Abstract
Nickel is an essential metal for Helicobacter pylori, as it is the co-factor of two enzymes crucial for colonization, urease and hydrogenase. Nickel is taken up by specific transporters and its intracellular homeostasis depends on nickel-binding proteins to avoid toxicity. Nickel trafficking is controlled by the Ni(II)-dependent transcriptional regulator NikR. In contrast to other NikR proteins, NikR from H. pylori is a pleiotropic regulator that depending on the target gene acts as an activator or a repressor. We systematically quantified the in vivo Ni2+-NikR response of 11 direct NikR targets that encode functions related to nickel metabolism, four activated and seven repressed genes. Among these, four targets were characterized for the first time (hpn, hpn-like, hydA and hspA) and NikR binding to their promoter regions was demonstrated by electrophoretic mobility shift assays. We found that NikR-dependent repression was generally set up at higher nickel concentrations than activation. Kinetics of the regulation revealed a gradual and temporal NikR-mediated response to nickel where activation of nickel-protection mechanisms takes place before repression of nickel uptake. Our in vivo study demonstrates, for the first time, a chronological hierarchy in the NikR-dependent transcriptional response to nickel that is coherent with the control of nickel homeostasis in H. pylori.
INTRODUCTION
Helicobacter pylori is a bacterial pathogen that colonizes the gastric mucosa of half of the world’s human population. Infection by H. pylori is associated with the development of ulcer disease, gastric carcinoma and MALT lymphoma (1,2). Nickel (Ni2+) is essential for H. pylori, because it is the co-factor of the enzyme urease required for resistance to the gastric acidity and for in vivo colonization (3). Urease catalyzes the hydrolysis of urea into ammonia and bicarbonate, the two buffering compounds that allow the bacterium to maintain intracellular neutrality. This enzyme is composed of the UreA and UreB subunits, is very abundant (up to 6% of total soluble cellular proteins) and requires 24 nickel ions per active enzymatic complex (4). Helicobacter pylori synthesizes another Ni2+-containing enzyme that is important for colonization; the [Ni-Fe] hydrogenase composed of HydA, HydB and HydC (5). This hydrogenase allows the utilization of hydrogen as an energy source by oxidizing the molecular hydrogen during respiratory-based energy production in the gastric mucosa (6). To ensure acid resistance and efficient host colonization, these two metallo-enzymes must be activated by nickel insertion (3,5). Their activities are dependent on the intracellular Ni2+ level that needs to be strictly controlled. Indeed, when excess Ni2+ accumulates, these ions inhibit growth and exhibit toxic effects by interfering with protein–metal binding and catalysis, and by generating reactive oxygen species (7). Consequently, H. pylori has to maintain a balance between uptake and intracellular storage of Ni2+ ions, as well as delivery to Ni2+-metalloenzymes when required.
In H. pylori, nickel acquisition from the external environment relies on FrpB4, an outer membrane (OM) transporter energized by the TonB machinery that is in addition involved in iron uptake (8). Nickel is transported through the cytoplasmic membrane by the high-affinity Ni2+ permease NixA (9).
Helicobacter pylori also possesses unique proteins involved in nickel storage and/or nickel delivery including three proteins that bind Ni2+. Hpn is a histidine-rich protein that represents up to 2% of the total proteins (10,11) and Hpn-like (Hpn2) is a histidine/glutamine-rich protein (12). Both proteins play an important role in Ni2+ detoxification and storage (11,13). Another abundant protein, HspA is a GroES co-chaperonine homolog that through its unique histidine- and cysteine-rich C-terminal extension sequesters nickel and acts as a specialized nickel chaperone for hydrogenase (14). Finally, nickel efflux is possible through a metal pump CznABC that functions to export nickel, zinc and cadmium across the cytoplasmic membrane (15).
Control of the critical Ni2+ homeostasis in H. pylori depends on the Ni(II)-dependent transcriptional regulator, NikR (16,17). NikR regulators typically contain a N-terminal DNA-binding motif of the ribbon–helix–helix (RHH) family (18,19) and a C-terminal tetramerization domain (20). In Escherichia coli, under conditions of high Ni2+ concentration, EcNikR represses the expression of a single target, the nikABCDE operon encoding a nickel ABC transporter (21), by binding to a well-defined palindrome in the corresponding promoter region (22). In contrast to EcNikR, NikR from H. pylori is a pleiotropic regulator. A H. pylori ΔnikR mutant displays an increased Ni2+ susceptibility and colonization attenuation in the mouse model (16,17,23). The H. pylori NikR regulator is original in that, depending on its target gene it acts in response to nickel either as an activator or a repressor. Transcriptomic analysis was performed to compare the H. pylori response to nickel of a wild-type (WT) strain and an isogenic ΔnikR mutant (17). NikR was found to regulate directly or indirectly the expression of genes involved in several functions including motility and chemotaxis, respiration, stress response and OM proteins. In addition, this transcriptomic analysis (17) identified differentially expressed genes involved in metal-related functions: (i) copper (copA2); (ii) iron (pfr, ceuE1 and fur, the latter encoding the iron-responsive transcriptional regulator); (iii) nickel (frpB4, hpn, hpn2, ureAB, hydABC and possibly fecA3); and (iv) both iron and nickel (exbB-exbD-tonB). Some of these genes have been identified as direct NikR targets by in vitro DNA–protein binding analysis. NikR directly activates the transcription of ureAB (24–26) and represses nikR, exbB-exbD-tonB, nixA, frpB4, fecA3 and fur in the presence of Ni2+ (17,26–30). Two NikR operators (binding sites) were identified for fecA3 and three for fur (28,30). In vitro binding of NikR to the hpn promoter region was shown by electrophoretic mobility shift assays (EMSA), but was not further characterized and nothing was known about the in vivo response of this gene to nickel (31).
In vitro characterization of the properties of the H. pylori NikR protein, including extensive structure–function analysis and the identification of high-affinity nickel binding sites, has been reported (31–34). Despite intense efforts (26,28,29) [for a review see (35)], the in vivo mode of action of NikR on its multiple targets is still poorly understood. In addition, previous in vivo studies have mostly focused on analysis of individual genes, some under non-physiological conditions (high nickel concentrations). Only one direct positively regulated target (ureA) has been characterized so far. In addition, nothing is known about the in vivo discrimination and response hierarchy of the positively or negatively NikR targets in response to nickel, although this is expected to strongly impact the control of Ni2+ homeostasis.
To address these questions, quantitative real-time PCR was used to systematically measure the in vivo transcriptional response to nickel of 11 genes (four activated and seven repressed) that are directly regulated by NikR and that encode functions related to nickel metabolism. To define the in vivo response to Ni2+- and/or NikR of these target genes, we measured activation or repression in response to different Ni2+ concentrations that did not interfere with H. pylori growth and established the kinetics of the responses. These data lead us to propose a model of a hierarchical NikR-mediated response to nickel in H. pylori where activation of protection mechanisms takes place as an initial response before repression of nickel uptake. This chronological tuning of the expression of the NikR-regulated genes reflects the ability of H. pylori to adapt to its gastric environment through transcriptional metalloregulation.
EXPERIMENTAL PROCEDURES
Strains and culture conditions
Helicobacter pylori strains were recovered from −80°C stocks on blood–agar base 2 (Oxoid) plates containing 10% horse blood and an antibiotics/fungicide cocktail consisting of 12.5 µg/ml vancomycin, 0.31 µg/ml polymyxin B, 6.25 µg/ml trimethoprim and 2.5 µg/ml fungizone, under microaerophilic conditions (Oxoid). Kanamycin (30 µg/ml) and/or chloramphenicol (10 µg/ml) were added when required. Liquid cultures were grown in brucella broth (BB) containing 0.2% cyclodextrin (BBβ) and the antibiotics cocktail. About 0.2 µM Ni2+ ions are estimated to be intrinsically present in the BB medium (36). The cultures were supplemented with NiCl2 at various concentrations as indicated (Supplementary Figure S1). We previously described the construction of the H. pylori 26695 ΔnikR mutant (32) and of the 26695 exbB-TAP reporter strain (8). Strain 26695 ΔnikR exbB-TAP was constructed by replacing the entire nikR gene by a non-polar kanamycin resistance cassette (KnR-NP) after natural transformation [as in (37)] with plasmid pILL690M (17) into strain 26695 exbB-TAP. The 26695 nixA::TAP CmR fusion was constructed as previously described (8,38) using the primers indicated in Supplementary Table S1. Strain 26695 ΔnikR nixA-TAP was obtained by inserting the nixA::TAP CmR cassette into the 26695 ΔnikR mutant (32). Correct chromosomal insertion of the non-polar kanamycin or chloramphenicol cassettes and correct allelic exchange were verified by Polymerase Chain Reaction (PCR).
mRNA extraction and qRT-PCR
Cultures of H. pylori (OD600nm = 0.6) were centrifuged at 4°C for 10 min at 3000g, RNA was extracted using the phenol-chloroform method as previously described (23). DNA was removed from RNA preparations by DNase I digestion with 5 U RNase-free DNase I Recombinant (Roche) for 20 min at 37°C followed by a second phenol-chloroform purification. A second DNase treatment was carried out with the Turbo DNA-free kit (Ambion) according to the manufacturers’ instructions. Total RNA was quantified on a NanoDrop spectophotometer and visualized on an ethidium bromide-stained agarose gel. Total RNA served as a template for cDNA synthesis using the AMV Reverse Transcriptase (Promega). Synthesis reactions were carried out following the manufacturer’s protocol, starting with 1 µg total RNA and 50 ng random hexamers (Roche) per 20 µl reaction mixture. cDNA was diluted to 100 ng/µl. RNA transcripts were quantified on a Applied Biosystems StepOnePlus PCR machine using Power SYBR Green PCR Master Mix (Applied Biosystems) in 20 µl reaction mixture containing 50 ng of total cDNA. For each target gene, primer pairs were chosen that generated a unique PCR product. In the case of the hydABC operon, the optimal primer pair was inside the hydB gene. The transcript levels were normalized to the level of the housekeeping ppk gene (encoding polyphosphate kinase, HP1010) as in (27). Experiments were performed with three independent RNA samples and each sample was assayed in triplicate. Supplementary Table S1 lists the primer sequences used for qRT-PCR.
Western blotting
Proteins from H. pylori were extracted using the Bugbuster protein-extraction reagent according to the manufacturers’ instructions (Novagen). Protein concentrations were measured with the Bradford assay (BioRad). Twenty micrograms of crude extracts were separated by 12.5% SDS–PAGE and blotted on a PVDF membrane (Millipore). NikR and UreA were detected with rabbit polyclonal antibody raised against NikR and UreA from H. pylori, respectively (17,39). To detect the ExbB-TAP and NixA-TAP fusion proteins (TAP: Tandem Affinity Purification tag) as in (8), the Peroxidase Anti-Peroxidase antibody (PAP, Sigma) was used. Binding of the Enhanced Chemiluminescence (ECL) anti-rabbit IgG coupled to peroxidase antibody (GE Health care) and PAP antibody were revealed with the ECL Plus reagent (Pierce).
Protein overexpression and purification
Native NikR was expressed in E. coli BL21 and purified by a combination of anion exchange and gel-filtration chromatographies, as in (32,40). Purity, integrity and metal content were assessed by SDS–PAGE, mass analysis and metallochromic assay, respectively (40). The purified protein was concentrated using Amicon centrifuge devices with a cutoff of 5 kDa, aliquoted and stored at −80°C in 20 mM Tris–HCl pH 7.4, 400 mM NaCl, 10% glycerol and 0.1 mM DTT. For EMSA, purified Hp-NikR was quickly thawed and the buffer exchanged against EMSA binding buffer using micro-bio spin 6 columns (Biorad).
EMSA
The entire intergenic regions or fragments of the regions situated upstream from the hpn, hpn2, hydA and hspA genes were PCR amplified with primers (listed in Supplementary Table S1, and shown in Supplementary Figure S2) using the proof-reading pfu turbo DNA polymerase (Stratagene). After purification with the Qiaquick PCR purification kit (Quiagen), 33 nM of these PCR products were labeled with γ-32P-ATP by incubation with T4 polynucleotide kinase (Fermentas) at 37°C for 30 min. Non-incorporated nucleotides were removed with a micro-bio spin 6 column (Biorad). Radiolabeled DNA fragments (450 pM) were incubated for 30 min with different concentrations of purified NikR at room temperature in 15 µl binding buffer. Binding buffer consisted of 20 mM Bis–Tris Borate pH 7.4, 50 mM KCl, 100 µM NiSO4, 3 mM MgCl2, 2.5% glycerol, 0.1% Triton X-100 and 1.5 µg ml−1 dIdC (as non-specific DNA binding competitor). Protein concentrations (expressed in subunit concentration) were calculated according to the estimated epsilon of the monomeric subunit of NikR (9770 M−1 cm−1). Native polyacrylamide gels at 5% (acrylamide:bisacrylamide, 29:1) prepared with 100 mM Bis–Tris Borate buffer, pH 7.4 and 100 µM MnCl2 (running buffer) were pre-run for 30 min at 50 V in running buffer before loading the samples. Complexes were resolved by running the gels for 20 or 40 min at 150 V, according to the fragment size. Radiolabeled signals were detected on a storage phosphor screen and visualized with a Typhoon 9400 scanner (Molecular Dynamics, Sunnyvale, CA, USA; excitation light 532 nm, emission filter 580 BP 30). Signals were quantified using ImageQuant software (Molecular Dynamics). A PCR product corresponding to a 325 bp internal fragment of the fur gene was used as a negative control. No shift was observed for the fur fragment, even at high concentrations of purified NikR protein indicating the absence of non-specific DNA binding. A 32-bp duplex containing the NikR binding site upstream from the ureA gene was used as a positif control to establish the protein–DNA binding conditions (Supplementary Figure S3).
RESULTS
Regulation of 11 NikR target genes by nickel
To characterize the in vivo transcriptional response to nickel and the dependence of this response on NikR, 11 H. pylori genes were selected. Seven were established direct NikR target genes that in response to nickel, are either activated like ureA (hp0073) or repressed like nixA (hp1077), fur (hp1027), exbB (hp1339), frpB4 (hp1512), fecA3 (hp1400) and nikR (hp1338). We studied four putative NikR target genes that encode proteins related to Ni2+ homeostasis in H. pylori. The hpn (hp1427), hpn2 (hp1432) and hydB (hp0632) genes were found to be deregulated in a ΔnikR mutant in a transcriptomic study (17) and hspA (hp0011) is a nickel chaperone (14). Transcription of these 11 genes was measured by quantitative real-time PCR (qRT–PCR) in the WT strain 26695 and in its isogenic ΔnikR mutant, each grown without added nickel or with NiCl2 (with the exception of nikR autoregulation that could not be tested in the mutant).
We first established defined, reproducible and physiological conditions of H. pylori nickel exposure in order to avoid an unwanted metal-stress response. Growth medium was BB (that contains intrinsically low nickel amounts, 0.2 µM) supplemented with β-cyclodextrin [instead of the classically used fetal bovine serum (FBS) that chelates nickel in an uncontrolled manner]. Upon addition of 5 or 10 µM NiCl2 to the medium, both WT and ΔnikR strains presented identical growth curves attesting of the lack of nickel toxicity (Supplementary Figure S1). These nickel concentrations are in the range of the human dietary nickel intake of around 4 µM that is estimated to be found in the host stomach (41). At 20 µM NiCl2, the highest concentration tested, a slight growth defect of the ΔnikR mutant was detected (Supplementary Figure S1).
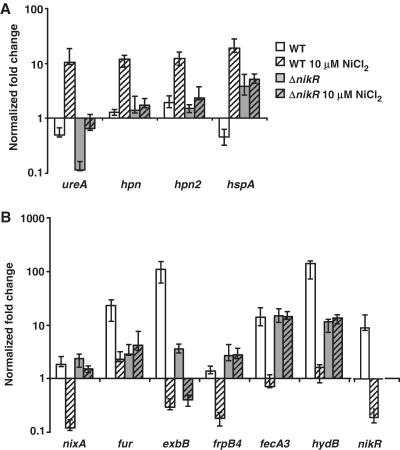
The mRNA levels of 11 selected genes were determined after overnight growth (16 h) with 10 μM NiCl2. In the WT strain, ureA, hpn, hpn2 and hspA were upregulated about 10-fold (Figure 1A, WT versus WT+10 μM NiCl2). Transcription of nixA, fur, exbB, frpB4, fecA3, hydB and nikR was reduced in the WT strain in response to Ni2+ supplementation by a factor of 15, 10, 369, 8, 19, 85 and 48, respectively (Figure 1B, WT versus WT+10 μM NiCl2).
Figure 1.
Nickel-dependent regulation of the NikR target genes in H. pylori WT 26695 and the isogenic ΔnikR mutant. Expression of the NikR-upregulated genes ureA, hpn, hpn2, hspA (A) and NikR-downregulated genes nixA, fur, exbB, frpB4, fecA3, hydB, nikR (B) was measured by qRT–PCR. The level of transcripts after overnight culture in BBβ without added NiCl2 or with a supplementation of 10 μM NiCl2 was determined relative to that of the level of ppk (hp1010) under the same conditions.
For the hpn, hpn2, nixA, frpB4, fecA3 and hydB genes, induction or repression was exclusively dependent on nickel-loaded NikR. Indeed, no significant deregulation was observed in a ΔnikR mutant without nickel (Figure 1A and B, WT versus ΔnikR) or with 10 μM NiCl2 (Figure 1A and B, ΔnikR versus ΔnikR+10 μM NiCl2). For the hspA, ureA, fur and exbB genes, additional levels of regulation were revealed in the ΔnikR mutant as compared with the WT strain. In the ΔnikR mutant, hpsA expression was enhanced independently of nickel. Downregulation of ureA, fur and exbB was observed in the ΔnikR mutant. Upon Ni2+ supplementation in the ΔnikR mutant, strong ureA activation and moderate exbB repression were observed suggesting that these targets are submitted to nickel regulation that is NikR independent.
Thus, each of the selected genes, encoding proteins related to the H. pylori nickel metabolism, is regulated by NikR in response to nickel and this with different intensities. Additional levels of regulation of the expression of some of these genes were revealed in the ΔnikR mutant. From now on, we will concentrate on the NikR-dependent regulation of the 11 targets.
Novel direct NikR targets
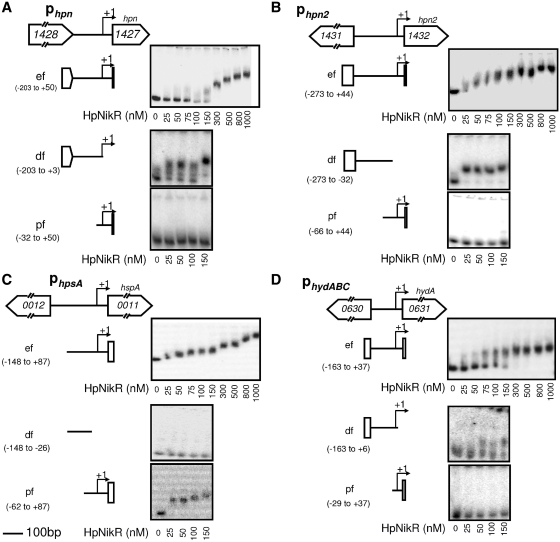
Our measurements revealed that the expression of hpn, hpn2 and hspA is activated while that of hydB is repressed by NikR upon nickel addition to the culture medium. To assess whether these regulatory effects were direct through interaction of NikR with the promoter regions (P) of these genes, native NikR protein was purified and tested using EMSA (with 100 µM NiSO4 in the binding buffer and 100 µM MnCl2 in the running buffer). The intergenic regions situated upstream from each of these genes hpn, hpn2, hspA and hydA (the first gene of the hydA-B-C operon) were investigated either as a whole entire fragment (ef) or as divided into a distal fragment (df) and a proximal fragment (pf) relative to the position of the transcription start sites (TSS) (Supplementary Figure S2). The TSS of hpn, hpn2 and hydA were after the study of Sharma et al. (42), that of hspA was previously determined (43).
In the presence of Ni(II) and Mn(II), purified NikR protein caused a migration shift of the entire DNA fragments (ef) of the four genes that was visible starting from NikR concentrations of 50–100 nM (Figure 2).
Figure 2.
EMSA of NikR binding to the hpn, hpn2, hspA and hydA promoter regions (Phpn, Phpn2, PhspA, PhydABC). The investigated intergenic regions preceding each gene are schematically represented: (A), region hp1428-hp1427 (upstream from hpn), (B) hp1431-hp1432 (upstream from hpn2), (C) hp0012-hp0011 (upstream from hspA) and (D) hp0630-hp0631 (upstream from hydA). TSS are marked by +1 and arrows. EMSA with native purified NikR are shown using the DNA fragments indicated on the left. For each gel, the first lane on the left presents the control without NikR protein. NikR was added to the samples at the nanomolar concentrations indicated below each gel.
For Phpn, the apparent dissociation constant (Kd) for NikR was estimated to be 100 nM, for PhydA was 90 ± 10 nM and for Phpn2 was even lower (Figure 2). The hspA promoter region exhibited different levels of migration as a function of protein concentration with a first shift at 25 nM [NikR], suggesting the existence of at least two NikR-binding sites (Figure 2C). Using smaller DNA fragments for Phpn and Phpn2, we exclusively observed gel shift with the distal DNA fragments (df) at NikR concentrations as low as 25 nM (Figure 2A and B). For hspA, gel shift was found exclusively with the pf extending from position −62 to +87 relative to the TSS (Figure 2C), while only the df that includes the TSS of hydA was shifted by the addition of NikR (Figure 2D).
In conclusion, we showed that the hpn, hpn2, hydABC and hspA genes are directly regulated by NikR upon increase in nickel concentration by specific binding of NikR to their respective promoter regions.
Response of NikR targets to increasing nickel concentrations
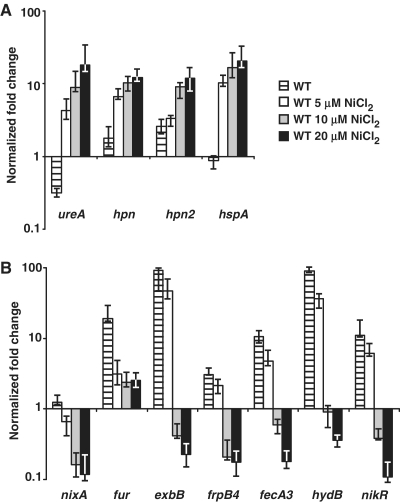
In order to quantify the impact of Ni2+ concentration on the activation and repression by NikR, qRT-PCR was used to follow transcription of the 11 genes in the WT strain grown overnight without added nickel or with increasing NiCl2 concentrations of 5, 10 and 20 μM. Under these conditions, regulation was observed at 10 µM for every gene either activated (ureA, hpn, hpn2 and hspA, see Figure 3A) or repressed (nixA, fur, exbB, frpB4, fecA3, hydB and nikR, see Figure 3B).
Figure 3.
Effects of increasing concentrations of nickel on the transcription of NikR target genes in H. pylori WT 26695 strain. Expression of the NikR-upregulated genes ureA, hpn, hpn2, hspA (A) and NikR-downregulated genes nixA, fur, exbB, frpB4, fecA3, hydB, nikR (B) was measured by qRT–PCR. The levels of transcripts were quantified after overnight culture in BBβ supplemented with 0, 5, 10 or 20 µM NiCl2. The housekeeping gene ppk (hp1010) was used as a reference.
Response to increasing nickel supplementation can be classified into two categories: (i) regulation already apparent at 5 µM (the lowest nickel concentration tested) for ureA, hpn, hspA, nixA and fur that, with the exception of fur, gradually increases with nickel concentration and (ii) regulation that requires at least 10 µM of nickel to be established for hpn2, exbB, fecA3, frpB4, hydB and nikR, a situation observed in six out of the seven repressed genes.
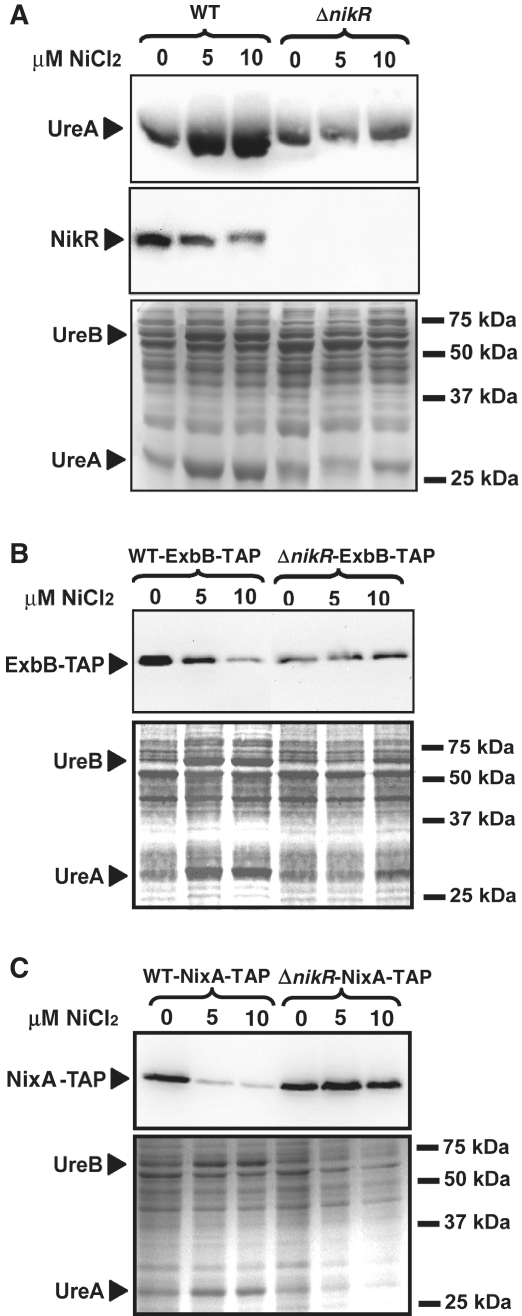
We then analyzed how the concentration-dependent nickel transcriptional regulation is translated into protein synthesis for one activated target, ureA and for three negatively regulated targets nikR, nixA and exbB (Figure 4). Western blots were performed on soluble protein fractions of WT and ΔnikR strains grown at different nickel concentrations and were revealed with specific polyclonal antibodies directed against UreA (39), NikR (17) and with a PAP antibody directed against the epitope-tagged ExbB-TAP and NixA-TAP versions [as described in (8)].
Figure 4.
Western blots of soluble proteins extracted from overnight grown H. pylori WT 26695 strain and the isogenic Δnik R mutant in response to increasing concentrations of supplemented NiCl2 (0, 5 or 10 µM NiCl2). Targeted proteins are UreA, NikR (A), ExbB-TAP (B) and NixA-TAP (C). Coomassie-stained gels are shown as loading controls.
As shown in Figure 4A, a strong increase in the amount of the UreA protein was already visible when the WT strain was grown with 5 µM NiCl2. Consistently, the nickel-dependent activation of the synthesis of both UreA and UreB subunits (expressed from the ureA-B operon) was visible on a Coomassie blue-stained SDS gel that served as a loading control (Figure 4A–C, lower panels). Again in agreement with the concentration-dependent transcriptional responses, diminished amounts of NikR and ExbB-TAP proteins were detected at 10 µM NiCl2 and at 5 µM for NixA-TAP (Figure 4). In the ΔnikR mutant, nickel-dependent regulation of UreA, ExbB-TAP and NixA-TAP synthesis was completely abolished (could not be assessed for the nikR gene).
In conclusion, we showed that the NikR targets responded differently to increasing nickel concentrations, with repression generally being set up at higher nickel concentrations than activation (with the exception of fur and to a lower extent of nixA). For four targets, the transcriptional regulation was confirmed by protein expression-level measurements.
Kinetics of NikR-dependent regulation
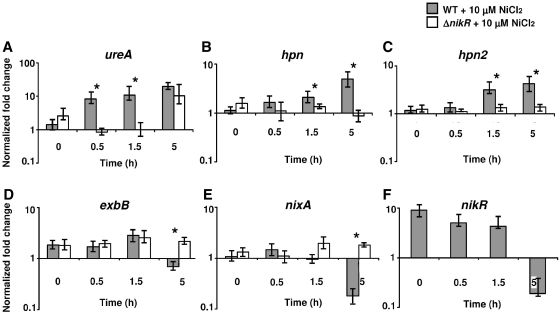
We further asked at what time the NikR regulation is taking place in response to nickel for the induced and repressed target genes. RNA from WT and ΔnikR strains was isolated at 0, 0.5, 1.5 and 5 h after addition of 10 μM NiCl2 and the transcripts of NikR target genes were quantified by qRT-PCR. Figure 5 presents the results for three NikR-activated genes, ureA, hpn and hpn2 (panels A–C) and three NikR-repressed genes, exbB, nixA and nikR (panels D–F). For each time point, we performed statistical analyses using Student’s t-test to compare the expression levels in the WT strain versus the ΔnikR mutant (not applicable for the nikR gene). Statistically significant differences (P-value < 0.02) are marked by a star in Figure 5.
Figure 5.
Kinetics of the nickel-dependent regulation of NikR target genes ureA (A), hpn (B), hpn2 (C), exbB (D), nixA (E) and nikR (F) in H. pylori WT 26695 strain and the ΔnikR mutant. The level of transcripts in bacteria cultures (OD600nm = 0.5) in BBβ was determined at 0, 0.5, 1.5 and 5 h after addition of 10 μM NiCl2, relative to that of the level of ppk (hp1010) under the same conditions. Statistically significant differences of the expression levels in the WT strain versus the ΔnikR mutant are marked by a star (P < 0.02, Student’s t-test).
NikR activation of ureA transcription was detected 30 min after nickel addition and the NikR-independent nickel regulation only after 5 h (Figure 5A). NikR-dependent induction of hpn and hpn2 was established 1.5 h after adding NiCl2 (Figure 5B and C). NikR-dependent repression was observed 5 h after nickel addition for exbB, for nixA and for the negative feedback of nikR (Figure 5D–F) as well as for fur (data not shown). Finally, repression of frpB4, fecA3, hydB and activation of hspA took place after 5 h (data not shown).
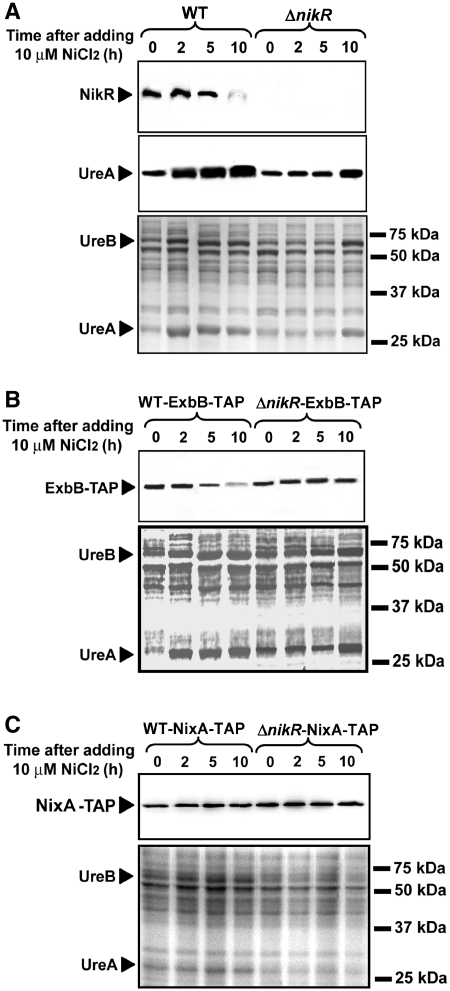
Western blots were performed under the same conditions to measure the amounts of UreA, NikR, NixA-TAP and ExbB-TAP proteins. Soluble proteins of H. pylori WT or ΔnikR strains were analyzed from samples taken at 0, 2, 5 and 10 h after 10 µM nickel addition. These longer kinetics were chosen to analyze the nikR regulation that was shown by qRT-PCR to take place after 5 h. Indeed, the amounts of NikR protein were strongly diminished 10 h after nickel addition (Figure 6A). For the UreA, and ExbB-TAP proteins, the results were consistent with the kinetics of transcriptional regulation (Figures 5 and 6). In the WT strain, UreA induction was established 2 h after nickel addition and the activation by nickel in the ΔnikR mutant was only visible at 10 h. The same observation could be made for both UreA and UreB proteins visible on Coomassie stained SDS gels of the same samples that served as loading controls (Figure 6). Finally, ExbB-TAP repression is observed 5 h after adding NiCl2, the effect being enhanced at 10 h. This response is strictly dependent on NikR because we observed no significant difference of the ExbB-TAP amounts in the ΔnikR mutant (Figure 6B). NixA-TAP protein amounts indicated that repression is only visible after exposure to 10 µM NiCl2 during >10 h and confirmed the absence of a NikR-independent response to nickel (Figures 4C and 6C). NikR-independent regulation of ureA observed by qRT-PCR (Figure 1) was confirmed at the protein level (Figures 4 and 6). In comparison with the WT strain, the ΔnikR mutant produces less UreA in the absence of nickel, while the amount of UreA is increased after 10 h exposure of this mutant to nickel (Figure 6A). ExbB synthesis is diminished in the ΔnikR mutant as compared with the WT strain, while the weak NikR-independent nickel repression measured by qRT-PCR is not visible on the western blots (Figure 4).
Figure 6.
Western blots of H. pylori WT 26695 and ΔnikR mutant proteins in response to 10 μM NiCl2. Samples were removed at 0, 2, 5 and 10 h after addition of supplemented nickel to exponential phase cultures cells (OD600nm = 0.5). The targeted proteins are UreA, NikR (A) ExbB-TAP (B) and NixA-TAP (C). Coomassie-stained gels are shown as loading controls.
Thus, the kinetics of the nickel-dependent NikR regulation vary with the target genes and, with the exception of hspA, the activation is set up long before repression.
DISCUSSION
The H. pylori genome is one-quarter of the size of the E. coli genome (44) and encodes a restricted number of transcriptional regulators. Consequently, several regulators have a pleiotropic role to allow H. pylori to survive in the harsh conditions of its gastric niche. The H. pylori NikR regulator that controls intracellular nickel homeostasis illustrates this property very well. In contrast to E. coli, where NikR has a single target, the H. pylori NikR controls the transcription of multiple genes in response to nickel and pH variations, nickel bio-availability being increased at low pH (17,45). In addition, H. pylori NikR acts in response to nickel, either as an activator or a repressor depending on its target gene. While metallation and in vitro DNA-binding properties of NikR have been extensively studied, its in vivo mode of action on its multiple targets is poorly understood. In the present study, we investigated systematically the in vivo response to nickel of 11 NikR targets in order to establish parameters such as the hierarchy and relative intensities of the responses and to progress in the understanding of the in vivo target discrimination.
First, growth conditions with low-nickel concentrations were established to avoid metal stress and to mimic more accurately physiological conditions. The Ni2+-NikR-dependent response was measured for direct targets, seven repressed and four activated genes. These included target genes that have not been previously characterized, one repressed operon hydABC (encoding the [NiFe] hydrogenase subunits) and three activated genes hpn2, hspA and hpn (encoding nickel-binding proteins). Using EMSA, we demonstrated specific binding of Ni2+-NikR to the promoter regions of these four targets hpn, hpn2, hydA and hspA. Apparent dissociation constant (Kd) for Phpn and Phpn2 were estimated to be around 100 nM using the entire intergenic region preceding these genes. This is comparable with the previously estimated Kd for Phpn (180 ± 70 nM) (31). Consistent with the binding site of a classical transcriptional activator, which functions by contacting RNA polymerase, retardation by NikR was retained exclusively with the ‘distal’ fragments comprising DNA regions upstream from the predicted hpn and hpn2 promoters. EMSA with the PhspA DNA fragment suggested two or more NikR-binding sites. While intermediate retardation is observed with the proximal fragment extending to 62-bp upstream from the TSS, the entire region is required for complete retardation suggesting cooperativity between the different NikR-binding sites. For the repressed target hydA, the estimated Kd was 90 ± 10 nM and strong retardation was solely observed with the entire fragment indicating that an extended region surrounding the TSS is required for efficient NikR binding.
Under our test conditions, a concentration of 10 µM NiCl2 is sufficient to induce the expression of ureAB, hpn, hpn2 and hspA and to repress the transcription of nixA, fur, exbB-exbD-tonB, frpB4, fecA3, hydABC and nikR in a NikR-dependent manner. Importantly, activation was already set up at 5 µM (with the exception of hpn2) and the intensity of the response varied with the target gene. The level of repression was in general stronger than that of activation (Figures 3 and 4). In a previous work, the addition of 100 µM nickel (that probably impeded H. pylori growth), was found to repress nixA expression about 2-fold after 5 min, was NikR-dependent and correlated with NixA protein amounts of 2-day-old bacteria (36). Davis et al. (27) quantified, in a WT H. pylori strain, the effect of nickel on the frpB4, ureA, fecA3, nixA and nikR transcripts. With the exception of nixA, the regulation factors were considerably lower than those of the present study possibly as a consequence of the test conditions (10 µM NiCl2 in BB with serum instead of β-cyclodextrin used here).
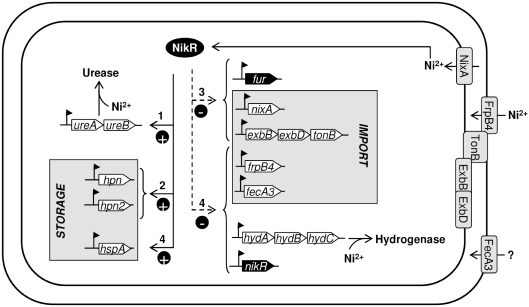
In order to determine the different steps in the cellular control of nickel homeostasis, we investigated the in vivo kinetics of the NikR response to the addition of 10 µM nickel with our 11 target genes (Figures 5 and 6). The results showed that transcriptional activation mostly takes place before repression. As summarized in Figure 7, we revealed a step-by-step regulation of the different members of the NikR regulon that fitted nicely with the biological functions of the proteins they encode.
Figure 7.
Regulation of NikR target genes involves an activation (filled line)/repression (dotted line) bimodal mechanism. The chronology of the Ni2+-dependent NikR regulation is indicated by numbers from 1 corresponding to a rapid response to 4 indicating a delayed response.
The first change to be observed in response to increased nickel concentration is the induction of ureAB transcription via NikR. As acid is known to increase metal bio-availability, enhanced nickel concentration might be an emergency signal to increase urease expression as an essential defense mechanism against acid stress. This answer is rapidly followed by an increase in the expression level of the genes coding for the nickel storage proteins Hpn and Hpn2 (10,11). These first steps allow H. pylori to control the intracellular nickel concentration in order to avoid metal toxicity and to store nickel that could possibly be delivered to urease in case of need.
During a third step, H. pylori decreases transport of nickel into the cytoplasm by repressing transcription of nixA, encoding the inner-membrane permease NixA that dictates the cytoplasmic nickel concentration (26,36,46) and by diminishing transcription of the exbB-exbD-tonB operon. This prevents the ExbB, ExbD and TonB proteins to transduce energy to the TonB-dependent transporters, FrpB4 for nickel uptake (8) and FecA3, for which a similar role is postulated but not demonstrated (29). This step shuts off the entrance of nickel both into the periplasm and in the cytoplasm. The expression of fur is also repressed by NikR. Then, expression of two OM TonB-dependent transporters was diminished, FrpB4 and FecA3 as well as the expression of the hydrogenase operon (hydABC). In the meanwhile, transcription of hspA, coding for a third nickel-binding protein is increased.
Thus, upon nickel exposure, several mechanisms essential to maintain nickel homeostasis are achieved by the three first steps (storage and inhibition of nickel import into the cytoplasm), then the OM-transporters FrpB4 and FecA3 become obsolete and are turned off. The hydrogenase production is at last also turned off, with a reduction of the energy production for the cell. The late activation of hspA that acts as an hydrogenase nickel-chaperone might correspond to a mechanism of compensation for the absence of the hydrogenase nickel sink (14). Finally, we postulate that nikR is the latest negatively feedback-regulated gene, as its expression is no longer necessary when nickel homeostasis equilibrium is reached.
To understand the complex regulation within the NikR regulon, one should keep in mind that the in vivo regulatory outcome in response to nickel is a consequence of multi-factorial modifications in intracellular nickel concentrations due to: (i) the nickel added to the growth medium, (ii) the changes in the amounts of the regulated proteins involved in nickel storage and transport and (iii) the modifications of the concentration of the NikR regulator itself. Accordingly, in vivo competition for nickel between NikR protein and the urease incorporation pathway has been reported (47).
The hierarchical regulation can be explained by different mechanisms: (i) the NikR transcriptional regulation is effective at a specific intracellular nickel concentration, Ni2+ levels need to reach a threshold to trigger regulation of NikR targets. Alternatively (ii) a specific concentration of nickel-bound NikR is needed to allow multiple bindings on target promoters, either because it has a weak affinity for the operator or because the regulator requires binding to more than one operators. We favor this latter model, since the three targets for which multiple NikR operators have been established in vitro (fecA3, nikR and hspA) were among those presenting a strongly delayed response in vivo. Effective in vitro regulation of fecA3 by NikR in response to nickel was shown to require two adjacent binding sites with high and low affinity (48). NikR has three binding sites on the fur promoter region (28) and we observed in the present work more than one NikR operator for hspA. Finally, (iii) the NikR protein may use differential affinities for the operators (possibly different consensus binding sites) to distinguish the genes it regulates. Interestingly, a recent in vitro work with purified NikR demonstrates that a two-tiered mode of DNA recognition allows the classification of NikR target promoters according to their dissociation constants (33). Using 80-fold more nickel than in our tests, NikR was found to have a nanomalor affinity for the ureA, nixA, frpB4 and fecA3 promoters and a micromolar affinity for exbB, nikR and fur promoters (33). These differences in affinity do not explain our in vivo data. In addition, we recently showed that: (i) when the metals used for protein activation were adjusted, the affinities of NikR for its targets remained in the nanomolar range; and (ii) an external metal-binding site (X sites), specific to H. pylori NikR, is required for efficient binding to NikR targets (34). The nature of the metal filling the X sites in vivo remains to be clarified, but it can be proposed that secondary metal-binding sites (X sites) interferes with the target recognition by NikR. Despite numerous analysis, only poorly defined NikR recognition consensus sequences have been proposed (TATWATT-N10-AATWATA) (28,29) and more recently TRWYA-N(15)-TRWYA (49). No sequence with significant homology to this consensus could be identified in the DNA sequences used for EMSA of our novel direct NikR targets. In the future, footprint assays should help to determine the exact position(s) of the binding of NikR to these promoter region sequences. Recently, Romagnoli et al. (30) reported an asymmetric mode of in vivo recognition of the fecA3 high-affinity NikR-binding site. They concluded that this is consistent with the lack of extended and conserved symmetry elements in all the NikR operators studied so far (30). In a very recent work, Benanti and Chivers (50) proposed that the sequence recognition specificity of Hp-NikR to its targets depends on a combination of half site, spacers and flanking regions sequences. In agreement with these conclusions and given the variety in the responses of the targets reported here, we hypothesize that there are several types of NikR-binding mechanisms, including single or multiple operators, and associated with different consensus and spacer sequences.
For four of our selected genes, additional levels of regulation were observed in the ΔnikR mutant. For the fur and exbB genes, we suspect that repression by the iron-reponsive regulator Fur is revealed by the absence of NikR. Indeed, fur negative auto-regulation and exbB-exbD-tonB repression by Fur have been reported in H. pylori (28). Interestingly, we found that both the expression of ureA and the synthesis of UreA and UreB proteins are enhanced in response to nickel independently of NikR, a regulation that takes place as late as 5 h after nickel addition. No gene encoding a homolog of the recently described alternative nickel sensor families [RcnR/CsoR (51)], Nur (52) or ArsR-SmtB (53) is found in the H. pylori genome. Thus, this novel control level could be mediated by another metallo-regulator, like Fur for instance. Alternatively, NikR-independent nickel regulation could be caused by direct metal binding on the mRNA of the corresponding operons. Additional studies are needed to establish the mechanisms underlying these regulations.
In conclusion, our data showed that in H. pylori one single regulator, NikR, activated by a metal ion, Ni2+, acts by an original bimodal (on or off) mechanism to hierarchically control its target genes and thereby maintains cellular nickel homeostasis essential for its survival. Moreover, nickel regulatory pathways are more complex than expected since they imply an additional NikR-independent mechanism. The hierarchy in the in vivo gene expression response to nickel is properly in agreement with the biological functions of the targets. This suggests a role for multiple NikR operators and opens interesting questions on the precise mechanisms of target discrimination by the pleiotropic NikR regulator in H. pylori.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
French National Research Agency (ANR-07-BLAN-0083), including post-doctoral stays to C.M. and C.B.; German Academic Exchange Service (DAAD) and European Network of Excellence PathoGenomics to K.S. Funding for open access charge: Institut pasteur.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Caroline Fauquant for her help in optimization of EMSA and Rafi Ahmad for bioinformatic analysis of the NikR-targets regions. Laurent Terradot and Cyril Dian are greatly acknowledged for support and discussions.
REFERENCES
- 1.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton KA, Gilbert JV, Joyce EA, Wanken AE, Thevenot T, Baker P, Plaut A, Wright A. In vivo complementation of ureB restores the ability of Helicobacter pylori to colonize. Infect. Immun. 2002;70:771–778. doi: 10.1128/iai.70.2.771-778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu LT, Mobley HL. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect. Immun. 1990;58:992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson JW, Maier RJ. Molecular hydrogen as an energy source for Helicobacter pylori. Science. 2002;298:1788–1790. doi: 10.1126/science.1077123. [DOI] [PubMed] [Google Scholar]
- 6.Maier RJ, Fu C, Gilbert J, Moshiri F, Olson J, Plaut AG. Hydrogen uptake hydrogenase in Helicobacter pylori. FEMS Microbiol. Lett. 1996;141:71–76. doi: 10.1111/j.1574-6968.1996.tb08365.x. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti SK, Bai C, Subramanian KS. DNA-protein crosslinks induced by nickel compounds in isolated rat lymphocytes: role of reactive oxygen species and specific amino acids. Toxicol. Appl. Pharmacol. 2001;170:153–165. doi: 10.1006/taap.2000.9097. [DOI] [PubMed] [Google Scholar]
- 8.Schauer K, Gouget B, Carriere M, Labigne A, de Reuse H. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Mol. Microbiol. 2007;63:1054–1068. doi: 10.1111/j.1365-2958.2006.05578.x. [DOI] [PubMed] [Google Scholar]
- 9.Bauerfeind P, Garner RM, Mobley LT. Allelic exchange mutagenesis of nixA in Helicobacter pylori results in reduced nickel transport and urease activity. Infect. Immun. 1996;64:2877–2880. doi: 10.1128/iai.64.7.2877-2880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert JV, Ramakrishna J, Sunderman FW, Jr, Wright A, Plaut AG. Protein Hpn: cloning and characterization of a histidine-rich metal-binding polypeptide in Helicobacter pylori and Helicobacter mustelae. Infect. Immun. 1995;63:2682–2688. doi: 10.1128/iai.63.7.2682-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge R, Watt RM, Sun X, Tanner JA, He QY, Huang JD, Sun H. Expression and characterization of a histidine-rich protein, Hpn: potential for Ni2+ storage in Helicobacter pylori. Biochem. J. 2006;393:285–293. doi: 10.1042/BJ20051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng YB, Zhang DM, Li H, Sun H. Binding of Ni2+ to a histidine- and glutamine-rich protein, Hpn-like. J. Biol. Inorg. Chem. 2008;13:1121–1131. doi: 10.1007/s00775-008-0397-0. [DOI] [PubMed] [Google Scholar]
- 13.Seshadri S, Benoit SL, Maier RJ. Roles of His-rich hpn and hpn-like proteins in Helicobacter pylori nickel physiology. J. Bacteriol. 2007;189:4120–4126. doi: 10.1128/JB.01245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauer K, Muller C, Carriere M, Labigne A, Cavazza C, De Reuse H. The Helicobacter pylori GroES cochaperonin HspA functions as a specialized nickel chaperone and sequestration protein through its unique C-terminal extension. J. Bacteriol. 2010;192:1231–1237. doi: 10.1128/JB.01216-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahler FN, Odenbreit S, Haas R, Wilrich J, Van Vliet AH, Kusters JG, Kist M, Bereswill S. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 2006;74:3845–3852. doi: 10.1128/IAI.02025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vliet AH, Poppelaars SW, Davies BJ, Stoof J, Bereswill S, Kist M, Penn CW, Kuipers EJ, Kusters JG. NikR mediates nickel-responsive transcriptional induction of urease expression in Helicobacter pylori. Infect. Immun. 2002;70:2846–2852. doi: 10.1128/IAI.70.6.2846-2852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras M, Thiberge JM, Mandrand-Berthelot MA, Labigne A. Characterization of the roles of NikR, a nickel-responsive pleiotropic autoregulator of Helicobacter pylori. Mol. Microbiol. 2003;49:947–963. doi: 10.1046/j.1365-2958.2003.03621.x. [DOI] [PubMed] [Google Scholar]
- 18.Chivers PT, Sauer RT. NikR is a ribbon-helix-helix DNA-binding protein. Protein Sci. 1999;8:2494–2500. doi: 10.1110/ps.8.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiter ER, Drennan CL. Ribbon-helix-helix transcription factors: variations on a theme. Nat. Rev. Microbiol. 2007;5:710–720. doi: 10.1038/nrmicro1717. [DOI] [PubMed] [Google Scholar]
- 20.Chivers PT, Sauer RT. NikR repressor: high-affinity nickel binding to the C-terminal domain regulates binding to operator DNA. Chem. Biol. 2002;9:1141–1148. doi: 10.1016/s1074-5521(02)00241-7. [DOI] [PubMed] [Google Scholar]
- 21.De Pina K, Desjardin V, Mandrand-Berthelot MA, Giordano G, Wu LF. Isolation and characterization of the nikR gene encoding a nickel-responsive regulator in Escherichia coli. J. Bacteriol. 1999;181:670–674. doi: 10.1128/jb.181.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chivers PT, Sauer RT. Regulation of high affinity nickel uptake in bacteria. Ni2+-dependent interaction of NikR with wild-type and mutant operator sites. J. Biol. Chem. 2000;275:19735–19741. doi: 10.1074/jbc.M002232200. [DOI] [PubMed] [Google Scholar]
- 23.Bury-Mone S, Thiberge JM, Contreras M, Maitournam A, Labigne A, De Reuse H. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 2004;53:623–638. doi: 10.1111/j.1365-2958.2004.04137.x. [DOI] [PubMed] [Google Scholar]
- 24.Abraham LO, Li Y, Zamble DB. The metal- and DNA-binding activities of Helicobacter pylori NikR. J. Inorg. Biochem. 2006;100:1005–1014. doi: 10.1016/j.jinorgbio.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Dosanjh NS, Hammerbacher NA, Michel SL. Characterization of the Helicobacter pylori NikR-P(ureA) DNA interaction: metal ion requirements and sequence specificity. Biochemistry. 2007;46:2520–2529. doi: 10.1021/bi062092w. [DOI] [PubMed] [Google Scholar]
- 26.Ernst FD, Kuipers EJ, Heijens A, Sarwari R, Stoof J, Penn CW, Kusters JG, van Vliet AH. The nickel-responsive regulator NikR controls activation and repression of gene transcription in Helicobacter pylori. Infect. Immun. 2005;73:7252–7258. doi: 10.1128/IAI.73.11.7252-7258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis GS, Flannery EL, Mobley HL. Helicobacter pylori HP1512 is a nickel-responsive NikR-regulated outer membrane protein. Infect. Immun. 2006;74:6811–6820. doi: 10.1128/IAI.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delany I, Ieva R, Soragni A, Hilleringmann M, Rappuoli R, Scarlato V. In vitro analysis of protein-operator interactions of the NikR and Fur metal-responsive regulators of coregulated genes in Helicobacter pylori. J. Bacteriol. 2005;187:7703–7715. doi: 10.1128/JB.187.22.7703-7715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst FD, Stoof J, Horrevoets WM, Kuipers EJ, Kusters JG, van Vliet AH. NikR mediates nickel-responsive transcriptional repression of the Helicobacter pylori outer membrane proteins FecA3 (HP1400) and FrpB4 (HP1512) Infect. Immun. 2006;74:6821–6828. doi: 10.1128/IAI.01196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romagnoli S, Agriesti F, Scarlato V. In vivo recognition of the fecA3 target promoter by Helicobacter pylori NikR. J. Bacteriol. 2011;193:1131–1141. doi: 10.1128/JB.01153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benanti EL, Chivers PT. The N-terminal arm of the Helicobacter pylori Ni2+-dependent transcription factor NikR is required for specific DNA binding. J. Biol. Chem. 2007;282:20365–20375. doi: 10.1074/jbc.M702982200. [DOI] [PubMed] [Google Scholar]
- 32.Dian C, Schauer K, Kapp U, McSweeney SM, Labigne A, Terradot L. Structural basis of the nickel response in Helicobacter pylori: crystal structures of HpNikR in Apo and nickel-bound states. J. Mol. Biol. 2006;361:715–730. doi: 10.1016/j.jmb.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 33.Dosanjh NS, West AL, Michel SL. Helicobacter pylori NikR’s interaction with DNA: a two-tiered mode of recognition. Biochemistry. 2009;48:527–536. doi: 10.1021/bi801481j. [DOI] [PubMed] [Google Scholar]
- 34.Bahlawane C, Dian C, Muller C, Round A, Fauquant C, Schauer K, De Reuse H, Terradot L, Michaud-Soret I. Structural and mechanistic insights into Helicobacter pylori NikR activation. Nucleic Acids Res. 2010;38:3106–3118. doi: 10.1093/nar/gkp1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danielli A, Scarlato V. Regulatory circuits in Helicobacter pylori: network motifs and regulators involved in metal-dependent responses. FEMS Microbiol. Rev. 2010;34:738–752. doi: 10.1111/j.1574-6976.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 36.Wolfram L, Haas E, Bauerfeind P. Nickel represses the synthesis of the nickel permease NixA of Helicobacter pylori. J. Bacteriol. 2006;188:1245–1250. doi: 10.1128/JB.188.4.1245-1250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bury-Moné S, Skouloubris S, Dauga C, Thiberge J-M, Dailidiene D, Berg DE, Labigne A, De Reuse H. Presence of active aliphatic amidases in Helicobacter species able to colonize the stomach. Infect. Immun. 2003;71:5613–5622. doi: 10.1128/IAI.71.10.5613-5622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stingl K, Schauer K, Ecobichon C, Labigne A, Lenormand P, Rousselle JC, Namane A, de Reuse H. In vivo interactome of Helicobacter pylori urease revealed by tandem affinity purification. Mol. Cell. Proteomics. 2008;7:2429–2441. doi: 10.1074/mcp.M800160-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Vanet A, Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect. Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fauquant C, Diederix RE, Rodrigue A, Dian C, Kapp U, Terradot L, Mandrand-Berthelot MA, Michaud-Soret I. pH dependent Ni(II) binding and aggregation of Escherichia coli and Helicobacter pylori NikR. Biochimie. 2006;88:1693–1705. doi: 10.1016/j.biochi.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Barceloux DG. Nickel. J. Toxicol. Clin. Toxicol. 1999;37:239–258. doi: 10.1081/clt-100102423. [DOI] [PubMed] [Google Scholar]
- 42.Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeisz S, Sittka A, Chabas S, Reiche K, Hackermuller J, Reinhardt R, et al. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464:250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- 43.Suerbaum S, Thiberge JM, Kansau I, Ferrero RL, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol. Microbiol. 1994;14:959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 44.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 45.van Vliet AH, Ernst FD, Kusters JG. NikR-mediated regulation of Helicobacter pylori acid adaptation. Trends Microbiol. 2004;12:489–494. doi: 10.1016/j.tim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Wolfram L, Bauerfeind P. Conserved low-affinity nickel-binding amino acids are essential for the function of the nickel permease NixA of Helicobacter pylori. J. Bacteriol. 2002;184:1438–1443. doi: 10.1128/JB.184.5.1438-1443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benanti EL, Chivers PT. An intact urease assembly pathway is required to compete with NikR for nickel ions in Helicobacter pylori. J. Bacteriol. 2009;191:2405–2408. doi: 10.1128/JB.01657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danielli A, Romagnoli S, Roncarati D, Costantino L, Delany I, Scarlato V. Growth phase and metal-dependent transcriptional regulation of the fecA genes in Helicobacter pylori. J. Bacteriol. 2009;191:3717–3725. doi: 10.1128/JB.01741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoof J, Kuipers EJ, van Vliet AH. Characterization of NikR-responsive promoters of urease and metal transport genes of Helicobacter mustelae. Biometals. 2010;23:145–159. doi: 10.1007/s10534-009-9275-7. [DOI] [PubMed] [Google Scholar]
- 50.Benanti EL, Chivers PT. Helicobacter pylori NikR exhibits distinct conformations when bound to different promoters. J. Biol. Chem. 2011;286:15728–15737. doi: 10.1074/jbc.M110.196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwig JS, Rowe JL, Chivers PT. Nickel homeostasis in Escherichia coli - the rcnR-rcnA efflux pathway and its linkage to NikR function. Mol. Microbiol. 2006;62:252–262. doi: 10.1111/j.1365-2958.2006.05369.x. [DOI] [PubMed] [Google Scholar]
- 52.Ahn BE, Cha J, Lee EJ, Han AR, Thompson CJ, Roe JH. Nur, a nickel-responsive regulator of the Fur family, regulates superoxide dismutases and nickel transport in Streptomyces coelicolor. Mol. Microbiol. 2006;59:1848–1858. doi: 10.1111/j.1365-2958.2006.05065.x. [DOI] [PubMed] [Google Scholar]
- 53.Campbell DR, Chapman KE, Waldron KJ, Tottey S, Kendall S, Cavallaro G, Andreini C, Hinds J, Stoker NG, Robinson NJ, et al. Mycobacterial cells have dual nickel-cobalt sensors: sequence relationships and metal sites of metal-responsive repressors are not congruent. J. Biol. Chem. 2007;282:32298–32310. doi: 10.1074/jbc.M703451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.