Abstract
Translation Initiator of Short 5′ UTR (TISU) is a unique regulatory element of both transcription and translation initiation. It is present in a sizable number of genes with basic cellular functions and a very short untranslated region (5′ UTR). Here, we investigated translation initiation from short 5′ UTR mRNAs with AUG in various contexts. Reducing 5′ UTR length to the minimal functional size increases leaky scanning from weak and strong initiators but hardly affects translation initiation and ribosomal binding directed by TISU. Ribosome interaction with TISU mRNA is cap dependent and involves AUG downstream nucleotides that compensate for the absent 5′ UTR contacts. Interestingly, eIF1 inhibits cap-proximal AUG selection within weak or strong contexts but not within TISU. Furthermore, TISU-directed translation is unaffected by inhibition of the RNA helicase eIF4A. Thus, TISU directs efficient cap-dependent translation initiation without scanning, a mechanism that would be advantageous when intracellular levels of eIF1 and eIF4A fluctuate.
INTRODUCTION
Regulation of mRNA translation occurs primarily at the initiation stage. The most crucial parameters for translation initiation are the m7G cap structure, the length and composition of the 5′ UTR, the context of the AUG-initiation codon, the poly(A) tail and the availability of translation initiation factors (1–3). Translation initiation of most eukaryotic mRNAs is thought to occur via a linear scanning of the 40S ribosomal subunit that stops at 5′-proximal AUG codon. The 40S ribosomal subunit occasionally skips the first AUG and initiates translation at a downstream (DS) AUG, a phenomenon known as leaky scanning. The extent of leaky scanning depends on the AUG-nucleotide context, the length of the 5′ UTR and the features of AUG downstream nucleotides (4,5). For mammalian mRNAs, the best-characterized translation initiation context is the Kozak element in which the most significant nucleotides are the purine (R) in position −3 and the G in position +4 relative to the A of the AUG. These two positions distinguish between a ‘strong’ or a ‘weak’ translation initiation that can prevent or allow leaky scanning, respectively (6).
Recently, we have identified an element (SAASATGGCGGC, in which S is C or G) called Translation Initiator of Short 5′ UTR (TISU), located downstream and close to the transcription start site (TSS) and controls the initiation rates of both transcription and translation. TISU is present in 4.5% of protein-encoding genes, most of them with an unusually short 5′ UTR (12 nt median length) (7). TISU genes are specifically enriched in mRNAs encoding for proteins involved in basic cellular functions such as respiration, protein metabolism and RNA synthesis. We found that TISU is essential for transcription and that its activity in transcription is mediated by the YY1 transcription factor (7). The ATG core of the TISU element and its flanking sequences, in addition to the −3 purine and the +4 G, create a strong translation-initiation context that has the ability to direct accurate translation initiation from a short 5′ UTR (7). The mechanism of TISU-directed translation initiation and the regulatory role it plays in translation are presently unknown.
For translation initiation, the 40S ribosomal subunit associates with several initiation factors (eIFs) and the initiator tRNA (Met-tRNAi), to form the 43S pre-initiation complex (PIC) (1–3). The 43S PIC is then recruited to the mRNA by eIF4F, a complex consisting of eIF4E, the m7G cap-binding subunit, eIF4A, an RNA helicase that unwinds the m7G cap-proximal 5′ UTR and eIF4G, a scaffold for eIF4E and eIF4A binding (3). The 43S PIC then scans the mRNA linearly checking for successive triplets as they enter the peptidyl (P)-site of the ribosome (4) until it encounters the first AUG that interact with the anticodon in Met-tRNAi through base pairing (8). This match arrests the scanning and releases the eIFs enabling the binding of the 60S ribosomal subunit to form the 80S initiation complex (9).
The key factor determining fidelity of translation initiation is eIF1 (10–12). It converts the 43S complex from an ‘open’ conformation that enables the recognition of any codon, to a ‘close’ conformation that restricts binding to an AUG codon in the proper sequence context (13). The role of the purine in position −3 and the G in position +4 is to stabilize the 48S following recognition of the initiation codon (14). However, if an AUG within a favorable context is situated 8 nt from the m7G cap, eIF1 promotes bypass of this AUG so that most of the ribosomes initiate instead at a downstream site (13). Consistent with this finding, a 5′ UTR with a length of at least 20 nt is needed for an efficient recognition of an AUG with a favorable context and further lengthening of an unstructured 5′ UTR significantly increases translation efficiency (15). These observations are in agreement with the finding that when the P-site of the 40S ribosomal subunit is situated on the AUG codon, the initiation complex forms contacts with the mRNA from 17-nt upstream and 11-nt downstream to the AUG (16).
In the present study, we revisited the role of AUG context, 5′ UTR length and translation initiation factors in regulation of translation initiation. We report that when the distance between m7G cap and AUG was reduced to 5 nt, fidelity and efficiency of translation initiation as well as 48S ribosome binding were maintained only with TISU, implying initiation without scanning. Using several assays, we established that recruitment of the initiation machinery to TISU is cap dependent. eIF1 inhibited cap-proximal AUG selection in either weak or strong contexts but not within a TISU context. Moreover, TISU-directed translation is less sensitive to inhibition of the RNA helicase, eIF4A. These results reveal a unique mode of cap dependent and scanning independent translation initiation through TISU, a mechanism that is likely to be functional when availability eIF1 and eIF4A changes.
MATERIALS AND METHODS
Plasmid construction
To generate a green fluorescent protein (GFP)-reporter gene suitable for translation assays in vitro and in vivo, the pEGFP-N1 vector (Clontech) was modified as follows: an oligonucleotide containing 35 repeats of adenosine was generated by polymerase chain reaction (PCR) and inserted via XbaI and AflII sites downstream of the GFP-coding sequence in order to generate poly(A) tail in the in vitro-synthesized mRNA. This oligonucleotide also contained SpeI site immediately upstream to AflII site. The multiple cloning site of the pEGFP-N1 was replaced with CAA repeats via HindIII and AgeI site to remove undesirable secondary structures. In addition, ScaI site was inserted into the vector, using the Quikchange Site-Directed Mutagenesis kit (Stratagene), at positions 538–543 upstream to the CMV TATA-box element. Then the CMV core promoter was replaced by a T7 promoter via ScaI and NheI sites. Various AUG contexts were constructed by inserting the appropriate oligonucleotide into Eco47III and BglII sites. The constructs bearing an AUG in a weak, strong or TISU contexts with 8 and 5 nt from the m7G cap were prepared by annealing oligonucleotidenucleotides containing T7 promoter and the desired ATG context at the specific location, and filling in with Klenow. The double-stranded fragments were then digested with BglII and cloned into the modified pEGFP through ScaI and BglII.
The secondary structure at position 6 or 15 downstream from the AUG of either TISU or a strong AUG context was previously described (17). This sequence forms a secondary structure with ΔG = −24 kcal/mol as determined by the RNA mfold algorithm (http://mfold.bioinfo.rpi.edu/cgi-bin/rna-form1.cgi). This sequence was inserted via BglII and HindIII sites into the modified pEGFP-N1. Then oligonucleotides-containing T7 promoter and an AUG within either TISU or a strong AUG context located 5 nt from the TSS of the T7 promoter were inserted via ScaI and BglII sites. The same sequence was also cloned close to the m7G cap before the AUG via NheI site. The constructs were linearized by SpeI prior to in vitro transcription.
The construct bearing the two frames of GFP was prepared as follows: a 153 amino acids fragment of the N′ of the EGFP containing a stop codon was cloned via XbaI site (2nd frame) into the modified pEGFP-N1 plasmid [containing the CAA repeats and the poly(A)]. Then the same fragment was inserted via AgeI and the upstream XbaI site that dose not flank with a Dam-methylation site, instead of the whole EGFP, to generate the first frame. Oligonucleotides bearing TISU with an AUG 5 nt far from the 5′-end, TISU with an AUG 18 nt far from the 5′-end and oligonucleotides bearing both TISU with an AUG 5 and 18 nt far from the 5′-end were annealed and cloned via AseI (filled in with klenow) and BglII sites.
For construction of eIF1-expression plasmids, the eIF1 cDNA was isolated by RT–PCR from HEK293T RNA and then cloned either in pET28a vector (Novagen) via Ecl136 and XhoI sites for bacterial expression or in the pCRUZ-HA vector (Santa Cruz Biotechnology, Inc.) via ScaI and Bgl II sites for expression in mammalian cells. eIF4A1 WT and dominant negative mutant were previously described (18). For expression in mammalian cells, eIF4A1 WT and mutant were subcloned into pCruz-HA via EcoRV and Bgl II sites.
Cell culture and antibodies
HEK293T and HeLa cell lines were used for this study. These cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Transfections of in vitro-synthesized mRNA were performed in six-well plates Lipofectamine Reagent (Invitrogen). Plasmid transfections were carried out using the standard CaPO4 method. Commercial antibodies against GFP and HA were previously described (7). The total (#9644) and non-phospho 4E-BP (#4923) specific antibodies are from Cell Signaling Technology.
Preparation of mRNA for in vitro and in vivo translation assays
For synthesis of capped mRNA, the constructs containing the T7 promoter were linearized and used with the RiboMAXTM Large Scale RNA Production Systems T7 (Promega) supplemented with either a Ribo m7G or an unmethylated (ApppG) Cap Analog (New England Biolabs). The reaction was stopped by addition of RQ1 RNase-Free DNaseI (Promega) and the mRNA extracted with phenol–chloroform and precipitated with ethanol. The capped mRNAs were denatured at 65°C for 10 min and then placed on ice for 2 min. mRNAs with the hair-pin structure were denatured at 70°C for 15 min in RNA structure buffer (10 mM Tris pH 7, 100 mM KCl and 10 mM MgCl2) and left to cool slowly to ∼30°C. The concentration of the synthesized mRNAs was determined and their integrity was confirmed by agarose gel electrophoresis (Supplementary Figure S1). mRNA transfection into HEK293T, HeLa and HepG2 cells were carried out as previously described (7).
In vitro and in vivo translation assays
In vitro translation was carried out in nuclease-treated Rabbit Reticulocyte Lysate (Promega; 25 µl total reaction volume) that was supplemented with 1 μg of in vitro synthesized capped mRNA and 35S-methionine. After incubation at 30°C for 90 min, 10% of each reaction was analyzed by 15% PAGE. For the in vivo translation assay 5–10 μg of the in vitro-transcribed mRNA and 5 μg of luciferase mRNA, as internal control, were denatured and co-transfected into 293T cells that had been previously seeded on 12-well plates, using 15 μg Lipofectamine Reagent (Invitrogen). Twenty-four hours after transfection total cell extracts were prepared. Transfection efficiency was normalized by measuring luciferase activity and normalized extracts were then subjected to western blotting using anti-GFP mAb.
To determine the effect of eIF1 and eIF4A on translation, we transfected into HEK293T cells in a six-well plate, 50 ng of each GFP reporter plasmid together with the indicated amounts of the translation-initiation factor. The amount of expression plasmid was kept constant with the empty expression plasmid. GFP levels were determined as described above. Since the CMV–Renilla reporter was found be refractory to the inhibition by the dominant negative mutant of eIF4A, we used it to normalize for transfection efficiency.
Toe printing
The toe-printing assay was performed as previously described (19). The sequencing reaction was carried out with the Sequenase Version 2.0 kit (USB corporation) using pEGFP-N1 vector as template. Results were visualized with a Phosphoimager (Fuji, BAS 2500)
Expression and purification of eIF1
eIF1 was purified from Escherichia coli BL21(DE3) bacteria transformed with eIF1-pET28a construct. Bacteria were grown in 2YT+ Kanamycin medium at 37°C up to OD (600)= 0.6. Then, IPTG (0.1 mM) was added and bacteria were harvested 4 h later. The samples were sonicated and the soluble fractions were purified on Nickel His Trap HP column (Qiagen) as recommended by the manufacturer.
RESULTS
TISU-mediated translation initiation operates without scanning
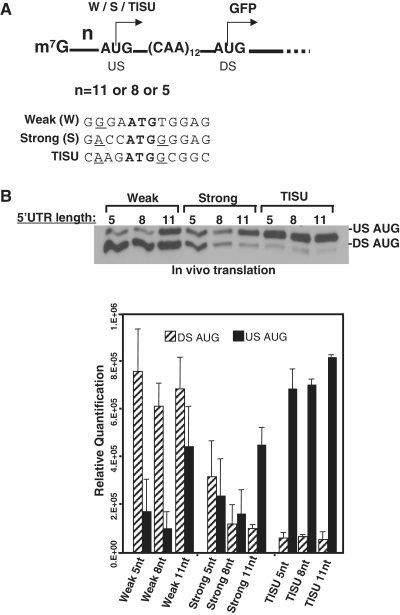
The scanning mechanism of translation initiation (9) postulates that the 43S ribosomal subunit enters at the 5′-end of the mRNA, aided by the m7G cap, and transverses the 5′ UTR until it encounters the first AUG codon. However, when the length of the 5′ UTR is shorter than 32 nt, there is leakage to a second downstream AUG (15) and the level of translation initiation decreases with reduced 5′ UTR length (20), most likely due to fewer contacts of the 43S ribosomal subunit with the mRNA (16). To determine the mechanism of translation initiation from short 5′ UTR mRNAs bearing various AUG contexts, we inserted three different sequences: a TISU element, a strong and a weak AUG contexts downstream of the T7 promoter and upstream of the GFP-coding sequence. For each of these elements, the distance between the m7G cap and the AUG was set to be 11, 8 or 5 nt (Figure 1). These constructs were transcribed with T7 polymerase and capped in vitro (Supplementary Figure S1), and the mRNAs were then co-transfected into cells together with a capped luciferase mRNA that was used to normalize for differences in transfection efficiency. The site of translation initiation was determined by immunoblot with GFP antibody. Reducing the 5′ UTR down to its shortest possible length, 5 nt hardly affected the efficiency and fidelity of TISU (Figure 1). In contrast, shortening the 5′ UTR when the AUG is within a strong or a weaker context substantially increased the leakage to the downstream AUG. The T at the +4 position in the reporter with the weak AUG context changed the encoded amino acid to tryptophane that may affect the reporter protein stability. We therefore compared the decay rates of the GFP reporters with either TISU or the weak initiator and found them to be very similar (Supplementary Figure S2). These findings suggest that translation through TISU is highly efficient with extremely short 5′ UTR length and can take place without scanning.
Figure 1.
(A) The sequences of TISU, strong and weak AUG contexts were cloned DS to a T7 promoter so that their AUG is in frame with the authentic GFP AUG and is distant from the m7G cap by 11, 8 or 5 nt. These constructs were used to synthesize capped mRNAs in vitro. (B) The in vitro synthesized mRNA described in (A) were co-transfected into 293T cells together with luciferase mRNA that serves to normalize transfection efficiency. Translation of the GFP and luciferase proteins were analyzed by immunoblot with GFP antibody and by luciferase assay, respectively. Representative blot of in vivo-translation experiments is shown in the upper panel. Initiation from the upstream AUG (US) produces a protein of 30 KDa and from the DS 27 KDa. Quantified results of three independent transfection experiments are shown at the bottom panel.
Ribosome binding to leader-less mRNA with TISU involves AUG downstream nucleotides
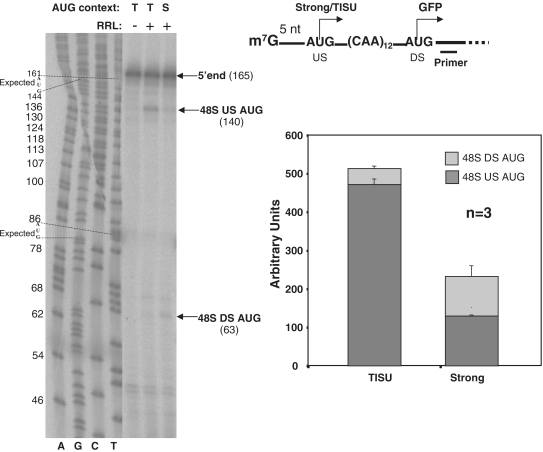
Considering that for accurate initiation the ribosome has to interact with 15–17 nt upstream of the AUG (16,21), which is impossible with a short 5′ UTR, TISU may provide an attachment site for the initiation complex without extensive 5′ UTR contacts. To test this, we analyzed binding by the 43S ribosome using toe-printing assays. mRNAs with 5-nt long 5′ UTR and with either TISU or a strong AUG context were incubated with rabbit reticulocyte lysate in the presence of a non-hydrolyzable GTP analog, to hold the initiation complex bound to the mRNA initiation site, and then subjected to primer extension analysis. Under these conditions, the reverse transcriptase is stalled when it reaches the boundary of the 48S binding. We found that when the 5′ UTR is only 5-nt long, the 48S ribosomal complex binds TISU with higher affinity than the strong element (Figure 2A). Under these conditions, the ribosome interferes with the reverse transcription, 17 nt downstream of the AUG (Figure 2A, US AUG) that is indicative of the proximity of this region to the border of ribosome interaction with the mRNA. With the strong context, an equivalent weak association of the 48S ribosome is detected with the upstream and the downstream AUGs (Figure 2A), as expected from leaky scanning.
Figure 2.
The 48S-ribosomal subunit binds to mRNAs-bearing TISU at the 5′-end with high affinity. (A) A toe-printing assay on mRNAs-bearing AUG within TISU (T) or strong (S) contexts. A schematic representation of the mRNA and the 32P-labeled primer used for toe-printing procedure is shown in the right. The constructs were in vitro transcribed and annealed with 32P-labeled primer. RRL was incubated with the non-hydrolyzable GTP analog, GMP-PNP, at 25°C for 10 min, then the mRNA was added for additional 10 min. Subsequently the location of the 48S on the mRNA was analyzed by primer extension as shown in the representative gel. The locations of 48S binding site boundary (∼15 nt DS of the AUG) are marked. A detailed description of the sequence of the analyzed region is shown in Supplementary Figure S3.
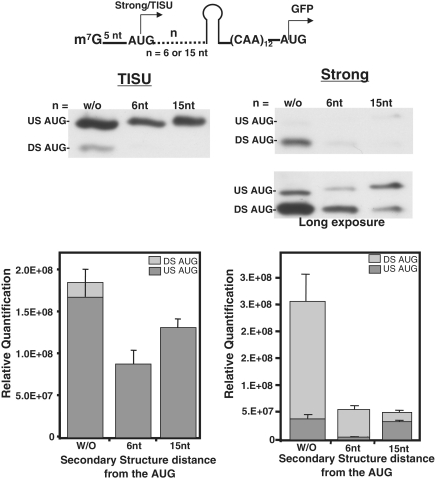
To further compare between TISU and other AUG context in leader-less mRNAs, we inserted a previously characterized secondary structure (17) 6 and 15 nt downstream from the AUG (Figure 3, upper panel). Secondary structure up to position 12 from the m7G cap is expected to interfere with ribosome binding to the mRNA (22–24). With long 5′ UTR secondary structure at position 2 or 8 downstream of the AUG had no significant effect on translation efficiency in vitro; but when positioned 14 nt downstream of the AUG translation, fidelity was increased (17). Secondary structure present at 6 nt downstream of the AUG (13 nt from the m7G cap), caused a ∼60% reduction in TISU-mediated translation, but for the strong AUG context, it was severely inhibitory (Figure 3). These results indicate that in leader-less mRNAs with both TISU and the strong context nucleotides beyond position six downstream of AUG are important for the binding of the ribosome to the mRNA and for AUG recognition without scanning. The fact that the effect of the secondary structure on translation mediated by TISU was moderate, further confirms that the AUG flanking sequences of TISU is a better context for the ribosome than the strong Kozak element (Figure 3). Secondary structures at position +15 are beyond ribosome binding site and enhance fidelity for both TISU and the strong AUG context in accordance with previous report (17), verifying that the effect observed in this experiment is indeed due to the secondary structure. These findings strongly suggest that the ribosome forms sequence-specific contacts with the TISU sequence as well as with non-specific nucleotides downstream of TISU to compensate for the lack of 5′ UTR contacts and facilitate translation without scanning.
Figure 3.
The effect of a moderate secondary structure on the efficiency and fidelity of translation from a strong and TISU AUG contexts located five nt from the m7G cap. (A) Schematic representation of a secondary structure positioned 6 or 15 nt DS of the AUG is shown on the top panel. The constructs with or without secondary structure were in vitro transcribed and then translated in vivo by transfection into 293T cells and translation efficiency was assayed by immunoblot with anti-GFP. Representative immunoblots of three independent experiments is shown. The graphs represent the average ± SD of the intensity of the upstream (30 KDa) and DS (27 KDa) translation site of 3–5 independent experiments (In the case of the strong AUG, the densitometry analysis was done using the blot from the long exposure).
Recruitment of the initiation complex to TISU mRNA is cap dependent
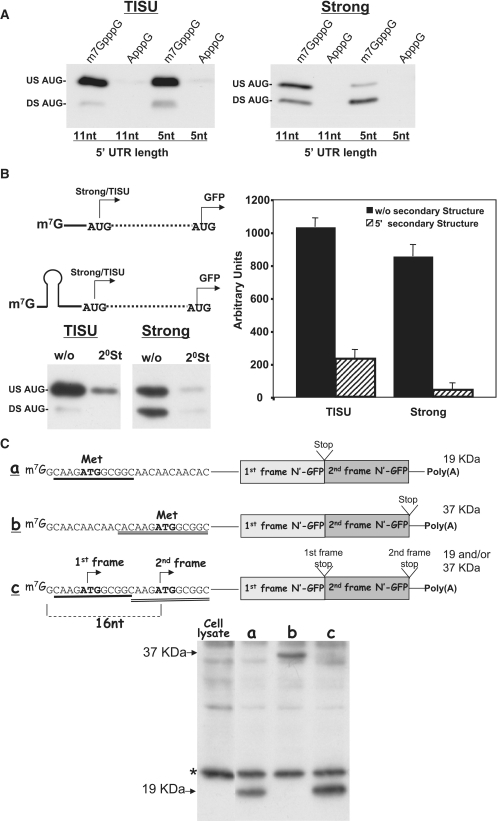
For accurate initiation, the ribosome has to interact with 15- to 17-nt upstream of the AUG (16,21), but TISU appears to provide an attachment site for the ribosome without extensive 5′ UTR contacts. The question arising is whether ribosome recruitment is direct as with IRES or is mediated by the m7G cap. To test this, we used GFP constructs bearing either a TISU element or a strong AUG context with 5′ UTR length of 5 and 11 nt. These constructs were transcribed with T7 polymerase and capped in vitro with either a m7GpppG cap or an unmethylated cap analog, ApppG that is known to retain mRNA stability but is not recognized by the translation machinery. These mRNAs were then co-transfected into cells together with a capped luciferase mRNA. After 24 h, the stability of the various reporter mRNA was determined by quantitative RT–PCR and translation was analyzed by immunoblot with GFP antibody. As expected the stability of the mRNAs with the methylated and unmethylated caps is very similar (Supplementary Figure S4). The presence of the TISU element seems to slightly reduce the recovery of the mRNA from cells, regardless of the type of the cap suggesting that TISU-containing mRNAs may be slightly less stable (Supplementary Figure S4). Translation initiation directed by both, TISU and the strong AUG context, was clearly seen when the mRNA was capped by the m7GpppG, but was almost undetected with the cap analog (Figure 4A).
Figure 4.
Translation initiation from TISU is dependent on 7mG cap. (A) mRNAs bearing AUG with either TISU or a strong contexts located either 5 or 11 nt from the 5′-termini were in vitro transcribed in the presence of m7GpppG cap or the unmethylated cap-analog ApppG. The mRNAs were translated in vivo by transfection into 293T cells. Cell lysate was prepared 24 hours following transfection and subjected to western blot using anti-GFP. Translation efficiency is shown in the representative blot of three independent experiments. (B) The effect of AUG upstream secondary structure on the efficiency and fidelity of translation from a strong and TISU AUG contexts. The upper-left panel shows a schematic representation of a secondary structure located upstream from the AUG within TISU or strong AUG context. The constructs with or without secondary structure were in vitro transcribed and then translated in vivo by transfection into 293T cells and translation efficiency was assayed by blotting with anti-GFP. Representative immunoblot for the strong and the TISU AUG contexts are shown in the bottom-left panel and the graphs represent the average ± SD of the intensity of the upstream (30 KDa) translation site of four independent experiments. (C) Recognition of TISU's AUG is dependent on the 5′-end of mRNA. The upper panel shows a schematic representation of mRNAs bearing either one or two TISU elements in tandem with the expected protein size translated from each AUG. The mRNAs were transcribed and capped in vitro and then were translated in vivo by transfection into 293T cells. Cell lysate was prepared 24 hours following tranfection and subjected to western blot using anti-GFP (lower panel). The positions of 19 and 37 KDa proteins are shown by arrows.
To examine further the dependency on mRNA 5′-end, we introduced a moderate secondary structure (ΔG = −24 kcal/mol) at the mRNA 5′-end between the m7G cap and a strong or TISU AUG contexts (Figure 4B) that would hamper recruitment of the initiation complex through the m7G cap. This insertion dramatically reduced translation efficiency through both AUG contexts. We also constructed plasmids bearing two TISU elements in tandem so that translation initiation from each element would generate a protein of different size as shown schematically in Figure 4C. If the ribosome interacts directly with TISU, we would expect to observe translation initiation from both elements. The results revealed that in the presence of two TISU elements, only the one that is close to the cap is utilized (Figure 4C), ruling out the possibility that the 48S ribosome could directly interact with TISU. These findings together show that TISU-directed translation initiation is dependent on the 7mG cap.
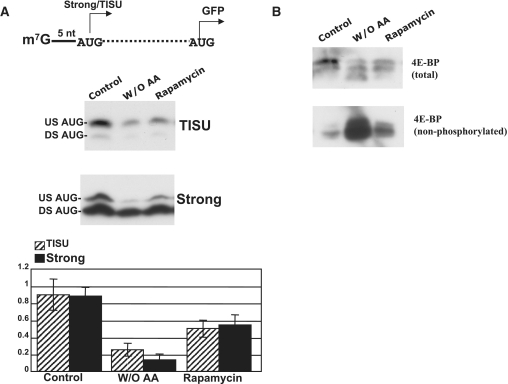
Amino acids that are essential for protein synthesis also play a regulatory role in cap-dependent translation initiation through activation of the mammalian target of rapamycin (mTOR), a protein kinase that relieves the cap-binding subunit, eIF4E, from inhibition by eIF4E-BP (3,25,26). We examined the activity of mRNAs with short 5′ UTR under conditions in which eIF4E is suppressed. HeLa cells were transfected with in vitro-transcribed mRNA that harbors either TISU or the strong AUG initiator preceded by a 5′ UTR of 5 nt. The cells were then incubated for 24 h either in a medium lacking amino acids or in a normal medium containing 20 nM rapamycin, a drug known to inhibit mTOR activity and the cap-binding factor eIF4E (26). mTOR phosphotylates eIF4E-BP to prevent it from inhibiting eIF4E. Both amino acid starvation and rapamycin treatment inhibited translation initiation regardless of the AUG context (Figure 5A). As expected, this inhibition was accompanied with a significant increase in eIF4E-BP non-phosphorylated (Figure 5B). The effect of this dose of rapamycin on the transfected reporter mRNAs is more severe than its effect on global translation that was monitored by polysomal profiling (Supplementary Figure S5). These findings show a clear dependence of TISU mRNA on amino acid availability and further support its reliance on the m7G cap.
Figure 5.
The activity of TISU is dependent on amino acid availability. (A) HeLa cells were transfected with in vitro-synthesized GFP–mRNA driven either by TISU or a strong initiation context preceded by a 5-nt long, 5′ UTR. Cells were incubated for 24 h either in full medium (control) or amino acid-free medium (W/O AA) or full medium containing 20 nM rapamycin. The serum used for cell growth was dialyzed prior to use to remove residual amino acids. The cells were harvested and GFP levels were detected by a GFP antibody. Representative western blots are shown and the graph at the bottom represents the average ± SD of the intensity of the upstream (30 KDa) translation site of four independent experiments. (B) Analysis of 4EBP phosphorylation status. Cell lysates from the experiment described in (A) were subjected to western blot with antibodies against total or antibodies specific to the un-phosphorylated form of 4EBP as indicated.
eIF1 effect on start-codon selection in short 5′ UTR mRNAs is dependent on AUG context
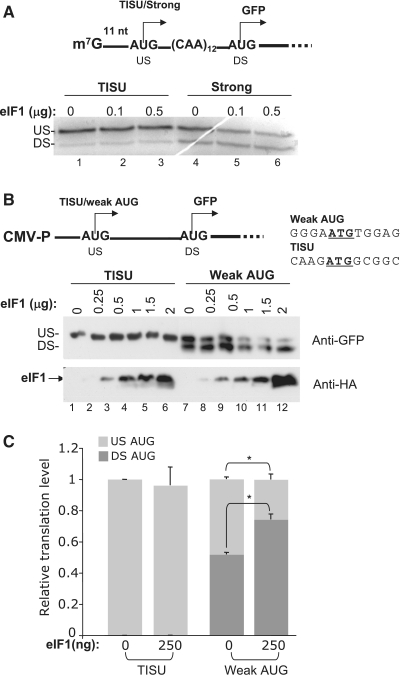
eIF1 has an important role in promoting scanning and start-codon selection (10–12,27). A previous study that examined eIF1 activity in vitro revealed that eIF1 stimulates ribosome leakiness when the AUG is in a context of a short 5′ UTR suggesting that eIF1 inhibits AUG recognition when the ribosome is unable to form sufficient contacts with AUG upstream nucleotides (13). Thus, it is likely that the level of eIF1 in the cell can modulate translation efficiency of mRNAs with short 5′ UTR. We compared the effect of eIF1 on translation efficiency and fidelity of mRNAs with a short 5′ UTR and either a strong AUG context or TISU. eIF1 was expressed in E. coli, purified and then added to in vitro-translation reaction with rabbit reticulocyte lysate. As shown in Figure 6A, increasing amounts of eIF1 had no effect on translation fidelity directed by TISU (lanes 1–3), but increasing amounts of eIF1 inhibited the initiation of the upstream AUG, but not that of the downstream AUG (lanes 4–6). This finding suggests that the ability of eIF1 to promote leaky scanning is not general but depends on the AUG context. To gain further support for this idea, we examined the effect of eIF1 on in vivo translation. For this purpose, we used the GFP-reporter gene driven either by TISU or by a weak AUG context, both with a short 5′ UTR. These plasmids were co-transfected into HEK293T cells with increasing amounts of eIF1 expression plasmid (Figure 6B). The results revealed that the mRNA with the weak AUG context was highly sensitive to eIF1 expression as in the presence of low eIF1 concentration utilization of the upstream AUG was inhibited while initiation from the downstream AUG increased (compare lane 7 to lanes 8 and 9 in Figure 6B and Figure 6C). At higher levels of eIF1, initiation from both, upstream and downstream AUGs, was inhibited. In contrast, eIF1 had no significant effect on TISU-mediated translation initiation that was primarily from the upstream AUG, at any concentration (lanes 1–6). These findings, which are consistent with the effect of eIF1 in vitro, show that TISU mRNAs differ from other mRNAs with short 5′ UTR in its resistance to the leakage promoting effect of eIF1.
Figure 6.
A. The influence of eIF1 on translation initiation in vitro from short 5′ UTR mRNA and distinct-AUG contexts. (A) schematic representation of the GFP-reporter gene with either TISU or a strong AUG context, both with 11 nucleotides, 5′ UTR. eIF1 was expressed in E. coli, purified and the indicated amounts were added to in vitro-translation reactions with the described constructs. Reactions with TISU and the strong AUG context are indicated at the top. (B) The effect of eIF1 on translation efficiency and accuracy in vivo. The upper panel shows a scheme of the GFP-reporter gene driven either by TISU or by a weak-AUG context, both with short 5′ UTR. The AUG-flanking sequence is shown. These reporters were transfected into HEK293T cells with increasing amounts of eIF1-expression plasmid as indicated, and the translation-initiation site was determined by immunoblot with GFP-specific antibody. US and DS denote upstream and downstream initiation site, respectively. eIF1 expression was analyzed by immunoblot using anti-HA antibody. (C) A graph representing translation directed by TISU or the weak-AUG reporter, GFP, in the absence or presence of low dose of eIF1 plasmid (250 ng), from three independent experiments (average ± SD). The overall translation without eIF1 was set to one. The relative intensity of the upstream translation site is presented by light grey bars and the DS-translation site by dark grey bars. The asterisks denotes statistically significant difference, P < 0.005.
TISU-mediated translation is refractory to eIF4A inhibition
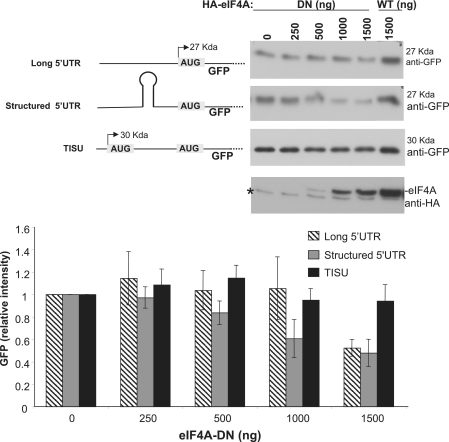
eIF4A, a subunit of the m7G cap-binding complex, eIF4F, is an RNA helicase that uses ATP to unwind secondary structures in the mRNA 5′ UTR (13,18). As most TISU-bearing mRNAs are characterized by a short 5′ UTR (7), one would expect TISU-mediated translation to be less dependent on eIF4A. To test this possibility, we utilized a previously characterized mutant of eIF4A that is deficient in helicase activity but can still be incorporated into the eIF4F complex thereby acting as a dominant negative mutant (18). We examined the effect of eIF4A mutant on three different types of GFP-reporter mRNAs. The first has a long and unstructured 5′ UTR, the second has a secondary structure embedded within long 5′ UTR and the third bears TISU in a context of a short 5′ UTR (Figure 7, upper left panel). An inherent problem with examining the effect of a general translation-initiation factor such as eIF4A is to find an internal control that would be unaffected by it. We screened several reporter plasmids for the effect of the dominant negative mutant of eIF4A and found that the Renilla luciferase (RL) from the pRL–CMV plasmid is not significantly affected by the dominant negative mutant of eIF4A in multiple experiments (Supplementary Figure S6), therefore this plasmid was chosen to serve as an internal normalizing control. HEK293T cells were transfected with the GFP and RL reporters described above together with either wild type or increasing amounts of eIF4A-dominant negative mutant expression plasmid. The expression of eIF4A wild-type and mutant was validated by immunoblot with anti-HA tag antibody (Figure 7). The results show that increasing amounts of the dominant negative mutant of eIF4A inhibited the expression of the reporters bearing either unstructured long or structured 5′ UTR, with the secondary structured 5′ UTR reporter being sensitive to eIF4A inhibition at lower concentrations (Figure 7). In contrast, TISU mRNA was the least affected by the eIF4A mutant. These findings suggest that TISU is likely to confer to the genes bearing it, resistance to fluctuations in eIF4A availability.
Figure 7.
The effect of a dominant negative mutant of eIF4A on translation. (A) schematic representation of the GFP-reporter genes is shown in the upper-left panel. The first has a long unstructured 5′ UTR, the second has a hair-pin structure within the 5′ UTR and the third has TISU in a context of a short 5′ UTR. HEK293T cells were transfected with these GFP reporters together with increasing amounts of eIF4A-dominant negative mutant (eIF4A-DN) or wild-type eIF4A-expression plasmids as indicated. The amount of expression plasmid was kept constant with the empty expression vector. RL under the CMV promoter was found to be refractory to eIF4A-DN (see Supplementary Figure S6) was used to normalize for transfection efficiency. GFP, eIF4A-DN and eIF4A expression were analyzed by immunoblot in which representative are shown on the upper right section. The asterisk in the eIF4A blot denotes a non-specific band. A graph representing the average of densitometric measurements of three independent transfection experiments is shown in the lower panel.
DISCUSSION
In this study, we carried out a detailed molecular analysis of the features characterizing translation initiation from short 5′ UTR mRNAs with AUG in various contexts. From our data, a unique mode of translation initiation through TISU has emerged. TISU guides cap-dependent translation initiation without scanning with high efficiency and fidelity. Moreover, TISU displays differential requirement for certain translation-initiation factors. These features clearly discriminate between TISU and other AUG contexts including the Kozak, which cannot support accurate translation initiation from leader-less mRNAs. This mechanism of translation initiation is likely to provide an advantage to TISU under certain physiological circumstances. The properties of TISU are unprecedented in the light of previous studies showing that an AUG within a favored sequence requires a 5′ UTR of at least 20 nt for accurate translation initiation (15). The most likely explanation for the 20 nt minimal length is that the 40S subunit of the ribosome forms contacts with ∼17-nt upstream of the AUG and 11-nt downstream to it (16).
Using toe-printing assays and examining the effect of a hairpin structure downstream to TISU (6 and 15 nt from the AUG), we deduce that in addition to the AUG-flanking sequences of TISU, the nucleotides downstream from the TISU element are important for binding by the 40S subunit of the ribosome to the mRNA. Interestingly, the same secondary structure did not exert an inhibitory effect when placed 2- and 8-nt downstream of the AUG in mRNA with longer 5′ UTR (17), suggesting that contacts between the 43S ribosome and nucleotides downstream of TISU in the mRNA, compensate for the lack of scanning and extensive contacts with nucleotides in the 5′ UTR. The effect of a secondary structure downstream of the AUG was substantially more severe for the strong Kozak element than the TISU in mRNAs with short 5′ UTR, confirming the requirements of both the TISU element and additional downstream nucleotides for binding by the ribosome to overcome the lack of 5′ UTR.
The characteristics of TISU described above raised the question of whether the translation mediated by TISU is cap dependent. We addressed this question by determining the translation efficiency of mRNA containing the unmethylated cap analog (ApppG) and by placing a secondary structure between the cap and TISU or by analyzing the site translation initiation when two TISU elements are placed in tandem. Our results revealed >90% reduction in translation efficiency from mRNA with an unmethylated cap analog compared with the m7G-capped mRNAs. Likewise an interfering secondary structure between the m7G cap and TISU diminished translation, and translation initiated exclusively from the TISU that is adjacent to the m7G cap. These findings together provide clear evidence that TISU-mediated translation is cap dependent.
The non-scanning nature and the short 5′ UTR length in TISU genes evoked the possibility that translation through TISU would be less dependent on eIF4A, an RNA helicase that unwinds secondary structures on the mRNA 5′ UTR and facilitates scanning (13,18). In accordance with this expectation, eIF4A seems to be dispensable for TISU-mediated translation from short 5′ UTR mRNA. It is possible that the recently identified RNA helicase, DHX29 that was shown to facilitate translation initiation from structured mRNAs is also not essential for translation of TISU mRNAs (28,29). However, we cannot rule out the possibility that the helicase activity of eIF4A may be required for TISU-mediated translation if the AUG is embedded within a secondary structure.
eIF1 facilitates AUG-codons discrimination by the initiation complex on the basis of their nucleotide context (favoring the Kozak sequence in the mRNA) and their location relative to the m7G cap (13). It promotes the dissociation of the 43S from non-AUG codons, from AUG codons in a poor context and from AUG codon very close to the 5′-end of the mRNA (13,30). In agreement with these observations, we found that eIF1 inhibited AUG selection even in a favored strong context when it was located 11 or 5 nt from the m7G cap. In contrast, eIF1 failed to inhibit initiation from TISU-AUG codon located close to the m7G cap. A possible way to explain the differential effect of eIF1 is based on kinetic considerations. The process of formation of the initiation complex on TISU may be much faster than the rate by which eIF1 induces its dissociation. With the TISU element close to the m7G cap, the 48S encounters a high affinity AUG context, immediately after its recruitment by the m7G cap-bound eIF4F that induces its rapid conversion into a ‘closed’ state (13). This is followed by eIF2-mediated GTP hydrolysis, release of eIF2-GDP and assembly of the large ribosomal subunit. Once the 80S complex is formed, it is not responsive to eIF1 anymore.
Protein synthesis, one of the most energy consuming processes of the cell, is highly sensitive to changes in nutrient concentration. Among the important agents influencing mRNA translation are amino acids and glucose that are known to modulate the activity of several translation-initiation factors that are critical for cap-dependent mRNA translation initiation (31). We show that the translation-initiation activity of TISU is diminished by amino acid deficiency, a stress that increases the ratio between the inactive and active m7G cap-binding subunit, eIF4E (3). This finding provides additional support for the cap-dependence of TISU-directed translation initiation.
It is reasonable that the differential effect of eIF1 on mRNAs with distinct initiation contexts would have a regulatory role. eIF1 mRNA was reported to be induced under certain stress conditions such as DNA damage and ER stress (32,33). An increase in eIF1 protein under certain physiological conditions could then inhibit initiation from mRNAs with a short 5′ UTR or poor-AUG contexts while mRNAs-bearing TISU may be refractory to its effect. However it remains to be determined whether elevated mRNA levels of eIF1 under these stresses lead to an increase in eIF1 protein, as mRNA translation is known to be inhibited under these stresses. It would therefore be interesting to examine the protein levels of eIF1 and the activity of TISU.
TISU is a regulatory element that controls both transcription and translation initiation of genes with basic cellular functions. Therefore, any perturbation in TISU's activity is likely to be deleterious to the cell. Future studies on the factor requirement for TISU's activity, its regulation under specific physiological settings and the role it plays in co-ordinating transcription and translation will lead to the discovery of additional molecular parameters governing its activity.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Yeda-Sela Center for Basic Research and the Y. Leon Benoziyo Institute for Molecular Medicine, both at the Weizmann Institute. Funding for open access charge: Yeda-Sela Center and Y. Leon Benoziyo Institute.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Sandra Moshonov for critical reading and editing the manuscript. R.D. is the incumbent of the Ruth and Leonard Simon Chair of Cancer Research.
REFERENCES
- 1.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell. Biol. 11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marintchev A, Wagner G. Translation initiation: structures, mechanisms and evolution. Q. Rev. Biophys. 2004;37:197–284. doi: 10.1017/S0033583505004026. [DOI] [PubMed] [Google Scholar]
- 3.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XQ, Rothnagel JA. 5′-untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation. Nucleic Acid Res. 2004;32:1382–1391. doi: 10.1093/nar/gkh305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozak M. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene. 2005;361:13–37. doi: 10.1016/j.gene.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Elfakess R, Dikstein R. A translation initiation element specific to mRNAs with very short 5′ UTR that also regulates transcription. PLoS ONE. 2008;3:e3094. doi: 10.1371/journal.pone.0003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cigan AM, Feng L, Donahue TF. tRNAi(met) functions in directing the scanning ribosome to the start site of translation. Science. 1988;242:93–97. doi: 10.1126/science.3051379. [DOI] [PubMed] [Google Scholar]
- 9.Unbehaun A, Borukhov SI, Hellen CU, Pestova TV. Release of initiation factors from 48S complexes during ribosomal subunit joining and the link between establishment of codon-anticodon base-pairing and hydrolysis of eIF2-bound GTP. Genes Dev. 2004;18:3078–3093. doi: 10.1101/gad.1255704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung YN, Maag D, Mitchell SF, Fekete CA, Algire MA, Takacs JE, Shirokikh N, Pestova T, Lorsch JR, Hinnebusch AG, et al. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev. 2007;21:1217–1230. doi: 10.1101/gad.1528307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell SF, Lorsch JR. Should I stay or should I go? Eukaryotic translation initiation factors 1 and 1A control start codon recognition. J. Biol. Chem. 2008;283:27345–27349. doi: 10.1074/jbc.R800031200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanda JS, Cheung YN, Takacs JE, Martin-Marcos P, Saini AK, Hinnebusch AG, Lorsch JR. eIF1 controls multiple steps in start codon recognition during eukaryotic translation initiation. J. Mol. Biol. 2009;394:268–285. doi: 10.1016/j.jmb.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pestova TV, Kolupaeva VG. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 2002;16:2906–2922. doi: 10.1101/gad.1020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisarev AV, Kolupaeva VG, Pisareva VP, Merrick WC, Hellen CU, Pestova TV. Specific functional interactions of nucleotides at key -3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes Dev. 2006;20:624–636. doi: 10.1101/gad.1397906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak M. A short leader sequence impairs the fidelity of initiation by eukaryotic ribosomes. Gene Expr. 1991;1:111–115. [PMC free article] [PubMed] [Google Scholar]
- 16.Pisarev AV, Kolupaeva VG, Yusupov MM, Hellen CU, Pestova TV. Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. EMBO J. 2008;27:1609–1621. doi: 10.1038/emboj.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl Acad. Sci. USA. 1990;87:8301–8305. doi: 10.1073/pnas.87.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svitkin YV, Pause A, Haghighat A, Pyronnet S, Witherell G, Belsham GJ, Sonenberg N. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA. 2001;7:382–394. doi: 10.1017/s135583820100108x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak M. Primer extension analysis of eukaryotic ribosome-mRNA complexes. Nucleic Acid Res. 1998;26:4853–4859. doi: 10.1093/nar/26.21.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak M. Effects of long 5′ leader sequences on initiation by eukaryotic ribosomes in vitro. Gene Expr. 1991;1:117–125. [PMC free article] [PubMed] [Google Scholar]
- 21.Steitz JA, Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3′ terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc. Natl Acad. Sci. USA. 1975;72:4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol. Cell Biol. 1989;9:5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier J, Sonenberg N. Photochemical cross-linking of cap binding proteins to eucaryotic mRNAs: effect of mRNA 5′ secondary structure. Mol. Cell Biol. 1985;5:3222–3230. doi: 10.1128/mcb.5.11.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelletier J, Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985;40:515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- 25.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 26.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 27.Pestova TV, Borukhov SI, Hellen CU. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature. 1998;394:854–859. doi: 10.1038/29703. [DOI] [PubMed] [Google Scholar]
- 28.Parsyan A, Shahbazian D, Martineau Y, Petroulakis E, Alain T, Larsson O, Mathonnet G, Tettweiler G, Hellen CU, Pestova TV, et al. The helicase protein DHX29 promotes translation initiation, cell proliferation, and tumorigenesis. Proc. Natl Acad. Sci. USA. 2009;106:22217–22222. doi: 10.1073/pnas.0909773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisareva VP, Pisarev AV, Komar AA, Hellen CU, Pestova TV. Translation initiation on mammalian mRNAs with structured 5′ UTRs requires DExH-box protein DHX29. Cell. 2008;135:1237–1250. doi: 10.1016/j.cell.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomakin IB, Kolupaeva VG, Marintchev A, Wagner G, Pestova TV. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev. 2003;17:2786–2797. doi: 10.1101/gad.1141803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proud CG. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- 32.Chin LS, Singh SK, Wang Q, Murray SF. Identification of okadaic-acid-induced genes by mRNA differential display in glioma cells. J. Biomed. Sci. 2000;7:152–159. doi: 10.1007/BF02256622. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh MS, Fernandez-Salas E, Yu M, Hussain A, Dinman JD, Peltz SW, Huang Y, Fornace AJ., Jr Cloning and characterization of a human genotoxic and endoplasmic reticulum stress-inducible cDNA that encodes translation initiation factor 1(eIF1(A121/SUI1)) J. Biol. Chem. 1999;274:16487–16493. doi: 10.1074/jbc.274.23.16487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.