Abstract
Muscle protein wasting occurs in human immunodeficiency virus (HIV)-infected individuals and is often the initial indication of acquired immunodeficiency syndrome (AIDS). Little is known about the alterations in muscle protein metabolism that occur with HIV infection. Nine subjects with AIDS wasting (CD4 < 200/mm3), chronic stable opportunistic infections (OI), and ≥10% weight loss, fourteen HIV-infected men and one woman (CD4 > 200/mm3) without wasting or OI (asymptomatic), and six HIV-seronegative lean men (control) received a constant intravenous infusion of [1-13C]leucine (Leu) and [2-15N]glutamine (Gln). Plasma Leu and Gln rate of appearance (Ra), whole body Leu turnover, disposal and oxidation rates, and [13C]Leu incorporation rate into mixed muscle protein were assessed. Total body muscle mass/fat-free mass was greater in controls (53%) than in AIDS wasting (43%; P = 0.04). Fasting whole body proteolysis and synthesis rates were increased above control in the HIV+ asymptomatic group and in the AIDS-wasting group (P = 0.009). Whole body Leu oxidation rate was greater in the HIV+ asymptomatic group than in the control and AIDS-wasting groups (P < 0.05). Fasting mixed muscle protein synthesis rate was increased in the asymptomatic subjects (0.048%/h; P = 0.01) but was similar in AIDS-wasting and control subjects (0.035 vs. 0.037%/h). Plasma Gln Ra was increased in AIDS-wasting subjects but was similar in control and HIV+ asymptomatic subjects (P < 0.001). These findings suggest that AIDS wasting results from 1) a preferential reduction in muscle protein, 2) a failure to sustain an elevated rate of mixed muscle protein synthesis while whole body protein synthesis is increased, and 3) a significant increase in Gln release into the circulation, probably from muscle. Several interesting explanations for the increased Gln Ra in AIDS wasting exist.
Keywords: acquired immunodeficiency syndrome-wasting syndrome, immune cell function, amino acid metabolism, metabolic complications, stable isotopes, mass spectrometry, leucine, glutamine
Infection with the human immunodeficiency virus (HIV-1) typically progresses to involuntary weight loss characterized by a preferential reduction in lean tissue mass (8, 9, 15, 20). The cause of HIV-associated wasting is important to understand because weight loss contributes to reduced immunocompetence in acquired immunodeficiency syndrome (AIDS), and mortality from AIDS wasting is correlated with the magnitude of lean tissue depletion (8, 14, 20, 30). Clarifying the mechanism for AIDS wasting will advance the development of anticatabolic and anabolic interventions.
The pathogenesis of AIDS wasting, in particular muscle protein wasting, is unknown. Mulligan et al. (24) reported that resting energy expenditure is directly related to the amount of HIV-1 mRNA in the circulation (viral burden). This suggests that a high viral load may actuate substrate metabolism. However, the specific metabolic perturbations and/or dysregulations responsible for AIDS-associated muscle protein wasting are unknown, especially with respect to muscle amino acid metabolism.
Macallan et al. (19) reported that the rate of leucine release from whole body protein was increased in AIDS wasting in both fasting and fed subjects. Selberg et al. (32) studied HIV-infected subjects with weight loss and poor nutritional intake and found an increased rate of whole body protein turnover using the oral [15N]glycine approach, but HIV-seronegative controls were not studied. These reports suggest that the rate of whole body protein turnover is increased in AIDS wasting. The rates of muscle protein synthesis in HIV-infected, AIDS-without weight loss, and AIDS-wasting subjects were determined by McNurlan et al. (23) using the [2H]phenylalanine flooding-dose technique. They reported that the fractional rate of muscle protein synthesis was not different between seronegative control subjects and among the three groups of HIV-infected subjects. However, the absolute rate of muscle protein synthesis (g/day) was not reported. If AIDS wasting is characterized by a loss of total body muscle mass, the absolute rate of muscle protein synthesis should be reduced in AIDS wasting. We hypothesized that whole body proteolysis would be increased, whereas muscle mass and muscle protein synthesis rate would be decreased in AIDS wasting. A constant intravenous infusion of [1-13C]leucine was used to trace whole body protein metabolism because it is an essential amino acid, is not synthesized de novo, and has traditionally been used to determine the rate of muscle protein synthesis in vivo (2, 3, 25, 36–38).
Skeletal muscle plays a primary role in maintaining nitrogen homeostasis during critical illness by releasing glutamine into the circulation. Whether this is true in AIDS wasting is unknown. Glutamine is the most abundant amino acid in the circulation and skeletal muscle free amino acid pool (10, 16). It is a primary transporter of amino nitrogen from skeletal muscle to other tissues as a precursor for urinary ammonia, nucleic acid, cyclic nucleotide synthesis, as an oxidative fuel and as a constituent of cellular proteins (16). About 70% of plasma glutamine rate of appearance (Ra) derives from skeletal muscle glutamine release (29), and about 40% of plasma glutamine Ra is due to direct release of glutamine from muscle protein breakdown (6, 10, 21, 27, 29). Plasma glutamine Ra reflects the rate of release of glutamine from protein (particularly muscle and liver) and the rate of de novo glutamine synthesis from other amino acid precursors, a portion of which are released during muscle proteolysis (29). Skeletal muscle glutamine release increases after trauma, surgery, sepsis, and catabolic hormone excess (4, 10, 16). In addition, glutamine is essential for the normal function of rapidly proliferating cells of the immune system (e.g., lymphocytes, macrophages, enterocytes, and thymocytes; Ref. 4). In HIV infection, the CD4 T lymphocytes become infected at a rapid rate (108–109 cells/day). CD4 and CD8 T lymphocytes must proliferate at an equally rapid rate to defend against invading pathogens and to replace infected CD4 T cells. Glutamine is a primary substrate for these T cells (16, 26–28). On the basis of these observations, we hypothesized that plasma glutamine Ra would be elevated in AIDS wasting.
We have determined plasma leucine turnover rate and glutamine Ra in subjects with AIDS wasting, HIV-infected subjects without wasting, and HIV-seronegative control subjects. We found a disproportionate loss of muscle mass, a higher plasma glutamine Ra, and an inappropriately low rate of muscle protein synthesis in subjects with AIDS wasting.
METHODS
Subjects
HIV-infected volunteers were recruited through the AIDS Clinical Trials Unit at Washington University School of Medicine and the Infectious Disease Clinic at Barnes Hospital (Table 1). All HIV-infected subjects were treated with at least one standard antiretroviral agent [azidothymidine (AZT or zidovudine), dideoxyinosine, dideoxycytidine, stavudine, and/or lamivudine] and were clinically stable on this therapy for at least 1 mo before study. On the basis of the severity of disease and wasting, the HIV-infected subjects were assigned to one of two groups: 1) asymptomatic subjects with <10% body weight loss within the previous year, CD4 lymphocyte counts >200 cells/mm3 (range 204–634 cells/mm3) measured within 30 days of study, and no history of opportunistic infection (n = 14 men, 1 woman), and 2) symptomatic subjects with involuntary, unexplained weight loss of at least 10% body weight within the previous year and CD4 lymphocyte counts <200 cells/mm3 (0–174 cells/mm3). All of these men (n = 9) had <2 watery stools/day, suggesting that malabsorption and gastrointestinal dysfunction were not the cause of wasting. All of these men had an active, stable, non-life-threatening AIDS-defining opportunistic infection (mycobacterium avium complex, cytomegalovirus retinitis, or esophagitis) at the time of study. Six healthy, HIV-seronegative (confirmed by ELISA and HIV antibody test) control subjects, matched for the body mass index of the asymptomatic subjects, were also studied as a comparison group. None of the subjects were taking anabolic agents (testosterone, dihydroepiandosterone, or growth hormone) or appetite stimulants (Megace or marinol) for at least 1 mo before study. The Human Studies Committee at Washington University School of Medicine approved the study, and all subjects provided informed consent before participating.
Table 1.
Descriptive characteristics
| Group | n | Age, yr | Ht, cm | Wt, kg | Body Mass Index, kg/m2 | Fat-Free Mass, kg | Muscle Mass, kg | CD4 Count, cells/mm3 | Years Known HIV+ |
|---|---|---|---|---|---|---|---|---|---|
| HIV seronegative | 6 | 30±3 | 182±2 | 73.6±3 | 22±1 | 58.6±1.8 | 31±1 | NA | NA |
| HIV+ asymptomatic | 15 | 37±2 | 174±2 | 70.6±4 | 23±1 | 52.0±2.7 | 27±2 | 426±37 | 5±1 |
| AIDS wasting | 9 | 40±2 | 181±2 | 63.4±3 | 19±1 | 52.9±1.5 | 23±2* | 78±34† | 7±1 |
Values are means ± SE. NA, not applicable.
P = 0.04 vs. control.
P < 0.001 vs. HIV+ asymptomatic.
Dietary control
Subjects were admitted to the General Clinical Research Center (GCRC) for a 48-h period. During this time, they consumed a flesh-free diet containing defined amounts of protein and calories. Flesh-free meals were employed to reduce the effects of dietary creatinine on 24-h urinary creatinine excretion measurements used to estimate whole body muscle mass (35). Before admission, a research dietitian surveyed each participant’s typical eating habits and designed the 2-day meal plan to provide 1.5 g protein · kg−1 · day−1 and 130–190 kJ (31–45 kcal) · kg body wt−1 · day−1. Of the total calories, ~20% derived from protein, ~50% from carbohydrate, and 30% from fat. The controlled intake did not differ in composition from the subject’s habitual intake (Table 2). Diet survey analyses of habitual intake indicated that the subjects in the three groups consumed similar and adequate amounts of macronutrients before study (Table 2). The meals were prepared in the Research Kitchen and served to the subjects on the GCRC. The subjects were instructed to eat all the food provided and no other food. Any small amount not consumed was weighed, and the daily intake record was corrected accordingly.
Table 2.
Participants’ habitual dietary intake
| Group | Dietary Intake, kcal · kg body wt−1 · day−1
|
||||
|---|---|---|---|---|---|
| Total | Protein | Carbohydrate | Fat | Alcohol | |
| HIV seronegative | 42±1 | 9.0±0.8 | 18±1 | 15±1 | 0.0±0.0 |
| HIV+ asymptomatic | 44±4 | 8.5±0.7 | 22±3 | 13±2 | 0.4±0.4 |
| AIDS wasting | 42±7 | 9.8±1.7 | 20±3 | 12±3 | 0.6±0.6 |
Values are means ± SE. No significant differences in habitual intake were observed between groups.
Whole body glutamine and leucine rates of appearance and muscle protein synthesis rate
After the evening meal on day 2 (at 1800), whole body and skeletal muscle amino acid kinetics were determined during an overnight (14-h) intravenous infusion of [1-13C]leucine and [2-15N]glutamine. The plasma glutamine Ra was determined using a constant intravenous infusion of [2-15N]glutamine (6.8 μmol · kg−1 · h−1, ~99 atom%; MassTrace, Woburn, MA) (6, 21, 29). A primed (7.58 μmol/kg) constant intravenous infusion (7.58 μmol · kg−1 · h−1) of [1-13C]leucine (~99 atom%; MassTrace) was used to estimate the rates of whole body protein breakdown, synthesis, and amino acid oxidation, using the reciprocal pool approach (2, 3, 22, 25, 31, 37), and to determine the in vivo rate of incorporation of [13C]leucine into skeletal muscle protein (2, 25, 36–38). Whole body and skeletal muscle amino acid kinetics were measured during an overnight fast (1900–0800).
In blood samples taken before and at 30-min intervals during the last 2.5 h of the amino acid tracer infusion, plasma α-ketoisocaproic acid (α-KIC) was isolated, derivatized (33), and analyzed by gas chromatography (GC)-electron impact-mass spectrometry (MS) to determine 13C enrichment. These measurements were performed on a Hewlett-Packard 5940 GC/MS system with selected ion monitoring of ions at m/z 232 and 233 at the appropriate retention times for the KIC-QTMS derivative (25, 31, 36–38). The plasma [α-13C]KIC enrichment value was used to calculate the rate of whole body protein breakdown (2, 3, 20, 22, 37). This value was also used as the precursor pool enrichment for the calculation of the fractional rate of mixed muscle protein synthesis (25, 33, 36–38).
In the same blood samples, plasma glutamine was isolated by using a modification of a described anion-exchange chromatographic method (5). [U-13C5]glutamine was added to the plasma before isolation and used as an internal standard for the quantification of plasma glutamine concentration (Cambridge Isotope Laboratories, Andover, MA). The glutamine was converted to the heptafluorobutyrylpropyl ester derivative and analyzed by GC-MS in negative ion chemical ionization mode. 15N enrichment was determined by selected ion monitoring of ions at m/z 407 and 408, and glutamine concentration was determined by monitoring ions at m/z 407 and 412 at the appropriate retention time.
Exhaled breath samples were collected into 20-ml evacuated tubes (Becton-Dickinson Vacutainer) before and at the end of the amino acid tracer infusion. Breath samples were analyzed for 13CO2 enrichment (m/z 45 and 44 ions) by use of isotope ratio MS (IRMS). The 13C enrichment values were used in conjunction with 15-min measurements of CO2 production (ml/min) made at the same time points with a Delta-Trac indirect calorimeter (Sensormedics, Yorba Linda, CA) to determine the rate of whole body leucine oxidation (2, 22). The difference between the measured whole body protein breakdown rate and leucine oxidation rate is the nonoxidative disposal rate of leucine, which represents the whole body protein synthesis rate (3, 22, 37).
To determine the in vivo rate of incorporation of leucine into mixed muscle protein, muscle protein [13C]leucine enrichment was measured using GC-combustion-IRMS (38) on two samples (~10–20 mg wet wt) removed from the vastus lateralis muscle. One sample was removed 90 min after the tracer amino acid infusion began, and a second muscle sample was removed from the contralateral vastus lateralis at the end of the infusion (14 h). Samples were prepared for GC-combustion-IRMS analysis as described (38).
Body composition
After an overnight fast, body fat and fat-free mass (FFM) were determined using a Hologic QDR-2000 enhanced-array whole body dual-energy X-ray absorptiometer (Waltham, MA). Whole body images were processed using Hologic software (version 5.64A). Estimates of total body muscle mass were derived by averaging two 24-h urine creatinine excretion values (35). Urinary creatinine was determined colorimetrically using a commercially available automated analyzer (Kodak Ektachem 700XR).
Statistics
All parameters were compared in the three groups by ANOVA. When this indicated a significant difference (P < 0.05), a Student-Newman-Keuls post hoc test was used to identify which of the three groups differed. Means ± SE are reported.
RESULTS
Body composition
Total body muscle mass was disproportionately low in the AIDS-wasting group (23 ± 2 kg; 36% body weight and 43% of FFM; Table 1). Muscle mass was higher in the HIV+ asymptomatic group (27 ± 2 kg; 38% body weight and 52% FFM), and highest in the HIV-seronegative control group (31 ± 1 kg; 42% body weight and 53% FFM; P = 0.04). When expressed per kilogram of FFM, there was a preferential loss of total body muscle mass in the AIDS-wasting group. There was a significant correlation between total body muscle mass and FFM (P < 0.002, r = 0.72). By convention, we expressed all amino acid kinetic parameters per kilogram of FFM. Expressing them per kilogram of total body muscle mass augmented the group differences observed.
Glutamine kinetics
The plasma glutamine Ra was higher in the AIDS-wasting group than in the other two groups (P < 0.001; Fig. 1). Plasma glutamine Ra was similar in HIV-seronegative controls and HIV+ asymptomatic subjects and within the normal range reported by others using a [15N]glutamine tracer (5, 6, 29). During the final 2 h of the 14-h infusion, the [2-15N]glutamine tracer had equilibrated in the plasma pool, and a plateau [15N]glutamine enrichment (mol% excess) in the plasma was achieved (Table 3). The coefficients of variation for the [15N]glutamine enrichments determined during the final 2 h of the infusion were 4.8, 2.8, and 5.6% for the control, HIV+ asymptomatic, and AIDS-wasting groups, respectively. Plasma glutamine concentration tended to be greater in the AIDS-wasting group (385 ± 29 μM) and HIV+ asymptomatic group (407 ± 43 μM) than in the HIV-seronegative controls (350 ± 31 μM; P > 0.05, NS).
Fig. 1.
Plasma glutamine rate of appearance [Ra, μmol glutamine · kg fat-free mass (FFM)−1 · h−1] in AIDS-wasting, HIV+ asymptomatic, and HIV-seronegative control subjects. ○, Individual subjects. *P < 0.001 vs. HIV+ asymptomatic and seronegative control groups.
Table 3.
Plasma [15N]glutamine enrichment during final 2 h of [2-15N]glutamine infusion
| Group | [15N]glutamine Enrichment, mol % excess
|
||||
|---|---|---|---|---|---|
| 12 h | 12.5 h | 13 h | 13.5 h | 14 h | |
| HIV seronegative | 2.28±0.15 | 2.60±0.22 | 2.50±0.21 | 2.48±0.17 | 2.51±0.27 |
| HIV+ asymptomatic | 2.73±0.15 | 2.83±0.13 | 2.88±0.16 | 2.69±0.15 | 2.73±0.10 |
| AIDS wasting | 1.67±0.13 | 1.56±0.17 | 1.79±0.13 | 1.78±0.08 | 1.76±0.14 |
Values are means ± SE.
Leucine kinetics
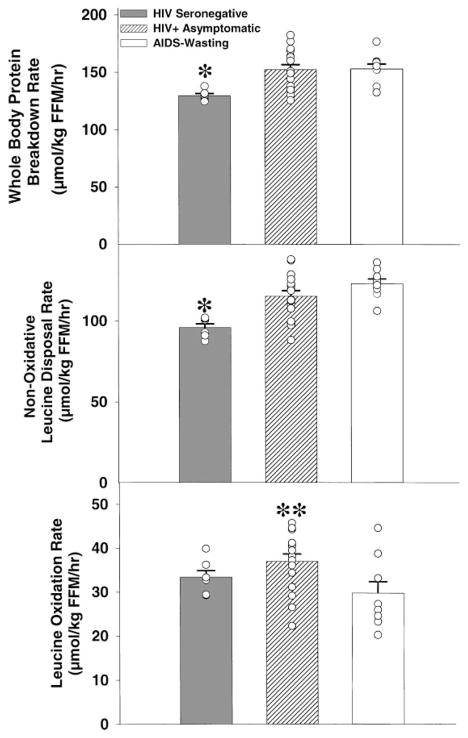
The whole body protein breakdown rate and nonoxidative disposal rate of leucine were greater (P = 0.009) in the HIV-infected subjects than in control subjects (Fig. 2). Similar findings have been reported by Macallan et al. (19). The rate of [13C] leucine oxidation was greater in the HIV+ asymptomatic group (P < 0.05) than it was in the AIDS-wasting and control groups. These kinetic measurements were made during an overnight fast, so whole body net leucine balance (synthesis minus breakdown) was negative in all three groups but most negative in the HIV+ asymptomatic group (−37 ± 1.7 vs. −30 ± 2.6 μmol · kg FFM−1 · h−1 in AIDS wasting; P < 0.05).
Fig. 2.
Fasting whole body leucine turnover rate (μmol leucine · kg FFM−1 · h−1) in AIDS-wasting, HIV+ asymptomatic, and HIV-seronegative control subjects. Whole body protein breakdown rate and nonoxidative leucine disposal rate (whole body protein synthesis rate) were elevated in HIV+ subjects compared with control. *P = 0.009 vs. HIV+ and AIDS groups. Leucine oxidation rate was greater in HIV+ asymptomatic. **P < 0.05 vs. AIDS wasting).
The fractional rate of mixed muscle protein synthesis was greater (P = 0.01) in the HIV+ asymptomatic group than in the AIDS-wasting group and the control group (0.048 ± 0.003%/h; Fig. 3). The fractional rate of mixed muscle protein synthesis was similar in the control and AIDS-wasting subjects (AIDS wasting = 0.035 ± 0.004%/h; control = 0.037 ± 0.002%/h). Compared with previous studies that used the [1-13C]leucine constant-infusion technique (2, 25, 33, 38), the rate of mixed muscle protein synthesis in the control group was on the low end of the normal range. This may be due to the change in diet imposed by the controlled-protein, flesh-free diet, or the 2 days of physical inactivity imposed by the GCRC admission. Regardless, the HIV-seronegative group was included in the study design to control for these effects, so that comparisons between these controls and the HIV-infected subjects are valid.
Fig. 3.

Fractional rate of mixed skeletal muscle protein synthesis in AIDS-wasting, HIV+ asymptomatic, and HIV-seronegative control subjects. *P = 0.01 vs. HIV-seronegative and AIDS-wasting groups.
The absolute rate of mixed muscle protein synthesis (g protein/h) was calculated from whole body muscle mass, the fractional rate of synthesis, and the assumption that muscle is 19% protein (2, 25). The absolute rate of mixed muscle protein synthesis was greater in the HIV+ asymptomatic group (2.4 ± 0.2 g protein/h) than in the AIDS-wasting group (1.5 ± 0.2 g protein/h; P = 0.01) and similar to that in the control group (2.2 ± 0.2 g protein/h). When the absolute rate of mixed muscle protein synthesis was expressed as a percentage of the whole body protein synthesis rate, this percentage was within the normal range (2, 25) in control (24 ± 2%) and HIV+ asymptomatic subjects (25 ± 2%) but was decreased in the AIDS-wasting subjects (15 ± 1%; P < 0.001). This indicates that muscle protein synthesis contributed less to the elevated rate of whole body protein synthesis in the AIDS-wasting subjects. Conversely, the synthesis of other body proteins (e.g., liver, enterocytes, and lymphocytes) must have contributed more to the increased rate of whole body protein synthesis in the AIDS-wasting group.
DISCUSSION
Our findings suggest the following. 1) AIDS wasting is associated with a preferential reduction in total body muscle mass. In the AIDS-wasting group, muscle mass represented a disproportionately small percentage of FFM and body weight than in the control group (Table 1). 2) HIV infection accelerated the rates of whole body proteolysis and synthesis when examined after an overnight fast and compared with HIV-seronegative subjects. This indicates that some body proteins are synthesized and some degraded at faster rates in HIV-infected subjects. This agrees with earlier reports and suggests that whole body protein turnover is accelerated in HIV-infected subjects when measured in the overnight-fasted condition (19, 20, 32). The increased rate of whole body leucine turnover requires additional energy expenditure and supports the finding that HIV infection is associated with a hypermetabolic state (24), especially with respect to amino acid metabolism. 3) The increased plasma glutamine Ra in AIDS wasting may reflect an increased rate of de novo glutamine synthesis in muscle and liver. This is an energy-requiring process and may contribute to the hypermetabolic condition.
These findings suggest that muscle amino acid kinetics may be different in patients with AIDS wasting and HIV+ asymptomatic subjects. In AIDS wasting, fasting whole body leucine balance was similar to that of HIV-seronegative subjects, but muscle protein synthesis contributed less to the rate of whole body protein synthesis. This may indicate that more amino acids are utilized for the synthesis of other proteins in the whole body and fewer are available for the synthesis of muscle protein. In HIV+ asymptomatic subjects, muscle protein synthesis rate was elevated and represented a normal proportion of the whole body protein synthesis rate. In this group, a tendency to lose muscle protein may be associated with the increased rate of whole body leucine oxidation. This may result in a more negative whole body leucine balance (Fig. 2).
The measurement of whole body leucine turnover rate using the [13C]leucine constant-infusion method reflects amino acid metabolism in all proteins in the body (2, 3, 22, 37), not one protein in particular. To better understand muscle amino acid metabolism in HIV disease, we examined in vivo amino acid incorporation into skeletal muscle protein. We found that the fractional rate of mixed muscle protein synthesis was greater in HIV+ asymptomatic subjects than in HIV-seronegative controls and AIDS-wasting subjects. Likewise, the absolute rate of muscle protein synthesis was greater in the HIV+ asymptomatic subjects than in the AIDS-wasting subjects. The increased rate of muscle protein synthesis may protect the HIV+ asymptomatic subjects from amino acid losses that may occur through an increased amino acid oxidation rate. The AIDS-wasting subjects appear to have a reduced capacity to utilize amino acids in muscle protein synthetic pathways. Instead, other rapidly synthesized proteins (e.g., immune cells, enterocytes, and acute-phase proteins) may utilize circulating amino acids at the expense of muscle protein. Taken collectively, the disproportionate reduction in total body muscle mass and the reduced rate of muscle protein synthesis relative to whole body protein synthesis suggest that AIDS muscle protein wasting results from an inability to sustain an elevated rate of muscle protein synthesis when whole body proteolysis and protein synthesis are increased.
These findings do not support an earlier study that reported reduced rates of whole body protein breakdown and synthesis in subjects infected with HIV (34). This discrepancy may be explained by the use of a different amino acid tracer methodology. The subjects received an oral [15N]glycine bolus dose with Ensure, and urine 15N excretion was measured while small quantities of Ensure were consumed throughout the 9-h study. The HIV-infected subjects’ dietary intake before this quasi-fed metabolic study was not controlled, and this may have influenced the measured rates of whole body protein metabolism. Not all of the HIV-infected subjects in the earlier studies were receiving antiretroviral therapy (i.e., AZT). The influence of antiviral medications on human muscle amino acid metabolism has not been reported. The possibility exists that these nucleoside analogs block mitochondrial DNA synthesis and uncouple muscle mitochondrial respiration, causing a myopathy similar to that characterized in AZT-treated rodents (17, 18).
We found that the fractional and absolute rates of muscle protein synthesis were decreased in AIDS-wasting subjects compared with HIV+ asymptomatic subjects. This does not agree with a recent report that HIV-seronegative control, HIV-infected, AIDS-no weight loss, and AIDS-wasting subjects have a similar rate of muscle protein synthesis measured by the amino acid flooding-dose technique. In this study, a large amount of [2H]phenylalanine (45 mg/kg) and unenriched phenylalanine was administered over a 10-min period, and the amount of [2H]phenylalanine incorporated into mixed muscle protein was determined 90 min later. Typically, the flooding-dose technique results in measured values for muscle protein synthesis rates that are approximately two times higher than the constant-infusion approach (33). As expected, our constant-infusion measurements of muscle protein synthesis rate in HIV-infected subjects are one-half those reported by McNurlan et al. (23). This, as well as imposed changes in dietary and physical activity patterns, may explain why we found differences in muscle protein synthesis not previously observed in HIV-infected subjects (23).
Muscle protein synthesis rate was determined by incorporation of [1-13C]leucine into a mixture of structural, contractile, mitochondrial, and enzymatic proteins in the muscle sample. Even though contractile proteins constitute 70–75% of the mixed muscle proteins, it is of interest to isolate individual muscle proteins (e.g., mitochondrial, sarcoplasmic, myosin heavy chain, and actin) from the muscle specimens and determine [13C]leucine incorporation into individual proteins with different synthesis rates (2). The expression of individual muscle proteins is regulated differently, and it is possible that they are affected differently in AIDS wasting. Identifying the muscle protein(s) most affected in AIDS wasting will assist in designing appropriate and efficacious anabolic and anticatabolic interventions.
It is important to note that we did not directly determine the rate of muscle proteolysis in the current study. The balance between the rates of muscle protein synthesis and proteolysis accounts for the magnitude of muscle protein wasting. A direct measure of muscle proteolysis would require the determination of amino acid balance (e.g., phenylalanine, tyrosine, and 3-meth-ylhistidine) across a large muscle group (e.g., thigh muscles) using arterial and venous cannulation and blood flow rate determinations. This procedure would have adversely affected subject recruitment into the current study.
The plasma leucine and glutamine kinetic measures were made after an overnight fast. In AIDS wasting, the disproportionately low muscle protein synthesis rate and increased rate of whole body leucine turnover may reflect a lack of sufficient amino acid availability to support muscle protein synthesis. Although unprecedented, proteolytic processes may be amplified by fasting in HIV-infected individuals more than they are in seronegative controls. We have not addressed the possibility that AIDS muscle wasting results from an inadequate increase in the rate of muscle protein synthesis during feeding. Experiments to determine how the rate of muscle protein synthesis is affected by feeding in AIDS wasting are of interest.
The reasons for the increased plasma glutamine Ra in AIDS wasting are unclear. We have previously reported that plasma glutamine-glutamate concentration in AIDS patients is not greater than in HIV-seronegative controls (13). We have confirmed this observation in the current study using an isotope-dilution MS determination of plasma glutamine concentration. Because glutamine did not accumulate in the circulation, glutamine uptake by some other tissue(s) must be increased to match the elevated plasma glutamine Ra in AIDS wasting. Possibilities include the following: 1) to provide glutamine as a fuel for colonocytes, enterocytes, and mucosal cells of the intestinal tract to maintain structural integrity, absorptive function and smooth muscle protein synthesis (10, 21) (In our studies, volunteers with obvious gastrointestinal dysfunction were excluded, but subclinical enteropathies might have existed); 2) to transfer excess nitrogen formed by proteolysis to the liver for urea synthesis to prevent excessive ammoniagenesis (6, 10, 21, 29); 3) to provide a gluconeogenic substrate to the kidneys (29) or to facilitate ammonia excretion to maintain acid-base balance (16); 4) to provide glutamine for hepatic glutathione synthesis to replenish levels that may be depleted by chronic illness [Several (7, 11, 12) but not all investigations (1) have supported administration of the antioxidant and glutathione precursor N-acetylcysteine to reduce oxidative damage in HIV disease]; and 5) to provide a substrate or a precursor for nucleic acid synthesis in quiescent and activated, rapidly proliferating T and B lymphocytes and macrophages known to avidly utilize glutamine (4, 26–28). Newsholme et al. (26–28) have reported that activated human lymphocytes have a 50% greater rate of utilization of glutamine than quiescent human lymphocytes in vitro. We found a 25% greater plasma glutamine Ra in AIDS-wasting subjects with advanced disease and 0–174 CD4 cells/mm3. It is conceivable that rapidly proliferating lymphocytes and macrophages utilize a portion of the elevated glutamine Ra in AIDS wasting. These possibilities need further investigation.
In summary, our findings suggest that AIDS wasting is associated with a preferential and disproportionate reduction in muscle mass. This may be caused by 1) a failure to sustain an increased rate of mixed muscle protein synthesis while whole body protein turnover is increased, 2) a dysregulation of endogenous amino acid metabolism away from muscle protein synthetic pathways and toward other pathways of amino acid metabolism (oxidation), and 3) a significant increase in glutamine release into the circulation. This may reduce the rate of muscle protein synthesis. The increased plasma glutamine Ra may reflect muscle injury and accelerated protein degradation. It may also reflect a regulated process whereby de novo synthesis of glutamine is increased to satisfy an elevated glutamine requirement by other tissues. These findings may indicate that glutamine is a conditionally essential nutrient in people living with HIV or AIDS.
Acknowledgments
We thank the study participants for their tolerance and altruism. Peggy Visio was the research dietician. Janet Voorhees was the nurse coordinator.
This work was supported by National Institutes of Health Grants DK-49393, RR-00954, RR-00036, and AI-25903 (Washington University AIDS Clinical Trials Unit).
References
- 1.Aillet F, Gougerot-Pocidalo MA, Virelizier JL, Israel N. Appraisal of potential therapeutic index of antioxidants on the basis of their in vitro effects on HIV replication in monocytes and interleukin-2-induced lymphocyte proliferation. AIDS Res Hum Retrovirol. 1994;10:405–411. doi: 10.1089/aid.1994.10.405. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal P, Ljungqvist O, Nair KS. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans. Am J Physiol. 272 doi: 10.1152/ajpendo.1997.272.1.E45. [DOI] [PubMed] [Google Scholar]; Endocrinol Metab. 1997;35:E45–E50. [Google Scholar]
- 3.Bier DM. Intrinsically difficult problems: the kinetics of body proteins and amino acids in man. Diabet Metab Rev. 1989;5:111–132. doi: 10.1002/dmr.5610050203. [DOI] [PubMed] [Google Scholar]
- 4.Calder PC. Glutamine and the immune system. Clin Nutr. 1994;13:2–8. doi: 10.1016/0261-5614(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 5.Darmaun D, Manary MJ, Matthews DE. A method for measuring both glutamine and glutamate levels and stable isotope enrichments. Anal Biochem. 1985;147:92–102. doi: 10.1016/0003-2697(85)90013-2. [DOI] [PubMed] [Google Scholar]
- 6.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol. 251 doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]; Endocrinol Metab. 1986;14:E117–E126. [Google Scholar]
- 7.Droge W. Cysteine and glutathione deficiency in AIDS patients: a rationale for the treatment with N-acetyl-cysteine. Pharmacology. 1993;46:61–65. doi: 10.1159/000139029. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld C. What causes wasting in AIDS? N Engl J Med. 1995;333:123–124. doi: 10.1056/NEJM199507133330210. [DOI] [PubMed] [Google Scholar]
- 9.Grunfeld C, Feingold KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- 10.Hall JC, Heel K, McCauley R. Glutamine. Br J Surg. 1996;83:305–312. doi: 10.1002/bjs.1800830306. [DOI] [PubMed] [Google Scholar]
- 11.Hammarqvist F, Luo JL, Cotgreave IA, Andersson K, Wernerman J. Skeletal muscle glutathione is depleted in critically ill patients. Crit Care Med. 1997;25:78–84. doi: 10.1097/00003246-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Herzenberg LA, Derosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortin GL, Landt M, Powderly WG. Changes in plasma amino acid concentrations in response to HIV-1 infection. Clin Chem. 1994;40:785–789. [PubMed] [Google Scholar]
- 14.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 15.Kotler DP, Wang J, Pierson RN., Jr Body composition in patients with the acquired immunodeficiency syndrome. Am J Clin Nutr. 1985;42:1255–1265. doi: 10.1093/ajcn/42.6.1255. [DOI] [PubMed] [Google Scholar]
- 16.Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48:297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nature Med. 1995;1:417–422. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 18.Lewis W, Gonzalez B, Chomyn A, Papoian T. Zidovudine induces molecular, biochemical, and ultrastructural changes in rat skeletal muscle mitochondria. J Clin Invest. 1992;89:1354–1360. doi: 10.1172/JCI115722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macallan DC, McNurlan MA, Milne E, Calder AG, Garlick PJ, Griffin GE. Whole-body protein turnover from leucine kinetics and the response to nutrition in human immunodeficiency virus infection. Am J Clin Nutr. 1995;61:818–826. doi: 10.1093/ajcn/61.4.818. [DOI] [PubMed] [Google Scholar]
- 20.Macallan DC, Noble C, Baldwin C, Foskett M, McManus T, Griffin GE. Prospective analysis of patterns of weight change in stage IV human immunodeficiency virus infection. Am J Clin Nutr. 1993;58:417–424. doi: 10.1093/ajcn/58.3.417. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DE, Campbell G. The effect of dietary protein intake on glutamine and glutamate nitrogen metabolism in humans. Am J Clin Nutr. 1992;55:963–970. doi: 10.1093/ajcn/55.5.963. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[13C]leucine. Am J Physiol. 238 doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]; Endocrinol Metab. 1980;1:E473–E479. [Google Scholar]
- 23.McNurlan MA, Garlick PJ, Steigbigel RT, DeCristofaro KA, Frost RA, Lang CH, Johnson RW, Santasier AM, Cabahug CJ, Fuhrer J, Gelato MC. Responsiveness of muscle protein synthesis to growth hormone administration in HIV-infected individuals declines with severity of disease. J Clin Invest. 1997;100:2125–2132. doi: 10.1172/JCI119747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulligan K, V, Tai W, Schambelan M. Energy expenditure in human immunodeficiency virus infection. N Engl J Med. 1997;336:70–71. doi: 10.1056/NEJM199701023360115. [DOI] [PubMed] [Google Scholar]
- 25.Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 254 doi: 10.1152/ajpendo.1988.254.2.E208. [DOI] [PubMed] [Google Scholar]; Endocrinol Metab. 1988;17:E208–E213. [Google Scholar]
- 26.Newsholme EA. The possible role of glutamine in some cells of the immune system and the possible consequence for the whole animal. Experientia. 1996;52:455–459. doi: 10.1007/BF01919315. [DOI] [PubMed] [Google Scholar]
- 27.Newsholme EA, Parry-Billings M. Properties of glutamine release from muscle and its importance for the immune system. J Parenter Enter Nutr. 1990;14(Suppl):63S–67S. doi: 10.1177/014860719001400406. [DOI] [PubMed] [Google Scholar]
- 28.Newsholme P, Costa Rosa LFBP, Newsholme EA, Curi R. The importance of fuel metabolism to macrophage function. Cell Biochem Function. 1996;14:1–10. doi: 10.1002/cbf.644. [DOI] [PubMed] [Google Scholar]
- 29.Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Glutamine: a major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J Clin Invest. 1995;95:272–277. doi: 10.1172/JCI117651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palenicek JP, Graham NMH, He YD, Hoover DA, Oishi JS, Kingsley L, Saah AJ, Armenian H, Farzadegan H, Kass N, Margolick J, McArthur J, Palenicek J, Taylor E, Phair JP, Chmiel JS, Cohen B, Ogorman M, Murphy R, Variakojis D, Wesch J, Wolinsky S, Detels R, Visscher B, Chen I, Dudley J. Weight loss prior to clinical AIDS as a predictor of survival. J Acquir Immune Defic Syndr Human Retrovirol. 1995;10:366–373. [PubMed] [Google Scholar]
- 31.Schwarz HP, I, Karl E, Bier DM. The alpha-keto acids of branched-chain amino acids: simplified derivatization for physiological samples and complete separation as quinoxalinols by packed column gas chromatography. Anal Biochem. 1980;108:360–366. doi: 10.1016/0003-2697(80)90600-4. [DOI] [PubMed] [Google Scholar]
- 32.Selberg O, Suttmann U, Melzer A, Deicher H, Muller MJ, Henkel E, McMillan DC. Effect of increased protein intake and nutritional status on whole-body protein metabolism of AIDS patients with weight loss. Metabolism. 1995;44:1159–1165. doi: 10.1016/0026-0495(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 33.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol. 262 doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]; Endocrinol Metab. 1992;25:E372–E376. [Google Scholar]
- 34.Stein TP, Nutinsky C, Condoluci D, Schluter MD, Leskiw MJ. Protein and energy substrate metabolism in AIDS patients. Metabolism. 1990;39:876–881. doi: 10.1016/0026-0495(90)90136-z. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZM, Gallagher D, Nelson ME, Matthews DE, Heymsfield SB. Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr. 1996;63:863–869. doi: 10.1093/ajcn/63.6.863. [DOI] [PubMed] [Google Scholar]
- 36.Watt PW, Lindsay Y, Scrimgeour CM, Chien PAF, Gibson JNA, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. p. 460. [Google Scholar]
- 38.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography/combustion isotope ratio mass spectrometry. Biol Mass Spectrom. 1992;21:486–490. doi: 10.1002/bms.1200211004. [DOI] [PMC free article] [PubMed] [Google Scholar]