Summary
Evolving under constant threat from invading microbes, macrophages have acquired multiple means of killing bacteria. In this issue of Cell Host & Microbe, Botella and colleagues describe a novel anti-microbial mechanism based on elevated levels of intraphagosomal Zn2+ and the corresponding induction of bacterial genes to ameliorate this host-derived stress.
There are few inter-organismal interactions that are more intimate than the one between a pathogen that causes a chronic infection and its host. Such an interaction molds the evolution of both partners so that all anti-microbial strategies acquired by the host are countered by avoidance or neutralization mechanisms developed by the pathogen. The “success” of either organism depends on the relative balance of these responses.
The paper by Botella and colleagues (2011) documents an anti-microbial mechanism that is induced in the macrophages upon infection by Mycobacterium tuberculosis (Mtb). The macrophage releases free Zn2+ from intracellular stores, which is then pumped into the newly-formed phagosome raising the intra-phagosomal Zn2+ concentration to levels that would limit growth of Mtb. In response, Mtb up-regulates expression of the P-type ATPase-encoding gene ctpC, which regulates the intra-bacterial levels of Zn2+ through efflux of the metal ion. Mtb deficient in expression of this transporter exhibited impaired intracellular survival. The key steps in this process are summarized in Figure 1.
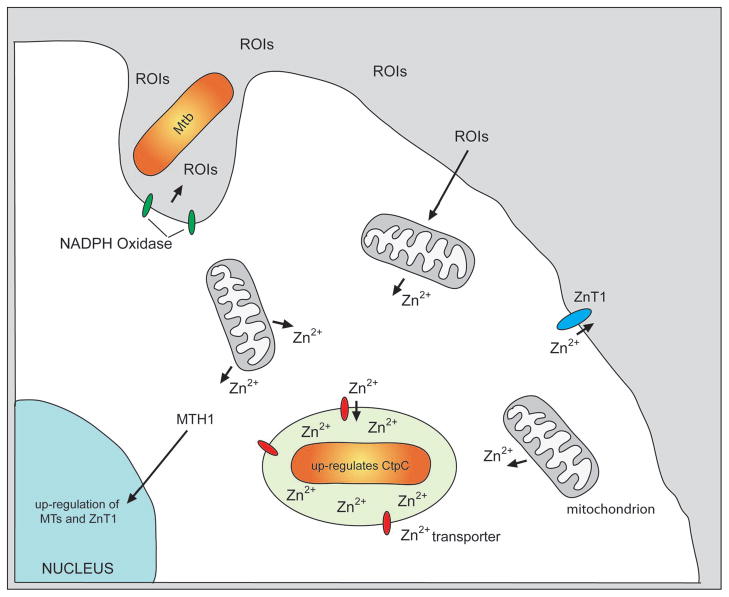
Figure 1.
This diagram illustrates the sequence of events that lead to increased Zn2+ concentration within the Mycobacterium tuberculosis-containing phagosome, and the subsequent up-regulation of both host and bacterial genes in response to free Zn2+. During phagocytosis of Mtb the NADPH Oxidase complex is formed on the phagosomal membrane and generates reactive oxygen intermediates (ROIs). These ROIs signal back on the host cell and induce release of free Zn2+ from intracellular stores, such as the mitochondria. The free Zn2+ is transported into the Mtb vacuole and other endosomal compartments through unknown Zn2+ transporters. Within the phagosome, this increase in free Zn2+, which is potentially toxic to Mtb, is countered through the up-regulation of the bacterial Zn2+ efflux pump CtpP. Within the host cell, the increase in cytoplasmic Zn2+ induces the migration of metal regulatory transcriptional factor 1 (MTH1) to the nucleus where it up-regulates transcription of genes encoding metallothioneins and the Zn2+ efflux pump ZnT1. ZnT1 is assembled in the plasmalemma and pumps free Zn2+ out of the infected cell.
Using Fluozin-3 (FZ3), a probe specific for free Zn2+, the authors demonstrated that Zn2+ is rapidly released from intracellular stores following infection with Mtb. A similar mobilization of intracellular Zn2+ stores was also observed upon infection with E. coli, suggesting that the macrophage was being stimulated through its innate immune receptors, such as Toll-like receptors. Intriguingly, known inhibitors of the NADPH oxidase, which generates the reactive oxygen intermediates (ROIs) induced during the phagocytosis of particles such as Mtb, blocked the release of free Zn2+, implicating reactive oxygen intermediates in the release of free Zn2+ (Andrews, 2000). The exact pathways leading to the mobilization of these intracellular stores of Zn2+ would be an interesting area for future study. Recently, it has been suggested that Mtb actively down-regulates the production of ROIs through bacterial NADH dehydrogenase activity, which has the potential to both reduce reactive oxygen species and the release of Zn2+ from the host cell mitochondria (Miller et al., 2010).
The authors exploited some elegant histochemistry to localize those regions enriched for free Zn2+ in both the macrophages and the intracellular bacteria at the ultrastructural level. Stoltenberg and colleagues developed an autometallographic technique whereby fixation in the presence of sodium sulfide induces the formation of zinc-sulfur crystals that can be enhanced by silver (Stoltenberg et al., 2005). Using this method Botella and co-workers observed that, following infection, silver crystals were visible in the host cell within the mitochondria, within endocytic compartments and associated with the inner face of the plasmalemma (Botella et al. 2011). In the bacterium they identified three distinct patterns of localization. Silver crystals were seen associated with the surface of the bacterium, within a region that likely corresponds to the periplasmic space, and within the cytoplasm of the bacterium. This latter localization was seen more frequently in bacterial cells that appeared compromised, implying the increased Zn2+ concentration correlated with bacterial death. This hypothesis was substantiated further by comparison between wild-type Mtb and a mutant bacterial strain deficient in expression of CtpC, a P-ATPase transporter associated with the efflux of Zn2+. When macrophages were infected with both strains there were significantly more zinc-sulfur crystals seen in the cytosol of the mutant.
The increased concentration of free Zn2+ in the bacteria-containing phagosomes is consistent with a previous study by Wagner and colleagues who documented elevated levels of metal ions in Mtb- and M. avium (Mac)-containing phagosomes as measured by hard x-ray microprobe (Wagner et al., 2005). The levels of both Fe2+ and Zn2+ exhibited interesting deviations from the extracellular concentrations. The Mtb and Mac-containing phagosomes exhibited elevated levels of Fe2+. This is consistent with previous reports demonstrating the cycling of the iron-binding protein transferrin through the Mtb-containing vacuole (Clemens and Horwitz, 1996; Sturgill-Koszycki et al., 1996), and transcriptional profile studies that indicate that Mtb is in an iron-replete environment (Homolka et al., 2010; Tailleux et al., 2008). Mtb defective in the synthesis of iron-chelating siderophores do not exhibit this increase suggesting that the bacterium is responsible for sequestering Fe2+ within the phagosome (Wagner et al., 2005). Treatment of macrophages infected with Mac with TNF-α, which impairs bacterial survival, neutralized the increase in intra-vacuolar Fe2+. In contrast, the concentration of Zn2+ was elevated most markedly in the phagosomes containing the avirulent mutant Mtb defective in siderophore production and in Mac-containing phagosomes following activation of the host cells with TNF-α (Wagner et al., 2005)33 From these data it is clear that Fe2+ concentration correlates positively with bacterial fitness, while Zn2+ concentration correlates negatively with bacterial fitness. However, the significance of these observations was not apparent at the time of the earlier publication by Wagner and colleagues but is brought sharply into focus by the current study of Botella and coworkers, who identify Zn2+-loading as a host-mediated anti-microbial response. The route by which Zn2+ gets into the phagosome remains to be determined.
Previous transcriptional profiling of both Mtb and the host cell in macrophage infection models had demonstrated the strong and coordinated up-regulation of expression of genes linked to heavy metal poisoning or the response to excess free metals in both cells (Homolka et al., 2010; Tailleux et al., 2008). Upon entry into macrophages, Mtb markedly up-regulated expression of several P-type ATPases commonly associated with the efflux of free metal ions such as Zn2+ or Cu2+, and the induction of expression of two of these genes, cptG and cptC, was enhanced by increased Zn2+ concentration in vitro. Mutant Mtb deficient in the expression of one of these putative transporters, CtpC, accumulated Zn2+ in culture, and as discussed previously, inside macrophages. On the host cell side, the macrophage showed enhanced expression of metal toxicity-linked genes such as the metallothioneins, and the Zn2+ exporter ZnT1. In addition, the infected cells demonstrated translocation of the metal-regulatory transcription factor 1 (MTF1) from the cytosol to the nucleus where it binds to the regulatory regions of known genes involved in controlling metal-mediated stress (Andrews, 2001). These data argue strongly that the release of free Zn2+, induced upon bacterial infection, is impacting the biology of both organisms.
All these data generate a compelling story of a novel anti-microbial mechanism and Mtb’s response to ensure its continued survival. However, one area where this story is less clear is the phenotype of the ctpC-deficient mutant. This mutant exhibited a reduced capacity to survive in human macrophages in culture and the phenotype could be complemented upon ectopic expression of ctpC. In contrast, the mutant lacked any phenotype upon infection of wild-type Balb/C mice. This indicated that either the bacteria had compensatory mechanisms that were not invoked in in vitro macrophage infections, or that there was a host difference between the mouse in vivo infections and the human macrophage infections in culture. There is a history of literature documenting the disparities between the induction of anti-microbial circuits in human versus mouse macrophages and this may simply be another divergence of note.
To demonstrate that the induction of free Zn2+ and its delivery to the phagosome has broad significance as an anti-microbial response, the authors looked at the survival profiles of wild-type E. coli versus a mutant strain in which the zinc efflux ATPase, ZntA, had been inactivated. Upon uptake into host phagocytes, both strains induced release of free Zn2+, which was seen to concentrate in the bacteria-containing vacuoles. The rate of killing of the wild-type bacteria was markedly slower than the zntA-deficient mutant. These data provide an independent validation of the hypothesis that the generation of free Zn2+ upon stimulation by microbial insult, coupled with its delivery into the phagosome, represents a novel strategy to eradicate intracellular pathogens.
Several obvious questions remain. How do the ROI’s induce release of Zn2+? Which transporters deliver the Zn2+ into the phagosome? How does Zn2+ intoxicate these bacteria? And what is the basis of the disparity between the human macrophage infections and the murine infection? However, none of these questions detract from the appeal of these data and the underlying hypothesis, whereby the concentration of the essential metal ion, Zn2+, is manipulated by the host to determine the success or failure of an infection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- Andrews GK. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14:223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- Botella H, Peyron P, Levillian F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere G, Waddell S, Foti M, Lugo-Villarino G, Gao Q, Marridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. P1-type ATPases mediate microbial resistance to zinc poisoning in human macrophages. Cell, Host and Microbe. 2011 doi: 10.1016/j.chom.2011.08.006. (In this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens DL, Horwitz MA. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184:1349–1355. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolka S, Niemann S, Russell DG, Rohde KH. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog. 2010;6:e1000988. doi: 10.1371/journal.ppat.1000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL, Velmurugan K, Cowan MJ, Briken V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 2010;6:e1000864. doi: 10.1371/journal.ppat.1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg M, Bruhn M, Sondergaard C, Doering P, West MJ, Larsen A, Troncoso JC, Danscher G. Immersion autometallographic tracing of zinc ions in Alzheimer beta-amyloid plaques. Histochem Cell Biol. 2005;123:605–611. doi: 10.1007/s00418-005-0787-0. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- Tailleux L, Waddell SJ, Pelizzola M, Mortellaro A, Withers M, Tanne A, Castagnoli PR, Gicquel B, Stoker NG, Butcher PD, et al. Probing host pathogen cross-talk by transcriptional profiling of both Mycobacterium tuberculosis and infected human dendritic cells and macrophages. PLoS One. 2008;3:e1403. doi: 10.1371/journal.pone.0001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Honer Zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]