Abstract
The majority of axons in the central nervous system (CNS) are eventually myelinated by oligodendrocytes, but whether the timing and extent of myelination in vivo reflect intrinsic properties of oligodendrocytes, or are regulated by axons, remains undetermined. Here, we use zebrafish to study CNS myelination at single-cell resolution in vivo. We show that the large caliber Mauthner axon is the first to be myelinated (shortly before axons of smaller caliber) and that the presence of supernumerary large caliber Mauthner axons can profoundly affect myelination by single oligodendrocytes. Oligodendrocytes that typically myelinate just one Mauthner axon in wild type can myelinate multiple supernumerary Mauthner axons. Furthermore, oligodendrocytes that exclusively myelinate numerous smaller caliber axons in wild type can readily myelinate small caliber axons in addition to the much larger caliber supernumerary Mauthner axons. These data indicate that single oligodendrocytes can myelinate diverse axons and that their myelinating potential is actively regulated by individual axons.
Keywords: CNS Myelination, In vivo imaging, Oligodendrocyte, Zebrafish
INTRODUCTION
The vast majority of axons in the vertebrate central nervous system are eventually myelinated by oligodendrocytes (Hildebrand et al., 1993), which facilitates rapid saltatory conduction along their length (Bakiri et al., 2011; Waxman and Swadlow, 1977), ensures their long-term viability (Nave, 2010) and contributes to functional regulation of nervous system plasticity (Fields, 2010). Although we know much about the early development of oligodendrocytes (Li et al., 2009; Richardson et al., 2006) and that oligodendrocytes can differentiate and express myelin proteins in the absence of axons in vitro (Barres et al., 1994; Knapp et al., 1987; Nawaz et al., 2009; Temple and Raff, 1986), the key question of whether the timing and extent of myelination in vivo represents intrinsic properties of oligodendrocytes or is regulated by the axons remains unanswered. Although it has been known for nearly 100 years that oligodendrocyte morphology correlates with the caliber of axons that they myelinate, whereby oligodendrocytes usually associate with either a small number of large caliber axons or a larger number of smaller axons (Bunge, 1968; Butt and Berry, 2000; Del Rio-Hortega, 1921; Del Rio-Hortega, 1928; Remahl and Hildebrand, 1990), a causal role for axons in establishing these relationships, although predicted (Ueda et al., 1999), remains to be formally demonstrated.
Here, we use zebrafish, which have recently become established as a powerful model for the study of glial cells and myelinated axons (Brosamle and Halpern, 2002; Buckley et al., 2010; Kazakova et al., 2006; Kirby et al., 2006; Li et al., 2007; Lyons et al., 2009; Monk et al., 2009; Pogoda et al., 2006; Takada and Appel, 2010), to study the role that axons play in regulating CNS myelination in vivo. As is the case in mammals, we show that single oligodendrocytes in zebrafish myelinate either a small number of large caliber axons or a larger number of smaller axons. We identify the very first axon myelinated in the zebrafish CNS as the very large caliber Mauthner axon, and show that increasing the number of Mauthner axons in vivo dramatically regulates myelination by single oligodendrocytes. In the presence of supernumerary Mauthner axons we observe oligodendrocytes that usually myelinate just one Mauthner axon myelinating many Mauthner axons. In addition, we observed oligodendrocytes that usually myelinate only smaller caliber axons readily myelinating both large caliber Mauthner axons as well as axons of much smaller caliber, which happens very rarely in wild types. These results show that oligodendrocytes can myelinate axons of very different size and that individual axons can regulate the myelinating potential and, thus, the morphology of single oligodendrocytes in vivo.
MATERIALS AND METHODS
Zebrafish
We used the following standard zebrafish strains and lines: AB, Golden and Tg(sox10(7.2):mRFP) (Kirby et al., 2006). For this study, we also generated Tg(mbp:EGFP-CAAX) and Tg(mbp:EGFP). All animals were maintained in accordance with UK Home Office guidelines.
Plasmid construction
A 2 kb fragment of mbp regulatory sequence (Jung et al., 2010) was amplified from genomic DNA of the AB strain using the following primers that contain att recombination sites (underlined): attB4_mbpF, GGGGACAACTTTGTATAGAAAAGTTGCAGATGCTGAGATGTGACTACTGCAAATGA; attB1R_mbpR, GGGGACTGCTTTTTTGTACAAACTTGGTTGATCTGTTCAGTGGTCTACAGTCTGGA.
Purified PCR products were recombined with pDONRP4-P1R (Invitrogen) using BP clonase (Invitrogen) to generate the 5′ element clone p5E_mbp. Tol2 transgenesis constructs were generated by recombination of the abovementioned and other donor clones, all of which are components of the Tol2kit (Kwan et al., 2007), with pDEST_Tol2_CG2 using LR clonase II Plus (Invitrogen).
DNA injections and generation of transgenic zebrafish
Fertilised eggs were co-injected with 1 nl of a solution containing 10 ng/μl plasmid DNA and 25 ng/μl tol2 transposase mRNA. Injected fish were analysed as mosaics or grown to adulthood to raise stable transgenic lines.
notch1a morpholino injection
We injected zebrafish embryos with 500 pg of a morpholino directed against a notch1a-specific splice junction (Ma and Jiang, 2007) to temporarily abrogate embryonic notch1a expression. We carried out RT-PCR to assay notch1a mRNA expression levels using the primers 5′-CTTCTGCACTTTCTGGAGATTTAAAGAAG-3′ (Ma and Jiang, 2007) and 5′-CACACGTCTGACCTGTGAAGC-3′.
hoxb1 mRNA injection
We injected zebrafish embryos with 50 pg of hoxb1 mRNA. The majority of hoxb1 mRNA-injected animals were morphologically normal and typically had two Mauthner axons on one side of the CNS and one on the contralateral side. A subset of hoxb1 mRNA-injected animals had morphological abnormalities as have been previously described (Hale et al., 2004), and were therefore excluded from further study.
Immunohistochemistry
Whole-mount antibody labelling was carried out using standard protocols. Anti-3A10 (Developmental Studies Hybridoma Bank) was used at a concentration of 1:200.
Confocal imaging
Embryonic and larval zebrafish were embedded in 1.5% low melting point agarose (Sigma) in embryo medium with tricaine. Imaging was performed at a Zeiss LSM710 confocal microscope. Quantification of cell and myelin sheath number was carried out on confocal z-stacks using unbiased stereological methods.
Transmission electron microscopy
Preparation of tissue was as carried out as described previously (Lyons et al., 2008). Microwave stimulation during tissue processing was carried out using a Panasonic microwave with `Inverter' technology. Images were taken with a Phillips CM120 Biotwin. Data analyses were carried out using Adobe Photoshop and ImageJ.
RESULTS
CNS myelination proceeds according to stereotyped gradients in vivo
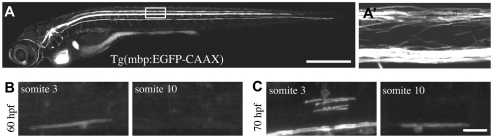
To define CNS myelination in vivo at single cell resolution, we generated transgenic constructs and stable transgenic lines to drive gene expression in myelinating glial cells of the zebrafish (Fig. 1A; see Fig. S1 in the supplementary material). Time-course analyses of fluorescent reporter lines generated using previously identified regulatory sequences of the myelin basic protein (mbp) gene (Jung et al., 2010) showed that the very first axon to be myelinated in the CNS is that of the large Mauthner neuron (Fig. 1B). The Mauthner cell is an individually identifiable reticulospinal neuron; each fish has two Mauthner neurons, one on each side of the midline of rhombomere 4 (Kimmel et al., 1982) and each Mauthner neuron projects a very large caliber axon along a stereotyped path in the ventral spinal cord (Jontes et al., 2000; Kimmel et al., 1982). mbp:EGFP-CAAX (which drives a membrane-tethered variant of EGFP) was first detected along proximal (anterior) parts of the Mauthner axon from about 60 hours post-fertilisation (hpf) and proceeded progressively towards more distal (posterior) parts of the axon over time (Fig. 1B,C). Myelination of axons in more dorsal regions of the spinal cord occurred soon after the appearance of myelination of the ventral Mauthner axon (Fig. 1C). Myelination of these more dorsally located axons also occurred in an anterior to posterior gradient (data not shown). These observations show that myelination occurs according to stereotyped anterior-posterior and ventral-dorsal gradients at the level of axonal tracts and individual axons.
Fig. 1.
Transgenic reporters reveal first axon myelinated in vivo in zebrafish CNS. (A) Lateral view of a stable Tg(mbp:EGFP-CAAX) zebrafish at 8 dpf. Myelinating glia of the CNS and PNS are labelled, as is the heart, which serves as a marker of transgenesis. (A′) Lateral view of the spinal cord (area indicated by box in A). Prominent myelinated tracts myelinated in the dorsal spinal cord and ventral spinal cord are apparent. (B) Lateral views of a stable Tg(mbp:EGFP-CAAX) zebrafish at 60 hpf show that the very first axon to be myelinated is the large Mauthner axon in the ventral spinal cord, which is first myelinated in the anterior spinal cord. (C) Lateral views of a stable Tg(mbp:EGFP-CAAX) zebrafish at 70 hpf. Myelination of the Mauthner axon has now commenced in the more posterior part of the spinal cord. At this stage, oligodendrocytes have started to myelinate axons in the dorsal spinal cord. Dorsal is up and anterior is to the left in all images. Scale bars: 500 μm in A; 20 μm in C.
Individual myelinating oligodendrocytes have diverse morphologies in vivo
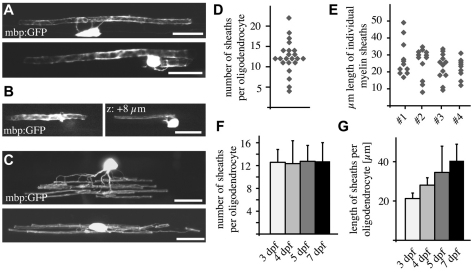
In order to visualise individual myelinating oligodendrocytes in vivo we injected wild-type embryos with plasmid DNA encoding mbp:EGFP or mbp:EGFP-CAAX and imaged them between 3 and 9 days post-fertilisation (dpf). We observed that the oligodendrocytes that associate with the very large Mauthner axon typically associate only with this axon (Fig. 2A). The vast majority of such cells (103/133) associated with just one Mauthner axon, whereas the remaining cells associated with the Mauthner axon on both sides of the embryonic midline (Fig. 2A). We also found that a very small proportion of wild-type oligodendrocytes (5%, 11/204) were capable of myelinating the Mauthner axon as well as axons of much smaller caliber (Fig. 2B). The position of oligodendrocytes that associate with the Mauthner axons was very stereotyped: the cell bodies always resided in the ventral spinal cord, almost always ventral to the Mauthner axon itself (Fig. 1B,C; Fig. 2A,B).
Fig. 2.
Single-cell analysis reveals morphological diversity of individual CNS oligodendrocytes. (A) Lateral views of single mbp:EGFP-expressing oligodendrocytes associating with one Mauthner axon (top) and both Mauthner axons (bottom). (B) Lateral view of a single mbp:EGFP-expressing oligodendrocyte associating with the large Mauthner axon (left) and an axon of much smaller caliber (right). (C) Lateral views of single oligodendrocytes associating with multiple axons in the dorsal spinal cord (top) and ventral spinal cord (bottom). (D) Myelin sheath number per oligodendrocyte (excluding those that myelinate the Mauthner axons) at 4 dpf. (E) Myelin sheath length in four sample oligodendrocytes (that do not associate with the Mauthner axon) at 4 dpf. (F) Average myelin sheath number per cell over time. This does not include oligodendrocytes that myelinate the Mauthner axon. (G) Average myelin sheath length per cell over time. This does not include oligodendrocytes that myelinate the Mauthner axon. Error bars represent s.d. Scale bars: 10 μm.
As expected, the vast majority of oligodendrocytes in the spinal cord extended multiple myelinating processes that associated with numerous axons of relatively small caliber compared with the Mauthner axons. These cells exhibited striking morphological diversity with respect to the number and length of their individual myelin sheaths (Fig. 2C-E). At 4 dpf, we found that the majority of such cells (14 of 22) had 11-14 myelinating processes, although process number varied between 4 and 22 (Fig. 2D). Also, the length of individual nascent myelin sheaths per cell was highly variable and ranged from 6 μm to 49 μm (Fig. 2E). The number of myelin sheaths per oligodendrocyte was relatively stable from 3-7 dpf, but average myelin sheath length per cell increased almost twofold over the same time period (Fig. 2F-G).
Supernumerary Mauthner axons are robustly myelinated in vivo
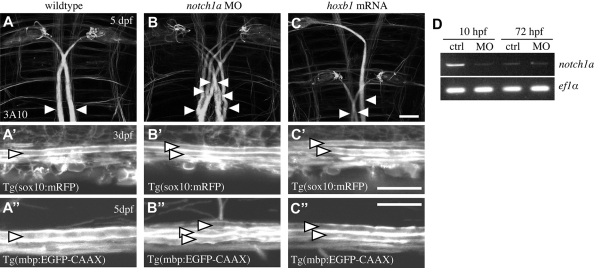
In order to test how individual large caliber axons might regulate CNS myelination, we generated animals with supernumerary Mauthner neurons using two independent genetic manipulations. Previous studies have shown that disruption of notch1a causes a mild neurogenic phenotype in zebrafish, characterised in part by the appearance of multiple Mauthner neurons (Gray et al., 2001). Such extra Mauthner neurons are born between 9 and 12 hpf, as in wild type, and extend axons to the posterior spinal cord, as in wild type (Liu et al., 2003). It is important to point out that the birth of these neurons and their axonal growth occurs long before the appearance of oligodendrocytes. Because Notch1 has been associated with oligodendrocyte development and myelination (Genoud et al., 2002; Hu et al., 2003; Park and Appel, 2003; Park et al., 2005; Wang et al., 1998), we sought to only temporarily reduce Notch1a function, and found that injection of 500 pg of a previously published morpholino (Ma and Jiang, 2007) was sufficient to generate animals with supernumerary Mauthner neurons and axons (Fig. 3A,B). The level of notch1a mRNA in such morphants is reduced relative to control at 10 hpf (when Mauthner neurons are born) but is indistinguishable from controls by 3 dpf (when extensive myelination commences) (Fig. 3D). In order to have an independent method to generate extra Mauthner axons we injected embryos with hoxb1 mRNAs (Fig. 3C), which has previously been shown to be capable of generating animals with ectopic Mauthner neurons by duplicating rhombomere 4 identity in the hindbrain (Hale et al., 2004).
Fig. 3.
Supernumerary Mauthner axons are ensheathed by myelinating glia. (A-C) Ventral views of the hindbrains of wild-type (A), notch1a morphant (B) and hoxb1 mRNA-injected (C) larvae at 5 dpf labelled with the 3A10 antibody, which recognises neurofilament-associated proteins. Mauthner axons are indicated by arrowheads. Scale bar: 20 μm. (A′-C′) Analysis of Tg(sox10:mRFP) (A′-C′) and Tg(mbp:EGFP-CAAX) (A′-C′) shows that wild-type (A′,A′), notch1a morphant (B′,B′) and hoxb1-injected (C′,C′) Mauthner axons are all ensheathed and myelinated. Arrowheads indicate Mauthner axons. Scale bars: 20 μm. (D) RT-PCR analyses show that levels of notch1a mRNA are reduced in morpholino (MO)-injected animals relative to controls at 10 hpf but not at 72 hpf.
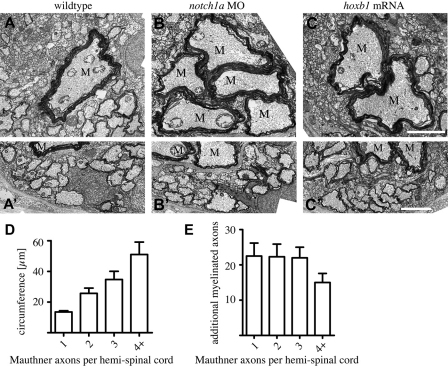
Examination of notch1a morphants and embryos injected with hoxb1 mRNA between 3 and 9 dpf using transgenic reporters (sox10:mRFP and mbp:EGFP-CAAX) and transmission electron microscopy showed that normal and supernumerary Mauthner axons were covered by glial membrane and robustly myelinated (Fig. 3; Fig. 4; data not shown). The Mauthner axon, like many axons in situ, is not circular in cross-sectional profile (Fig. 4). We therefore quantified the circumference(s) of Mauthner axon(s) in our analyses, because this variable (in combination with axon length) reflects the axonal surface available for potential interactions with oligodendrocytes. We saw that the summed circumference(s) of all Mauthner axon(s) per hemi-spinal cord increased almost linearly with the number of Mauthner axons present (Fig. 4D). In control animals that have one Mauthner axon on each side of the spinal cord, the average circumference per axon was 13.5±0.8 μm (mean±s.d., n=12) at 9 dpf. In animals with two Mauthner axons on one side of the spinal cord, the combined circumference was approximately doubled (26±3.5 μm, n=11, P<0.0001) and those with three on each side nearly tripled (35±5 μm, n=3, P<0.0001). In animals with even more Mauthner axons, their total circumference increased even further.
Fig. 4.
Supernumerary Mauthner axons are robustly myelinated. (A-C′) Transmission electron microscope images of transverse sections through the spinal cord of wild-type (A,A′), notch1a morphant (B,B′) and hoxb1 mRNA-injected (C,C′) zebrafish larvae at 9 dpf shows that all Mauthner axons are robustly myelinated (A-C) and that there is a similar number of axons myelinated in the ventral spinal cord despite the presence of supernumerary Mauthner (M) axons (A′-C′). Scale bars: 2 μm. (D) Total circumference of Mauthner axon(s) as a function of the number of Mauthner axons present per hemi-spinal cord. (E) Number of myelinated axons, excluding Mauthner axon(s), present in the ventral spinal cord as a function of the number of Mauthner axons present per hemi-spinal cord. Error bars represent s.d.
In order to test whether myelination of extra Mauthner axons occurred at the expense of other axons, we counted the total number of myelinated axons (excluding the Mauthner axons) in control and experimental ventral spinal cords (Fig. 4E). Control animals with one Mauthner axon on each side of the spinal cord had an average of 23 additional myelinated axons (±4, n=10). In animals with two Mauthner axons on each side of the spinal cord there were 22 additional axons myelinated (±4, n=10, P=0.58) as was also the case in animals with three Mauthner axons (n=3, P=0.71). These data show that the presence of even a threefold increase in Mauthner axon circumference does not affect the number of other axons myelinated in the ventral spinal cord. There was, however, a slight decrease in the number of myelinated axons when there were four or more Mauthner axons present in one side of the ventral spinal cord (15±3, n=4, P=0.0038).
Together, our results show that in two genetically independent manipulations oligodendrocytes can myelinate extra large caliber axons that are not present in their normal wild-type environment and can do so without affecting myelination of other axons. These observations beg the question as to how oligodendrocytes respond to the presence of extra axons and their consequent additional axonal surface area.
Supernumerary Mauthner axons do not regulate myelinating oligodendrocyte number
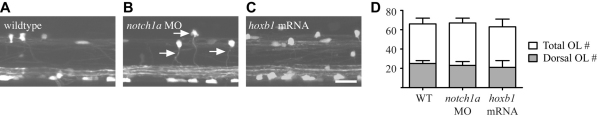
In order to test whether additional Mauthner axons regulate early stages of oligodendrocyte development, we examined the number and distribution of oligodendrocytes in notch1 morphants and animals with hoxb1 mRNA overexpression. We found no significant differences in oligodendrocyte number in the spinal cords of animals with supernumerary Mauthner axons relative to controls over a 425 μm stretch of the spinal cord centred at the mid-trunk level: control animals had an average of 66 mbp:EGFP-expressing oligodendrocytes (±6, n=6) at 5 dpf; notch1a morphants an average of 67 (±5, n=10, P=0.72); and hoxb1 mRNA-injected animals an average of 63 (±8, n=9, P=0.45) (Fig. 5). Wild-type and supernumerary Mauthner axons are localised to the ventral spinal cord, as are the oligodendrocytes that typically myelinate them (Fig. 1), and given that the Mauthner axon is the first myelinated, we wanted to test the possibility that the distribution of oligodendrocytes within the spinal cord might be affected by the presence of supernumerary Mauthner axons in the ventral spinal cord. We saw, however, that the number of oligodendrocyte cell bodies located specifically in the dorsal spinal cord was almost identical in wild type as in animals with extra Mauthner axons (control: 25±3, n=6; notch1a morphants: 23±4, n=10, P=0.31; hoxb1 mRNA-injected animals: 21±7, n=9, P=0.21) (Fig. 5D).
Fig. 5.
Presence of supernumerary Mauthner axons does not affect oligodendrocyte number or distribution. (A-C) Lateral views of the spinal cords in Tg(mbp:EGFP)-expressing wild-type (A), notch1a morphant (B) and hoxb1 mRNA-injected (C) animals shows that oligodendrocyte number and distribution are not affected by the presence of supernumerary Mauthner axons. Arrows point to oligodendrocytes in the dorsal spinal cord with projections to the ventral spinal cord. Compare with Fig. 7B. Scale bar: 20 μm. (D). Total oligodendrocyte number per 425 μm length of tissue in the mid-trunk spinal cord of wild-type, notch1a morphant and hoxb1 mRNA-injected animals and, in grey, the number of oligodendrocytes specifically located in the dorsal domain of the spinal cord. Error bars represent s.d.
Supernumerary Mauthner axons regulate the morphology of single oligodendrocytes
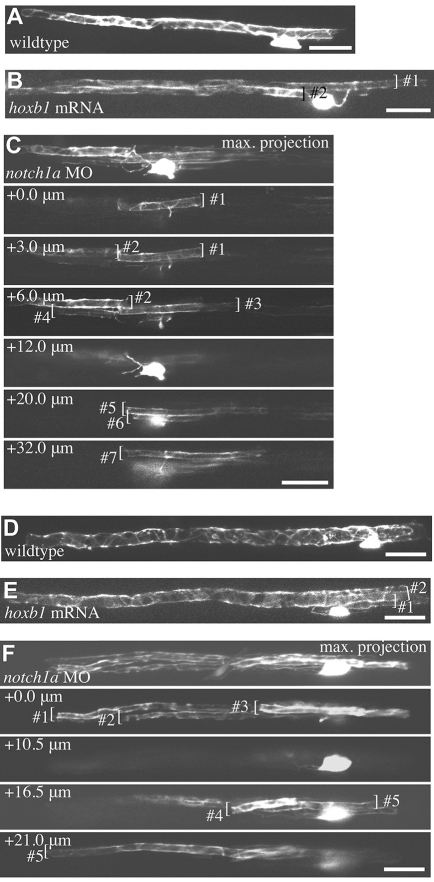
In order to elucidate precisely how supernumerary Mauthner axons regulate single myelinating oligodendrocytes, we injected plasmid DNA encoding mbp:EGFP or mbp:EGFP-CAAX into notch1a morphants and into embryos injected with hoxb1 mRNA. Whereas ∼80% of the oligodendrocytes that myelinated the Mauthner axon in wild type associated with only one Mauthner axon, we found no such oligodendrocytes in notch1a morphants. Instead, we saw that all oligodendrocytes associated with at least two Mauthner axons (n=22) and that the majority of these associated with at least three Mauthner axons (15/22) (Fig. 6). In one case, we saw an oligodendrocyte that associated with seven Mauthner axons (Fig. 6C). In animals injected with hoxb1 mRNA we also found that the vast majority of oligodendrocytes associated with more than one Mauthner axon (16/21) (Fig. 6). In wild-type animals, the average total length of myelin sheaths along Mauthner axon(s) made by single oligodendrocytes was 150±47 μm, in notch1a morphants was 256±57 μm (P<0.0001) and in hoxb1 mRNA-injected animals was 187±85 μm (P=0.05) at 5 dpf. By 9 dpf, the average total lengths were 192±53 μm in wild type, 419±95 μm (P<0.0001) in notch1a morphants and 249±107 μm (P=0.09) in hoxb1 mRNA-injected animals. Together with the fact that the total circumference of supernumerary Mauthner axons far exceeds the circumference of individual wild-type Mauthner axons (Fig. 4D), it is clear that individual oligodendrocytes in animals with supernumerary Mauthner axons myelinate a far greater axonal surface area than do wild-type oligodendrocytes.
Fig. 6.
Individual oligodendrocytes myelinate multiple supernumerary Mauthner axons. (A-F) All images are of live single mbp:EGFP-expressing oligodendrocytes in the spinal cord of larvae at 5 dpf (A-C) and 9 dpf (D-F). (A,D) Wild-type oligodendrocytes associated with single Mauthner axons. (B,E) Oligodendrocytes in hoxb1 mRNA-injected animals with myelin sheaths on two Mauthner axons. (C,F) Oligodendrocytes in notch1a morphants that make seven (C) and five (F) myelin sheaths on supernumerary Mauthner axons. Maximum intensity projections and individual confocal z-sections are indicated for clarity. Individual myelin sheaths indicated by brackets. Scale bars: 20 μm.
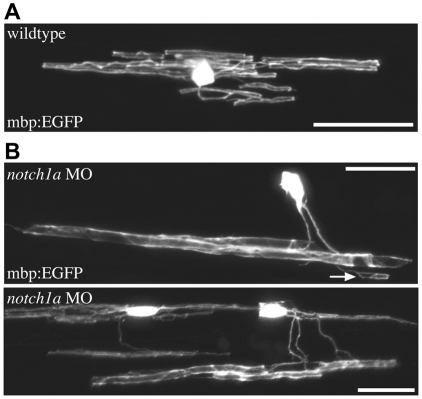
In addition to identifying oligodendrocytes that myelinated a greater number of Mauthner axons than normal, we observed that oligodendrocytes in the ventral spinal cord can myelinate supernumerary Mauthner axons as well as axons of relatively smaller caliber (data not shown). Remarkably, we also saw oligodendrocytes located in dorsal regions of the spinal cord, which typically only ever myelinate small caliber axons, extend processes to the ventral spinal cord to associate with supernumerary Mauthner axons, and in many cases also myelinate much smaller caliber axons (Fig. 7). This was never observed in wild-type animals but occurred readily in both notch1 morphants and embryos injected with hoxb1 mRNA, and at both 5 dpf and 9 dpf, which shows this was a stable association. This dramatic observation shows that individual oligodendrocytes can readily myelinate axons of very different size and that axons profoundly affect the myelination capacity of single oligodendrocytes.
Fig. 7.
Individual oligodendrocytes readily myelinate supernumerary Mauthner axons and smaller caliber axons. (A) In wild type, oligodendrocytes in the dorsal spinal cord myelinate only small caliber axons. (B) Oligodendrocytes in the dorsal spinal cords of animals with supernumerary Mauthner axons can myelinate the large Mauthner axons in the ventral spinal cord in addition to smaller caliber axons (e.g. arrow). Scale bars: 20 μm.
DISCUSSION
Here, we use zebrafish to characterise and manipulate CNS myelination in vivo. Zebrafish are ideally suited to cellular analyses at high resolution owing to their small size, optical transparency, rapid early development, accessibility and relative simplicity compared with mammals, in which the vast majority of studies of myelinated axons have been carried out. By analysing the morphology of single zebrafish oligodendrocytes in vivo using fluorescent transgenes we recapitulate key observations made in mammals nearly 100 years ago by Pio Del Rio-Hortega (Del Rio-Hortega, 1921; Del Rio-Hortega, 1928), which showed that oligodendrocytes typically myelinate either a small number of large caliber axons or a larger number of smaller caliber axons in situ. By virtue of being able to visualise myelination from its very onset at single cell resolution in vivo, we identify the large caliber Mauthner axon as the first to be myelinated in the zebrafish CNS, and show that this individual axon is myelinated along a proximal to distal gradient. We make use of two independent genetic manipulations to generate animals that have extra large caliber Mauthner axons and show that these axons can dramatically affect oligodendrocyte behaviour. Our manipulation of large caliber axon number shows that individual oligodendrocytes can readily myelinate axons of vastly different caliber in vivo and that myelination cues are, therefore, independent of axon subtype, a result that was predicted by previous transplantation studies in which oligodendrocytes derived from the optic nerve (which contains primarily small caliber axons) were capable of myelinating axons in the spinal cord (which contains axons of different sizes) (Fanarraga et al., 1998).
Our data that the presence of supernumerary large caliber Mauthner axons does not affect oligodendrocyte number are, at first glance, at odds with a previous study which showed that an increase in optic nerve axon number caused a concomitant increase in oligodendrocyte number (Burne et al., 1996). Indeed, although recent evidence has suggested that cell-cell interactions between neighbouring oligodendrocytes might contribute to regulation of their distribution and number (Kirby et al., 2006), there is extensive evidence from previous studies that axons can regulate oligodendrocyte number by affecting their proliferation and survival (Barres and Raff, 1999). However, it is important to point out that in the study of Burne et al. total axonal number was increased nearly twofold, whereas our manipulation simply increases the number of very large caliber axons during the onset of myelination and has a negligible effect on overall axon number. We do not, therefore, rule out a general role for axons in regulating oligodendrocyte number, but show instead that individual axons can dramatically regulate the myelination potential of single oligodendrocytes.
Although our data indicate that individual axons regulate CNS myelination in vivo, we do not know the identity of the causative axonal signal(s). Previous molecular characterisation has suggested that removal of inhibitory axonal cues is a pre-requisite for CNS myelination. Axons initially express high levels of polysialylated neural cell adhesion molecule (PSA-NCAM) on their surfaces, and myelination commences only when PSA-NCAM levels are reduced (Charles et al., 2000; Fewou et al., 2007; Jakovcevski et al., 2007; Keirstead et al., 1999). Analyses of the transmembrane protein Lingo1 (Lee et al., 2007; Mi et al., 2005) also suggest that it too might function on axons to inhibit myelination. In the peripheral nervous system (PNS) there is strong evidence that axonal Neuregulin 1 type III serves as an instructive cue for myelination (Michailov et al., 2004; Taveggia et al., 2005). Although Neuregulin 1 type III is not required for CNS myelination in vivo, it can stimulate hypermyelination of CNS axons in vivo (Brinkmann et al., 2008), which indicates that oligodendrocytes can respond to instructive axonal cues. It will be interesting to see whether future studies will identify instructive axonal signals required for CNS myelination.
Our data that single oligodendrocytes faced with supernumerary large caliber Mauthner axons can myelinate a larger axonal surface area than they normally do during development prompts the question of whether there is a maximum amount of axonal surface that any single oligodendrocyte can myelinate. If single oligodendrocytes myelinate close to their maximum capacity, e.g. by adulthood, then unless every oligodendrocyte is replaced following demyelination after injury or disease, incomplete repair might be inevitable. Our demonstration that axon size can regulate oligodendrocyte morphology is also relevant to the remyelination of axons. Given the significant cross-sectional growth of myelinated axons that takes place after the onset of myelination, it is likely that the caliber of any axon demyelinated long after development will be significantly larger than when it was first myelinated. This discrepancy in target caliber might mean that the morphology of remyelinating oligodendrocytes will be different than during development. It is possible that during remyelination more individual oligodendrocytes make fewer myelin sheaths on such larger caliber axons (Blakemore, 1974). If this is indeed the case, remyelination might necessitate the employment of a greater number of oligodendrocytes than are required to myelinate the same complement of axons during development. It is, however, also possible that oligodendrocytes faced with such relatively large caliber axons during remyelination do not regulate their morphology dramatically, but rather extend less myelin around individual axons. This possibility could explain the fact that remyelinated axon profiles are often surrounded by thin myelin (Blakemore, 1974). Future studies of demyelination and remyelination may benefit from high-resolution analyses that can assess precisely how much axonal surface is myelinated or how much myelin is made by individual oligodendrocytes.
In summary, our study shows that individual axons can profoundly regulate the myelinating potential of single oligodendrocytes, which has implications for the formation and regulation of myelinated axons throughout life and for mechanisms that may need to be considered during their repair.
Supplementary Material
Acknowledgments
We thank Carl Tucker, John Mullins, Patricia Smart and Sebastien Rider for fishroom support; Bruce Appel, Koichi Kawakami, Jiang Yun Jin, Hae-Chul Park and Victoria Prince for sharing reagents; Crerar hotels for free confocal access; and Stephen Mitchell for TEM assistance. Very special thanks to Chi-Bin Chien and the Chien laboratory for the Tol2kit. We are grateful to Thomas Becker, Peter Brophy, Ben Emery, Kelly Monk, Alya Raphael, William Talbot, Anna Williams and members of the Lyons, ffrench-Constant and Brophy laboratories for critical reading of the manuscript.
Footnotes
Funding
This work was supported by a David Phillips Fellowship from the BBSRC, an Innovative grant from the UK MS Society and an International Reintegration Grant to D.A.L., an FCT doctoral studentship to R.G.A. and a Wellcome Trust Programme Grant and UK MS Society Centre award to C.ff.-C. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.071001/-/DC1
References
- Bakiri Y., Karadottir R., Cossell L., Attwell D. (2011). Morphological and electrical properties of oligodendrocytes in the white matter of the corpus callosum and cerebellum. J. Physiol. 589, 559-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Raff M. C. (1999). Axonal control of oligodendrocyte development. J. Cell Biol. 147, 1123-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Lazar M. A., Raff M. C. (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097-1108 [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. (1974). Pattern of remyelination in the CNS. Nature 249, 577-578 [DOI] [PubMed] [Google Scholar]
- Brinkmann B. G., Agarwal A., Sereda M. W., Garratt A. N., Muller T., Wende H., Stassart R. M., Nawaz S., Humml C., Velanac V., et al. (2008). Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosamle C., Halpern M. E. (2002). Characterization of myelination in the developing zebrafish. Glia 39, 47-57 [DOI] [PubMed] [Google Scholar]
- Buckley C. E., Marguerie A., Roach A. G., Goldsmith P., Fleming A., Alderton W. K., Franklin R. J. (2010). Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology 59, 149-159 [DOI] [PubMed] [Google Scholar]
- Bunge R. P. (1968). Glial cells and the central myelin sheath. Physiol. Rev. 48, 197-251 [DOI] [PubMed] [Google Scholar]
- Burne J. F., Staple J. K., Raff M. C. (1996). Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J. Neurosci. 16, 2064-2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A. M., Berry M. (2000). Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum. J. Neurosci. Res. 59, 477-488 [DOI] [PubMed] [Google Scholar]
- Charles P., Hernandez M. P., Stankoff B., Aigrot M. S., Colin C., Rougon G., Zalc B., Lubetzki C. (2000). Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 97, 7585-7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Hortega P. (1921). Estudios sobre la neuroglia. La glia de escasas radiaciones oligodendroglia. Bol. Real Soc. Esp. Hist. Nat. 21, 63-92 [Google Scholar]
- Del Rio-Hortega P. (1928). Tercera aportacion al conocimiento morfologico e interpretacion funcional de la oligodendroglia. Mem. Real Soc. Esp. Hist. Nat. 14, 40-122 [Google Scholar]
- Fanarraga M. L., Griffiths I. R., Zhao M., Duncan I. D. (1998). Oligodendrocytes are not inherently programmed to myelinate a specific size of axon. J. Comp. Neurol. 399, 94-100 [PubMed] [Google Scholar]
- Fewou S. N., Ramakrishnan H., Bussow H., Gieselmann V., Eckhardt M. (2007). Down-regulation of polysialic acid is required for efficient myelin formation. J. Biol. Chem. 282, 16700-16711 [DOI] [PubMed] [Google Scholar]
- Fields R. D. (2010). Neuroscience. Change in the brain’s white matter. Science 330, 768-769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud S., Lappe-Siefke C., Goebbels S., Radtke F., Aguet M., Scherer S. S., Suter U., Nave K. A., Mantei N. (2002). Notch1 control of oligodendrocyte differentiation in the spinal cord. J. Cell Biol. 158, 709-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M., Moens C. B., Amacher S. L., Eisen J. S., Beattie C. E. (2001). Zebrafish deadly seven functions in neurogenesis. Dev. Biol. 237, 306-323 [DOI] [PubMed] [Google Scholar]
- Hale M. E., Kheirbek M. A., Schriefer J. E., Prince V. E. (2004). Hox gene misexpression and cell-specific lesions reveal functionality of homeotically transformed neurons. J. Neurosci. 24, 3070-3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C., Remahl S., Persson H., Bjartmar C. (1993). Myelinated nerve fibres in the CNS. Prog. Neurobiol. 40, 319-384 [DOI] [PubMed] [Google Scholar]
- Hu Q. D., Ang B. T., Karsak M., Hu W. P., Cui X. Y., Duka T., Takeda Y., Chia W., Sankar N., Ng Y. K., et al. (2003). F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115, 163-175 [DOI] [PubMed] [Google Scholar]
- Jakovcevski I., Mo Z., Zecevic N. (2007). Down-regulation of the axonal polysialic acid-neural cell adhesion molecule expression coincides with the onset of myelination in the human fetal forebrain. Neuroscience 149, 328-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes J. D., Buchanan J., Smith S. J. (2000). Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat. Neurosci. 3, 231-237 [DOI] [PubMed] [Google Scholar]
- Jung S. H., Kim S., Chung A. Y., Kim H. T., So J. H., Ryu J., Park H. C., Kim C. H. (2010). Visualization of myelination in GFP-transgenic zebrafish. Dev. Dyn. 239, 592-597 [DOI] [PubMed] [Google Scholar]
- Kazakova N., Li H., Mora A., Jessen K. R., Mirsky R., Richardson W. D., Smith H. K. (2006). A screen for mutations in zebrafish that affect myelin gene expression in Schwann cells and oligodendrocytes. Dev. Biol. 297, 1-13 [DOI] [PubMed] [Google Scholar]
- Keirstead H. S., Ben-Hur T., Rogister B., O’Leary M. T., Dubois-Dalcq M., Blakemore W. F. (1999). Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J. Neurosci. 19, 7529-7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Powell S. L., Metcalfe W. K. (1982). Brain neurons which project to the spinal cord in young larvae of the zebrafish. J. Comp. Neurol. 205, 112-127 [DOI] [PubMed] [Google Scholar]
- Kirby B. B., Takada N., Latimer A. J., Shin J., Carney T. J., Kelsh R. N., Appel B. (2006). In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9, 1506-1511 [DOI] [PubMed] [Google Scholar]
- Knapp P. E., Bartlett W. P., Skoff R. P. (1987). Cultured oligodendrocytes mimic in vivo phenotypic characteristics: cell shape, expression of myelin-specific antigens, and membrane production. Dev. Biol. 120, 356-365 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099 [DOI] [PubMed] [Google Scholar]
- Lee X., Yang Z., Shao Z., Rosenberg S. S., Levesque M., Pepinsky R. B., Qiu M., Miller R. H., Chan J. R., Mi S. (2007). NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J. Neurosci. 27, 220-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lu Y., Smith H. K., Richardson W. D. (2007). Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J. Neurosci. 27, 14375-14382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., He Y., Richardson W. D., Casaccia P. (2009). Two-tier transcriptional control of oligodendrocyte differentiation. Curr. Opin. Neurobiol. 19, 479-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. S., Gray M., Otto S. J., Fetcho J. R., Beattie C. E. (2003). Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit. J. Neurosci. 23, 8159-8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D. A., Naylor S. G., Mercurio S., Dominguez C., Talbot W. S. (2008). KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome. Development 135, 599-608 [DOI] [PubMed] [Google Scholar]
- Lyons D. A., Naylor S. G., Scholze A., Talbot W. S. (2009). Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat. Genet. 41, 854-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Jiang Y. J. (2007). Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 3, e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Miller R. H., Lee X., Scott M. L., Shulag-Morskaya S., Shao Z., Chang J., Thill G., Levesque M., Zhang M., et al. (2005). LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745-751 [DOI] [PubMed] [Google Scholar]
- Michailov G. V., Sereda M. W., Brinkmann B. G., Fischer T. M., Haug B., Birchmeier C., Role L., Lai C., Schwab M. H., Nave K. A. (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700-703 [DOI] [PubMed] [Google Scholar]
- Monk K. R., Naylor S. G., Glenn T. D., Mercurio S., Perlin J. R., Dominguez C., Moens C. B., Talbot W. S. (2009). A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325, 1402-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K. A. (2010). Myelination and support of axonal integrity by glia. Nature 468, 244-252 [DOI] [PubMed] [Google Scholar]
- Nawaz S., Kippert A., Saab A. S., Werner H. B., Lang T., Nave K. A., Simons M. (2009). Phosphatidylinositol 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J. Neurosci. 29, 4794-4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. C., Appel B. (2003). Delta-Notch signaling regulates oligodendrocyte specification. Development 130, 3747-3755 [DOI] [PubMed] [Google Scholar]
- Park H. C., Boyce J., Shin J., Appel B. (2005). Oligodendrocyte specification in zebrafish requires notch-regulated cyclin-dependent kinase inhibitor function. J. Neurosci. 25, 6836-6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda H. M., Sternheim N., Lyons D. A., Diamond B., Hawkins T. A., Woods I. G., Bhatt D. H., Franzini-Armstrong C., Dominguez C., Arana N., et al. (2006). A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev. Biol. 298, 118-131 [DOI] [PubMed] [Google Scholar]
- Remahl S., Hildebrand C. (1990). Relation between axons and oligodendroglial cells during initial myelination I. The glial unit. J. Neurocytol. 19, 313-328 [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Kessaris N., Pringle N. (2006). Oligodendrocyte wars. Nat. Rev. Neurosci. 7, 11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada N., Appel B. (2010). Identification of genes expressed by zebrafish oligodendrocytes using a differential microarray screen. Dev. Dyn. 239, 2041-2047 [DOI] [PubMed] [Google Scholar]
- Taveggia C., Zanazzi G., Petrylak A., Yano H., Rosenbluth J., Einheber S., Xu X., Esper R. M., Loeb J. A., Shrager P., et al. (2005). Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S., Raff M. C. (1986). Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell 44, 773-779 [DOI] [PubMed] [Google Scholar]
- Ueda H., Levine J. M., Miller R. H., Trapp B. D. (1999). Rat optic nerve oligodendrocytes develop in the absence of viable retinal ganglion cell axons. J. Cell Biol. 146, 1365-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Sdrulla A. D., diSibio G., Bush G., Nofziger D., Hicks C., Weinmaster G., Barres B. A. (1998). Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63-75 [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Swadlow H. A. (1977). The conduction properties of axons in central white matter. Prog. Neurobiol. 8, 297-324 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.