Abstract
Plants use volatile terpene compounds as odor cues for communicating with the environment. Fleshy fruits are particularly rich in volatiles that deter herbivores and attract seed dispersal agents. We have investigated how terpenes in citrus fruit peels affect the interaction between the plant, insects, and microorganisms. Because limonene represents up to 97% of the total volatiles in orange (Citrus sinensis) fruit peel, we chose to down-regulate the expression of a limonene synthase gene in orange plants by introducing an antisense construct of this gene. Transgenic fruits showed reduced accumulation of limonene in the peel. When these fruits were challenged with either the fungus Penicillium digitatum or with the bacterium Xanthomonas citri subsp. citri, they showed marked resistance against these pathogens that were unable to infect the peel tissues. Moreover, males of the citrus pest medfly (Ceratitis capitata) were less attracted to low limonene-expressing fruits than to control fruits. These results indicate that limonene accumulation in the peel of citrus fruit appears to be involved in the successful trophic interaction between fruits, insects, and microorganisms. Terpene down-regulation might be a strategy to generate broad-spectrum resistance against pests and pathogens in fleshy fruits from economically important crops. In addition, terpene engineering may be important for studying the basic ecological interactions between fruits, herbivores, and pathogens.
Plants produce a wide variety of secondary metabolites, many of which are volatile compounds that are released by leaves, flowers, fruits, and roots. These compounds serve as signals between plants and within distal parts of the same plant (Baldwin et al., 2006). They are also involved in protecting the plant against abiotic stress (Gershenzon and Dudareva, 2007), defending the plant against pests and pathogens (Bednarek and Osbourn, 2009; Vickers et al., 2009), and attracting herbivore predators (Kessler and Baldwin, 2001; Degenhardt et al., 2009) and pollinators (Kessler et al., 2008). Volatile compounds that are emitted by flowers greatly contribute to the plant’s reproductive success and survival in natural ecosystems (Kessler et al., 2008). In addition, fruits are generally rich in terpene compounds that determine their specific bouquet and may attract mutualists and repel antagonists, as in animal-pollinated flowers (Junker and Blüthgen, 2010). Flavor volatiles in plants (particularly in fruits) are linked to human selection of genotypes and their use for nutritional, health, or industrial purposes (Goff and Klee, 2006).
It is widely accepted that the primary function of terpene compounds in immature fruit is to defend against all types of potential consumers. Changes in these substances occur during maturation, in combination with changes in texture, taste, and color. These changes are necessary to attract frugivorous animals for fruit predation and seed dispersal (Janzen, 1977; Herrera, 1982; Sallabanks and Courtney, 1992). Fruit traits are thought to evolve in response to the sum of selective pressures exerted by mutualists and antagonists (Whitney and Stanton, 2004; Cazetta et al., 2008). Nonetheless, proof that a specific fruit terpene acts as an attractant or repellent for specific pests or pathogens has not been obtained (Dudareva and Pichersky, 2008).
In the last decade, a series of important studies have been published on plant volatiles as repellents of pests and as attractants of herbivore predators (Arimura et al., 2000; De Moraes et al., 2001; Aharoni et al., 2003). The results from these studies seem to suggest that it may be possible to modulate plant volatile emission through metabolic engineering to improve the plant’s defense against pests. The overexpression of the precursor for a linalool/nerolidol synthase from strawberry (Fragaria spp.) in transgenic Arabidopsis (Arabidopsis thaliana) led to accumulation of high levels of linalool and consequently to the induction of resistance against aphids (Aharoni et al., 2003). The overexpression of this transgene in mitochondria of Arabidopsis leads to the accumulation of nerolidol and a derived homoterpene, 4,8-dimethyl-1,3(E),7-nonatriene, which attract insect carnivore predators that are natural enemies of pest mites (Kappers et al., 2005). In addition, the overexpression of the gene encoding a sesquiterpene synthase from corn (Zea mays), the terpene synthase clone 10, in transgenic Arabidopsis plants attracts parasitic wasps due to the emission of high levels of sesquiterpenes, which are normally released when the larvae of these wasps chew the leaves (Schnee et al., 2006). More recently, the overexpression of the gene of a (E)-β-caryophyllene synthase from oregano (Origanum vulgare) in transgenic corn makes the roots attract nematodes that protect the plant from beetles (Degenhardt et al., 2009). The transgenic overexpression of a precursor gene of a pachulol synthase in tobacco (Nicotiana tabacum) together with the farnesyl diphosphate synthase, a precursor of sesquiterpenes, leads to high accumulations of pachulol and 13 other sesquiterpenes, which make the plants highly resistant to larvae of insect pests (Wu et al., 2006).
The role of different terpenoid compounds in pathogen resistance is well documented, particularly in forest trees, but the overexpression of precursors of these genes as a biotechnology strategy for plant protection has not yet been reported (Trapp and Croteau, 2001).
In summary, the use of metabolic engineering to induce resistance against biotic agents represents an alternative technology to the use of expensive and highly toxic fungicides, bactericides, and pesticides. The use of this technology could also result in increased product quality.
The external colored peel of citrus fruits, known as the flavedo, is embedded with thousands of oil glands containing terpene volatile compounds. (+)-Limonene is the most abundant of these compounds (97% of total terpene in orange (Citrus sinensis) fruits; Dugo and Di Giacomo, 2002). The extraordinarily high amount of limonene that accumulates in orange oil glands suggests an important biological role for this terpene compound in fruit aroma and in the plant’s interactions with the environment. Recently, cDNAs for monoterpene synthases have been isolated from citrus, including several (+)-limonene synthases (Lücker et al., 2002; Shimada et al., 2004). The genetic modification of tobacco plants with three of these monoterpene synthases and their subsequent combination in one plant by crossing, showed that it was possible to increase the amount and alter the composition of monoterpenoids produced in those plants (Lücker et al., 2004).
To determine whether the accumulation of limonene in fruits has a defensive function in planta, we manipulated the terpene content in oil glands with an antisense (AS) down-regulation of the (+)-limonene synthase gene in mature sweet orange plants (cv Navelina). Unexpectedly, transgenic fruits were resistant to economically important fungal and bacterial citrus pathogens and showed the repulsion of a major citrus insect pest.
RESULTS AND DISCUSSION
Molecular Characterization and Volatile Composition of Transgenic Plants
Transgenic plants expressing a citrus limonene synthase gene (CitMTSE1) in the AS orientation were generated, and integration of the transgene was confirmed by both PCR and Southern-blot analyses of the genomic DNA (Supplemental Fig. S1). AS transformants and their fruits were visually indistinguishable from those transformed with an empty vector (EV) and wild-type plants. Moreover, fruit quality traits (weight and volume, color index, acidity, maturity index, juice volume, and vitamin C content) were not affected by this genetic modification. The transgenic lines AS1, AS3, AS6, and AS7 were further investigated.
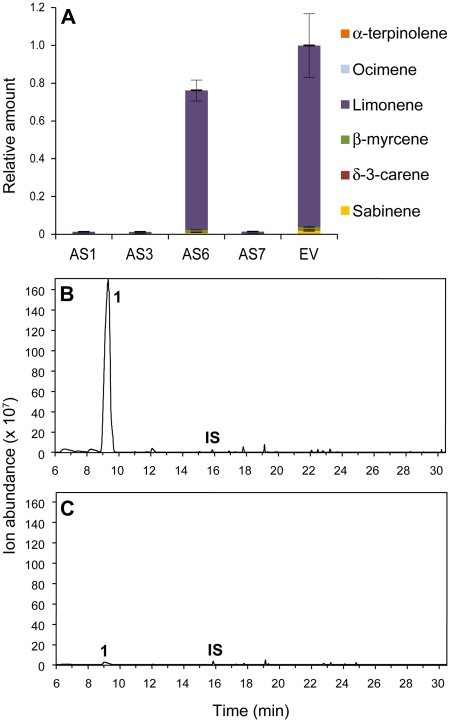
Total terpene profiles in fruit peels from the AS lines showed a range of phenotypes, with slight (AS6) to strong (AS1, AS3, and AS7) decreases in limonene accumulation compared to fruits from the EV control (Fig. 1). The accumulation of other monoterpenes, sesquiterpenes, and monoterpene aldehydes also decreased, whereas the level of monoterpene alcohols increased. Thus, lines AS1, AS3, and AS7 produced at least 85 and 50 times less (+)-limonene and β-myrcene, respectively, than the EV control but increased the production of monoterpene alcohols (more than 10 times for β-citronellol and nerol) and some esters (more than 3 times for geranyl acetate; Supplemental Table S1), likely due to a partial redirection of the pathway. Down-regulation of monoterpenes other than limonene might be also explained by the formation of multiple products from a single monoterpene synthase (Lücker et al., 2002; Shimada et al., 2004).
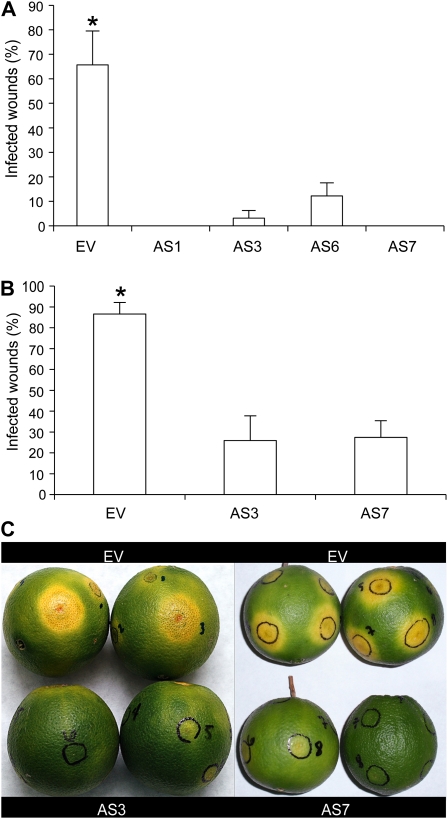
Figure 1.
Phenotypes of orange flavedo in AS and control-treated (EV) Navelina sweet orange plants. A, Relative amount of individual terpenes is presented as a percentage (given as a fraction of unity) area of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one. Data represent mean values ± sem and are derived from at least five fruits per plant. B and C, Representative total ion chromatograms of the volatile profile for orange fruit flavedo from EV (B) and AS7 transgenic plants (C). Peaks number one and IS correspond to limonene and the internal standard (2-octanol), respectively.
Response of Citrus Pathogens to Limonene Down-Regulation in Transgenic Fruits
To test whether terpene down-regulation confers resistance or susceptibility to different citrus pathogens, transgenic AS fruits were challenged with Penicillium digitatum, a fungus that causes the green mold rot in citrus fruits, and Xanthomonas citri subsp. citri, the bacterium that causes citrus canker disease. Terpene down-regulation in AS flavedo was confirmed in samples taken 5 d after inoculation (Supplemental Figs. S2 and S3).
P. digitatum causes the most damaging postharvest disease of citrus fruits worldwide. It does not cause the decay of other noncitrus fruits or vegetables. The etiology of the disease is well understood. Dormant Penicillium spores present on the fruit’s surface become active if the peel is injured. The spores germinate rapidly and colonize the injured tissue. Citrus fruit volatiles play an important role in host recognition by P. digitatum. Flavedo oil from several citrus species and volatiles emitted from injured oranges were reported to stimulate in vitro germination of P. digitatum conidia (Droby et al., 2008). In the case of citrus fruit, volatiles are released from ruptured oil glands following mechanical wounding, facilitating the infection process (Droby et al., 2008). Inhibitory effects have also been attributed to citrus monoterpenes, however, including limonene and derivatives (Ben-Yehoshua et al., 2008).
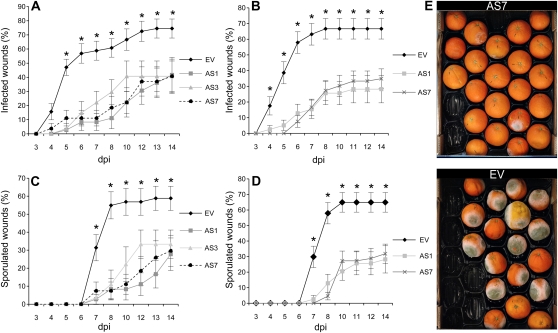
When mature AS and EV fruits were inoculated with P. digitatum, the percentage of infected wounds and wounds with spores in EV fruits 8 d postinoculation were 60.8% and 54.9%, respectively, but only 18.5% and 7.4%, in AS7 fruits. Results with AS1 and AS3 lines were similar, with no significant difference found in the area under the disease progress curve (AUDPC) for infected wounds (P < 0.05; Fig. 2). To assess whether the reduced content of limonene and other terpenes causes an increased susceptibility to other nonpathogenic fungi, we inoculated AS and EV fruits with Penicillium minioluteum. No infection occurred in either AS or EV fruits, indicating that general terpene down-regulation does not alter the interaction with other nonspecialized microorganisms.
Figure 2.
Transgenic expression of CitMTSE1 in the AS orientation in orange plants confers fungal resistance. A to E, Evolution of the disease caused by the fungus P. digitatum in mature orange fruits inoculated with 1 × 104 spores mL−1: percentage of infected (A and B) and sporulated (C and D) wounds in orange fruits of EV and AS lines in two consecutive fruiting seasons—season 1 (A and C) and season 2 (B and D). Results are the average ± sem (n ≥ 10). dpi, Days postinoculation. *, P < 0.05 using Fisher’s Protected lsd test. We repeated all experiments at least twice and obtained similar results. E, AS and EV fruits 8 d after inoculation.
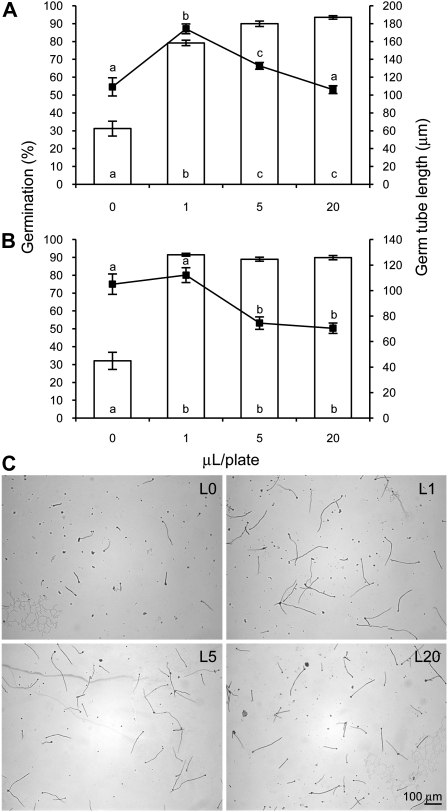
To study whether the resistance phenotype was related to limonene down-regulation or was indirectly induced as a consequence of increased monoterpene alcohols, P. digitatum challenge assays were performed in vitro with pure (+)-limonene and nerol. Results showed that both compounds had a pronounced stimulatory effect on germination of P. digitatum spores directly related to their concentration (Fig. 3). Germ tube elongation response was much higher with limonene at low concentrations, while nerol at high levels had just a slight inhibitory effect (Fig. 3).
Figure 3.
Effect of the monoterpenes limonene (A) and nerol (B) in petri dish assay on percent germination (□) and growth (▪) of P. digitatum. Results are average of three microscopic fields of different colonies containing at least 30 spores each ± sem (n = 15). Treatments with different letters are significantly different at P < 0.05 using Fisher’s Protected lsd test. C, Images shown are light micrographs at 10× magnification of germinating spores in different concentrations of limonene. Scale bar indicates 100 μm.
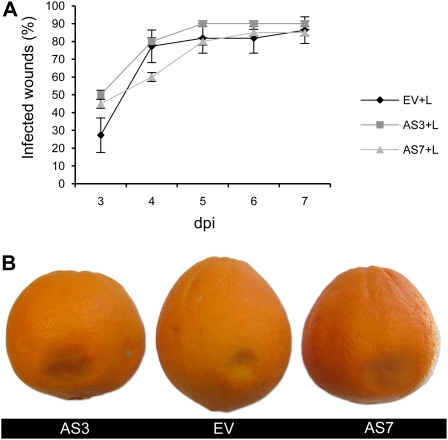
To provide further evidence that down-regulation of limonene was directly responsible for the resistance response, AS and EV orange fruits were supplemented with limonene and then inoculated with P. digitatum. The percentage of infected wounds in EV and AS3 fruits 4 d postinoculation were 77.3% and 80.0%, respectively. Results with AS7 were similar, with no significant difference found for infected wounds (P < 0.05; Fig. 4). This confirmed the critical importance of limonene accumulation levels on fruit susceptibility to P. digitatum.
Figure 4.
Supplementation of pure limonene to the peel of EV and AS fruits confers early infection by the fungus P. digitatum. A and B, Evolution of the disease caused by the fungus P. digitatum in mature orange fruits inoculated with 1 × 104 spores mL−1. A, Percentage of infected wounds in orange fruits of EV and AS lines with 5 μL of limonene applied to the wound. Results are the average ± sem (n = 10). dpi, Days postinoculation. No significant differences were found at P < 0.05 using Fisher’s Protected lsd test. B, AS and EV fruits 4 d after inoculation. [See online article for color version of this figure.]
We also challenged fruits with X. citri subsp. citri, an economically important citrus pathogen that reduces fruit yield and quality and causes quarantine restrictions for the movement of fresh fruit from affected areas (Graham et al., 2004). This bacterium enters the host plant tissues through stomates and wounds and multiplies in the lesions in leaves, stems, and mainly in the fruits. All aboveground tissues of the citrus plant are maximally susceptible to infection by X. citri subsp. citri during the last half of the expansion phase of growth (Graham et al., 2004). The percentage of infected wounds in green fruits inoculated with the bacterium at 4 weeks postinoculation was 65.7% in EV fruit, whereas few infections were observed in inoculated AS fruits (P < 0.05; Fig. 5). Peel pieces from lesions of inoculated AS and EV fruits yielded X. citri subsp. citri colonies when cultivated in an appropriate medium. This result suggests that the presence of a threshold amount of limonene may be necessary for the bacterium to establish infection in citrus fruits. However, we cannot rule out in this case that other up- or down-regulated compound/s in AS fruits may contribute to the resistance phenotype observed.
Figure 5.
Transgenic expression of CitMTSE1 in the AS orientation in orange plants confers bacterial resistance. A and B, Number of wounds with symptoms after inoculation of green mature orange EV and AS fruits with 106 CFU mL−1 of the bacterium X. citri subsp. citri in two consecutive seasons. Results are the average ± sem (n ≥ 10). *, P < 0.05 using Fisher’s Protected lsd test. We repeated all experiments at least twice and obtained similar results. C, AS and EV fruits at 4 weeks postinoculation.
To assess whether this response is genotype dependent, both pathogens were inoculated onto Pineapple sweet orange fruits with or without terpene down-regulation. The results paralleled those of the Navelina sweet orange (Fig. 6; Supplemental Table S2). This finding suggests that disease resistance is directly correlated with limonene down-regulation. Thus, this control strategy could be extended to other citrus species and varieties that accumulate high levels of limonene in the flavedo, such as most sweet oranges, mandarins (Citrus reticulata), grapefruits (Citrus paradisi), and their hybrids. It would be worth testing whether this resistance phenotype could be extended to other important bacterial and fungal citrus pathogens. The relationship between terpenoid production and the activation of defense mechanisms is not fully understood and further research is required. Recent work has shown that glucosinolates, a group of secondary metabolites that are important for preventing damage caused by herbivores in brassicas, are required for the plant’s defense against certain pathogens (Bednarek et al., 2009; Clay et al., 2009).
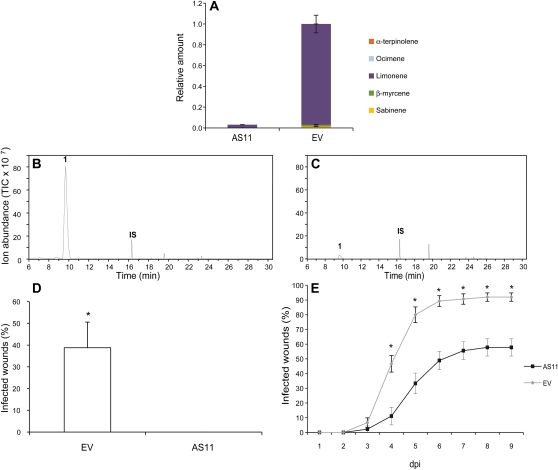
Figure 6.
Phenotypes and behavioral responses of orange flavedo to pathogens in AS and control-treated (EV) Pineapple sweet orange plants. A, Relative amounts of individual terpenes are presented as a percentage area relative to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one. Data represent mean values ± sem and are derived from at least five fruits per plant. B and C, Representative total ion chromatograms of the volatile profile of orange fruit flavedo from EV (B) and AS11 transgenic plants (C). Peaks number one and IS correspond to limonene and the internal standard (2-octanol), respectively. D, Number of infected wounds at 4 weeks after inoculation of EV and AS fruits with the bacterium X. citri. E, Evolution of the disease caused by the fungus P. digitatum and the percentage of infected wounds in the orange fruits of the EV and AS lines. Results are average ± sem (n ≥ 20).*, P < 0.05 using Student’s t test.
Response of a Citrus Insect Pest to Limonene Down-Regulation in Transgenic Fruits
There is evidence to suggest that limonene and other terpene compounds of the citrus peel confer partial resistance in fruits to the Mediterranean fruit fly, medfly (Ceratitis capitata), a major pest of citrus species worldwide (Papachristos and Papadopoulos, 2009), and to other tephritid pests (Back and Pemberton, 1915). We assessed the behavioral response of medfly to terpene down-regulation in sweet orange fruits by no-choice and two-choice flight tunnel assays.
No-choice assays showed that the oviposition response of medfly females after three days of exposure to AS or EV fruits was similar (Supplemental Fig. S4), supporting the notion that medfly females are capable of counteracting the hypothetical deterrent effect induced by the high levels of essential oils that are present in orange flavedo.
It has been suggested that the acquisition of a certain aroma in the flavedo is responsible for increased mating success of medfly males, as demonstrated by exposing entire rooms of mass-reared medfly males to the aroma of orange oil (Shelly et al., 2008). Flight tunnel assays with medfly males exposed to different pure synthetic compounds [(+)-limonene, nerol, and citronellol] in disk assays revealed a preference for these monoterpenes over control water, being limonene the most attractant one (Supplemental Fig. S5). Accordingly, we examined how the behavioral response of medfly males could be affected by the modification of the terpene profile in AS fruits. Flight tunnel assays with medfly males exposed to AS and EV fruits showed that males were more attracted to EV than to AS fruits in green (19% versus 5%, P < 0.05; Supplemental Fig. S6) and mature fruits (32% versus 2%, P < 0.05; Fig. 7), suggesting that limonene emission attracts the male flies. Moreover, when medfly males in the field were exposed to AS versus EV fruits together (Supplemental Movie S1), they were strongly attracted to and landed preferentially on EV fruits. The addition of pure limonene to the peel of mature AS fruits confirmed that this compound was responsible for this behavior in cage assays, because AS fruits were so attractive as EV fruits to medfly males under these conditions (Fig. 8). This olfactory-mediated flight behavior might decrease the mating success of those medfly males exposed to AS fruit in the field.
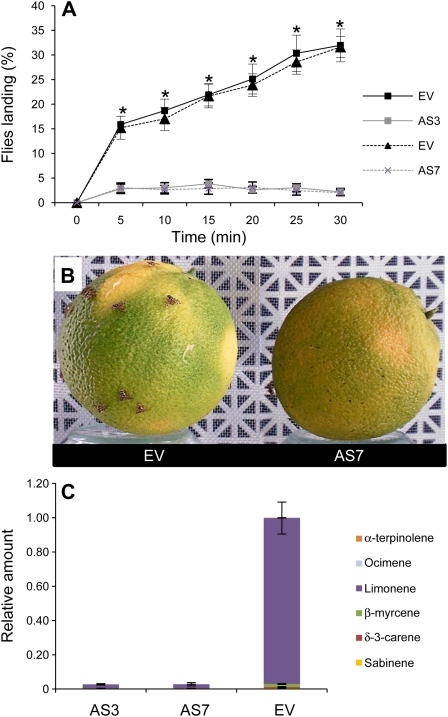
Figure 7.
Terpene-mediated response of medfly males exposed to AS and EV fruits in flight tunnel assays. A, Mean percentage of flies landing on AS and EV control fruits. Results are average ± sem (n = 10). *, P < 0.05 using Student’s t test. We repeated all experiments with two different AS lines and obtained similar results. B, Terpene-mediated landing of flies on EV (left) and AS7 (right) fruits. C, Relative amounts of individual terpenes are presented as a percentage area of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one. Data represent mean values ± sem and are derived from at least five fruits per plant.
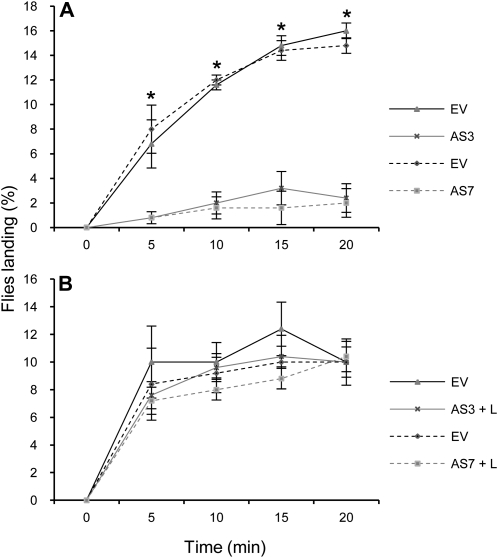
Figure 8.
Terpene-mediated response of medfly males exposed to AS and EV fruits in cage assays. A, Mean percentage of flies landing on AS and EV control fruits. Results are average ± sem (n = 10). *, P < 0.05 using Student’s t test. B, Mean percentage of flies landing on AS supplemented with 100 μL of pure limonene (L) and EV control fruits. Results are average ± sem (n = 10). No significant differences were found at P < 0.05 using Student’s t test. We repeated all experiments with two different AS lines and obtained similar results.
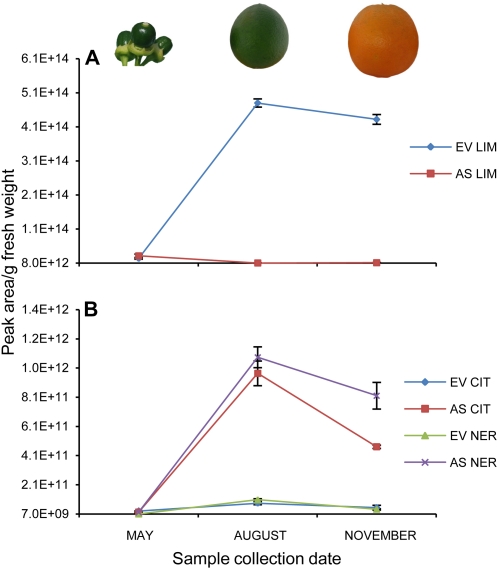
The effects of limonene down-regulation in fruit flavedo on medfly attraction and fungal and bacterial infections strongly indicate that the high accumulation of this monoterpene in the peel of citrus fruits is required for the success of the fruit. In nature, limonene content is usually low in orange fruits during the 2 to 3 months postanthesis; it then drastically increases when the fruit is still green but contains seeds and remains at high level until the fruit becomes fully mature (Fig. 9; Kekelidze et al., 1989; Dugo and Di Giacomo, 2002). In contrast to the view that animal dispersers of fleshy fruit seeds compete with microbes for food resources (Janzen, 1977; Herrera, 1982; Cipollini and Levey, 1997), our data indicate that once a fruit has completely developed seeds, it advertises its condition to potential legitimate dispersers by inducing changes in terpene volatile signals, which also serve to attract specialized insects and microorganisms. In this way they could indirectly increase seed dispersal by providing a nutritional benefit to vertebrates that eat insect-infested or pathogen-infected fruits (Sallabanks and Courtney, 1992; Cazetta et al., 2008). Dispersal could occur when the terpene-rich peel barrier is broken, making the seeds more accessible to terrestrial mammals, or by releasing volatiles that attract specialized vertebrates. This peel would otherwise be toxic or a deterrent for seed-dispersing animals. It has been recently reported that the attraction of birds to heavily insect-infested trees is directly correlated with the emission of several specific terpene compounds (Mäntylä et al., 2008).
Figure 9.
Evolution of citrus peel volatiles during EV and AS fruit development. A, Limonene (LIM) variation. B, Citronellol (CIT) and nerol (NER) variation.
Our results provide a more comprehensive view of the potential coevolution of fruit terpene volatiles that act as signals in multiple trophic chains for insect herbivores and pathogens and seed-dispersing vertebrates. Moreover, these results demonstrate that genetic engineering of volatile terpenoids represents a promising method for developing broad-spectrum resistance or tolerance to pests and pathogens in fleshy fruits and potentially in other economically important crops.
MATERIALS AND METHODS
Citrus Transformation
Mature transformants from orange (Citrus sinensis) plants (cv Navelina and cv Pineapple) were generated as previously described (Rodríguez et al., 2008). A binary vector (pBI121FLM) was constructed containing the limonene synthase gene from satsuma mandarin (Citrus unshiu Mark.; CitMTSE1, accession AB110636) in an AS orientation under the control of the cauliflower mosaic virus 35S promoter and the nopaline synthase gene (NOS) terminator using standard restriction and ligation DNA techniques. The T-DNA of this binary vector also included the neomycin phosphotransferase II gene (nptII) driven by the NOS promoter and terminator sequences (Supplemental Fig. S1A). The binary plasmid pBI121FLM was used as the vector system for transforming EV control plants (Supplemental Fig. S1B).
PCR and Southern-Blot Analysis
Standard PCR techniques were used to detect the limonene synthase gene construct sequence. The primers used were 5′-ATCTCCACTGACGTAAGGGATGACG-3′ (p35S) and 5′-ATGTCTTCTTGCATTAATCCCT-3′ (CitMTSE1). Reactions were performed in 25 μL containing 1 μL of DNA (50 ng μL−1), 200 μm deoxynucleotide triphosphates, 3 mm MgCl2, 50 mm KCl, 20 mm Tris-HCl pH 8.4, 0.25 μm of each primer, and 0.5 units of Taq DNA polymerase (Roche). Reactions were subjected to 35 cycles of 0.5 min at 95°C, 0.5 min at 58°C, and 2 min at 72°C for the CitMTSE1 gene. Amplified DNA was detected with UV light after electrophoresis on 1% agarose-ethidium bromide gels. Genomic DNA was isolated from leaves as previously described (Dellaporta et al., 1983). To detect CitMTSE1, Southern-blot experiments were performed on samples digested with 20 μg of HindIII, separated on 1% agarose gels, and blotted onto nylon membranes (Hybond-N+, Amersham Pharmacia). Filters were probed with digoxigenin-labeled (DIG-11-dUTP; Roche Diagnostics) fragments of the 35S promoter prepared by PCR, fixed by UV irradiation, and detected by chemiluminescence with the substrate disodium 3-(4-methoxyspiro [1,2-dioxetane-3,2’-{5′-chloro}tricyclo {3.3.1.13,7}decan]-4-yl)phenyl phosphate (Roche Diagnostics).
Chemicals
Synthetic compounds used in the assays and as references for identification of the citrus volatiles were: (R)-(+)-limonene (99%), nerol (97%), citronellol (95%), and 2-octanol all supplied by Sigma-Aldrich.
Extraction of Volatiles and Gas Chromatography-Mass Spectrometry Analysis
Flavedo tissue was obtained from orange fruits, immediately frozen in liquid nitrogen, and stored at −80°C until extraction. A Thermo Trace GC Ultra coupled to a Thermo DSQ mass spectrometer with electron ionization mode at 70 eV was used. Frozen ground material (200 mg) was weighed in screw-cap Pyrex tubes and then immediately 3 mL of cold pentane and 25 μg of 2-octanol were added as an internal standard. Samples were homogenized on ice for 30 s with a Yellowline homogenizer (model DI 25). The suspension was vortexed for 15 s, and 3 mL of MilliQ water were added. The sample was further vortexed for 30 s and centrifuged at 1,800g for 10 min at 4°C. The organic phase was recovered with a Pasteur pipette, and the aqueous phase reextracted two more times with 3 mL of pentane. A 2-μL aliquot of the pooled organic phases was directly injected into the gas chromatograph-mass spectrometer for volatile analysis; at least two extractions for each sample were performed.
The ion source and the transfer line were set to 200°C and 260°C, respectively. Volatile compounds were separated on a HP-INNOWax (Agilent J&C Columns) column (30 m × 0.25 mm i.d. × 0.25 μm film). The column temperatures were programmed as follows: 40°C for 5 min, raised to 150°C at 5°C min−1, then raised to 250°C at 20°C min−1 and held for 2 min at 250°C. The injector temperature was 220°C. Helium was the carrier gas at 1.5 mL min−1 in the splitless mode. Electron impact mass spectra were recorded in the 30 to 400 amu range with a scanning speed of 0.5 scans−1. Compounds were identified by matching the acquired mass spectra with those stored in the reference libraries (Wiley6 and National Institute of Standards and Technology) or from authentic standard compounds when available.
Data were quantified by integrating the peak areas of total ion chromatograms and normalizing to the recovery rate of the internal standard (2-octanol). The data in Figures 1, 6, 7, and Supplemental Figures S2 and S3 represent relative amounts of individual terpenes and are presented as a percentage area of each terpene (given as a fraction of unity) with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one. The data in Supplemental Tables S1 and S2 represent fold-changes for each volatile in the AS lines relative to the EV line. Negative values indicate a decrease and positive values an increase of the specific volatile with respect to the reference EV line. Values represent at least two independent experiments and are shown as means ± se (sem).
Inoculation of Fruit with Penicillium digitatum and Penicillium minioluteum
AS and EV mature oranges cv Navelina and cv Pineapple were used throughout this study. P. digitatum isolate NAV-7 and P. minioluteum isolate GAA-2 were obtained from the culture collection of the Laboratory of Pathology, Postharvest Technology Center, Instituto Valenciano de Investigaciones Agrarias (IVIA). Fruits were used immediately after harvest and were surface disinfected (1-min immersion in a sodium hypochlorite solution [4 gL−1]), rinsed with fresh water, and left to air dry at room temperature before inoculation.
The concentration of the spore suspension was measured with a hemocytometer and adjusted to 1 × 104 spores mL−1 by dilution with sterile water. Fruit inoculation with P. digitatum or P. minioluteum was conducted as described previously (Palou et al., 2001). Spore suspensions were prepared from 7- to 10-d-old cultures on potato dextrose agar (Difco) incubated in the dark at 25°C. Spores were removed from sporulating colonies with a sterile loop and suspended in Tween 80 (0.05% w/v) in sterile distilled water. After vigorous agitation for 3 min in a vortex mixer, the remaining mycelial fragments were removed by filtration through two layers of cheesecloth.
A petri dish assay system was developed to assess the effect of different concentrations of synthetic compounds limonene and nerol on spore germination and germ tube development as previously described (Droby et al., 2008).
Oranges were inoculated by immersing a stainless steel rod with a probe tip 1 mm in width and 2 mm in length into the spore suspension and wounding the rind once in the equator. The wound penetrated the albedo tissue but not the juice sacs, simulating natural infection. Three different rind sites around the equator of each fruit were inoculated. Different fruits were used for each fungus. For the assays of limonene supplementation, 5 μL of the pure compound were allowed to penetrate in the wound and the same procedure for inoculation was performed. For each season and treatment, replicates of 10 to 25 fruit per transgenic line were used. Inoculated fruit were placed on plastic cavity trays on open cardboard trays that prevent fruit contact and incubated at 20°C and 80% relative humidity (RH) for two weeks. Disease incidence and sporulation, which are considered to be the number of infected wounds and sporulated infections, respectively, and disease severity, which is measured by the lesion diameter, were checked daily. Severity values were used to calculate the AUDPC (de Capdeville et al., 2002). Data on disease severity (AUDPC) and arcsine-transformed data on the percentage of infected wounds and sporulated lesions were subjected to the ANOVA using Statgraphics v.5.1 software (Manugistics Inc.). When appropriate, Fisher’s Protected lsd test (P < 0.05) was used to separate the means.
Inoculation of Fruit with Xanthomonas citri subsp. citri
AS and EV green oranges, the most susceptible developmental stage (cv Navelina and cv Pineapple), were used throughout this study. Fruits were used immediately after harvest and were ethanol surface disinfected, rinsed with fresh water, and left to air dry at room temperature before inoculation. The inoculum was prepared with strain 306 of Xanthomonas citri subsp. citri at 106 colony-forming units (CFU) mL−1 isolated in Brazil and obtained from the culture collection of the Laboratory of Bacteriology, IVIA. Inoculations were performed as previously reported (Viloria et al., 2004). Fruits were inoculated using a 1-mL syringe without a needle with phosphate-buffered bacterial suspensions at 106 CFU mL−1 obtained from an overnight culture in nutrient broth (Difco) or with phosphate buffer alone as control. Using a stainless steel rod equipped with a top, all lesions were ensured to be 1-mm depth; all wounds penetrated the flavedo tissue. Fruits were punctured at five to eight inoculation points. Lesions were evaluated at 7, 15, and 30 d after inoculation. Between 15 and 20 fruits per repetition of each transgenic line were used.
Inoculated fruits were placed in plastic trays covered with transparent film in a temperature- and humidity-controlled incubator (28°C/80% RH, respectively) for 4 weeks until symptoms of an infection halo and canker lesions were visible. Disease incidence was estimated by measuring the number of developed lesions, the number of fruits with a developed halo, and the diameter of this halo. Fisher’s Protected lsd test (P < 0.05) was used to evaluate the data on the percentage of infected wounds.
Insect Assays
Larvae and adults of medfly (Ceratitis capitata) were obtained from a laboratory population maintained at IVIA since 2002. Larvae were reared on an artificial diet as previously described (San Andrés et al., 2007). After emergence, adults were kept in ventilated Perspex cages (20 × 20 × 20 cm) and fed with a mixture of sugar and hydrolyzed yeast (Saccharomyces cerevisiae; Biokar Diagnostics Co.; 4:1, w:w) and water until they were 5 d old. Larvae and adult flies were maintained in an environmental chamber at 25°C ± 2°C, 60% ± 10% RH, and 16:8 h (light:dark) photoperiod.
No-choice assays were performed in an insecticide-free greenhouse at 26°C ± 2°C day temperatures, with a relative humidity between 60% to 80%. The experimental arena consisted of aluminum-framed cages of 150 × 150 × 90 cm covered with gauze. Groups of 100 5-d-old adults (50 males and 50 females) were released inside each cage containing a single plant with one fruit. Flies were fed as described before and allowed to lay eggs for 3 d.
For two-choice flight assays, a laboratory Plexiglass tunnel model OLFM-WT (Analytical Research Systems) measuring 180 × 60 × 60 cm producing a laminar flow of air was used to compare AS against EV fruit. Air was pulled through the chamber at 0.2 m s−1 connected to the downwind end. Air exiting the chamber was directly removed. In addition, this tunnel contained inlet and outlet vents to bring new air into the room from the outside and remove air from the room to the outside. Fruits were placed in the upwind end of the tunnel, and flies were released at the downwind opening. For two-choice cage assays, cages measuring 50 × 30 × 30 cm were used to compare AS against EV fruit. For paper assays, 30 μL of pure compounds were applied to 8-cm-diameter filter paper disks. For each assay, 50 5-d-old medfly males were released from the downwind end of the flight tunnel or inside the cages and allowed to respond freely between 20 and 30 min. Both EV and AS fruits were placed in the peel eight times with a 2-mm-long steel rod. All assays were performed at 25°C ± 2°C, under fluorescent lights (2,000 lx). In cage assays, 100 μL of synthetic limonene was used to cover the AS fruit. Ten to 20 replications were conducted in all experiments.
The oviposition response, number of punctures per fruit, number of pupae per fruit, and percentage of emergence data from no-choice assays were compared using Mann-Whitney U test (P < 0.05), and a t test of arcsine-transformed data were performed to examine the mean percentage of male medflies landing on the transgenic (AS) and control (EV) fruits in the two-choice assays.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AB110636.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Molecular analyses of Navelina (AS1-AS7) and Pineapple (AS11) citrus plants transformed with the limonene synthase gene in an AS orientation.
Supplemental Figure S2. Relative amounts of individual terpenes in fruits used for the assays, presented as a percentage area (fraction of unity) of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one in P. digitatum assays.
Supplemental Figure S3. Relative amounts of individual terpenes in fruits used for the assays, presented as a percentage area (fraction of unity) of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of one in X. citri subsp. citri assays.
Supplemental Figure S4. Oviposition response of medfly females in no-choice assays after 3 d of exposure to the orange odor of AS and EV plants.
Supplemental Figure S5. Terpene-mediated response of medfly males exposed to pure synthetic monoterpene compounds in flight tunnel assays.
Supplemental Figure S6. Terpene-mediated response of medfly males exposed to AS and EV green fruits in flight tunnel assays.
Supplemental Table S1. Fold-change of volatiles in AS transgenics compared to EV control Navelina sweet orange mature flavedo.
Supplemental Table S2. Fold-change of volatiles in AS transgenics compared to EV control Pineapple sweet orange mature flavedo.
Supplemental Movie S1. Behavior of sterile medfly males exposed to EV fruit (left) and AS3 fruit (right) under field conditions.
Supplementary Material
Acknowledgments
We are grateful to Drs. Jaime Primo-Millo and Vicente Navarro (Centro de Ecología Química Agrícola, Universidad Politécnica de Valencia) for the wind tunnel facilities, Josep E. Peris and Clara Montesinos (IVIA) for technical assistance, and Dr. Pedro Moreno (IVIA) for his critical review of the manuscript.
References
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15: 2866–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406: 512–515 [DOI] [PubMed] [Google Scholar]
- Back EA, Pemberton CE. (1915) Susceptibility of citrus fruits to the attack of the Mediterranean fruit fly. J Agric Res 3: 311–330 [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. (2006) Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311: 812–815 [DOI] [PubMed] [Google Scholar]
- Bednarek P, Osbourn A. (2009) Plant-microbe interactions: chemical diversity in plant defense. Science 324: 746–748 [DOI] [PubMed] [Google Scholar]
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106 [DOI] [PubMed] [Google Scholar]
- Ben-Yehoshua S, Rodov V, Nafussi B, Feng X, Yen J, Koltai T, Nelkenbaum U. (2008) Involvement of limonene hydroperoxides formed after oil gland injury in the induction of defense response against Penicillium digitatum in lemon fruit. J Agric Food Chem 56: 1889–1895 [DOI] [PubMed] [Google Scholar]
- Cazetta E, Schaefer HM, Galetti M. (2008) Does attraction to frugivores or defense against pathogens shape fruit pulp composition? Oecologia 155: 277–286 [DOI] [PubMed] [Google Scholar]
- Cipollini ML, Levey DJ. (1997) Why are some fruits toxic? Glycoalkaloids in Solanum and fruit choice by vertebrates. Ecology 78: 782–798 [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Capdeville G, Wilson CL, Beer SV, Aist JR. (2002) Alternative disease control agents induce resistance to blue mold in harvested ‘red delicious’ apple fruit. Phytopathology 92: 900–908 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH. (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410: 577–580 [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ. (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106: 13213–13218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta S, Wood J, Hicks J. (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1: 19–21 [Google Scholar]
- Droby S, Eick A, Macarisin D, Cohen L, Rafael G, Stange R, McColum G, Dudai N, Nasser A, Wisniewski M, et al. (2008) Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol Technol 49: 386–396 [Google Scholar]
- Dudareva N, Pichersky E. (2008) Metabolic engineering of plant volatiles. Curr Opin Biotechnol 19: 181–189 [DOI] [PubMed] [Google Scholar]
- Dugo G, Di Giacomo A. (2002) Citrus: The Genus Citrus. Medicinal and Aromatic Plants—Industrial Profiles Series. Taylor & Francis Group, CRC Press, New York [Google Scholar]
- Gershenzon J, Dudareva N. (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3: 408–414 [DOI] [PubMed] [Google Scholar]
- Goff SA, Klee HJ. (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311: 815–819 [DOI] [PubMed] [Google Scholar]
- Graham JH, Gottwald TR, Cubero J, Achor DS. (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5: 1–15 [DOI] [PubMed] [Google Scholar]
- Herrera CM. (1982) Defense of ripe fruit from pests: its significance in relation to plant-disperser interactions. Am Nat 120: 218 [Google Scholar]
- Janzen DH. (1977) Why fruits rot, seeds mold, and meat spoils. Am Nat 111: 691 [Google Scholar]
- Junker RR, Blüthgen N. (2010) Floral scents repel facultative flower visitors, but attract obligate ones. Ann Bot (Lond) 105: 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. (2005) Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Kekelidze NA, Lomidze EP, Janikashvili MI. (1989) Analysis of terpene variation in leaves and fruits of Citrus unshiu Marc. during ontogenesis. Flavour Fragrance J 4: 37–41 [Google Scholar]
- Kessler A, Baldwin IT. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. (2008) Field experiments with transformed plants reveal the sense of floral scents. Science 321: 1200–1202 [DOI] [PubMed] [Google Scholar]
- Lücker J, El Tamer MK, Schwab W, Verstappen FW, van der Plas LH, Bouwmeester HJ, Verhoeven HA. (2002) Monoterpene biosynthesis in lemon (Citrus limon): cDNA isolation and functional analysis of four monoterpene synthases. Eur J Biochem 269: 3160–3171 [DOI] [PubMed] [Google Scholar]
- Lücker J, Schwab W, van Hautum B, Blaas J, van der Plas LHW, Bouwmeester HJ, Verhoeven HA. (2004) Increased and altered fragrance of tobacco plants after metabolic engineering using three monoterpene synthases from lemon. Plant Physiol 134: 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntylä E, Alessio GA, Blande JD, Heijari J, Holopainen JK, Laaksonen T, Piirtola P, Klemola T. (2008) From plants to birds: higher avian predation rates in trees responding to insect herbivory. PLoS ONE 3: e2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou L, Smilanick JL, Usall J, Viñas I. (2001) Control of postharvest blue and green molds of oranges by hot water, sodium carbonate, and sodium bicarbonate. Plant Dis 85: 371–376 [DOI] [PubMed] [Google Scholar]
- Papachristos DP, Papadopoulos NT. (2009) Are citrus species favorable hosts for the Mediterranean fruit fly? A demographic perspective. Entomol Exp Appl 132: 1–12 [Google Scholar]
- Rodríguez A, Cervera M, Peris J, Peña L. (2008) The same treatment for transgenic shoot regeneration elicits the opposite effect in mature explants from two closely related sweet orange (Citrus sinensis (L.) Osb.) genotypes. Plant Cell Tissue Organ Cult 93: 97–106 [Google Scholar]
- Sallabanks R, Courtney SP. (1992) Frugivory, seed predation, and insect-vertebrate interactions. Annu Rev Entomol 37: 377–400 [DOI] [PubMed] [Google Scholar]
- San Andrés V, Ortego F, Castañera P. (2007) Effects of gamma-irradiation on midgut proteolytic activity of the mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Arch Insect Biochem Physiol 65: 11–19 [DOI] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103: 1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly TE, War M, Favela A. (2008) Exposing entire adult holding rooms containing sterile male Mediterranean fruit flies to orange oil increases the mating success of those males in field-cage trials. Fla Entomol 91: 686–689 [Google Scholar]
- Shimada T, Endo T, Fujii H, Hara M, Ueda T, Kita M, Omura M. (2004) Molecular cloning and functional characterization of four monoterpene synthase genes from Citrus unshiu Marc. Plant Sci 166: 49–58 [Google Scholar]
- Trapp S, Croteau R. (2001) Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52: 689–724 [DOI] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5: 283–291 [DOI] [PubMed] [Google Scholar]
- Viloria Z, Drouillard DL, Graham JH, Grosser JW. (2004) Screening triploid hybrids of ‘Lakeland’ limequat for resistance to citrus canker. Plant Dis 88: 1056–1060 [DOI] [PubMed] [Google Scholar]
- Whitney KD, Stanton ML. (2004) Insect seed predators as novel agents of selection on fruit color. Ecology 85: 2153–2160 [Google Scholar]
- Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. (2006) Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotechnol 24: 1441–1447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.