Abstract
Bundle sheath extensions (BSEs) are key features of leaf structure with currently little-understood functions. To test the hypothesis that BSEs reduce the hydraulic resistance from the bundle sheath to the epidermis (rbe) and thereby accelerate hydropassive stomatal movements, we compared stomatal responses with reduced humidity and leaf excision among 20 species with heterobaric or homobaric leaves and herbaceous or woody life forms. We hypothesized that low rbe due to the presence of BSEs would increase the rate of stomatal opening (V) during transient wrong-way responses, but more so during wrong-way responses to excision (Ve) than humidity (Vh), thus increasing the ratio of Ve to Vh. We predicted the same trends for herbaceous relative to woody species given greater hydraulic resistance in woody species. We found that Ve, Vh, and their ratio were 2.3 to 4.4 times greater in heterobaric than homobaric leaves and 2.0 to 3.1 times greater in herbaceous than woody species. To assess possible causes for these differences, we simulated these experiments in a dynamic compartment/resistance model, which predicted larger Ve and Ve/Vh in leaves with smaller rbe. These results support the hypothesis that BSEs reduce rbe. Comparison of our data and simulations suggested that rbe is approximately 4 to 16 times larger in homobaric than heterobaric leaves. Our study provides new evidence that variations in the distribution of hydraulic resistance within the leaf and plant are central to understanding dynamic stomatal responses to water status and their ecological correlates and that BSEs play several key roles in the functional ecology of heterobaric leaves.
Stomata close in response to changes in water status throughout the soil-plant-atmospheric continuum, including declines in soil water status (Davies and Zhang, 1991; Comstock and Mencuccini, 1998), reduced xylem hydraulic conductance proximal to leaves (Saliendra et al., 1995), and increased evaporative water loss (Mott and Parkhurst, 1991). Stomatal dynamics and closure in response to atmospheric drought can contribute to adaptation to dry habitats (Franks and Farquhar, 1999; Cunningham, 2004). It is unclear, however, where water status is sensed or how it is transduced into changes in stomatal conductance (gs). The similarity of stomatal responses to hydraulic supply and demand (Saliendra et al., 1995; Comstock and Mencuccini, 1998; Cochard et al., 2002) suggests that part of the regulation of gs results from negative feedback from water status within the leaf (Buckley, 2005). The resolution of these questions may require greater focus on functional aspects of leaf anatomy, in particular the hydraulic coupling of the epidermis with the rest of the leaf.
During natural transpiration, water potentials may vary greatly over small spatial scales within leaves, because of large and varying resistances in the pathways for water flow to the sites of evaporation (Mott, 2007; Zwieniecki et al., 2007; Peak and Mott, 2011). Some of this variation involves xylem hydraulic resistance within the leaf (Nardini et al., 2003; Brodribb and Holbrook, 2004; Sack et al., 2004; Lo Gullo et al., 2005). However, there can be substantial resistance downstream of xylem conduits. For example, among 10 tropical forest tree species, 11% to 74% of leaf hydraulic resistance (rleaf) resided outside the xylem (Sack et al., 2005). Helianthus annuus showed strong diurnal variation in rleaf, with half the resistance extravascular and modulated by irradiance and circadian rhythms, probably via changes in aquaporin expression and/or activation (Nardini et al., 2005). Reduction of rleaf by light coincided with the up-regulation of aquaporins in Juglans regia (Cochard et al., 2007). Other data (Zwieniecki et al., 2007) suggest that rleaf downstream of the xylem includes at least two components, representing flow into separate “fast” and “slow” pools of water at low and high resistance, respectively. Zwieniecki et al. (2007) proposed categorizing leaves based on the extent to which the epidermis and/or mesophyll are hydraulically sequestered from the xylem.
Evidence is mixed about the importance of these phenomena for stomatal function. Changes in water potential caused by reduced humidity can be propagated laterally within a leaf, inducing changes in gs in areas that experience constant humidity (Mott et al., 1997; Mott and Franks, 2001). Similarly, stomata can respond to changes in transpiration rate in distant regions of the same leaf (Buckley and Mott, 2000) or plant (Pataki et al., 1998; Pepin et al., 2002; Brooks et al., 2003). Long-distance responses include transient wrong-way opening movements similar to those generated by local hydraulic perturbations, which suggests that the long-distance responses are mediated by hydraulic signals. However, Mott (2007) reported that stomatal responses to humidity were independent on the two surfaces of amphistomatous leaves of Vicia faba and Xanthium strumarium: when humidity was changed at one surface only, stomata on that surface responded but stomata on the other surface did not. This suggests that changes in water potential near one surface may not propagate to the opposite surface, which in turn suggests that stomata in opposing epidermes on broad leaves might be hydraulically sequestered from one another and from the xylem. Peak and Mott (2011) hypothesized that guard cells themselves are hydraulically sequestered from the epidermis and instead sense humidity in the stomatal pore directly. An alternative explanation is that the hydraulic resistance between the epidermis and the bulk leaf tissue, including the xylem, can be very large, consistent with the observation that epidermal turgor pressure responded to humidity at the perturbed surface but not at the unperturbed surface in V. faba (Mott, 2007; turgor was not measured for X. strumarium).
The hydraulic resistance between the epidermis and the rest of the leaf may vary strongly across species, and especially between homobaric and heterobaric leaves. The latter possess bundle sheath extensions (BSEs) that connect the epidermis and vascular bundles (Wylie, 1952). BSEs can help distribute light through thicker leaves, enhancing photosynthesis (Nikolopoulos et al., 2002), and they may limit lateral CO2 diffusion within the leaf (Terashima, 1992; Morison et al., 2007). BSEs are more common in xeric, high-light species and upper canopy trees (Kenzo et al., 2007) and in deciduous woody species than in herbs (Wylie, 1952; McClendon, 1992). Scoffoni et al. (2008) found that the reduction of rleaf by light was much stronger in heterobaric than homobaric species (averaging 58% versus 20%, respectively). This suggests the BSEs are in the hydraulic pathway and can provide additional flow paths that may reduce rleaf in heterobaric leaves relative to homobaric leaves. Thus, as Wylie (1952) hypothesized based on their anatomy, BSEs may contribute significantly to water flow in heterobaric leaves by facilitating water transport to the epidermis. It is surprising that the role of BSEs in stomatal control has received so little attention.
Well-known features of stomatal behavior can provide insight about the roles of BSEs in leaf hydraulics and the control of water loss. In most angiosperm leaves, stomatal closure following either increased evaporative demand or reduced water supply is preceded by opening movements known as “wrong-way responses” (WWRs; Fig. 1). The WWR is caused by an initial reduction in epidermal back pressure on stomata (Darwin, 1898; Cowan, 1972), whereas the subsequent closure (“right-way response” [RWR]) results from a slower but larger decline in guard cell turgor pressure that may be caused by solute release from guard cells (Losch and Schenk, 1978; Ehret and Boyer, 1979; Grantz and Zeiger, 1986; Buckley and Mott, 2002a, 2002b; Powles et al., 2006). The rate of stomatal opening during the WWR depends on the time constant for changes in epidermal turgor pressure, Pe. The rate of change of Pe is:
 |
where ψb, ψe, and ψm are water potentials of the bundle sheath, epidermis, and mesophyll; rbe and rme are the resistances to the epidermis from the bundle sheath and mesophyll, respectively; E is transpiration rate and fe is the epidermal transpiration fraction; and εe and ve are epidermal elastic modulus and volume, respectively (for a derivation of Eq. 1, see Supplemental Data S2). Equation 1 suggests that stomatal opening rate due to reduced Pe will scale with 1/rbe for a step change in supply (ψb) and with fe for a step change in demand (E). However, increased demand may also reduce ψb, so the opening rate following a change in E may also scale with 1/rbe, but to a lesser extent.
Figure 1.
A representative response of stomatal conductance (gs) of Olea europea to a step increase in evaporative demand from 15 to 25 mmol mol−1 (decrease in relative humidity from 52% to 20%) at t1, followed by leaf excision at the petiole at t4. The points in time and corresponding gs values used to calculate the WWR and RWR parameters (t1–t6 and g1, g2, g4, and g5; note that g3 ≡ g1 and g6 ≡ g4) as described in “Materials and Methods” are represented diagrammatically.
This predicts that low rbe should increase WWR opening rates for both supply and demand perturbations, but more so for supply responses. Thus, we hypothesized that the rates of stomatal opening during excision WWRs (Ve) and humidity WWRs (Vh), as well as their ratio (Ve/Vh), will be greater in heterobaric than homobaric leaves. Similarly, because soil to leaf hydraulic resistance is greater in woody than herbaceous species (Turner et al., 1984; Mencuccini, 2003; Sack et al., 2003), we hypothesized that Ve, Vh, and Ve/Vh will be greater in herbaceous than woody species. To assess the degree and functional significance of hydraulic sequestration of the epidermis as affected by BSEs and plant growth habit, we tested these hypotheses by measuring stomatal responses to humidity and leaf excision in leaves of 10 homobaric and 10 heterobaric species, each group containing five herbaceous and five woody species (Table I). In addition, because our hypotheses arose from inspection of Equation 1, they may not account for some effects (e.g. ψb, Pe, and πe will change during the time period used to calculate Ve and Vh); therefore, we assessed the theoretical plausibility of our hypotheses by simulating our experiments in a dynamic compartment-resistance model of water flow and stomatal control (diagrammed in Fig. 1 and presented in detail in Supplemental Data S1).
Table I. Species used in this study.
| Species | Family |
| Heterobaric, woody | |
| Umbellularia californica | Lauraceae |
| Vitis vinifera | Vitaceae |
| Photinia × fraseri | Rosaceae |
| Nerium oleander | Apocynaceae |
| Viburnum opulus ‘Roseum’ | Adoxaceae |
| Heterobaric, herbaceous | |
| Helianthus annuus | Asteraceae |
| Hordeum vulgare | Poaceae |
| Phaseolus vulgaris | Fabaceae |
| Solanum lycopersicum | Solanaceae |
| Glycine max | Fabaceae |
| Homobaric, woody | |
| Hedera helix | Araliaceae |
| Olea europea | Oleaceae |
| Cotoneaster horizontalis. | Rosaceae |
| Euonymus fortunei ‘Colorata’ | Celastraceae |
| Ligustrum japonicum ‘Texanum’ | Oleaceae |
| Homobaric, herbaceous | |
| Vicia faba | Fabaceae |
| Nicotiana tabacum | Solanaceae |
| Tradescantia pallida | Commelinaceae |
| Chlorophytum comosum ‘Variegatum’ | Agavaceae |
| Ocimum basilicum | Lamiaceae |
RESULTS
Transient WWRs to humidity and leaf excision were observed in 51 of 58 leaves and 19 of 20 species studied. One of three individuals of Ocimum basilicum and Phaseolus vulgaris failed to show WWRs to humidity, and one of three individuals of Chlorophytum comosum and Euonymus fortunei and all three individuals of Tradescantia pallida showed no WWR to leaf excision. Where WWRs were observed, we found wide ranges for most WWR parameters (for definitions of parameters, see “Materials and Methods”). The humidity and excision WWR sizes (Wh and We) varied 29- and 484-fold across species, respectively. The WWR response lengths (Lh and Le) varied 15.9- and 18.8-fold, respectively. The rates of stomatal opening (Vh and Ve) varied 100- and 190-fold, respectively. The size of steady-state humidity responses (Rh) varied by 109-fold. Similarly wide variation was observed in these parameters when they were expressed relative to initial gs. The Wh,rel and We,rel varied 107- and 61-fold, respectively; Vh,rel and Ve,rel varied 66- and 149-fold, respectively; and Rh,rel varied 40-fold. These results are given in detail by species in Supplemental Table S1 and summarized by group (leaf type and life form) in Table II. A substantial part of the total variance in the data set, ranging from 22% to 60%, occurred within species (i.e. among replicates of the same species) for all parameters except Vh, Ve, and Rh,rel (Table II).
Table II. Means ± se of stomatal response parameters measured in heterobaric and homobaric species, and results of ANOVA (species nested within homobaric versus heterobaric nested within woody versus herbaceous).
| Trait | Symbol | Units | Variancea | Woody | Herbaceous | Heterobaric | Homobaric | Simulatedb | |||
| 1× rbe | 10× rbe | 20× rbe | |||||||||
| Initial stomatal conductance | g1 | mol m−2 s−1 | 44c | 0.071 ± 0.012c | 0.120 ± 0.021 | 0.108 ± 0.022c | 0.087 ± 0.016 | 0.083 | 0.074 | 0.067 | |
| Relative RWR to humidity | Rh | % | 37d | 29.3 ± 2.8e | 21.8 ± 2.1 | 22.9 ± 2.2d | 28.2 ± 3.0 | 25.9 | 27.6 | 29.1 | |
| WWR parameters | |||||||||||
| Rate of openingf | Humidity | Vh/10−5 | mol m−2 s−2 | 22d | 8.58 ± 1.45c | 19.7 ± 7.3 | 20.2 ± 7.3c | 8.1 ± 1.2 | 3.7 | 4.2 | 4.5 |
| Excision | Ve/10−5 | mol m−2 s−2 | 27d | 13.2 ± 9.9g | 41.0 ± 17.5 | 41.7 ± 15.8g | 9.4 ± 1.9 | 13.2 | 9.8 | 7.2 | |
| Relative size | Humidity | Wh,rel | % | 51c | 43.6 ± 19.4e | 24.3 ± 5.93 | 42.8 ± 19.5d | 25.0 ± 5.7 | 8.9 | 10.9 | 13.1 |
| Excision | We,rel | % | 46g | 62.5 ± 16.7d | 113 ± 79 | 138 ± 77g | 37.7 ± 9.1 | 19.0 | 18.3 | 17.4 | |
| Length | Humidity | Lh | min | 45c | 9.70 ± 1.73c | 6.97 ± 1.48 | 7.73 ± 1.32e | 8.94 ± 1.94 | 7.2 | 7.0 | 7.0 |
| Excision | Le | min | 60g | 7.89 ± 1.57e | 5.57 ± 1.17 | 7.00 ± 1.30d | 6.46 ± 1.56 | 4.9 | 5.0 | 5.3 | |
| Ratio of sizes | We/Wh | – | 57c | 2.22 ± 0.36c | 4.73 ± 2.10 | 4.77 ± 2.08e | 2.18 ± 0.46 | 2.14 | 1.68 | 1.33 | |
| Ratio of opening rates | Ve/Vh | – | 56c | 1.59 ± 0.31e | 3.22 ± 1.34 | 3.23 ± 1.20e | 1.40 ± 0.24 | 3.58 | 2.32 | 1.62 | |
Percentage of variance occurring within rather than among species.
Simulations used the model described in the Supplemental Data and the parameter values in Table III, except rbe, which was increased by factors of 10 and 20 in the simulations under the headings 10× rbe and 20× rbe, respectively.
P < 0.01.
P > 0.05.
P < 0.05.
Actual opening rates (mol m−2 s−2) were 10−5 times those given in the table.
P < 0.001.
WWR Kinetics
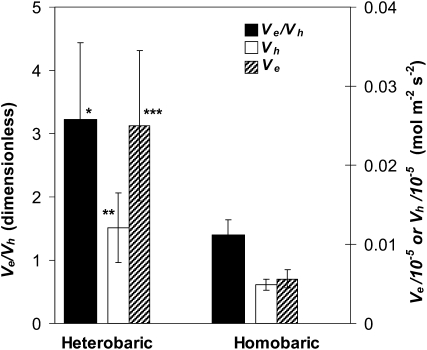
Heterobaric and homobaric species showed strong differences in WWR parameters. Consistent with our hypothesis, the ratio of the rates of stomatal opening during WWRs to excision and humidity (Ve/Vh) was more than twice as large for heterobaric than homobaric species (3.23 ± 1.20 versus 1.40 ± 0.24; P < 0.05; means ± se; Fig. 3; Table II). This difference arose because opening during WWRs to excision was 4.4 times faster in heterobaric than homobaric leaves (Ve/10−5 = 41.7 ± 15.8 versus 9.4 ± 1.9 mol m−2 s−2; P < 0.001; Fig. 3) and despite the fact that opening during WWRs to humidity was also 2.5 times faster in heterobaric leaves (Vh/10−5 = 20.2 ± 7.3 versus 8.1 ± 1.2 mol m−2 s−2; P < 0.01; Fig. 3). Notably, faster opening was not consistently associated with shorter WWRs: although humidity WWRs were shorter in heterobaric than homobaric species (P < 0.05), excision WWRs did not differ significantly in length (Table II).
Figure 3.
Rates of opening during WWRs to humidity (Vh; white bars) and leaf excision (Ve; hatched bars) and the ratio of those rates (Ve/Vh; black bars) in heterobaric and homobaric species. n = 10 species and 29 experiments in each group. Values shown are means ± se. * P < 0.05, ** P < 0.005, *** P < 0.001.
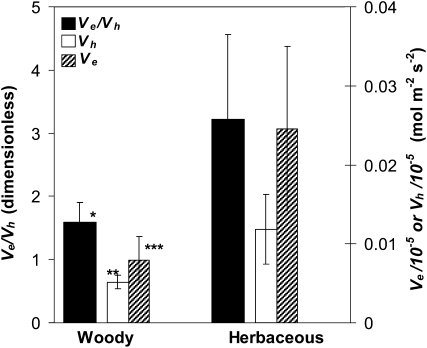
We also found that the hypothesized differences in WWR kinetics between woody and herbaceous species, Vh, Ve, and Ve/Vh, were 2.3, 3.1, and 2.0 times greater, respectively, in herbaceous than woody plants (P < 0.005, P < 0.001, and P < 0.05, respectively; Fig. 4; Table II). In this case, faster opening was associated with shorter humidity and excision WWRs (P < 0.005 and P < 0.05, respectively; Table II).
Figure 4.
Rates of opening during WWRs to humidity (Vh; white bars) and leaf excision (Ve; hatched bars) and the ratio of those rates (Ve/Vh; black bars) in woody and herbaceous species. n = 10 species and 29 experiments in each group. Values shown are means ± se. * P < 0.05, ** P < 0.005, *** P < 0.001.
The faster opening we observed in heterobaric than homobaric and in herbaceous relative to woody species was linked partly, but not entirely, with higher initial gs (Table II). Thus, Vh and Ve were correlated with g1, with relationships that appeared to be exponential (equations Vh = 0.00242 e8.2472∣·g1, r2 = 0.402; and Ve = 0.00334 e6.7698∣·g1, r2 = 0.145; P < 0.001 and P = 0.005, respectively; Supplemental Fig. S1). We determined whether BSEs and life form affected Ve and Vh independently of g1 by determining the residuals of Vh and Ve against g1 from these equations and testing for differences in these residuals using the nested ANOVAs (see “Materials and Methods”). For Vh and Ve, the residuals differed between heterobaric and homobaric (P = 0.064 and P < 0.001, respectively) and between woody and herbaceous species (P < 0.005 and P < 0.001, respectively). Supplemental Figure S2 shows mean residuals by species, leaf type, and life form. These findings indicated differences in rates of stomatal opening between leaf and plant types that were independent of differences in g1.
WWR and RWR Sizes
The sizes of WWRs differed between plant types in some cases. Thus, WWRs in response to excision were 3.7 times larger in heterobaric than homobaric species (We,rel = 138% ± 78% versus 38% ± 9%, respectively; P < 0.001; Fig. 5), but WWRs in response to humidity did not differ significantly in size between heterobaric and homobaric species (Wh,rel = 43% ± 20% versus 25% ± 6%, respectively; Fig. 5; Table II). Humidity WWRs were significantly larger in woody than herbaceous species (44% ± 19% versus 24% ± 6%, respectively; P < 0.05; Fig. 6; Table II). However, the ratio of WWR sizes was significantly greater in heterobaric than homobaric (4.77 ± 2.08 versus 2.18 ± 0.46; P < 0.05) and in herbaceous than woody plants (4.73 ± 2.10 versus 2.22 ± 0.36; P < 0.005; Fig. 5; Table II).
Figure 5.
Relative sizes of WWRs to humidity (Wh,rel; white bars) and leaf excision (We,rel; hatched bars) and the ratio of those sizes (We/Wh; black bars) in heterobaric and homobaric species. n = 10 species and 29 experiments in each group. Values shown are means ± se. * P < 0.05, *** P < 0.001. ns, Nonsignificant.
Figure 6.
Relative sizes of WWRs to humidity (Wh,rel; white bars) and leaf excision (We,rel; hatched bars) and the ratio of those sizes (We/Wh; black bars) in herbaceous and woody species. n = 10 species and 29 experiments in each group. Values shown are means ± se. * P < 0.05, ** P < 0.005. ns, Nonsignificant.
Steady state or RWRs in response to humidity were significantly larger in woody than herbaceous species (Rh,rel = 29.3 ± 2.8 versus 21.8 ± 2.1; P < 0.05; Table II), but heterobaric and homobaric species did not differ significantly (Table II).
Modeling Analysis
Using the baseline parameter set (Table III), in which the resistance from the bundle sheath to the epidermis, rbe, was assumed equal to that between the bundle sheath and the mesophyll, the model predicted Ve/Vh = 3.58 (1× rbe under “Simulated” in Table II). Using the same parameters but with resistance to the epidermis increased 10- or 20-fold (10× rbe and 20× rbe under “Simulated” in Table II), the model predicted Ve/Vh = 2.32 and 1.62, respectively. All three simulations predicted larger WWRs in response to excision than humidity, but this difference declined as rbe increased (We/Wh = 2.14, 1.68, and 1.33 at 1×, 10×, and 20× rbe, respectively). The absolute rates of opening and WWR sizes predicted by the model were smaller than we observed (Table II).
Table III. Variables and parameters in the leaf model used in this study.
| Parameter/Variable Name | Symbol | Units | Baseline Value |
| Dynamic state variables | |||
| Epidermal volume | ve | m3 m−2leaf | – |
| Guard cell volume | vg | m3 m−2leaf | – |
| Mesophyll volume | vm | m3 m−2leaf | – |
| Bundle sheath volume | vb | m3 m−2leaf | – |
| Guard cell osmotic content | ng | mol m−2leaf | – |
| Calculated state variables (i = e, g, b, m) | |||
| Turgor pressure | Pi | MPa | – |
| Osmotic pressure | πi | MPa | – |
| Water potential | ψi | MPa | – |
| Stomatal conductance to water vapor | gs | mol air m−2 s−1 | – |
| Transpiration rate | E | mmol water m−2 s−1 | – |
| Parameters | |||
| Hydraulic resistances (leaf area basis) | |||
| Leaf | rleaf | MPa m2 s mmol−1 | 0.08 |
| Soil-leaf xylem | rsx | MPa m2 s mmol−1 | 0.232 or ∞a |
| Extraxylar | rox | MPa m2 s mmol−1 | 0.032 |
| Bundle sheath-mesophyll | rbm | MPa m2 s mmol−1 | 0.0116 |
| Xylem-bundle sheath | rxb | MPa m2 s mmol−1 | 0.0116 |
| Bundle sheath-epidermis | rbe | MPa m2 s mmol−1 | 0.0232 |
| Mesophyll-epidermis | rme | MPa m2 s mmol−1 | 0.0232 |
| Epidermis-guard cell | reg | MPa m2 s mmol−1 | 0.126 |
| Values at incipient plasmolysis | |||
| Leaf symplastic volume | vtotal | m3 m−2leaf | 1.3 × 10−4 |
| Epidermal volume fraction | veo/vtotal | – | 0.31 |
| Bundle sheath volume fraction | vbo/vtotal | – | 0.062 |
| Guard cell volume fraction | vgo/vtotal | – | 0.0085 |
| Mesophyll volume fraction | vmo/vtotal | – | 0.62 |
| Epidermal osmotic pressure | πeo | MPa | 0.6 |
| Mesophyll osmotic pressure | πmo | MPa | 0.6 |
| Bundle sheath osmotic pressure | πbo | MPa | 0.6 |
| Miscellaneous | |||
| Pressure-conductance scaling factor | χ | mol air m−2 s−1 MPa−1 | 0.11 |
| Guard cell pressure-volume coefficients | c1, c2 | MPa, – | 4.1, 6.7 |
| Epidermal pressure-volume coefficient | c3, c4 | MPa | −13.4, 7.4 |
| Maximum mesophyll elastic modulus | εm | MPa | 15.3 |
| Guard cell osmoregulatory rate constant | α | mol s−1 MPa−1 | 0.005 |
| Hydroactive sensitivity [∂(πg,ss − πe)/∂Pe] | B | – | 4 |
| Fraction of evaporation from epidermis | fe | – | 0.5 |
| Fraction of evaporation from mesophyll | fm | – | 0.5 |
| Fraction of evaporation from bundle sheath | fb | – | 0 |
| Fraction of evaporation from guard cells | fg | – | 0 |
| Leaf-air water mole fraction difference | Δw | mmol water mol−1 air | 15 or 25b |
rsx was changed to infinity to simulate leaf excision.
Δw was changed from 15 to 25 mmol mol−1 to simulate reduced humidity.
We assessed parameter sensitivity by a series of simulations (Fig. 7) in which individual parameter values were doubled relative to their baseline values in Table III. This analysis suggested that Ve/Vh should be sensitive to changes in several parameters, including rbe. Specifically, increases in epidermal osmotic pressure at incipient plasmolysis (πeo), guard cell volume fraction (vgo/vtotal), or maximum mesophyll elastic modulus (εm) would strongly increase Ve/Vh, whereas increases in the rate constant for guard cell osmoregulation (α), the sensitivity of guard cell osmotic pressure to epidermal turgor at steady state (B), or the soil-leaf xylem resistance (rsx) would strongly reduce Ve/Vh. Doubling epidermal transpiration fraction (fe) reduced Ve/Vh by a similar degree as doubling rbe. Ve/Vh was minimally sensitive to other parameters. The absolute sensitivities of Ve and Vh differed widely among parameters. Notably, doubling α had no effect on Vh but reduced Ve by over 30%. Doubling symplastic volume (vtotal) reduced both Vh and Ve by about 45%. The similar effects of fe and rbe on Ve/Vh had different causes: fe increased Vh with little effect on Ve, whereas rbe reduced Ve with little effect on Vh. Increasing πeo enhanced Ve much more than Vh; the opposite was true for B (Fig. 7).
Figure 7.
Sensitivity of rates of opening during WWRs to humidity (Vh; white bars at right) and leaf excision (Ve; hatched bars at right) and the ratio of those rates (Ve/Vh; black bars at left) to doubling of individual parameter values in the model described in the Supplemental Data. All parameters except the one listed on a given row in this figure took on the baseline values given in Table III. Parameters are ordered with respect to their effect on Ve/Vh. b.s., Bundle sheath; g.c., guard cell.
We also assessed the sensitivity of Ve/Vh to a wider range of rbe values (Fig. 7). These simulations used a range of values of B and α, because we could not estimate these parameters from the literature yet sensitivity analysis found them to be important in WWR kinetics. Simulated values of Ve/Vh coincided with observed means for heterobaric and homobaric species at approximately 2.5 and 26 times the baseline value of rbe, respectively, under baseline values of B (4) and α (0.005 s−1); at approximately 1 and 16 times baseline rbe for high B (6); and at 6 and 26 times baseline rbe for low α (0.0025). (Similar trends were predicted at low B [2] and high α [0.01], but in neither case could simulations span the range of observed mean Ve/Vh values for both leaf types if rbe were constrained to 30 times baseline.) From these three ranges, we tentatively estimated that a 4- to 16-fold reduction of rbe by BSEs (26/6 ≈ 4, 26/2.5 ≈ 10, 16/1 = 16) could explain observed differences in WWR kinetics between heterobaric and homobaric leaves.
We repeated the preceding analysis (data not shown) by increasing rsx to estimate the change in rsx needed to reduce Ve/Vh by half, as observed for woody plants in comparison with herbaceous plants. For high α (0.01 s−1), a 6-fold increase in rsx reduced Ve/Vh by half. At other values of α and B, doubling rsx reduced Ve/Vh by 12% to 20%; however, further increases in rsx caused sustained oscillations in gs (data not shown).
DISCUSSION
We hypothesized that BSEs and growth habit would impact strongly on stomatal responses. Thus, BSEs in heterobaric leaves should increase the rates of stomatal opening during transient WWRs to Ve and Vh, but more so for excision, such that the ratio Ve/Vh should be greater in heterobaric than homobaric leaves. Our hypotheses were based on inspection of Equation 1 in the introduction and therefore unavoidably omitted some dynamic complexity. However, a more rigorous analysis based on a dynamic compartment-resistance model (Figs. 7 and 8) also predicted that Ve and Ve/Vh should be larger in plants with low rbe.
Figure 8.
Sensitivity of the ratio of opening rates during WWRs to leaf excision and humidity (Ve/Vh) to variation in the hydraulic resistance from the bundle sheath to the epidermis (rbe) in the model described in the Supplemental Data. The ordinate is multiples of rbe relative to its baseline value in Table III (0.0232 MPa m−2 s−1 mmol−1). The solid line shows baseline values for all parameters other than rbe; dashed lines show simulations assuming values of guard cell osmotic sensitivity to epidermal turgor (B) different from baseline values, as shown; dotted lines show simulations using values of guard cell osmoregulation rate constant (α) different from baseline values, as shown. Areas shown in gray are observed values (means ± 1 se) of Ve/Vh for heterobaric (HE) and homobaric (HO) species.
Our results supported these hypotheses for the impacts of BSEs. The Ve, Vh, and the ratio Ve/Vh were greater in heterobaric than homobaric leaves, as predicted. These results suggest that BSEs reduce the sensitivity of epidermal turgor (Pe) to changes in either hydraulic supply or demand for the epidermis, but more so for changes in hydraulic supply. We also predicted larger Ve, Vh, and Ve/Vh in herbaceous than woody plants because of the smaller total resistance of water supply to the epidermis in woody species. Our results supported those predictions as well.
Our data and model analyses support the hypothesis that BSEs substantially reduce hydraulic resistance to the epidermis. To estimate the size of that effect, we determined how widely rbe needed to vary to reproduce the mean values of Ve/Vh observed for heterobaric and heterobaric leaves (Fig. 8). On this basis, we estimated that a 4- to 16-fold reduction in rbe by BSEs could explain our observations. This is consistent with previous estimates of the extravascular fraction of rleaf (30%–50%; Sack et al., 2005) and of the effects of high irradiance on rleaf in heterobaric leaves (reductions of 58% versus 20%, respectively; Scoffoni et al., 2008), which suggest that BSEs reduce rbe by 5- to 24-fold (this is derived in Supplemental Data S5).
Effect of Parameters Other Than rbe on WWR Properties
Comparison of our results with the model suggested that parameters other than rbe may also vary systematically between heterobaric and homobaric leaves. Parameter sensitivity analysis showed that increasing total symplastic volume per unit leaf area (vtotal) would reduce both Vh and Ve independent of their ratio. This is also evident from inspection of Equation 1, and it reflects the ability of volume to buffer changes in turgor pressure. One study did in fact find thicker leaves in homobaric than heterobaric woody species (Liakoura et al., 2009), which is consistent with higher vtotal and thus with our finding of smaller Ve and Vh in homobaric species. Another possibility is that πeo and B covary and are larger in heterobaric leaves (these are, respectively, epidermal osmotic pressure at incipient plasmolysis and the sensitivity of guard cell osmotic gradient to Pe in the steady state), as these parameters both increase Ve and Vh but have opposing effects on their ratio Ve/Vh (Fig. 7).
Sensitivity analysis indicated four other parameters with greater effects than rbe on Ve/Vh: guard cell volume fraction (vgo/vtotal), maximum mesophyll elastic modulus (εm), guard cell osmoregulatory rate constant (α), and soil-leaf xylem resistance (rsx). Like πeo and B, vgo and α have strong but opposing effects on Ve/Vh in the model. However, α modulates rates of adjustment in guard cell osmotic content, which vgo translates into osmotic pressure; thus, one may expect α and vgo to covary, which would tend to cancel both parameters’ effects on Ve/Vh. Doubling εm increased Ve/Vh by 10%, so a 10-fold increase in εm could potentially explain the observed approximately 100% difference in Ve/Vh between heterobaric and homobaric leaves. This is unlikely, however, because even doubling εm increased it to 30.6 MPa, which exceeds the range of values commonly reported in plants (Niinemets, 2001; Saito and Terashima, 2004). Increases in another parameter, rsx, reduced the absolute magnitudes of both Ve and Vh, with a greater impact on Ve than Vh. These effects are qualitatively identical to those of rbe and can be understood on a similar basis.
Doubling the epidermal transpiration fraction (fe) reduced Ve/Vh by a similar degree as doubling rbe. This arose mainly from increased Vh in the case of fe but from decreased Ve in the case of rbe (Fig. 7). This bears out the analysis of Equation 1, which suggested that increased fe and decreased rbe should accelerate stomatal opening after demand and supply perturbations, respectively. The actual value of fe is unknown and highly debated (Tyree and Yianoulis, 1980; Maier-Maercker, 1983; Grantz, 1990). Our analysis suggests that if fe differs systematically with leaf type, one would expect larger fe in homobaric than in heterobaric leaves. That seems reasonable, as BSEs prevent evaporation from the epidermal cells that they subtend. However, evaporation from BSEs likely cannot explain trends in Ve/Vh with leaf type, because the effect of bundle sheath transpiration fraction (fb) was negligible in the model (data not shown).
Other parameters generally had smaller effects than rbe on Ve, Vh, and Ve/Vh. Doubling the resistance from the bundle sheath to the mesophyll (rbm) slightly increased Ve/Vh due to a small increase in Ve and decrease in Vh. These effects are opposite those of rbe, and this is because the ratio rbe/rbm essentially partitions water supply between the epidermis and mesophyll. Epidermal volume fraction (veo/vtotal) very slightly reduced both Ve and Vh, consistent with Equation 1, and bundle sheath volume fraction (vbo/vtotal) had negligible effects on Ve and Vh. Xylem to bundle sheath resistance (rxb) had effects qualitatively identical to, but much smaller than, those of rsx (i.e. reduced Ve and Vh and increased Ve/Vh), which is logical, given that rsx and rxb are in series and proximal to the bundle sheath. Increased epidermis to guard cell resistance (reg) slightly increased Ve but not Vh. Vh was minimally affected because reg has two opposing effects: it slows both the hydropassive and hydroactive declines in guard cell turgor (which increase and decrease opening rate, respectively) by impeding water movement. However, whereas the humidity WWR peaks when the hydroactive response overcomes the epidermal mechanical advantage, the excision WWR peaks when the epidermis reaches zero turgor, which means hydroactive kinetics have less influence on Ve than on Vh.
The model assumes a linear dependence of gs on guard cell turgor, whereas gs (and WWR size) may be mechanically limited to a maximum upper value. Thus, if heterobaric and homobaric species differed systematically in the proximity of their initial gs to that theoretical maximum, this could introduce a bias in WWR size and, transitively, in the parameters of WWR kinetics. Although we did not attempt to quantify maximum gs per se, we sought to minimize variations in the proximity of initial to maximum gs by measuring all leaves at the same high irradiance (600 μmol m−2 s−1, which exceeded that prevailing in the greenhouse during plant growth and acclimation prior to measurement).
The Cause of Wide Variation in WWR Parameters
Although we found strong effects of leaf type on most WWR parameters, there was large variation in those parameters, both among and within species. This variation could not be explained by differences in growing or measurement conditions. Some of this variation may relate to the mechanical advantage (m) of epidermal cells, which varies among and within species, the latter in relation to changing Pe (Franks et al., 1995, 1998; Franks and Farquhar, 2007). Technical challenges have limited measurements of m to a few species. We note that one would expect the qualitative effects of Pe-dependent changes in m to be consistent among replicate leaves from a given species; however, we found that WWR parameters often diverged even within species in this study, which suggests variation in factors other than m.
Notably, variation in WWR parameters has been reported previously. Powles et al. (2006) reported consistent differences in excision WWR size and duration between Photinia × fraseri individuals kept outdoors versus in a glasshouse in the days prior to measurement. They used a modeling analysis to attribute those effects to differences in the lag time preceding the guard cell hydroactive response. They also found that WWR size and length were positively correlated, as we did in this study. Those findings suggest that the time constant for relaxation of xylem-epidermis water potential gradients is correlated with the lag time preceding the guard cell hydroactive response (otherwise, longer WWRs would also be smaller). Thus, variation in WWR such as observed in these studies is consistent with variation in the hydraulic resistance of flow pathways proximal to the guard cells, including rbe and reg.
Some within-species variation in WWR kinetics may be genetic in origin. Sinclair et al. (2008) measured rleaf and excision responses in three genotypes of Glycine max, and one genotype had distinctively slower WWRs than the others. Interestingly, the “slow” genotype also displayed larger rleaf, a more nearly homeostatic transpiration rate at high Δw (larger Rh,rel), and failure of xylem hydration to mitigate either of those effects. Those results are consistent with our hypothesis that greater extravascular hydraulic resistance should slow stomatal movements after supply perturbations. They also suggest that extravascular resistance is substantial and can vary within species.
Differences in the Hydraulic Connection of the Epidermis to the Rest of the Leaf: Implications for Stomatal Control
WWRs were shorter in heterobaric leaves. This suggests that BSEs help reduce transient water loss after increases in evaporative demand. This may mitigate transient declines in leaf water potential and the associated risk of xylem cavitation, which would otherwise require further stomatal closure, limiting carbon gain. Thus, BSEs could enhance drought tolerance, which is consistent with their greater ecological distribution in high irradiance than in shade species (Kenzo et al., 2007).
Notably, not all land plants exhibit WWRs: they are apparently absent among extant ferns and lycophytes, which are also insensitive to abscisic acid (Brodribb and McAdam, 2011), a hormone known to induce stomatal closure in response to progressive soil drought in most seed plants (Davies et al., 1987; Raschke, 1987). This suggests that stomatal hydromechanics are fundamentally different in seedless plants and seed plants: whereas the former have strictly passive control of gs in relation to water status, seed plants require metabolic or other mechanisms to amplify passive changes in guard cell turgor to overcome the epidermal mechanical advantage (Buckley, 2005). Not surprisingly, then, guard cell osmoregulation in seed plants is highly complex and adaptable (Zeiger et al., 2002; Nilson and Assmann, 2007). Our finding that BSEs can affect stomatal dynamics, together with evidence that extravascular resistance is large and dynamic (Nardini et al., 2005; Sack et al., 2005; Cochard et al., 2007) and located partly in BSEs (Scoffoni et al., 2008), suggests that BSEs may contribute to the regulatory challenges posed by seed plants’ unique stomatal mechanics.
One likely role for a variable BSE resistance would be to reversibly amplify changes in epidermal water potential caused by changes in evaporative demand or water supply. “Apparent feed-forward” behavior of stomata (Franks et al., 1997), in which water loss actually declines at high evaporative demand, may be necessary for optimal stomatal control under certain conditions (Buckley, 2005). Feed-forward could result from increased hydraulic resistance (Oren et al., 1999; Buckley and Mott, 2002b; Buckley, 2005), perhaps via modulation of rbe. Variation in rbe could also help reconcile the observation that stomata respond independently to humidity in the two epidermes of amphistomatous leaves (Mott, 2007) with other data that showed strong hydraulic coupling of stomatal behavior over much greater distances (Heath and Russell, 1954; Schulze and Kuppers, 1979; Mott et al., 1997; Buckley and Mott, 2000). Clarity on this issue awaits further study on the phenomenology of rbe in relation to the external and leaf environment.
Differences in Steady-State Humidity Responses
This study also provided measurements of steady-state stomatal responses (RWRs) to reduced humidity. Relative RWR size (Rh,rel) did not differ significantly between heterobaric and homobaric leaves, but it was greater in woody than herbaceous species. This is consistent with an earlier study comparing humidity responses in six nonwoody and seven woody species (Franks and Farquhar, 1999). For a step change from Δwlow to Δwhigh in our model, Rh,rel is:
 |
where M is net epidermal mechanical advantage, χ is a turgor-to-conductance scaling factor, and R is the effective hydraulic resistance from the soil to the epidermis. (Eq. 2 is derived in Supplemental Data S3.) Thus, greater Rh,rel may reflect greater R, a more sensitive guard cell response (B), smaller mechanical advantage, or higher stomatal density (which largely determines χ) in woody plants. The latter is unlikely, as high χ also implies high initial gs, whereas we found lower g1 in woody than herbaceous plants. The model predicted greater Rh,rel in plants with greater rsx, rxb, rbe, and fe (all of which influence R) and greater B, confirming Equation 2, and smaller Rh,rel in plants with high πeo (data not shown). However, the effects of rsx and B were about 25 to 30 times greater than those of rbe and fe, and the next largest effects were 30 times smaller still, suggesting that RWRs in response to humidity are primarily affected by R and B. As whole plant resistance is generally greater in woody species (Turner et al., 1984; Mencuccini, 2003; Sack et al., 2003), we suspect that greater R is the most likely cause for larger RWRs in woody than herbaceous species.
CONCLUSION
We found that rates of stomatal opening during transient WWRs were greater in heterobaric than in homobaric leaves and that this difference was greater during excision than humidity responses. Theoretical analysis showed this pattern to be consistent with the hypothesis that BSEs reduce hydraulic resistance between the bundle sheath and epidermis (rbe). We estimated rbe to be on the order of 4 to 16 times greater in homobaric than heterobaric leaves by applying a theoretical model to our results; we estimated a similar range (5 to 24 times) from published measurements of effects of light on leaf hydraulic resistance in heterobaric versus homobaric leaves. Our results further support the view that BSEs substantially affect the hydraulic and stomatal functioning of leaves. Furthermore, we found stronger steady-state humidity responses in woody than herbaceous species, consistent with their greater hydraulic resistance. These findings indicate that the coupling of bulk leaf and xylem water status with the epidermis impacts the dynamics of stomatal responses to dry air and that leaf tissue variation can have strong impacts on these responses across species and life forms.
MATERIALS AND METHODS
Plant Material
Plants of diverse herbaceous and woody species (Table I) were obtained from local nurseries in Sonoma County, California, or grown from seed in the Sonoma State University Burr Greenhouse. Plants were acclimated in the greenhouse (64%–92% relative humidity and 21°C ± 3°C) at least 10 d prior to measurements. One healthy, young, fully expanded leaf from each plant was measured for stomatal responses for three plants per species, except Glycine max and Ligustrum japonicum, for which one leaf from each of two plants were measured. Herbaceous plants grown from seed were sown in 10-cm-square pots filled with soil:vermiculite:perlite (8:1:1) and provided slow-release fertilizer (Osmocote). Other plants were kept in the 4- or 20-L pots and soils in which they were purchased from nurseries. No plants were root bound.
Gas Exchange
Stomatal conductance was measured using an open-flow, single-pass gas-exchange system. Gases from tanks (Praxair) were mixed with mass flow controllers (FMA-5400 series; Omega) and partially humidified by bubbling through degassed, deionized water before injecting 1% CO2 in air from a tank (Praxair) with a mass flow controller. The gas stream was then split. Part passed through the reference cell of an infrared gas analyzer (IRGA; Li-Cor 7000; Li-Cor) and a chilled-mirror dewpoint hygrometer (General Eastern DEW-10; General Electric), and the remainder was passed through a mass flowmeter (FMA-2809; Omega) and a sealed chamber containing the leaf before entering the IRGA sample cell. Pressures were monitored with a bubbling manometer proximal to the IRGA and balanced by adjusting flows with valves.
The leaf chamber (of nickel-plated aluminum) used two high-speed fans to circulate air over the leaf, producing a boundary layer conductance of 0.85 mol air m−2 s−1. The chamber lid was a glass window (12.7 × 8.0 cm) in a high-density polyethylene frame. Leaf temperature was controlled by circulating water from a water bath through channels in the chamber body and monitored with two copper-constantan thermocouples appressed to the lower leaf surface. The photosynthetic photon flux density at the leaf surface was measured with a GaAsP photodiode (G1118; Hamamatsu) previously calibrated to a quantum sensor (LI-190; Li-Cor).
Experimental Protocol
Each experiment measured a humidity response followed by an excision response for the same leaf (Fig. 1). A plant was brought to the laboratory in the morning (7:00–8:30 am), and one healthy, young, fully expanded leaf was clamped in the chamber and allowed to equilibrate at 600 μmol m−2 s−1 photosynthetic photon flux density, 25°C leaf temperature, 15 mmol mol−1 leaf-air water vapor mole fraction difference (Δw; =52% relative humidity), and 370 μL L−1 ambient CO2 (ca). Stomatal conductance (gs) was logged every second. After gs reached a steady state (defined as 10 min without a directional trend), Δw was increased to 25 mmol mol−1 (=20% relative humidity) in a single step by adjusting the mixing ratio of humidified and dry air with mass flow controllers. After gs reached a new steady state, the leaf was excised at the petiole and gas exchange was logged for an additional 60 min.
Correction for Mixing and Desorption Lags
After humidity is reduced, mixing lags and vapor desorption can affect water vapor differential for a time. To correct for this, we measured time courses of IRGA output after a step reduction in humidity identical to that applied in leaf experiments with and without the leaf chamber in the sample line. We characterized the lag due to chamber mixing and desorption by comparing sample signals with and without the chamber and fit a two-phase exponential model to their difference between 1 and 5 min after the humidity change (r2 = 0.9987); that model showed a half-time of 9 s and a 95% time of 51 s. We characterized differences in sample versus reference line mixing lags by comparing sample and reference signals without the chamber and fit a second-order polynomial to their difference between 1 and 5 min after the change (r2 = 0.994; P < 0.0001). For each leaf experiment, we discarded data from less than 1 min after the Δw change and applied the corrections to measured water vapor differentials over the remaining time course.
Calculation of Parameters Describing Stomatal Responses
We quantified features of stomatal movements during WWRs and RWRs as described below and illustrated in Figure 1. For humidity responses, we recorded the initial value of gs (g1) at the time of the humidity change (t1), the time and value of gs at the peak of the WWR (t2 and g2), and the time at the end of the WWR (t3, defined as the time at which gs first dropped below g1; note that g3 ≡ g1). For excision responses, we recorded the gs value (g4) at the time of excision (t4), the time and gs value at the WWR peak (t5 and g5), and the time at the end of the WWR (t6). We calculated the absolute size of each WWR (W) as Wh = g2 − g1 for humidity and We = g5 − g4 for excision and the length of each WWR (L) as Lh = t2 − t1 and Le = t5 − t4. We calculated the rate of opening during each WWR (V) as Vh = Wh/Lh and Ve = We/Le. We also calculated the size of humidity RWRs (Rh) as Rh = g1 − g4; we did not attempt to quantify an RWR to excision. To normalize for differences in initial gs, we also calculated percentage relative sizes of WWRs and RWRs and opening rates (Wrel, Rrel, and Vrel) by dividing Wh, Rh, Vh, and Ve by g1 and We by g4 and multiplying by 100. Data for each trait were analyzed using a nested ANOVA, with species nested within heterobaric versus homobaric leaf type and nested within woody versus herbaceous life form (Minitab release 15). Data were incremented by 1 and log transformed before analyses to allow the inclusion of zero values and to increase normality and heteroscedasticity (Sokal and Rohlf, 1995). Additionally, as WWR parameters were linked with g1 (see “Results”), we determined whether BSEs and life form affected Ve and Vh independently of g1. We determined correlations of Ve and Vh on g1 and tested for differences in the residuals of those correlations using the nested ANOVAs (Sokal and Rohlf, 1995).
Dynamic Model of Water Flow and Stomatal Control
We simulated water flows, stomatal movements, and transpiration in leaves subjected to the experimental protocol described above using a physical model based on the conservation of mass in an explicit compartment/resistance network (Fig. 2). Complete details and parameter estimation are given in the Supplemental Data and briefly summarized here.
Figure 2.
Resistance/compartment diagram used to derive the dynamic model of water flow and stomatal control described in “Materials and Methods” and the Supplemental Data. The liquid phase resistances between the soil (s), xylem (x), bundle sheath and BSEs (b), epidermal (e), mesophyll (m), and stomatal guard cell (g) compartments are depicted, as are the fractional evaporation of water from the mesophyll, epidermis, and guard cells (arrows).
The model contains five state variables: symplastic volumes for four leaf water pools (mesophyll, epidermis, guard cells, and the bundle sheath, including BSEs in heterobaric species) and guard cell osmotic content (osmotic contents in other pools are assumed constant). The volumes determine turgor and osmotic pressures passively. Flows are calculated from water potential differences and resistances among pools. Each pool supports a fixed fraction of total evaporation (Table III), which is proportional to VPD and stomatal aperture. Aperture is a function of guard cell and epidermal turgor pressures. Xylem capacitance is assumed negligible, so xylem water potential is quasi-steady state with respect to other pools. The only active process is dynamic modulation of guard cell osmotic content to seek a “target” value of osmotic pressure (πg) proportional to epidermal turgor (Pe), which overcomes the epidermal mechanical advantage and causes a negative steady-state relationship between gs and VPD. WWRs arise because Pe necessarily declines before πg. The existence of this hypothesized “hydroactive feedback” is supported by a great deal of evidence (Losch and Schenk, 1978; Ehret and Boyer, 1979; Grantz and Zeiger, 1986; Buckley and Mott, 2002a, 2002b; Powles et al., 2006) and is the basis of previous models (Haefner et al., 1997; Buckley et al., 2003).
We derived a baseline set of parameter values (given in Table III) from the literature, for Vicia faba where possible, as described in Supplemental Data S4. V. faba is a homobaric species in our study and is common in the stomatal literature. The parameter that captures the hypothesized role of BSEs (rbe, bundle sheath-epidermis resistance) has not been directly measured. Therefore, we initially assumed rbe to be equal to the resistance from the bundle sheath to the mesophyll (rbm), and the baseline parameters represent a heterobaric leaf. However, we varied rbe among simulations to assess its potential role in stomatal dynamics.
We used this model to simulate our experimental protocol and assessed the sensitivity of predicted WWR kinetics to parameters in the model relative to a simulation using the baseline parameter set.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Correlations of opening rates with initial conductance.
Supplemental Figure S2. Residuals from correlations of opening rates with initial conductance.
Supplemental Table S1. Detailed wrong-way response parameters by species.
Supplemental Data S1. Description of the dynamic compartment/resistance model.
Supplemental Data S2. Derivation of Equation 1 in the text (rate of change of epidermal turgor pressure).
Supplemental Data S3. Derivation of Equation 2 in the text (relative size of steady-state humidity response).
Supplemental Data S4. Estimation of baseline parameter values used in the model.
Supplemental Data S5. Estimation of irradiance-dependent change in extravascular resistance in heterobaric leaves.
Supplementary Material
Acknowledgments
We thank undergraduate research students Bethany Calloway, John Kim, Nick Steele, Brandon Stevens, Ryan Stickney, Heather Vice, and Aimee Yates for assistance with data collection and Dan Crocker for statistical advice.
References
- Brodribb TJ, Holbrook NM. (2004) Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant Cell Environ 27: 820–827 [Google Scholar]
- Brodribb TJ, McAdam SAM. (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Brooks JR, Schulte PJ, Bond BJ, Coulombe R, Domec JC, Hinckley TM, McDowell N, Phillips N. (2003) Does foliage on the same branch compete for the same water? Experiments on Douglas-fir trees. Trees Struct Funct 17: 101–108 [Google Scholar]
- Buckley TN. (2005) The control of stomata by water balance. New Phytol 168: 275–292 [DOI] [PubMed] [Google Scholar]
- Buckley TN, Mott KA. (2000) Stomatal responses to non-local changes in PFD: evidence for long-distance hydraulic interactions. Plant Cell Environ 23: 301–309 [Google Scholar]
- Buckley TN, Mott KA. (2002a) Dynamics of stomatal water relations during the humidity response: implications of two hypothetical mechanisms. Plant Cell Environ 25: 407–419 [Google Scholar]
- Buckley TN, Mott KA. (2002b) Stomatal water relations and the control of hydraulic supply and demand. Prog Bot 63: 309–325 [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD. (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1785 [Google Scholar]
- Cochard H, Coll L, Le Roux X, Améglio T. (2002) Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol 128: 282–290 [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. (2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol 143: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock J, Mencuccini M. (1998) Control of stomatal conductance by leaf water potential in Hymenoclea salsola (T & G), a desert subshrub. Plant Cell Environ 21: 1029–1038 [Google Scholar]
- Cowan IR. (1972) Oscillations in stomatal conductance and plant functioning associated with stomatal conductance: observations and a model. Planta 106: 185–219 [DOI] [PubMed] [Google Scholar]
- Cunningham SC. (2004) Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Trees Struct Funct 18: 399–407 [DOI] [PubMed] [Google Scholar]
- Darwin F. (1898) Observations on stomata. Philos Trans R Soc Lond Ser B Biol Sci 190: 531–621 [Google Scholar]
- Davies WJ, Metcalfe JC, Schurr U, Taylor G, Zhang J. (1987) Hormones as chemical signals involved in root to shoot communication of effects of changes in soil environment. Hoad GV, Lenton JR, Jackson MB, Atkin R, eds, Hormone Action in Plant Development: A Critical Appraisal. Butterworths, London, pp 201–216 [Google Scholar]
- Davies WJ, Zhang JH. (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42: 55–76 [Google Scholar]
- Ehret DL, Boyer JS. (1979) Potassium loss from stomatal guard cells at low water potentials. J Exp Bot 30: 225–234 [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD. (1997) The apparent feedforward response of stomata to air vapour pressure deficit: information revealed by different experimental procedures with two rainforest trees. Plant Cell Environ 20: 142–145 [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD. (1998) A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ 21: 94–100 [Google Scholar]
- Franks PJ, Cowan IR, Tyerman SD, Cleary AL, Lloyd J, Farquhar GD. (1995) Guard cell pressure/aperture characteristics measured with the pressure probe. Plant Cell Environ 18: 795–800 [Google Scholar]
- Franks PJ, Farquhar GD. (1999) A relationship between humidity response, growth form and photosynthetic operating point in C3 plants. Plant Cell Environ 22: 1337–1349 [Google Scholar]
- Franks PJ, Farquhar GD. (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantz DA. (1990) Plant response to atmospheric humidity. Plant Cell Environ 13: 667–679 [Google Scholar]
- Grantz DA, Zeiger E. (1986) Stomatal responses to light and leaf-air water vapor pressure difference show similar kinetics in sugarcane and soybean. Plant Physiol 81: 865–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefner JW, Buckley TN, Mott KA. (1997) A spatially explicit model of patchy stomatal responses to humidity. Plant Cell Environ 20: 1087–1097 [Google Scholar]
- Heath OVS, Russell J. (1954) Studies in stomatal behaviour. VI. An investigation of the light responses of wheat stomata with the attempted elimination of control by the mesophyll. J Exp Bot 5: 1–15 [Google Scholar]
- Kenzo T, Ichie T, Watanabe Y, Hiromi T. (2007) Ecological distribution of homobaric and heterobaric leaves in tree species of Malaysian lowland tropical rainforest. Am J Bot 94: 764–775 [DOI] [PubMed] [Google Scholar]
- Liakoura V, Fotelli MN, Rennenberg H, Karabourniotis G. (2009) Should structure-function relations be considered separately for homobaric vs. heterobaric leaves? Am J Bot 96: 612–619 [DOI] [PubMed] [Google Scholar]
- Lo Gullo MA, Nardini A, Trifilò P, Salleo S. (2005) Diurnal and seasonal variations in leaf hydraulic conductance in evergreen and deciduous trees. Tree Physiol 25: 505–512 [DOI] [PubMed] [Google Scholar]
- Losch R, Schenk B. (1978) Humidity responses of stomata and the potassium content of guard cells. J Exp Bot 29: 781–787 [Google Scholar]
- Maier-Maercker U. (1983) The role of peristomatal transpiration in the mechanism of stomatal movement. Plant Cell Environ 6: 369–380 [Google Scholar]
- McClendon JH. (1992) Photographic survey of the occurrence of bundle-sheath extensions in deciduous dicots. Plant Physiol 99: 1677–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencuccini M. (2003) The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ 26: 163–182 [Google Scholar]
- Morison JIL, Lawson T, Cornic G. (2007) Lateral CO2 diffusion inside dicotyledonous leaves can be substantial: quantification in different light intensities. Plant Physiol 145: 680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA. (2007) Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant Cell Environ 30: 1444–1449 [DOI] [PubMed] [Google Scholar]
- Mott KA, Denne F, Powell J. (1997) Interactions among stomata in response to perturbations in humidity. Plant Cell Environ 20: 1098–1107 [Google Scholar]
- Mott KA, Franks PJ. (2001) The role of epidermal turgor in stomatal interactions following a local perturbation in humidity. Plant Cell Environ 24: 657–662 [Google Scholar]
- Mott KA, Parkhurst DF. (1991) Stomatal responses to humidity in air and helox. Plant Cell Environ 14: 509–515 [Google Scholar]
- Nardini A, Salleo S, Andri S. (2005) Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot). Plant Cell Environ 28: 750–759 [Google Scholar]
- Nardini A, Salleo S, Trifilo P, Lo Gullo MA. (2003) Water relations and hydraulic characteristics of three woody species co-occurring in the same habitat. Ann For Sci 60: 297–305 [Google Scholar]
- Niinemets U. (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82: 453–469 [Google Scholar]
- Nikolopoulos D, Liakopoulos G, Drossopoulos I, Karabourniotis G. (2002) The relationship between anatomy and photosynthetic performance of heterobaric leaves. Plant Physiol 129: 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM. (2007) The control of transpiration: insights from Arabidopsis. Plant Physiol 143: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schafer KVR. (1999) Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ 22: 1515–1526 [Google Scholar]
- Pataki DE, Oren R, Katul G, Sigmon J. (1998) Canopy conductance of Pinus taeda, Liquidambar styraciflua and Quercus phellos under varying atmospheric and soil water conditions. Tree Physiol 18: 307–315 [DOI] [PubMed] [Google Scholar]
- Peak D, Mott KA. (2011) A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ 34: 162–178 [DOI] [PubMed] [Google Scholar]
- Pepin S, Livingston NJ, Whitehead D. (2002) Responses of transpiration and photosynthesis to reversible changes in photosynthetic foliage area in western red cedar (Thuja plicata) seedlings. Tree Physiol 22: 363–371 [DOI] [PubMed] [Google Scholar]
- Powles JE, Buckley TN, Nicotra AB, Farquhar GD. (2006) Dynamics of stomatal water relations following leaf excision. Plant Cell Environ 29: 981–992 [DOI] [PubMed] [Google Scholar]
- Raschke K. (1987) Action of abscisic acid on guard cells. Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 253–279 [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. (2003) The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ 26: 1343–1356 [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. (2004) Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiol 134: 1824–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. (2005) Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol 167: 403–413 [DOI] [PubMed] [Google Scholar]
- Saito T, Terashima I. (2004) Reversible decreases in the bulk elastic modulus of mature leaves of deciduous Quercus species subjected to two drought treatments. Plant Cell Environ 27: 863–875 [Google Scholar]
- Saliendra NZ, Sperry JS, Comstock JP. (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula-occidentalis. Planta 196: 357–366 [Google Scholar]
- Schulze ED, Kuppers M. (1979) Short-term and long-term effects of plant water deficits on stomatal response to humidity in Corylus avellana L. Planta 146: 319–326 [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Pou A, Aasamaa K, Sack L. (2008) The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant Cell Environ 31: 1803–1812 [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook NM. (2008) Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiol Plant 132: 446–451 [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. (1995) Biometry: The Principles and Practice of Statistics in Biological Research, Ed 3. W.H. Freeman, New York [Google Scholar]
- Terashima I. (1992) Anatomy of nonuniform leaf photosynthesis. Photosynth Res 31: 195–212 [DOI] [PubMed] [Google Scholar]
- Turner NC, Spurway RA, Schulze ED. (1984) Comparison of water potentials measured by in situ psychrometry and pressure chamber in morphologically different species. Plant Physiol 74: 316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Yianoulis P. (1980) The site of water evaporation from sub-stomatal cavities, liquid path resistances and hydroactive stomatal closure. Ann Bot (Lond) 45: 175–193 [Google Scholar]
- Wylie RB. (1952) The bundle sheath extension in leaves of dicotyledons. Am J Bot 39: 645–651 [Google Scholar]
- Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu J. (2002) The guard cell chloroplast: a perspective for the twenty-first century. New Phytol 153: 415–424 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Brodribb TJ, Holbrook NM. (2007) Hydraulic design of leaves: insights from rehydration kinetics. Plant Cell Environ 30: 910–921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.