Abstract
Diabetic nephropathy is becoming an increasingly important cause of morbidity and mortality worldwide owing to the increasing prevalence of type 2 diabetes, largely driven by increasing obesity. There is considerable evidence that obesity, hypertension and other elements of the metabolic syndrome also contribute to the progression of renal disease independent of diabetes. How they interact and contribute to diabetic nephropathy, however, is not completely understood. Clinical diabetic nephropathy is preceded by an increase in glomerular filtration rate (GFR), microalbuminuria and glomerular hypertrophy. Poor glycemic control and elevated systolic blood pressure exacerbate proteinuria and renal injury that may culminate in end-stage renal disease. A similar sequence of events may lead to obesity-related renal disease even in the absence of diabetes. This article compares and contrasts factors involved in the development of glomerular hemodynamic and kidney pathological processes associated with diabetes and obesity.
Introduction
Diabetes is the most common cause of end-stage renal disease (ESRD) worldwide and is associated with increased cardiovascular risk, high morbidity and mortality [1–2]. While the increasing prevalence of ESRD is to some extent due to better survival of diabetic patients with chronic renal disease as a result of more effective treatments, it is mostly due to the dramatic increase in the prevalence of type 2 diabetes. The growing prevalence of obesity is a major driving force for the continued increase in the prevalence of type 2 diabetes [3] as well as the cluster of risk factors that make up the metabolic syndrome, including hypertension, insulin resistance and dyslipidemia. These disorders are intertwined and likely interact to increase the severity of chronic renal disease. Thus, understanding the complex interrelationships between these factors is of vital importance for our understanding of the pathophysiology of diabetic nephropathy and development of effective regimens for its prevention and treatment.
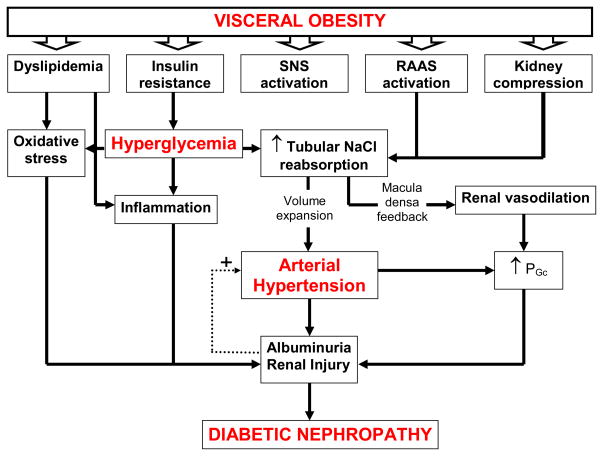
Clinical diabetic nephropathy evolves in a sequence of stages beginning with initial increases in GFR and intraglomerular capillary pressure (PGc), glomerular hypertrophy and microalbuminuria [4–5]. Poor glycemic control and elevated systolic blood pressure (BP) further exacerbate the disease progression to proteinuria, nodular glomerulosclerosis and tubulointerstitial injury and a decline in GFR which can eventually lead to ESRD [6–7]. The development of diabetic nephropathy is commonly thought to result from the cumulative interactions among multiple metabolic and hemodynamic factors which activate common intracellular signaling pathways that in turn trigger the production of cytokines and growth factors, leading to renal disease. However, the current consensus on what triggers the development of diabetic nephropathy and how it progresses is evolving in the face of worldwide epidemics of obesity, diabetes and kidney disease. This article reviews and Figure 1 summarizes potential mechanisms by which obesity, diabetes, hypertension and elements of the metabolic syndrome may either cause or exacerbate the progression of nephropathy.
Figure 1.
Interaction between metabolic and hemodynamic pathways in the pathophysiology of diabetic nephropathy.
Abbreviations: SNS: sympathetic nervous system; RAAS, renin angiotensin aldosterone system; PGc, intraglomerular capillary pressure.
Role of hypertension in nephropathy associated with obesity, metabolic syndrome and diabetes
Basic, clinical and population research indicate that visceral obesity, the main driver for type 2 diabetes and metabolic syndrome, raises BP [8–9]. Population studies indicate that excess weight gain may account for 78 percent of primary (essential) hypertension in men and 65 percent in women [10]. Moreover, the relationship between body mass index (BMI) and BP is nearly linear in diverse populations throughout the world [11]. Clinical studies also indicate that weight loss reduces BP in most hypertensive subjects and is effective in primary prevention of hypertension 9.
Visceral, but not subcutaneous, obesity appears to induce hypertension initially by increasing renal tubular reabsorption and causing a hypertensive shift of renal-pressure natriuresis through multiple mechanisms including activation of the sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS), as well as physical compression of the kidneys [12–13]. The hypertension that ensues, as well as the increases in PG, and GFR, and the metabolic abnormalities (e.g. dyslipidemia, hyperglycemia) likely interact to cause renal injury. A similar sequence of events may lead to renal injury in diabetes, irrespective of obesity, suggesting that hypertension plays a key role in obesity and diabetes-associated renal injury. Further supporting the central role of hypertension in renal injury is the fact that progressive renal injury only occurs when hypertension is superimposed on obesity or diabetes [14]. The importance of rigorous BP control for treating diabetic nephropathy is recognized in current guidelines, with a recommended target of BP less than 130/80 mmHg [15]. Multiple studies have clearly shown the protective effect on the kidneys of reducing BP in diabetes. In fact, tight BP control in diabetic patients may slow progression of nephropathy to a greater extent than tight control of blood glucose [16].
Effects of obesity, metabolic syndrome and diabetes on glomerular hemodynamics
One of the earliest renal changes in obese humans [17] and in obese dogs fed a high fat diet for only 5–6 weeks [13] is increased GFR. A likely explanation for increased GFR in obesity is increased salt reabsorption by the proximal tubule or loop of Henle, leading to tubuloglomerular feedback (TGF) mediated reduction in afferent arteriolar resistance, increased PGc and increased GFR [18]. The increased GFR initially serves as a compensatory response that permits restoration of salt balance despite continued increases in tubular reabsorption but, over the long-term, contributes to renal injury, especially when combined with elevated BP. The TGF-mediated dilation of afferent arterioles and attendant impairment of renal autoregulation permit increases in BP to be transmitted to the glomerular capillaries causing even greater increases in PGc and glomerular injury than would occur with comparable increases in BP in kidneys of non-obese, non-diabetic subjects [19].
Renal vasodilation and increases in GFR and PGc also occur early in the development of diabetes and are associated with a high risk for development of overt diabetic nephropathy [20]. Hyperglycemia is presumed to contribute to the development of glomerular hyperfiltration through mechanisms similar to those occurring in obesity, although the precise mechanisms underlying increased GFR remain inconclusive. Reduced delivery of salt to the macula densa, as a consequence of increased proximal reabsorption of glucose and sodium, may reduce afferent arteriolar resistance and increase PGc and GFR via attenuated TGF [21–23]. Also, afferent vasodilation and efferent vasoconstriction in response to circulating or locally formed vasoactive factors (e.g. angiotensin II) produced in response to hyperglycemia or shear stress may promote diabetic glomerular hyperfiltration [24–25].
Even though the mechanisms explaining the increase in GFR in diabetes and obesity uncomplicated by diabetes may be similar, the factors that trigger TGF-mediated renal vasodilation and glomerular hyperfiltration are different and some studies suggest that hyperglycemia and obesity may have at least partially additive effects on glomerular hemodynamics [19].
Since the metabolic syndrome is by definition a clustering of several metabolic factors and hypertension, it is often difficult to separate the effects of each element on glomerular hemodynamics and progression of renal injury. Experimental studies, however, provide some clues. For example, mice lacking the gene for the melanocortin-4 receptor are obese, hyperinsulinemic and hyperleptinemic but normotensive at 55 weeks of age [14]. These animals have moderately increased GFR and only modest albuminuria compared with WT mice; however, their GFR and albuminuria increased further when rendered hypertensive following treatment with N(G)-nitro-L-arginine methyl ester (L-NAME). These data suggest that elevations in BP exacerbate obesity-related glomerular hyperfiltration and albuminuria, further supporting the concept of an additive, or perhaps synergistic, effect of various components of obesity, metabolic syndrome, diabetes and hypertension on glomerular hemodynamics.
It is not clear, however, how these same factors contribute to the decline in GFR characteristic of advanced diabetic nephropathy. Few studies have examined this issue due to the fact that most experimental models of diabetes-related renal injury never really develop overt nephropathy and are in a permanent state of glomerular hyperfiltration. Obesity, metabolic syndrome and diabetes are states of low-grade inflammation and oxidative stress, all of which may lead to kidney damage, progressive loss of nephrons and decline in GFR over time with aging. Another element of the metabolic syndrome, hyperlipidemia, has been linked to reductions in GFR in diabetic nephropathy, especially in the latter stages of the disease. Numerous clinical trials have pointed to the importance of lipid control in preserving GFR in patients with diabetes [26]. However, further studies are needed to determine if the beneficial effects of lipid lowering agents in diabetic nephropathy are due to improvement in the lipid profile or if there are other renoprotective effects.
Effects of obesity, metabolic syndrome and diabetes on albuminuria
Diabetic nephropathy and elements of the metabolic syndrome including insulin resistance and hyperinsulinemia correlate with the development of microalbuminuria early in the disease process [27–28]. Microalbuminuria, in turn, signifies increased risk of progression to ESRD and cardiovascular disease [29]. The development of microalbuminuria in diabetic nephropathy was traditionally thought to stem from damage to the glomerular filtration barrier as a consequence of increases in BP which are transmitted to the glomeruli, raising PG and GFR, and/or hyperglycemia-associated inflammation and oxidative stress [27]. An alternative, or perhaps complementary, explanation is that diabetes also impairs proximal tubular reabsorption of albumin which filters across the glomerular barrier [30].
Hyperlipidemia is known to be a risk factor for the development of albuminuria in patients with diabetes [31]. Further supporting the importance of hyperlipidemia in diabetic nephropathy is the fact that treatment with lipid lowering agents improves proteinuria. However, the effectiveness of statins on albuminuria in overt diabetic nephropathy has not been established.
Effects of obesity, metabolic syndrome and diabetes on glomerular injury
There are several similarities in the histological appearance of glomeruli from diabetic and obese individuals and both have early structural changes in the kidney that accompany hyperfiltration and microalbuminuria. The glomeruli of patients with diabetes are characterized by glomerular hypertrophy, widening of the glomerular basement membrane, mesangial expansion, podocytopenia leading to nodular (Kimmelstiel-Wilson) glomerulosclerosis. Similarly, obesity-associated renal injury is characterized by glomerulomegaly, mesangial expansion, podocytopenia leading to focal glomerulosclerosis [19].
Given the similarities in the histological appearance of the glomerulus from diabetic and obese subjects, it is not surprising that the mechanisms leading to these changes are also similar. Diabetes and obesity are both states of low-grade inflammation associated with macrophage infiltration into the adipose tissue and the kidney. The infiltrating macrophages become a source of a whole host of pro- inflammatory cytokines including TNF-α, interleukin-6, and MCP-1 [32]. Furthermore, increased adiposity triggers the release of adipokines into the circulation that in turn may cause renal injury via production of reactive oxygen species. Persistent hyperglycemia also activates vasoactive hormonal pathways including the RAAS and endothelin. These in turn activate common second messenger signaling pathways such as protein kinase C (PKC) and MAP kinase (MAPK) and transcription factors such as nuclear factor-κB (NF-κB) that lead to the alteration in gene expression of a plethora of growth factors and cytokines such as transforming growth factor-beta (TGF-β). TGF-β is a key player in promoting podocyte apoptosis, mesangial cell proliferation and extracellular matrix synthesis, cellular events that are important in the development of diabetes and obesity-associated glomerular injury [33]. Hyperglycemia and associated metabolic disturbances also cause mitochondrial dysfunction and enhanced generation of ROS, which directly alter the expression of key proteins and cytokines causing renal injury.
Kidneys of obese individuals often have glomerular/mesangial lipid deposits (foam cells) present, which supports the concept of lipotoxicity, i.e. lipid-induced renal injury [19]. One of the mechanisms by which hyperlipidemia promotes glomerular injury is through renal upregulation of sterol-regulatory element-binding proteins (SREBP-1 and 2), which in turn promotes podocyte apoptosis and mesangial cell proliferation and cytokine synthesis.
Concluding remarks
Data from basic and clinical studies suggest that obesity, hypertension, hyperglycemia, hyperlipidemia and other elements of the metabolic syndrome are highly interrelated and contribute to the development and progression of diabetic nephropathy. Given their close relationship, it is often difficult to dissect out their individual effects. It is likely that multiple metabolic abnormalities act in concert to initially cause renal vasodilation, glomerular hyperfiltration and albuminuria that, in turn, effect glomerular pathology, especially when combined with increased BP, and that ultimately progresses to diabetic nephropathy.
Acknowledgments
The authors acknowledge the financial support of NIH/NHLBI (PO1HL51971 to J.E. Hall) and NIH/NIDDK (RO1DK075832 to C. Maric).
References
- 1.de Zeeuw D, Ramjit D, Zhang Z, Ribeiro AB, Kurokawa K, Lash JP, Chan J, Remuzzi G, Brenner BM, Shahinfar S. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int. 2006;69:1675–1682. doi: 10.1038/sj.ki.5000326. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Macia M, Ruggenenti P. Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J Am Soc Nephrol. 2006;17:S90–97. doi: 10.1681/ASN.2005121324. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004;286:F8–15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 5.Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol. 2003;23:194–199. doi: 10.1053/anep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 6.Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens. 2003;12:273–282. doi: 10.1097/00041552-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Leon CA, Raij L. Interaction of haemodynamic and metabolic pathways in the genesis of diabetic nephropathy. J Hypertens. 2005;23:1931–1937. doi: 10.1097/01.hjh.0000188415.65040.5d. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5:386–392. doi: 10.1007/s11906-003-0084-z. [DOI] [PubMed] [Google Scholar]
- 9.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 10.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 12.Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11:41–54. doi: 10.1053/j.arrt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 14.do Carmo JM, Tallam LS, Roberts JV, Brandon EL, Biglane J, da Silva AA, Hall JE. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R803–812. doi: 10.1152/ajpregu.00187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Buren PN, Toto R. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011;18:28–41. doi: 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:847–849. doi: 10.1161/CIRCULATIONAHA.110.960120. [DOI] [PubMed] [Google Scholar]
- 17.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 19.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685–696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 20.Yip JW, Jones SL, Wiseman MJ, Hill C, Viberti G. Glomerular hyperfiltration in the prediction of nephropathy in IDDM: a 10-year follow-up study. Diabetes. 1996;45:1729–1733. doi: 10.2337/diab.45.12.1729. [DOI] [PubMed] [Google Scholar]
- 21.Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol. 2009;111:30–38. doi: 10.1159/000208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods LL, Mizelle HL, Hall JE. Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am J Physiol. 1987;252:F65–73. doi: 10.1152/ajprenal.1987.252.1.F65. [DOI] [PubMed] [Google Scholar]
- 23.Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 2010;200:3–10. doi: 10.1111/j.1748-1716.2010.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherney DZ, Scholey JW, Miller JA. Insights into the regulation of renal hemodynamic function in diabetic mellitus. Curr Diabetes Rev. 2008;4:280–290. doi: 10.2174/157339908786241151. [DOI] [PubMed] [Google Scholar]
- 25.Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens. 2010;19:85–90. doi: 10.1097/MNH.0b013e32833240fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 27.Jauregui A, Mintz DH, Mundel P, Fornoni A. Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr Opin Nephrol Hypertens. 2009;18:539–545. doi: 10.1097/MNH.0b013e32832f7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer IH, Sibley SD, Kestenbaum B, Sampson JN, Young B, Cleary PA, Steffes MW, Weiss NS, Brunzell JD. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol. 2007;18:235–243. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, Shahinfar S, Gleim GW, Weir MR, Brenner BM, de Zeeuw D. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18:1540–1546. doi: 10.1681/ASN.2006050445. [DOI] [PubMed] [Google Scholar]
- 30.Comper WD, Russo LM. The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens. 2009;18:336–342. doi: 10.1097/MNH.0b013e32832cb96a. [DOI] [PubMed] [Google Scholar]
- 31.Rutledge JC, Ng KF, Aung HH, Wilson DW. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol. 2010;6:361–370. doi: 10.1038/nrneph.2010.59. [DOI] [PubMed] [Google Scholar]
- 32.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 33.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15:S55–57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]