Abstract
Hemodynamic monitoring has become a real challenge in the intensive care unit. As an integrative parameter for oxygen supply/demand, venous oxygen saturation (SvO2) provided by pulmonary artery catheterization is one of the most popular parameters to assess the adequacy of cardiac output. However, technical limitations and potential iatrogenic complications constitute important limits for a widespread use. Regular central venous catheters coupled with a fiberoptic lumen for central venous oxygen saturation (ScvO2) monitoring have been proposed as a surrogate for SvO2 monitoring. The purpose of the present article is to review the physiological backgrounds of circulation, the pathophysiology of circulatory failure and subsequent venous oxygen saturation alterations, and finally the merits and the limits of the use of ScvO2 in different clinical situations.
1. Introduction
Hemodynamic monitoring has become a common practice in the intensive care unit. Besides blood pressure measurement, most industrial efforts have concentrated on providing devices for cardiac output monitoring. However, adequate adaptation of these macrohemodynamic parameters is somehow challenging. Indeed, as cardiac output is an adaptive parameter, it is always difficult to judge whether a given value at a given time for a given patient is appropriate or not. Similarly, which value should be considered an appropriate goal for blood pressure, considering regional perfusion specificities (e.g., autoregulation or flow/pressure dependency), patient's age, history of hypertension, and so on. Therefore, considering that O2 supply to the tissue is the basic objective, intensivists have been trying to find out an integrative parameter that would be more suitable to globally assess hemodynamic status of their patients. As a surrogate for evaluating O2 demand/supply adequacy, central oxygen venous saturation (ScvO2) has become a popular parameter. As explained for the dummies, oxygen venous saturation is interpreted as a bank statement at the end of the month: “if the balance is negative, you can consider two explanations: you spend too much money or you earn not enough.” The aim of the present paper is precisely to critically analyze the physiological basements for such an interpretation, the data that support its use in clinical practice, and finally the limits that should be kept in mind while using such a parameter at the bedside.
2. Physiological Background
2.1. Normal Circulation Physiology
One of the main goals of blood circulation is to ensure oxygen supply to organs and tissues. The determinants of arterial oxygen delivery (DO2) are
cardiac output (CO);
arterial content in oxygen (CaO2).
The arterial content in oxygen has 2 components.
The main component is oxygen bound to hemoglobin (Hb).
The secondary component is dissolved oxygen.
The first one depends on hemoglobin concentration, hemoglobin affinity for oxygen (which varies for Hb isotypes and with environmental conditions such as temperature, pH, or 2.3 DPG concentrations), and, therefore, Hb oxygen saturation. The second component depends on arterial partial pressure of oxygen (PaO2) and is considered as negligible because of the very solubility coefficient of oxygen in plasma. It is then possible to set the equations:
CaO2 = (Hb × 1.34 × SaO2) + (0.003 × PaO2),
DO2 = CO × CaO2.
By ignoring the dissolved oxygen component, we get
DO2 = CO × 1,34 × SaO2.
Arterial blood is then deoxygenated in tissues. Tissue oxygen extraction depends on their demand but also on their ability for oxygen extraction. Therefore, after peripheral oxygen extraction, venous oxygen content depends on arterial content and tissue oxygen extraction.
2.2. Pathophysiology of Circulatory Failure
Shock is one of the leading causes of admission in the intensive care unit. It is usually defined as a mean arterial pressure (MAP) <60 mmHg or a systolic arterial pressure (SAP) <90 mmHh, or a decrease in SAP greater than 40 mmHg as compared to the usual SAP [1]. For many years, hemodynamic management has focused on “macrocirculatory” parameters such as blood pressure or cardiac output. Though the magnitude of macrocirculatory disorders is well known to be related to prognosis [2], its optimization seems mandatory [3] but insufficient [4]. Indeed, in septic shock patients, Sakr et al. observed that after 24 h hours of intensive care, the values of MAP, cardiac index (CI), and central venous pressure (CVP) did not discriminate survivors from nonsurvivors.
Hence, shock can be defined as a macrohemodynamic instability leading to an inappropriate oxygen supply/demand balance. Schematically, as represented in Figure 1, any fall in DO2 is initially compensated by an increase in tissue oxygen extraction (EO2), explaining that tissue VO2 is initially maintained. However, when tissue oxygen extraction capacity is overtaken, oxygen consumption begins to fall and lactate concentration increases, indicating a switch of the cellular metabolism from aerobic glycolysis to cytoplasmic anaerobic glycolysis. This threshold immediately precedes the onset of clinical organ failures.
Figure 1.
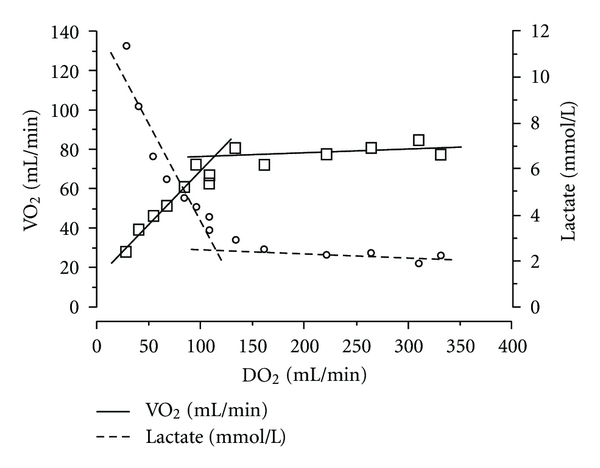
Evolution of oxygen consumption when oxygen delivery decreases. From Vincent and De Backer [5] with permission. Note the presence of a DO2 threshold located at approximately 100 mL/min. Below this value, oxygen consumption begins to fall and lactate concentration increases, indicating a switch from aerobic to anaerobic metabolism.
Considering such a pathophysiological scheme, hemodynamic support in shock should aim at correcting macrocirculatory but also microcirculatory parameters in order to avoid any local fall in O2 supply below this crucial threshold. Therefore, a parameter such as venous oxygen saturation (SvO2), that should reflect the inadequacy in oxygen supply, might be of great help.
2.3. Physiological Determinants for Venous Oxygen Saturation
Oxygen extraction in the tissues can be mathematically defined as follows:
EO2 = CO × (CaO2 − CvO2),
EO2 = VO2/DO2,
with CvO2 being venous oxygen content and VO2 being oxygen consumption.
Then, venous oxygen saturation can then be calculated using the following formula:
SvO2 = SaO2 − (VO2/(CO × Hb × 1.34)).
Hence, any decrease in venous oxygen saturation should be explained by
a decrease in SaO2;
a decrease in cardiac output;
a decrease in hemoglobin level;
an increase of oxygen consumption (VO2).
Thus, providing that SaO2, oxygen consumption, and haemoglobin level are in normal ranges, SvO2 can be used as a surrogate for cardiac output.
Likewise, if
EO2 = CO × (CaO2 − CvO2),
EO2 = VO2/DO2,
then,
EO2 = (SaO2 − SvO2)/SaO2.
Consequently, when SaO2 = 100%, then EO2 = 1 − SvO2 and SvO2 = 1 − EO2.
Then, SvO2 is also a good surrogate for EO2.
In shock, decrease in tissue oxygen supply is mostly related to a decrease in tissue blood flow, would it be relative (as in distributive shocks) or real (as in hemorrhagic shock). The first recommended measure in international guidelines for shock resuscitation consists in optimizing cardiac output by repeated fluid challenges [6], in order to correct oxygen supply/demand imbalance. In this aspect, SvO2 measurements could help guiding fluid challenges in shock patients.
3. SvO2—ScvO2: Is It the Same?
The reference technique to assess the adequacy of oxygen supply is the mixed venous oxygen saturation (SvO2), provided by pulmonary artery catheter (a.k.a. Swan-Ganz catheter) [7]. However, limitations related to difficulties of insertion and placement, but also to potential complications related with such a catheter, lead to a substantial decrease in its use. In the meantime, industrials have developed regular central venous catheter coupled with a fiberoptic lumen for continuous haemoglobin saturation monitoring. Placed through a jugular of a subclavian vein, at the confluent of the superior vena cava and the right auricle, such catheters actually monitor the central venous oxygen saturation (ScvO2) [8].
However, one should ask whether SvO2 and ScvO2 provide the same information. Actually, taken in pulmonary artery, SvO2 is a surrogate for global tissue oxygenation, whereas ScvO2 essentially reflects the oxygenation of the upper part of the body (head, neck upper limbs, and upper part of the trunk) and of a lower proportion of the lower part of the body (lower part of the trunk and lower limbs), depending on the exact position of the catheter's extremity. Anyhow, ScvO2 does not include venous blood coming from coronary sinus commonly located in the right auricle. Thus, taken at the confluent of the vena cava in the right auricle (i.e., upstream from the coronary sinus), ScvO2 does not include myocardial oxygenation. On the contrary, SvO2 concerns venous blood from pulmonary artery, that is, by definition, after the coronary venous sinus. Such a difference might highly impact the observed values, given that (1) venous blood from the coronary sinus, with a saturation of oxygen close to 40%, is the most deoxygenated venous blood of the body [9] and (2) that in critically ill patients, myocardial oxygen supply/demand imbalance is likely to occur.
4. ScvO2: A Validated Monitoring Parameter
4.1. Experimental Validation
Many studies have compared the ScvO2 and SvO2 values in the same patients (Table 1). Most of them showed a good correlation between ScvO2 and SvO2 and a similar trend in the temporal evolution. In 1989, Reinhart et al. [10] reported, in a dog model, a correlation coefficient between ScvO2 and SvO2 of 0.96. In this study, the two values exhibited less than 5% difference in 77% of the cases. Later on, Reinhart et al. [11] confirmed their results in ICU patients: ScvO2 and SvO2 had similar evolution in 90% of the cases and had a correlation coefficient of 0.81 (P < 0.001). Similarly, Martin et al. [12] reported a parallel evolution of ScvO2 and SvO2 in 75% of the cases. Considering such results, it seems that ScvO2 and especially its evolution over time could be used as an interesting surrogate for SvO2 monitoring. However, the impact of ScvO2 monitoring on the prognosis of critically ill patients remained to be demonstrated.
Table 1.
Summary of the studies comparing SvO2 and ScvO2 in humans or in experimental models.
| Author (year) | Type of patients (n) | Conclusion | Correlation coefficient |
|---|---|---|---|
| Tahvanainen et al. [13] (1982) | Intensive care (42) | ScvO2 = SvO2 | NC |
| Wendt et al. [14] (1990) | Intensive care (19) | ScvO2 ~ SvO2 | 0,78 |
| Kong et al. [15] (1990) | Kidney failure (8) | ScvO2 ~ SvO2 | NC |
| Berridge et al. [16] (1992) | Intensive care (51) | ScvO2 = SvO2 | 0,92 |
| Herrera et al. [17] (1993) | Thoracic surgery (23) | ScvO2 = SvO2 | NC |
| Pieri et al. [18] (1995) | Major surgery (39) | ScvO2 ≠ SvO2, nonsubstituable | 0,90 |
| Ladakis et al. [19] (2001) | Intensive care (61) | ScvO2 = SvO2 | 0,94 |
| Reinhart et al. [11] (2004) | Intensive care (32) | ScvO2 ~ SvO2 | 0,81 |
| Chawla et al. [20] (2004) | Intensive care (53) | ScvO2 > SvO2 | 0.88 |
| Dueck et al. [21] (2005) | Neurosurgery (70) | ScvO2 ≠ SvO2, substituable evolution | ≥0,75 |
| Ho et al. [22] (2010) | Intensive care | ScVO2 ≠ SvO2, nonsubstituable | NC |
4.2. Clinical Validation
Some authors, therefore, focused on evaluating the connection between SvcO2 and prognosis and especially the benefits turnoff considering SvcO2 optimization as a goal for resuscitation. Pearse et al. [23] observed in a cohort of 118 postoperative patients from major surgery that a decrease in ScvO2 during the first 8 hours was associated with an increase in 28-day morbidity and mortality. Consistently, Futier et al. [24] showed in major abdominal surgery that a ScvO2 <70% was associated with postoperative complications. In addition, ScvO2 seems to be a reliable and sensitive parameter to detect hemorrhage in trauma patients admitted to the Emergency Room [25], while other series suggest that ScvO2 could be a prognosis marker in myocardial infarction [26], acute heart failure [27], as well as in severe sepsis patients [28].
But the great clinical advantage related to early ScvO2 has been suggested by Rivers et al. [29]. Indeed, these authors reported that, in severe sepsis patients, an early and aggressive therapy that aimed at normalizing in the first hours the values of ScvO2 MAP and CVP achieved a reduction in in-hospital mortality from 46.5% to 30.5% (relative risk 0.58 (0.38–0.87), P = 0.009). These results were later confirmed by two large studies [30, 31] conducted, respectively, on 15,022 and 330 patients that both showed a mortality reduction related to the implementation of ScvO2 as a resuscitation goal. Though Levy's et al. study [30] failed to show any survival improvement specifically related to ScvO2 implementation, the global target implementation did (lactate measure, blood culture before antibiotics, broad spectrum antibiotics, fluid and vasopressors, CVP >8 mmHg, and ScvO2 >70%). This could be partly explained by the fact that, among those 6 resuscitation targets, ScvO2 >70% was the less commonly achieved, both after the first quarter of patients was included and after the final quarter of patients was included (resp., in 13.3% and 24.3% of the cases). Recently, Jones et al. [32] showed, in 300 septic shock patients, that the mortality of patients who benefited from a ScvO2 goal-directed therapy was low (23% (17–30%)) and similar to those who were treated using a lactate clearance goal-directed therapy (17% (11–24%)).
ScvO2 is considered as a suitable prognosis factor in many clinical situations in the critically ill patients. The Surviving Sepsis Campaign [33], gathering all European guidelines regarding severe sepsis and sepsis shock patients management, suggested including ScvO2 as a goal parameter in the first 6 hours of management (ScvO2 >70%).
5. ScvO2 Limits
5.1. Theoretical Limits
The first limit of using ScvO2 refers to its ignorance of the coronary sinus venous blood saturation. As the extremity of the ScvO2 catheter usually stands upstream from the joining point of coronary sinus in the right auricle, the ScvO2 value does not take into account the myocardial oxygen supply/demand adequacy. As myocardial oxygen extraction is physiologically basically high, venous coronary blood is one of the most deoxygenated venous bloods [9] of the body. This explains that the value of mixed venous blood saturation of oxygen (SvO2), which actually takes into account venous coronary blood, is usually lower than the ScvO2. Moreover, any major increase in myocardial oxygen consumption could lead to a critical myocardial oxygen extraction that would have no impact on ScvO2 monitoring. Besides, ScvO2, just as SvO2, is a global oxygenation parameter. So any local change in tissue oxygenation is at risk of being “diluted” in the rest of venous blood and then becoming undetectable. Similarly, in the case of a drop in regional venous saturation responsible for a drop of ScvO2, it would not be possible to assess the affected territory without further exploration. Then, theoretically, the distal extremity of the central venous catheter is supposed to be placed at the joining point of vena cava and the right auricle to enable a suitable assessment of tissue oxygenation of inferior and superior territories. However, checking the position of the catheter's distal extremity with chest X-ray is not accurate enough. Moreover, as venous saturation from the superior vena cava is systematically lower than inferior vena cava, any variation in the position of the catheter's tip could have a major influence on the measures and therefore lead to ScvO2 misinterpretation. Ultimately, as previously reported, ScvO2 depends on tissue oxygen extraction and hemoglobin affinity for oxygen. Experiments report that septic patients could suffer from a decrease in oxygen extraction capacity [34, 35], a rise in capillary shunt [34], as well as changes in hemoglobin affinity for oxygen [36]. All these changes may alter the theoretical relationship between SvcO2, and cardiac output, such as ScvO2 interpretation, to guide hemodynamic therapy becomes more complex.
5.2. Clinical Limits
First of all, one could argue that ScvO2 measurement requires a central venous catheter, which is an invasive technique, exposing patients to complications such as infection or hemorrhage. However, central venous lines are often needed for critically patients and could therefore be used for ScvO2 monitoring. However, in severe sepsis and septic shock, tissue hypoperfusion should lead to particularly low ScvO2 values, as observed by Rivers et al. in the early stage of sepsis. However, after the first hours of resuscitation, this situation is rarely met [37], and ScvO2 values tend to be paradoxically normal or even raised. This could be explained by the physiological modification induced by sepsis and previously described (decrease in tissue oxygen extraction capacity, rise of capillary shunt, and changes in hemoglobin affinity for oxygen). Consistently, in such situations, the agreement between SvO2 and ScvO2 seems much less satisfactory, especially in the context of septic shock [22, 38, 39]. Besides, ScvO2 clinical validation is mainly based on one single study [29], which is a single centre study, and its results are still controversial. As a matter of fact, van Beest et al. [37], in a Dutch prospective multicenter study, reported that only 1% of the patients meeting the inclusion criteria required by Rivers et al. [29] had a ScvO2 <50%. Ho et al. [40], in a retrospective study, as well as the ARISE group (Australian Resuscitation of Sepsis Evaluation), in a multicenter study [41], reported an in-hospital mortality of 26–28% in patients who did not benefit from an early goal-directed therapy but that met the inclusion criteria for Rivers' trial. This mortality rate is much lower than the one observed by Rivers in his control group. Finally, the low CVP values (5-6 mmHg) observed by Rivers et al. suggest that their patients were probably highly hypovolemic.
5.3. Global versus Regional Circulation
If global hemodynamic optimization is considered as an essential prerequisite to ensure adequate tissue perfusion, it may not be always sufficient to avoid the development of organ failure. The poor accuracy for global oxygen venous saturation monitoring to detect changes in regional oxygenation has been well described in animal models [42–44]. For instance, Legrand et al. [42] recently showed in a rat model that LPS-induced endotoxemia could induce alterations in microvascular perfusion and oxygenation in the renal cortex in rats, which appeared to be only weakly dependent on systemic and renal macrohemodynamic alterations. Consistently, Vallet et al. [44] and Lagoa et al. [43] reported, in endotoxemic dogs, that after resuscitation skeletal VO2 is maintained when blood flow within the gut is significantly disturbed with mucosal hypoxia. In human beings, as described by Sakr et al. [4]. global hemodynamic parameters fail to discriminate survivors from nonsurvivors after 24 hours of intensive care in septic shock patients. One illustrative example is the lack of accuracy of global SvO2 to detect cerebral venous desaturations [45]. In this perspective, global ScvO2 might face some limitations with respect to local inadequacy in the DO2/VO2 balance. Indeed, local SvO2 might not be detected by global oxygen saturation monitoring, the signal being diluted among a global normally saturated venous blood. Therefore, regional SvO2 could be an interesting supplementary target parameter. However, while regional SVO2 monitoring might be feasible at the bedside for some organ, such as jugular venous oxygen monitoring [46–48], it is much more difficult for others such as the kidney or the gut, for example. In such situation, some alternative parameters for regional monitoring could be of interest.
6. Candidate Parameter to Reflect Regional Inadequate Oxygen Supply
As for now, no biological or technical parameter has been proved to directly reflect regional oxygen supply inadequacy. Nevertheless, some parameters appear to be good surrogate candidate, such as tissue oxygen saturation (StO2) and regional carbon dioxide partial pressure (pCO2).
6.1. Tissue Oxygen Saturation (StO2)
StO2 can be estimated by near-infrared spectroscopy (NIRS) using the differential absorption properties of oxygenated and deoxygenated hemoglobin. Near-infrared light (wave length 680–800 nm) easily crosses biological tissue and is only absorbed by hemoglobin, myoglobin, and oxidized cytochrome, but the contribution of myoglobin and oxidized cytochrome in light absorption is very low [49, 50]. Light tissue penetration is dependant on the space between the illumination fiber and the detection fiber. With a 25 mm space, 95% of the light signals detected come from a 0–23 mm depth.
The steady StO2 value is a reflection of oxygen saturation of the haemoglobin present in the tissue volume crossed by the near-infrared light, containing a mix of arteriolar, capillary, and venous blood. It is then a complicated integrative parameter, but it has been shown to be correlated to the microcirculation state and is therefore considered as an acceptable parameter for tissue perfusion [51].
During shock from various origins, the relationship between StO2 values at the forearm and the prognosis has been extensively studied during the past decade [52–55]. During shock states, as StO2 drops correlate with fall in central venous, mixed venous oxygen saturation, or oxygen delivery [56–59], StO2 seems to be a good marker of regional DO2/VO2 imbalance, with the advantage of being applicable to different regional territories such as the brain [60], the liver [61], or the muscle [62], for example. However, this technique suffers some limitations, the major one being its poor sensitivity to rule out tissue hypoperfusion [55]. In order to improve its sensitivity, vascular occlusion tests (transient upper arm arterial occlusion with a pneumatic cuff) have been proposed [63]. By continuously monitoring StO2 during the test, a pattern curve is obtained with an initial decrease of StO2 during occlusion, followed after cuff deflation by an increase of StO2 usually transiently reaching higher values than baseline (hyperemic response) before returning to baseline. The slope of the decreasing part of the curve is the StO2 desaturation rate and is correlated to the tissue oxygen consumption, whereas the slope of the increasing part of the curve is the StO2 recovery rate and is correlated to the quality of the microvascular bedside [64].
6.2. Carbon Dioxide Partial Pressure
Regional capnography relies on the principle that cellular oxygen consumption through oxidative phosphorylation produces proportional amount of carbon dioxide. In this perspective, any decrease in blood flow would result in a CO2 accumulation detected by a capnograph. Tissue pCO2 could then be used as a surrogate for regional blood flow and oxygen consumption combined [65]. However, regional pCO2 is difficult to interpret, because CO2 production also depends on cellular metabolism level and arterial glucose concentration. This probably explains the fact that, despite appealing, this parameter is still rarely used in clinical practice.
7. Conclusion
In conclusion, ScvO2 measurement seems to be an interesting tool, especially in the early phase of shock to guide fluid management and blood transfusion or inotropic support. Nevertheless, a large knowledge of its determinants and the physiology of circulation seem to be essential to ensure a reliable interpretation in clinical practice. When ScvO2 is low, it reflects an adaptive mechanism to an unsuitable supply in oxygen and should lead doctors to understand the reasons for it and to propose an appropriate optimization strategy. As well, in clinical situations such as septic shock, after the first hours of management, a “normal” or even a high ScvO2 can be falsely reassuring.
Conflict of Interests
The author declares that there is no conflict of interests.
References
- 1.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Valles J, Rello J, Ochagavia A, Garnacho J, Alcala MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123(5):1615–1624. doi: 10.1378/chest.123.5.1615. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management: International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Medicine. 2007;33(4):575–590. doi: 10.1007/s00134-007-0531-4. [DOI] [PubMed] [Google Scholar]
- 4.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent-microcirculatory alterations are associated with organ failure and death in patients with septic shock. Critical Care Medicine. 2004;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, De Backer D. Oxygen transport—the oxygen delivery controversy. Intensive Care Medicine. 2004;30(11):1990–1996. doi: 10.1007/s00134-004-2384-4. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Weil MH. Fluid challenge revisited. Critical Care Medicine. 2006;34(5):1333–1337. doi: 10.1097/01.CCM.0000214677.76535.A5. [DOI] [PubMed] [Google Scholar]
- 7.Reinhart K, Bloos F. The value of venous oximetry. Current Opinion in Critical Care. 2005;11(3):259–263. doi: 10.1097/01.ccx.0000158092.64795.cf. [DOI] [PubMed] [Google Scholar]
- 8.Scheinman MM, Brown MA, Rapaport E. Critical assessment of use of central venous oxygen saturation as a mirror of mixed venous oxygen in severely ill cardiac patients. Circulation. 1969;40(2):165–172. doi: 10.1161/01.cir.40.2.165. [DOI] [PubMed] [Google Scholar]
- 9.Miyairi T, Miwa T, Takayama T, Ka K, Itoh K. Continuous monitoring of coronary sinus oxygen saturation during warm heart surgery. Journal of Thoracic and Cardiovascular Surgery. 1994;108(4):795–796. [PubMed] [Google Scholar]
- 10.Reinhart K, Rudolph T, Bredle DL, Hannemann L, Cain SM. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest. 1989;95(6):1216–1221. doi: 10.1378/chest.95.6.1216. [DOI] [PubMed] [Google Scholar]
- 11.Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Medicine. 2004;30(8):1572–1578. doi: 10.1007/s00134-004-2337-y. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Auffray JP, Badetti C, Perrin G, Papazian L, Gouin F. Monitoring of central venous oxygen saturation versus mixed venous oxygen saturation in critically ill patients. Intensive Care Medicine. 1992;18(2):101–104. doi: 10.1007/BF01705041. [DOI] [PubMed] [Google Scholar]
- 13.Tahvanainen J, Meretoja O, Nikki P. Can central venous blood replace mixed venous blood samples? Critical Care Medicine. 1982;10(11):758–761. doi: 10.1097/00003246-198211000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Wendt M, Hachenberg T, Albert A, Janzen R. Mixed venous versus central venous oxygen saturation in intensive medicine. Anasth Intensivther Notfallmed. 1990;25(1):102–106. [PubMed] [Google Scholar]
- 15.Kong CH, Thompson FD, Imms FJ. Cardiac output and oxygen uptake in patients with renal failure. Clinical Science. 1990;78(6):591–596. doi: 10.1042/cs0780591. [DOI] [PubMed] [Google Scholar]
- 16.Berridge JC. Influence of cardiac output on the correlation between mixed venous and central venous oxygen saturation. British Journal of Anaesthesia. 1992;69(4):409–410. doi: 10.1093/bja/69.4.409. [DOI] [PubMed] [Google Scholar]
- 17.Herrera A, Pajuelo A, Morano MJ, Ureta MP, Gutierrez-Garcia J, de las Mulas M. Comparison of oxygen saturations in mixed venous and central blood during thoracic anesthesia with selective single-lung ventilation. Revista Española de Anestesiología y Reanimación. 1993;40(6):349–353. [PubMed] [Google Scholar]
- 18.Pieri M, Brandi LS, Bertolini R, Calafà M, Giunta F. Comparison of bench central and mixed pulmonary venous oxygen saturation in critically ill postsurgical patients. Minerva Anestesiologica. 1995;61(7-8):285–291. [PubMed] [Google Scholar]
- 19.Ladakis C, Myrianthefs P, Karabinis A, et al. Central venous and mixed venous oxygen saturation in critically ill patients. Respiration. 2001;68(3):279–285. doi: 10.1159/000050511. [DOI] [PubMed] [Google Scholar]
- 20.Chawla LS, Zia H, Gutierrez G, Katz NM, Seneff MG, Shah M. Lack of equivalence between central and mixed venous oxygen saturation. Chest. 2004;126(6):1891–1896. doi: 10.1378/chest.126.6.1891. [DOI] [PubMed] [Google Scholar]
- 21.Dueck MH, Klimek M, Appenrodt S, Weigand C, Boerner U. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103(2):249–257. doi: 10.1097/00000542-200508000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ho KM, Harding R, Chamberlain J, Bulsara M. A comparison of central and mixed venous oxygen saturation in circulatory failure. Journal of Cardiothoracic and Vascular Anesthesia. 2010;24(3):434–439. doi: 10.1053/j.jvca.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Changes in central venous saturation after major surgery, and association with outcome. Critical Care. 2005;9(6):R694–R699. doi: 10.1186/cc3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Futier E, Robin E, Jabaudon M, et al. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Critical Care. 2010;14(5, article R193) doi: 10.1186/cc9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scalea TM, Hartnett RW, Duncan AO, et al. Central venous oxygen saturation: a useful clinical tool in trauma patients. The Journal of Trauma. 1990;30(12):1539–1543. [PubMed] [Google Scholar]
- 26.Hutter AM, Jr., Moss AJ. Central venous oxygen saturations: value of serial determinations in patients with acute myocardial infarction. The Journal of the American Medical Association. 1970;212(2):299–303. doi: 10.1001/jama.212.2.299. [DOI] [PubMed] [Google Scholar]
- 27.Ander DS, Jaggi M, Rivers E, et al. Undetected cardiogenic shock in patients with congestive heart failure presenting to the emergency department. The American Journal of Cardiology. 1998;82(7):888–891. doi: 10.1016/s0002-9149(98)00497-4. [DOI] [PubMed] [Google Scholar]
- 28.Rady MY, Rivers EP, Martin GB, Smithline H, Appelton T, Nowak RM. Continuous central venous oximetry and shock index in the emergency department: use in the evaluation of clinical shock. American Journal of Emergency Medicine. 1992;10(6):538–541. doi: 10.1016/0735-6757(92)90178-z. [DOI] [PubMed] [Google Scholar]
- 29.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England Journal of Medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 30.Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Medicine. 2010;36(2):222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Critical Care Medicine. 2007;35(4):1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 32.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. The Journal of the American Medical Association. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 34.Elbers PWG, Ince C. Bench-to-bedside review: mechanisms of critical illness—classifying microcirculatory flow abnormalities in distributive shock. Critical Care. 2006;10(4, article 221) doi: 10.1186/cc4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy RJ, Deutschman CS. Cytochrome c oxidase dysfunction in sepsis. Critical Care Medicine. 2007;35(9):S468–S475. doi: 10.1097/01.CCM.0000278604.93569.27. [DOI] [PubMed] [Google Scholar]
- 36.Lehot JJ, Elarby C, Vallon JJ, Motin J. Oxyhaemoglobin dissociation curve and 2,3-diphosphoglycerate in septic shock. Annales Francaises d’Anesthesie et de Reanimation. 1984;3(2):85–89. doi: 10.1016/s0750-7658(84)80002-7. [DOI] [PubMed] [Google Scholar]
- 37.van Beest PA, Hofstra JJ, Schultz MJ, Boerma CE, Spronk PE, Kuiper MA. The incidence of low venous oxygen saturation on admission to the intensive care unit: a multi-center observational study in The Netherlands. Critical Care. 2008;12(2, article R33) doi: 10.1186/cc6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopterides P, Bonovas S, Mavrou I, Kostadima E, Zakynthinos E, Armaganidis A. Venous oxygen saturation and lactate gradient from superior vena cava to pulmonary artery in patients with septic shock. Shock. 2009;31(6):561–567. doi: 10.1097/SHK.0b013e31818bb8d8. [DOI] [PubMed] [Google Scholar]
- 39.Varpula M, Karlsson S, Ruokonen E, Pettilä V. Mixed venous oxygen saturation cannot be estimated by central venous oxygen saturation in septic shock. Intensive Care Medicine. 2006;32(9):1336–1343. doi: 10.1007/s00134-006-0270-y. [DOI] [PubMed] [Google Scholar]
- 40.Ho BCH, Bellomo R, McGain F, et al. The incidence and outcome of septic shock patients in the absence of early-goal directed therapy. Critical Care. 2006;10(3, article R80) doi: 10.1186/cc4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The outcome of patients with sepsis and septic shock presenting to emergency departments in Australia and New Zealand. Critical Care and Resuscitation. 2007;9(1):8–18. [PubMed] [Google Scholar]
- 42.Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Journal of Intensive Care Medicine. 2011;37(9):1534–1542. doi: 10.1007/s00134-011-2267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagoa CE, de Figueiredo LF, Cruz RJ, Silva E, Rocha e Silva M. Effects of volume resuscitation on splanchnic perfusion in canine model of severe sepsis induced by live Escherichia coli infusion. Critical Care. 2004;8(4):R221–R228. doi: 10.1186/cc2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallet B, Lund N, Curtis SE, Kelly D, Cain SM. Gut and muscle tissue PO2 in endotoxemic dogs during shock and resuscitation. Journal of Applied Physiology. 1994;76(2):793–800. doi: 10.1152/jappl.1994.76.2.793. [DOI] [PubMed] [Google Scholar]
- 45.Nagdyman N, Fleck T, Barth S, et al. Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children. Intensive Care Medicine. 2004;30(3):468–471. doi: 10.1007/s00134-003-2101-8. [DOI] [PubMed] [Google Scholar]
- 46.Feldman Z, Robertson CS. Monitoring of cerebral hemodynamics with jugular bulb catheters. Critical Care Clinics. 1997;13(1):51–77. doi: 10.1016/s0749-0704(05)70296-7. [DOI] [PubMed] [Google Scholar]
- 47.Gayle MO, Frewen TC, Armstrong RF, et al. Jugular venous bulb catheterization in infants and children. Critical Care Medicine. 1989;17(5):385–388. doi: 10.1097/00003246-198905000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Gibbs EL, Lennox WG, Nims LF, Gibbs FA. Arterial and cerebral venous blood: arterial-venous differences in man. The Journal of Biological Chemistry. 1942;144(2):325–332. [Google Scholar]
- 49.Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiologica Scandinavica. 2000;168(4):615–622. doi: 10.1046/j.1365-201x.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 50.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. Journal of Applied Physiology. 1994;77(6):2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 51.Podbregar M, Mozina H. Skeletal muscle oxygen saturation does not estimate mixed venous oxygen saturation in patients with severe left heart failure and additional severe sepsis or septic shock. Critical Care. 2007;11(1, article R6) doi: 10.1186/cc5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohn SM, Nathens AB, Moore FA, et al. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. The Journal of Trauma. 2007;62(1):44–54. doi: 10.1097/TA.0b013e31802eb817. [DOI] [PubMed] [Google Scholar]
- 53.Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Medicine. 2007;33(9):1549–1556. doi: 10.1007/s00134-007-0739-3. [DOI] [PubMed] [Google Scholar]
- 54.Leone M, Blidi S, Antonini F, et al. Oxygen tissue saturation is lower in nonsurvivors than in survivors after early resuscitation of septic shock. Anesthesiology. 2009;111(2):366–371. doi: 10.1097/ALN.0b013e3181aae72d. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez A, Lisboa T, Martin-Loeches I, et al. Mortality and regional oxygen saturation index in septic shock patients: a pilot study. The Journal of Trauma. 2011;70(5):1145–1152. doi: 10.1097/TA.0b013e318216f72c. [DOI] [PubMed] [Google Scholar]
- 56.Heyer L, Mebazaa A, Gayat E, et al. Cardiac troponin and skeletal muscle oxygenation in severe post-partum haemorrhage. Critical Care. 2009;13, supplement 5, article S8 doi: 10.1186/cc8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mesquida J, Gruartmoner G, Martínez ML, et al. Thenar oxygen saturation (StO2) and invasive oxygen delivery measurements in critically ill patients in early septic shock. Shock. 2011;35(5):456–459. doi: 10.1097/SHK.0b013e3182094ab9. [DOI] [PubMed] [Google Scholar]
- 58.Mesquida J, Masip J, Gili G, Artigas A, Baigorri F. Thenar oxygen saturation measured by near infrared spectroscopy as a noninvasive predictor of low central venous oxygen saturation in septic patients. Intensive Care Medicine. 2009;35(6):1106–1109. doi: 10.1007/s00134-009-1410-y. [DOI] [PubMed] [Google Scholar]
- 59.Mulier KE, Skarda DE, Taylor JH, et al. Near-infrared spectroscopy in patients with severe sepsis: correlation with invasive hemodynamic measurements. Surgical Infections. 2008;9(5):515–519. doi: 10.1089/sur.2007.091. [DOI] [PubMed] [Google Scholar]
- 60.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. British Journal of Anaesthesia. 2009;103, supplement 1:i3–i13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 61.Nahum E, Skippen PW, Gagnon RE, Macnab AJ, Skarsgard ED. Correlation of transcutaneous hepatic near-infrared spectroscopy readings with liver surface readings and perfusion parameters in a piglet endotoxemic shock model. Liver International. 2006;26(10):1277–1282. doi: 10.1111/j.1478-3231.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 62.Girardis M, Rinaldi L, Busani S, Flore I, Mauro S, Pasetto A. Muscle perfusion and oxygen consumption by near-infrared spectroscopy in septic-shock and non-septic-shock patients. Intensive Care Medicine. 2003;29(7):1173–1176. doi: 10.1007/s00134-003-1805-0. [DOI] [PubMed] [Google Scholar]
- 63.Mayeur C, Campard S, Richard C, Teboul JL. Comparison of four different vascular occlusion tests for assessing reactive hyperemia using near-infrared spectroscopy. Critical Care Medicine. 2011;39(4):695–701. doi: 10.1097/CCM.0b013e318206d256. [DOI] [PubMed] [Google Scholar]
- 64.Lima A, van Bommel J, Sikorska K, et al. The relation of near-infrared spectroscopy with changes in peripheral circulation in critically ill patients. Critical Care Medicine. 2011;39(7):1649–1654. doi: 10.1097/CCM.0b013e3182186675. [DOI] [PubMed] [Google Scholar]
- 65.Vallée F, Mateo J, Dubreuil G, et al. Cutaneous ear lobe PCO2 at 37°C to evaluate microperfusion in patients with septic shock. Chest. 2010;138(5):1062–1070. doi: 10.1378/chest.09-2690. [DOI] [PubMed] [Google Scholar]


