ significantly to that of 5′LUC3′ RNA. (C) The mutation to the LRA shown in (A) was introduced into a plasmid encoding replication-competent H77ΔE1/p7 HCV RNA. Wild-type and mutant RNA were introduced into Huh7 cells and HCV RNA levels after 5 days were determined by northern blotting following sequestration or overexpression of miR-122. γ-actin RNA is shown as a loading control. The LRA mutation did not affect HCV RNA abundance or the response to miR-122.
The Ago proteins are required for miR-122 to activate translation via the HCV 5′-UTR
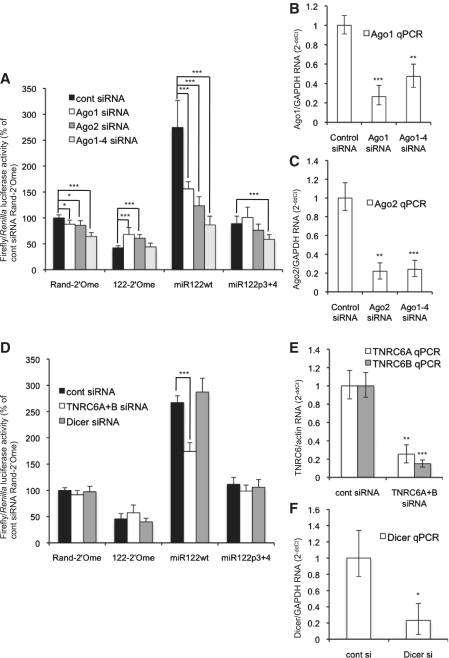
miRNA repression via a 3′-UTR requires an Ago protein, of which there are four in mammals, to function as part of the miRISC (3). The role for Ago proteins in regulation via 5′-UTR sites is less clear, as a miRNA that activates translation via a 5′-UTR site functions independently of Ago (16). Depletion of Ago proteins by RNAi resulted in inhibition of HCV replication (35) and Ago2 was shown to be necessary for miR-122 to stimulate both translation and replication of HCV (36). However, a recent study showing that miR-122 overexpression overcame the inhibition of HCV replication following Ago RNAi suggested an Ago-independent mechanism might operate (33). To test whether Ago proteins are necessary for miR-122 to activate translation in our system, we used RNAi to deplete Ago1 or Ago2 individually, or all four Ago proteins together, in Huh7 cells. The cells were then transfected with 5′LUC3′ RNA, with sequestration or overexpression of miR-122 (Figure 7A). RNAi was effective (Figure 7B and C) and depletion of Ago1, Ago2, or Ago1–4 led to a considerable reduction in translation activation by miR-122 compared to a control non-targeting siRNA transfection. The data are expressed relative to the Rand-2′Ome control in cells transfected with control siRNA and show that basal luciferase activity following sequestration of miR-122 did not decrease following RNAi to the Ago proteins (122-2′Ome, Figure 7A). This indicates that the Ago proteins are specifically required for miR-122 to activate translation in 5′LUC3′ and do not have other effects on HCV IRES-driven translation.
Figure 7.
Ago proteins are required for miR-122 to activate translation via the HCV 5′UTR. (A) Huh7 cells were transfected with siRNAs directed against Ago1, Ago2 or all four Ago mRNAs before transfection with 5′LUC3′ RNA and the Renilla luciferase control RNA and sequestration or overexpression of miR-122. Depletion of the Ago proteins reduced translational activation by miR-122. Average firefly/Renilla luciferase activity of three independent triplicate experiments is shown as a percentage of the Rand-2′Ome control in control siRNA-treated cells. (B) qPCR was carried out to detect Ago1 mRNA following RNAi to Ago1 or Ago1–4. Data are shown as 2−ΔΔCt relative to a GAPDH mRNA control as a percentage of the value obtained on control siRNA transfection and are an average of three independent experiments. (C) As (B), except that qPCR was used to detect Ago2 mRNA following RNAi to Ago2 or Ago1–4. RNAi against both Ago1 and Ago2 was effective. (D) Similar to (A), except that siRNAs directed against both TNRC6A and TNRC6B, or against Dicer, were used. RNAi to TNRC6A + B, but not Dicer, reduced activation by miR122wt. (E) qPCR was used to detect TNRC6A and TNR6CB mRNA. Data are expressed as 2−ΔΔCt relative to actin mRNA as an average of two independent RNAi experiments and indicate that RNAi was effective. (F) qPCR was used to detect Dicer mRNA following RNAi and average 2−ΔΔCt values of three independent experiments are shown relative to GAPDH mRNA. RNAi was effective. All error bars represent standard deviation. (*P < 0.01; **P < 0.001; ***P < 0.0001 compared to control siRNA).
The Ago proteins are involved in miRNA biogenesis and stability (37) and we observed a reduction in the level of endogenous miR-122 following Ago1–4 RNAi that might explain the reduction in regulation of 5′LUC3′ translation (data not shown). However, miR122wt was overexpressed in these experiments as a duplex, thus bypassing much of the miRNA biogenesis pathway, and substantially raised the cellular levels of miR-122 whether or not Ago1–4 RNAi was carried out (data not shown). Ago1–4 RNAi inhibited translation activation by overexpressed miR122wt (Figure 7A), implying that the requirement for the Ago proteins in this regulation is due to their function within the RISC and not their role in miR-122 biogenesis. This was supported by our observation that RNAi to the miRNA biogenesis enzyme Dicer did not affect regulation of 5′LUC3′ translation by miR-122 (Figure 7D) despite effective depletion (Figure 7F). We also tested the role of the GW182 proteins (TNRC6A-C in mammals), as these are essential for miRNA-mediated repression of gene expression (2). We used siRNAs targeting both TNRC6A and TNRC6B, a combination that effectively derepresses miRNA-targeted mRNAs (26). RNAi was effective (Figure 7E) and reduced the translational activation by miR122wt (Figure 7D). Depletion of these proteins did not have as much effect on miR-122-mediated regulation of 5′LUC3′ translation as Ago1–4 RNAi, so it is not clear whether TNRC6 proteins are essential for this regulation.
DISCUSSION
This study has expanded our understanding of the features governing the interaction between miR-122 and HCV RNA and the effects of miRNAs binding to 5′-UTR sites. Translational regulation in this system is a highly specialized process that is dependent on specific regions of both the HCV IRES and miR-122. It also requires uncapped RNA, which implies that miRNAs binding to 5′-UTR sites in capped cellular mRNAs are unlikely to use a similar mechanism of translational activation.
The inhibitory effect of a 5′ m7G cap that we observe is likely to explain the lack of regulation observed in plasmid DNA transfections (Figure 1C). As the miR-122 binding sites are very close to the 5′-end of the RNA, it is possible that the eIF4F protein factor complex recruited by the cap impedes interaction of miR-122-RISC with the RNA. It is also possible that the helicase eIF4A, which is part of eIF4F, could release bound miR-122 from the RNA. The stronger inhibition of the response to miR-122 in RNA bearing a poly (A) tail in addition to an ARCA cap could be explained by changes in the structure of the cap-binding complex when a poly (A) tail allows mRNA circularization. Although the HCV IRES is highly structured and would be expected to act as a barrier to scanning ribosomes, it is possible that this capped, polyadenylated mRNA might undergo cap-dependent translation initiation during which the IRES is unwound and would thus lose miR-122 regulation, for which the HCV IRES is necessary (Figure 2C). Current evidence indicates that HCV RNA is uncapped and has a phosphorylated 5′-end (27). The requirement for uncapped RNA for miRNA-dependent translational stimulation suggests that similar regulation would not occur in the context of cellular mRNAs, but has potential applicability to other uncapped viral RNAs. It is possible that cellular mRNAs in which translation is driven by an IRES might be subject to similar regulatory mechanisms if the miRNA binding sites were sufficiently distant from the cap to prevent interference by eIF4F.
Interestingly, we saw no repression of translation by either miR-122 or miR-21 binding to the 5′-UTR sites in our reporters following DNA or capped mRNA transfection (Supplementary Figure 4, and data not shown). This is in contrast to a study that showed repression mediated by miRNAs binding to 5′-UTR sites upstream of the HCV IRES in a reporter plasmid (12). The plasmid used by Lytle et al. (12) contained four miRNA binding sites, whereas our reporter only has two sites, which might explain this discrepancy. The number of sites in a 5′-UTR may be an important factor in allowing miRNA repression, as Drosophila miR-2 effectively represses translation via six, but not one or two, 5′-UTR sites in a reporter plasmid (13). However, a human cytomegalovirus (HCMV) miRNA predominantly targets 5′-UTRs of cellular mRNAs and mediates translational repression, for which single sites appear to be sufficient (14). This suggests that specific features of the target site or the miRNA may allow miRNAs to mediate repression via 5′-UTR sites.
Our results strongly imply that translation activation via the 5′-UTR requires specific features of both the miRNA and the target RNA. This regulation shows a requirement for the 3′ region of miR-122 that is independent of target interaction (Figure 3F). The importance of nucleotides 16 and 21–23 of miR-122 for translation regulation via both seed matches parallel the roles for these nucleotides in regulation of HCV replication observed by Machlin et al. (33) (Figure 3F). It has generally been thought that miRNAs function to recruit a common miRISC to distinct target sequences, based primarily on seed complementarity. However, recent evidence suggests that sequence features within a miRNA may have important roles in addition to target interaction. Some miRNAs are subject to 3′ modification, including miR-122, which has a single adenosine added to the 3′-end by GLD-2 that increases its stability (38). It is possible that there may be additional functional roles for this modification that are not achieved by miRNAs with different 3′-ends. The miR-122 3′-end may have a role in the recruitment of specific miRISC proteins or cofactors that mediate translation activation, or may form intra- or inter-molecular RNA–RNA interactions that are important for function, or affect subcellular localization. Further experimentation will be important to establish the role of the miR-122 3′-end in this regulation. The highly conserved sequence of many miRNAs, including miR-122, which has an identical sequence in humans and zebrafish, implies that features of the miRNA other than the seed sequence are important for function. It will be intriguing to see whether other miRNA 3′-ends allow specialized functions and whether the miR-122 3′-end is necessary for the regulation of additional miR-122 targets.
We find that the Ago proteins are required for miR-122 to activate translation via the HCV 5′-UTR (Figure 7A). This observation is supported by a recent study demonstrating that Ago2 is necessary for miR-122 to stimulate translation of HCV replicons (36), although the role of Ago proteins in miR-122-mediated regulation of HCV remains controversial (33). TNRC6 proteins also contribute to translation activation by miR-122, but do not have as great an effect on this process as Ago proteins (Figure 7D). This partial effect might be due to incomplete depletion of the proteins or to compensation by TNRC6C, although RNAi to TNRC6A and TNRC6B was sufficient to inhibit miRNA-mediated repression (26). The exact role of the TNRC6 proteins and the question of whether Ago proteins function as part of a specialized miRISC are currently under investigation.
The HCV IRES is a further major feature that is important for miR-122 to stimulate translation via a 5′-UTR (Figure 2C). The inability of the closely related CSFV IRES to substitute effectively for the HCV IRES suggests that IRES features other than the mechanism of translation initiation determine effective regulation by miR-122. The HCV and CSFV IRESs have numerous differences in sequence and a few differences in secondary structure (29), so it is possible that miR-122 regulates the formation of an HCV IRES-specific structure or interacts with HCV IRES-specific RNA sequence. miR-122 was shown to act by disrupting an inhibitory RNA structural motif in vitro (22), but we do not see any evidence for this in cells (Figure 6B and C). It is unlikely that miR-122 functions by disrupting a direct interaction between its binding sites and a different part of the HCV IRES, as translational stimulation can be effectively maintained when the miR-122 binding region is extensively mutated (Figures 3 and 4). It remains possible that miR-122 binding may mediate a switch to a more active conformation of the IRES in a manner that requires sequence or structural elements of HCV IRES domain III/IV. A second possibility is that one or more protein factors that bind specifically to the HCV IRES are involved in regulation by miR-122. We observe some miR-122-mediated translation stimulation when translation is driven by the CSFV or FMDV IRES. This suggests that features within the HCV IRES allow enhancement of a basal level of miRNA regulation, or that two distinct regulatory processes occur, only one of which requires the HCV IRES.
In general, there are few sequence elements within nucleotides 1–45 of HCV RNA that are necessary for miR-122 to stimulate translation. Both seed matches can be mutated to bind miR-21 and allow effective regulation by miR21/122 (Figure 3D) and activation of translation is not inhibited by mutagenesis of the spacer or deletion of SLI. An important exception is a requirement for nucleotides 2–4 of HCV RNA to allow translation stimulation via seed match A (Figure 4B), which is likely to be due to base-pairing to nucleotides 14–16 of miR-122. The location of the miR-122 binding sites relative to the 5′-end of the RNA and the HCV IRES does not appear to influence translational stimulation by miR-122 (Figure 5), but it is possible that additional increases in the distance between these elements might affect regulation.
The reporter system used in this study has been very useful in allowing us to test the roles of specific features of the RNA, such as the IRES, in allowing miR-122 to stimulate translation via the HCV 5′-UTR. It has now been established that miR-122 activates HCV IRES-driven translation in the context of a replicating virus, but that this translational regulation is not sufficient to explain the effect of the miRNA on viral replication (21). The other mechanism(s) by which miR-122 regulates HCV replication remains obscure, as new synthesis of HCV RNA measured by thiouridine labeling was not affected by miR-122 (39). It is possible that miR-122 interaction with HCV RNA changes during the viral life cycle, which would have consequences for experimental observations. Subcellular localization of both miR-122 and HCV RNA may also be important in governing the interaction and its effects. Our observations suggest that the requirements for translation activation are less stringent than those for full regulation of viral replication. Mutation of the spacer between the miR-122 seed matches dramatically reduces HCV replication but does not inhibit translation activation by miR-122 (24,33) (Figure 4D), while miR21/122 activates translation of a reporter RNA with miR-21 seed matches, but does not allow replication of m21A + B mutant HCV RNA (Figure 3D and E). This may be because extensive mutagenesis of the miR-122 binding region has other detrimental effects on the HCV life cycle, such as inhibiting recruitment of the viral RNA-dependent RNA polymerase (RdRP) to negative strand RNA. It is also possible that the requirements for the miR-122-HCV interaction are different in a replicating virus to those in a reporter RNA. Although the reporter system has limitations in determining the interactions that actually occur during viral replication, it has allowed us to establish in detail the minimum RNA requirements for miR-122 to interact functionally with the HCV 5′-UTR, in isolation from other roles for the HCV 5′-UTR in viral replication. This research represents an important step forward in our understanding of the mechanistic details of an essential host–virus interaction and reveals the importance of specialized features in determining miRNA activity.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR online.
FUNDING
Biotechnology and Biological Sciences Research Council, David Phillips Fellowship BB/F02360X/1 (to C.L.J.). Funding for open access charge: University of Nottingham.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Martin Bushell, David Heery and Anne Willis for critical reading of the manuscript and Richard Jackson and Stan Lemon for providing reagents.
REFERENCES
- 1.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 3.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol. Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr, Jungkamp AC, Munschauer M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnall-Levin M, Zhao Y, Perrimon N, Berger B. Conserved microRNA targeting in Drosophila is as widespread in coding regions as in 3′UTRs. Proc. Natl Acad. Sci. USA. 2010;107:15751–15756. doi: 10.1073/pnas.1006172107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009;16:144–150. doi: 10.1038/nsmb.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 9.Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14:872–877. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elcheva I, Goswami S, Noubissi FK, Spiegelman VS. CRD-BP protects the coding region of betaTrCP1 mRNA from miR-183-mediated degradation. Mol. Cell. 2009;35:240–246. doi: 10.1016/j.molcel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl Acad. Sci. USA. 2008;105:14879–14884. doi: 10.1073/pnas.0803230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl Acad. Sci. USA. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moretti F, Thermann R, Hentze MW. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA. 2010;16:2493–2502. doi: 10.1261/rna.2384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grey F, Tirabassi R, Meyers H, Wu G, McWeeney S, Hook L, Nelson JA. A viral microRNA down-regulates multiple cell cycle genes through mRNA 5′UTRs. PLoS Pathog. 2010;6:e1000967. doi: 10.1371/journal.ppat.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Tsai NP, Lin YL, Wei LN. MicroRNA mir-346 targets the 5′-untranslated region of receptor-interacting protein 140 (RIP140) mRNA and up-regulates its protein expression. Biochem. J. 2009;424:411–418. doi: 10.1042/BJ20090915. [DOI] [PubMed] [Google Scholar]
- 17.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 18.Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 19.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Junemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jangra RK, Yi M, Lemon SM. miR-122 Regulation of hepatitis C virus translation and infectious virus production. J Virol. 2010;84:6615–6625. doi: 10.1128/JVI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Toledano R, Ariza-Mateos A, Birk A, Martinez-Garcia B, Gomez J. In vitro characterization of a miR-122-sensitive double-helical switch element in the 5′ region of hepatitis C virus RNA. Nucleic Acids Res. 2009;37:5498–5510. doi: 10.1093/nar/gkp553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc. Natl Acad. Sci. USA. 2006;103:2310–2315. doi: 10.1073/pnas.0510727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jopling CL, Schutz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Huntzinger E, Braun JE, Heimstadt S, Zekri L, Izaurralde E. Two PABPC1-binding sites in GW182 proteins promote miRNA-mediated gene silencing. EMBO J. 29:4146–4160. doi: 10.1038/emboj.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Yamaji M, Hosaka M, Kishine H, Hijikata M, Shimotohno K. Analysis of the 5′ end structure of HCV subgenomic RNA replicated in a Huh7 cell line. Intervirology. 2005;48:104–111. doi: 10.1159/000081736. [DOI] [PubMed] [Google Scholar]
- 28.Barreau C, Dutertre S, Paillard L, Osborne HB. Liposome-mediated RNA transfection should be used with caution. RNA. 2006;12:1790–1793. doi: 10.1261/rna.191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CU. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilipenko EV, Pestova TV, Kolupaeva VG, Khitrina EV, Poperechnaya AN, Agol VI, Hellen CU. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 2000;14:2028–2045. [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 32.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machlin ES, Sarnow P, Sagan SM. Masking the 5′ terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex. Proc. Natl Acad. Sci. USA. 108:3193–3198. doi: 10.1073/pnas.1012464108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YK, Lee SH, Kim CS, Seol SK, Jang SK. Long-range RNA-RNA interaction between the 5′ nontranslated region and the core-coding sequences of hepatitis C virus modulates the IRES-dependent translation. RNA. 2003;9:599–606. doi: 10.1261/rna.2185603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl Acad. Sci. USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson JA, Zhang C, Huys A, Richardson CD. Human Ago2 is required for efficient miR-122 regulation of HCV RNA accumulation and translation. J. Virol. 2011;85:2342–2350. doi: 10.1128/JVI.02046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, Baba T. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev. 2009;23:433–438. doi: 10.1101/gad.1761509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman KL, Sarnow P. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by microRNA miR-122 involves distinct mechanisms. J. Virol. 2010;84:666–670. doi: 10.1128/JVI.01156-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.