Abstract
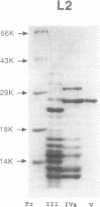
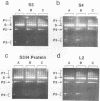
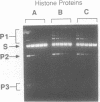
We report the purification of four proteins from Escherichia coli that stimulate or inhibit inter- and/or intramolecular recombination promoted by the yeast plasmid-encoded FLP protein. The proteins are identified as the ribosomal proteins S3 (27 kDa), L2 (26 kDa), S4 (24 kDa), and S5 (16 kDa), on the basis of N-terminal sequence analysis. The S3 protein is found to be identical to H protein, an E. coli histone-like protein that is related to histone H2A immunologically and by virtue of amino acid content. The H protein/S3 identity is based on co-migration on polyacrylamide gels, heat stability, amino acid analysis, and effects on FLP-promoted recombination. These results are relevant to current studies on the structure of the E. coli nucleoid. Since the H protein has previously been found associated with the E. coli nucleoid, the results indicate that either (a) some ribosomal proteins serve a dual function in E. coli, or, more likely, (b) ribosomal proteins can and are being mis-identified as nucleoid constituents.
Full text
PDF





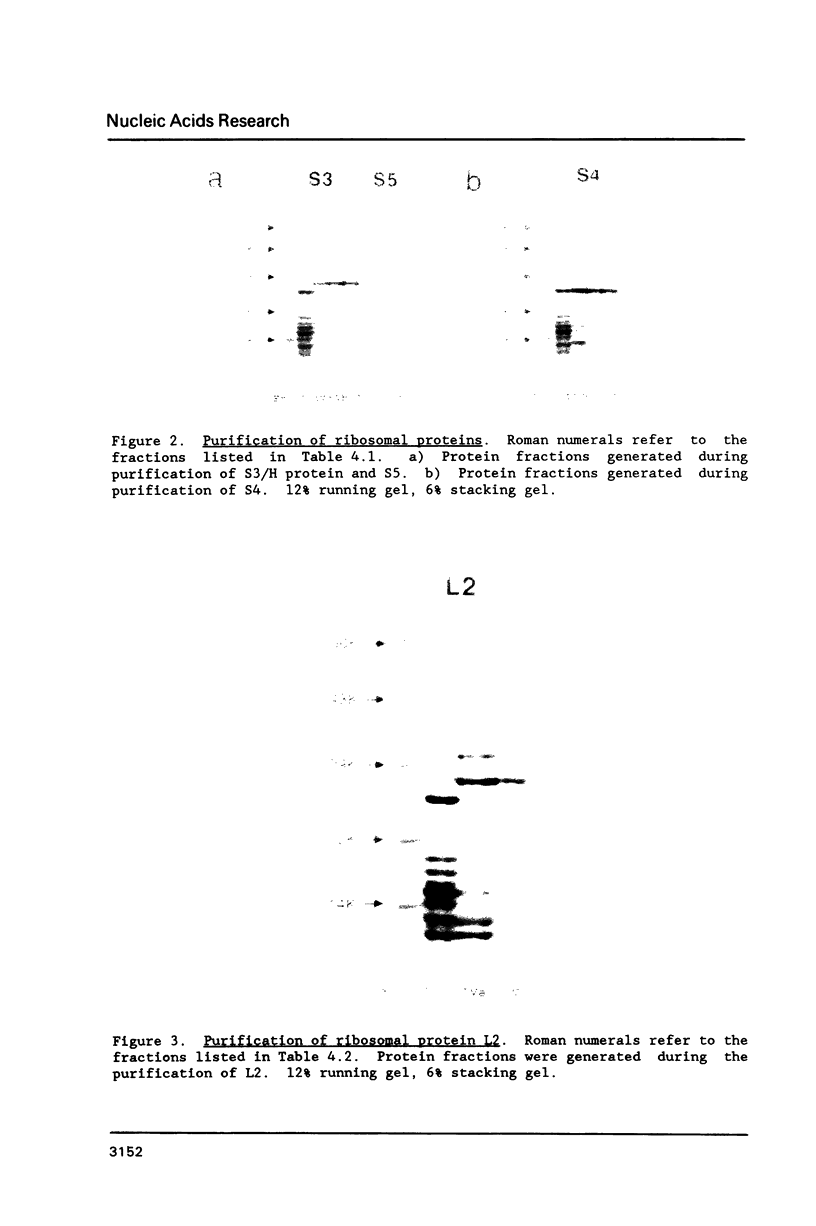
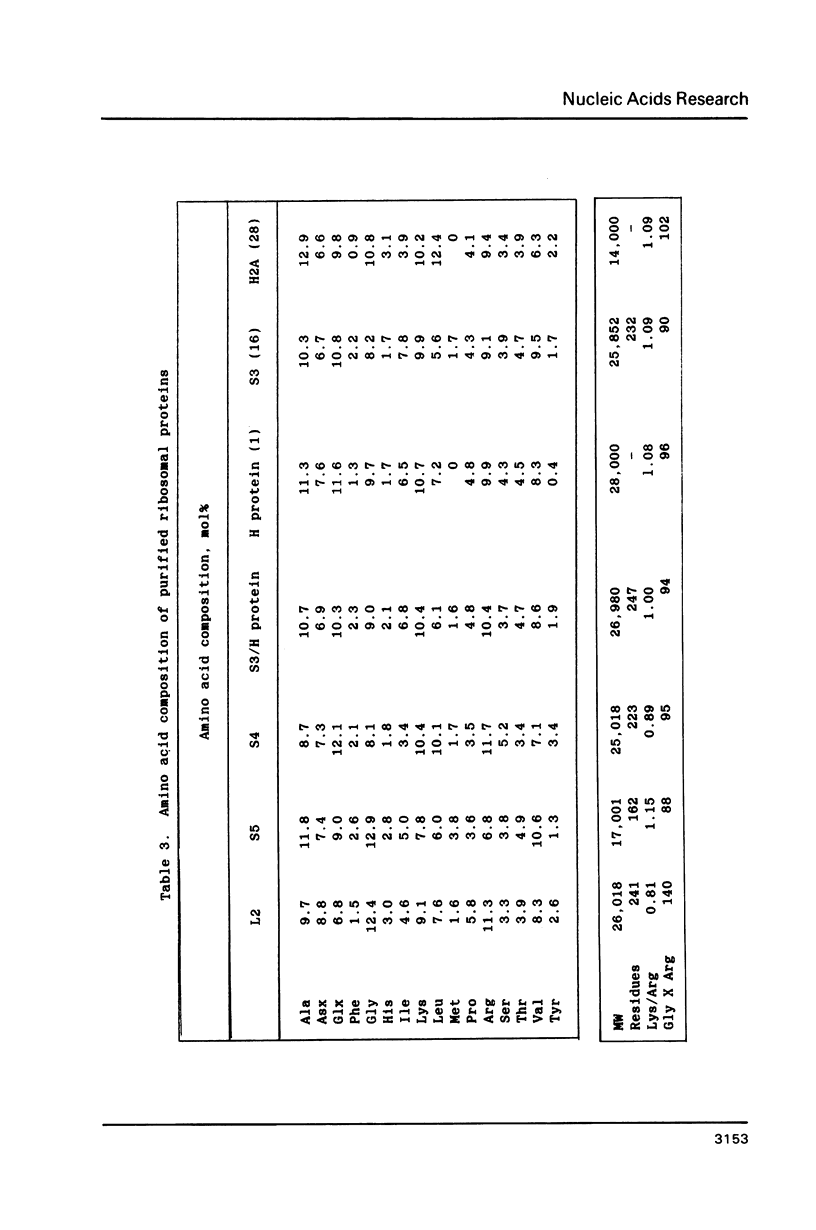

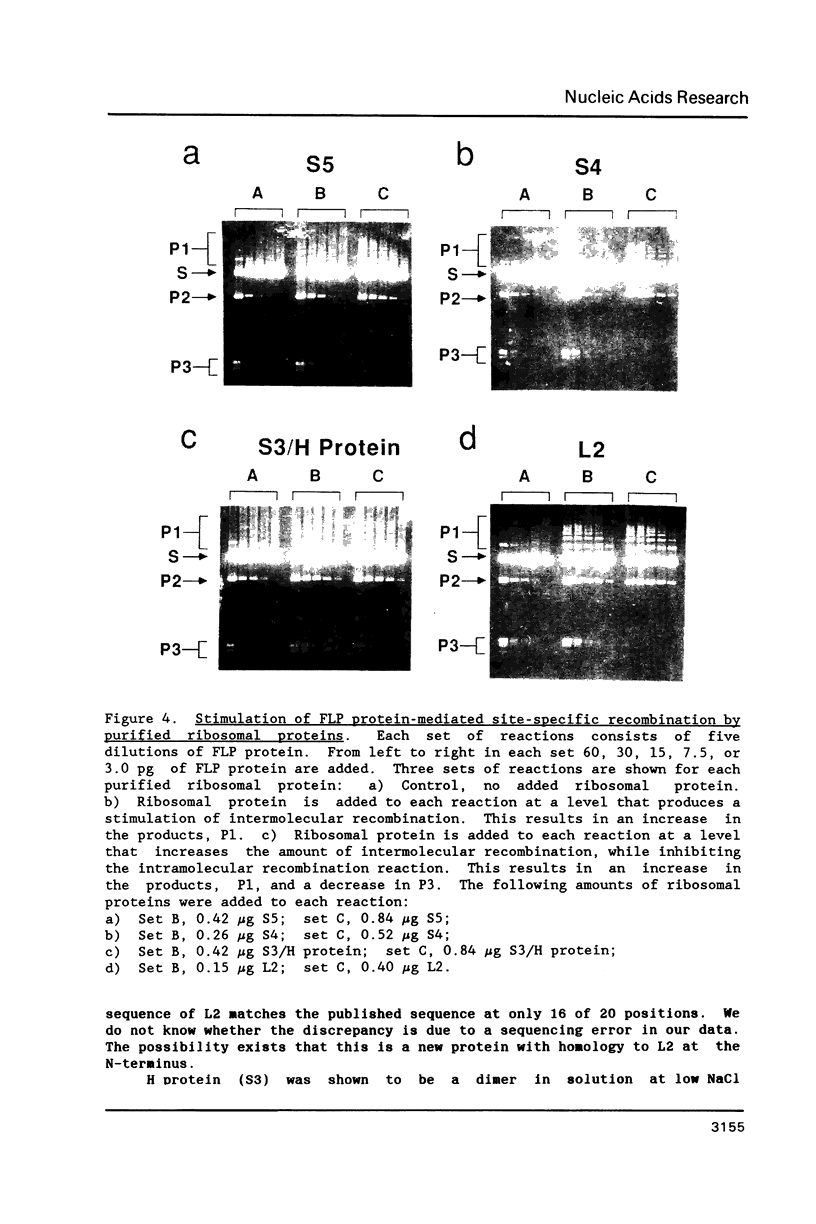


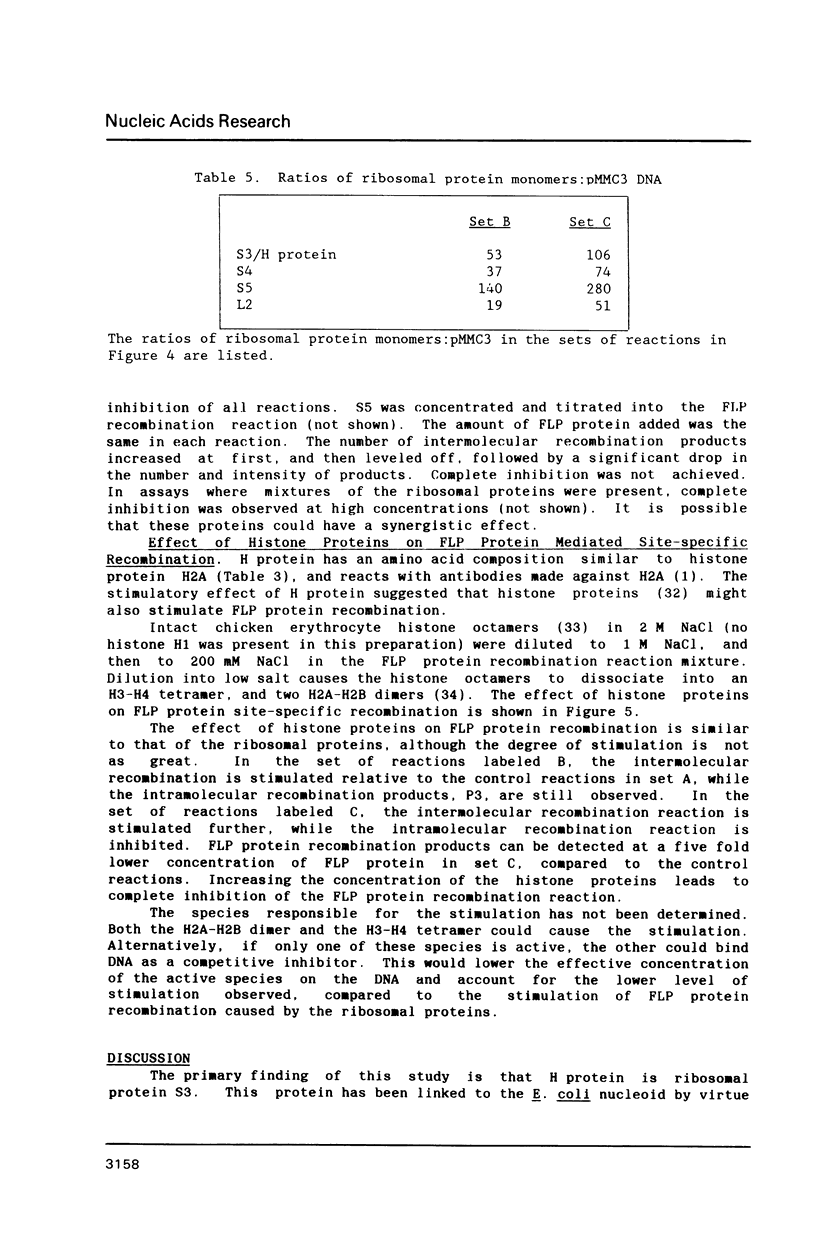



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell D., Davis G., Gosink M., Post L., Nomura M., Kestler H., Zengel J. M., Lindahl L. Nucleotide sequence of the alpha ribosomal protein operon of Escherichia coli. Nucleic Acids Res. 1985 Jun 11;13(11):3891–3903. doi: 10.1093/nar/13.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brauer D., Röming R. The primary structure of protein S3 from the small ribosomal subunit of Escherichia coli. FEBS Lett. 1979 Oct 15;106(2):352–357. doi: 10.1016/0014-5793(79)80531-1. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Guarascio V. R., Jayaram M. Recombination within the yeast plasmid 2mu circle is site-specific. Cell. 1982 May;29(1):227–234. doi: 10.1016/0092-8674(82)90107-6. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M. The FLP protein of the yeast 2-microns plasmid: expression of a eukaryotic genetic recombination system in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4223–4227. doi: 10.1073/pnas.80.14.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978 Nov 14;17(23):4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Sadowski P. D. The FLP protein of the 2-micron plasmid of yeast. Inter- and intramolecular reactions. J Biol Chem. 1985 Oct 5;260(22):12328–12335. [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Slonimski P. P. Circular DNA of a yeast episome with two inverted repeats: structural analysis by a restriction enzyme and electron microscopy. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3030–3034. doi: 10.1073/pnas.73.9.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Lutz H., Kornberg A. Novel histone H2A-like protein of escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5097–5101. doi: 10.1073/pnas.77.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. F., Simon M. I. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986 Aug 15;46(4):531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- Lutz H., Von Meyenburg K., Hübscher U. Quantitation with monoclonal antibodies of Escherichia coli H protein suggests histone function. J Bacteriol. 1985 Jun;162(3):1005–1007. doi: 10.1128/jb.162.3.1005-1007.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Leon L., Gates C. A., Attwood J. M., Wood E. A., Cox M. M. Purification of the FLP site-specific recombinase by affinity chromatography and re-examination of basic properties of the system. Nucleic Acids Res. 1987 Aug 25;15(16):6469–6488. doi: 10.1093/nar/15.16.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Leon L., Senecoff J. F., Bruckner R. C., Cox M. M. Site-specific genetic recombination promoted by the FLP protein of the yeast 2-micron plasmid in vitro. Cold Spring Harb Symp Quant Biol. 1984;49:797–804. doi: 10.1101/sqb.1984.049.01.090. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Nash H. A. Direct role of the himA gene product in phage lambda integration. Nature. 1981 Apr 9;290(5806):523–526. doi: 10.1038/290523a0. [DOI] [PubMed] [Google Scholar]
- Morris N. R. A comparison of the structure of chicken erythrocyte and chicken liver chromatin. Cell. 1976 Dec;9(4 Pt 1):627–632. doi: 10.1016/0092-8674(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Structure and properties of the bacterial nucleoid. Cell. 1982 Oct;30(3):667–669. doi: 10.1016/0092-8674(82)90269-0. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Klug A. Studies of nucleosome structure. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):493–501. doi: 10.1101/sqb.1983.047.01.059. [DOI] [PubMed] [Google Scholar]
- Schiltz E., Reinbolt J. Determination of the complete amino-acid sequence of protein S4 from Escherichia coli ribosomes. Eur J Biochem. 1975 Aug 15;56(2):467–481. doi: 10.1111/j.1432-1033.1975.tb02253.x. [DOI] [PubMed] [Google Scholar]
- Serdyuk I. N., Zaccai G., Spirin A. S. Globular conformation of some ribosomal proteins in solution. FEBS Lett. 1978 Oct 15;94(2):349–352. doi: 10.1016/0014-5793(78)80974-0. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Vetter D., Andrews B. J., Roberts-Beatty L., Sadowski P. D. Site-specific recombination of yeast 2-micron DNA in vitro. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7284–7288. doi: 10.1073/pnas.80.23.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. S., Kimura M., Dijk J. On a sequence similarity between ribosomal protein S5 and DNA binding protein II. FEBS Lett. 1985 Mar 25;182(2):249–252. doi: 10.1016/0014-5793(85)80308-2. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Greuer B. The primary structure of protein S5 from the small subunit of the Escherichia coli ribosome. FEBS Lett. 1978 Nov 1;95(1):91–98. doi: 10.1016/0014-5793(78)80059-3. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Nagata A., Kano Y., Imamoto F. Isolation and characterization of nucleoid proteins from Escherichia coli. Mol Gen Genet. 1984;196(2):217–224. doi: 10.1007/BF00328053. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Zurawski S. M. Structure of the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1985 Jun 25;13(12):4521–4526. doi: 10.1093/nar/13.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]