1. ABSTRACT
Immune system functions require blood leukocytes to continuously traffic throughout the body and repeatedly cross endothelial barriers (i.e., diapedese) as they enter (intravasation) and exit (extravasation) the circulation. The earliest studies to directly characterize diapedesis in vivo suggested co-existence of two distinct migratory pathways: between (para-cellular) and through (trans-cellular) individual endothelial cells. However, in the absence of conclusive in vitro observations, the latter pathway remained poorly accepted. The recent emergence of unambiguous in vitro reports of trans-cellular diapedesis has begun to illuminate mechanisms for this pathway and has renewed interest in its physiological roles. A thorough reevaluation of the existing literature reveals a large number of studies documenting significant use of the trans-cellular pathway in diverse in vivo settings. These include constitutive trafficking in bone marrow and lymphoid organs as well as upregulated extravasation in peripheral tissues during inflammation. Here we collectively summarize these in vivo observations alongside the emerging in vitro data in order to provide a framework for understanding the settings, mechanisms and roles for the trans-cellular route of diapedesis.
Keywords: Diapedesis, Endothelium, Leukocyte, Migration, Para-cellular, Podosome, Transmigration, Trans-cellular, Trafficking, Review
2. INTRODUCTION
In order to perform their immune functions of patrolling for and eliminating pathogens, leukocytes (i.e., lymphocytes and innate immune cells such as neutrophils, monocytes, macrophages and dendritic cells) must continually traffic throughout all compartments of the body (1). The vascular circulation and lymphatic system serve as the main conduits for leukocyte movement. These systems are lined by monolayers of endothelial cells that serve as the principal barrier between the vascular/lymphatic circulation and the underlying tissues. Thus, trafficking of leukocytes requires their repeated crossing of endothelium (i.e., diapedesis) as they enter (intravasation) and exit (extravasation) the circulation. It is recognized that this process represents a critical and rate-limiting component of both constitutive and inflammation-specific trafficking and in this way is an important therapeutic target for immune-mediated and inflammatory disease (1, 2).
Diapedesis, especially during extravasation, has been intensely investigated. The specialized post-capillary venules of secondary lymphoid organs (SLO; i.e., high endothelial venules (HEV)) constitutively express adhesion molecules and chemokines that support entry of circulating naive and subsets of memory lymphocytes (1). Similarly, the endothelium of post-capillary venules in most inflamed peripheral tissues (or capillaries for inflamed lung and liver) upregulates adhesion molecules and chemokines that support diapedesis of effector/memory lymphocytes, as well as innate immune cells (3). In both settings, extravasation begins with the accumulation of circulating leukocytes on the luminal surface of the endothelium through a well-characterized multi-step adhesion and activation cascade (3, 4). This is initiated by transient rolling-type interactions mediated by the selectin family of adhesion molecules, which facilitate sensing of endothelial chemokines. This in turn triggers high affinity interaction of leukocyte integrin receptors (e.g., LFA-1, Mac1 and VLA-4) with their endothelial ligands (e.g., ICAM-1, ICAM-2, and VCAM-1) resulting in leukocyte arrest via firm adhesion (5). Subsequently, lymphocytes undergo actin-dependent spreading, polarization and integrin-dependent lateral migration on the luminal surface of the endothelium. This activity seems to allow leukocytes the ability to search out sites permissive for endothelial barrier penetration (6, 7). Finally, the leukocyte must formally breach and migrate across the endothelium, a process formally referred to as ‘diapedesis’. This last and critical step remains only partially characterized and has long been a subject of controversy (8–10).
Though significant heterogeneity exists (11, 12), the endothelium of the vascular circulation is principally a monolayer of endothelial cells growing on an abluminal matrix (i.e., basement membrane). Junctions between endothelial cells are elaborate and complex, with different adherens, tight and gap junction zones, each formed from distinct molecular components and reinforced by interactions with the cortical actin cytoskeleton (13) (Figure 1). Though clear distinctions have been characterized, lymphatic and vascular endothelium share the same principal features (14, 15). In this way, vascular and lymphatic endothelia form selectively permeable barriers between the circulation and the underlying tissues. The problem for leukocytes of how to cross such barriers has two solutions: 1) disassembly of the intercellular junction and formation of a para-cellular gap (i.e., para-cellular diapedesis), or 2) formation of a trans-cellular pore directly through an individual endothelial cell (i.e., trans-cellular diapedesis).
Figure 1.
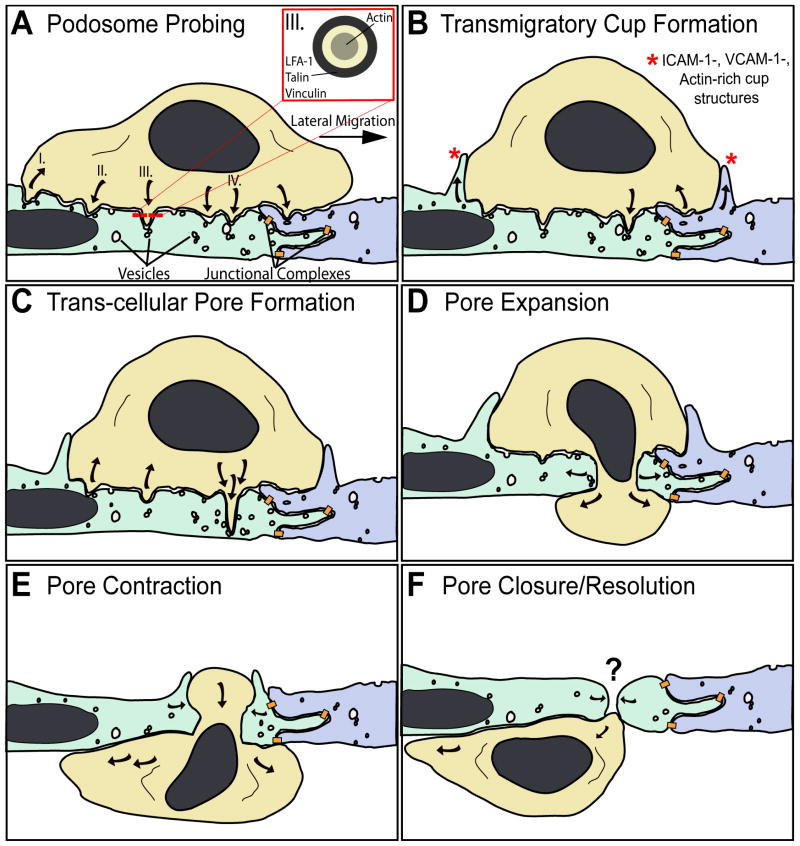
Mechanisms for trans-cellular diapedesis. The schematic summarizing basic morphologic features broadly observed in vivo and in vitro as a leukocyte (tan) progressively migrates across the endothelium through a trans-cellular pore. A small segment of endothelium is depicted in which two individual endothelial cells are distinguished by green and blue coloring. Locations where specific junctional adhesion complexes (i.e., tight, adherens and gap junctions) form are indicated (orange). A. Podosome Probing. The schematic depicts a ‘snapshot’ of a lymphocyte laterally migrating toward an intact inter-endothelial junction. During migration dozens of actin-dependent podosome-like protrusions dynamically form (downward pointing arrows) and retract (upward pointing arrows), concomitantly forcing endothelial invaginations termed ‘podo-prints’. This dynamic protrusion behavior is thought serve in migratory pathfinding as a means of ‘probing’ the endothelial surface for sites permissive to trans-cellular diapedesis. I. Shows a trailing edge podosome retracting. II. Shows a podosome protruding into, and being frustrated by, the rigid nuclear lamina. III. Shows a podosome and its cross-section (inset), highlighting the peripheral LFA-1 integrin/talin/vinculin zone and the actin-rich core. IV. Highlights a specific podosome that progressively extends to become an ‘invasive podosome’ and facilitate trans-cellular pore formation in panels B and C. Endothelial vesicles, VVO and caveolae (‘vesicles’) are seen enriched near or directly fused to podo-prints. B. Transmigratory Cup Formation. Overlapping temporally with podosome probing (A), endothelial cells proactively protrude actin-dependent, ICAM-1/VCAM-1-enriched protrusions (*) that embrace adherent leukocytes, forming ‘transmigratory cups’ that are thought to facilitate transition from lateral to trans-endothelial migration. Note many podosomes-like protrusions have or are retracting while one continues to protrude. C. Trans-cellular pore formation. At permissive sites a podosome-like protrusions progressively extend, transitioning to ‘invasive podosomes’, which forces the endothelial apical and basal plasma membrane into close opposition thereby facilitating initial trans-cellular pore formation for diapedesis. Active SNARE complex-dependent fusion of endothelial vesicle at the site of protrusion may facilitate this process. D. Pore expansion. The leukocyte progressively pushes across the trans-cellular pore causing expansion of its diameter to as much as 5 microns. E. Pore contraction. As the leukocyte completes diapedesis the pore contracts, maintaining close endothelial cell-leukocyte contacts. F. Pore closure/resolution. The leukocyte finally, exits the pore completely. Substantial in vitro and in vivo data support the existence of rapid resealing of the vacated pore. However, no details currently exist on the mechanisms of this important process.
The very first studies to directly address mechanisms for diapedesis in vivo (using electron microscopy (EM)) demonstrated evidence for both para- and trans-cellular routes (16–21). Although largely unappreciated, many subsequent studies demonstrated significant utilization of both routes in a wide range of in vivo settings (as reviewed in Section 3, below). However, the lack of clear evidence for trans-cellular diapedesis in initial studies using cultured in vitro endothelial models (22–27) seemed, for the most part, to overshadow the previous in vivo observations, as well as preclude mechanistic investigation of this pathway.
Recently, the first in vitro observations of trans-cellular diapedesis (as reviewed in Sections 4 and 5, below) have begun to illuminate mechanisms for this process and have brought renewed interest in its physiologic functions. In an effort to more broadly understand the settings, mechanisms and roles for this pathway, we attempt here to collectively review all of the existing in vivo and in vitro data for trans-cellular diapedesis. While discussing some of the in vivo observations and mechanistic characterization of para-cellular diapedesis, as a matter of focus, these topics (which have been reviewed extensively elsewhere (2, 9, 22–24)) will not be covered here in detail.
3. IN VIVO STUDIES OF TRANS-CELLULAR DIAPEDESIS
During immune surveillance various types of blood leukocytes traffic throughout almost all recesses of the body (1). The life cycle of most leukocytes or their progenitors starts in the bone marrow where they originate and then migrate into the circulation. Newly intravasated lymphoid T cell progenitors immediately home to the thymus and eventually reenter the vascular circulation as mature naive T lymphocytes. These then join B lymphocytes in constitutive and repeated cycles of migration into SLO (e.g., lymph nodes, Peyer’s patches, spleen and tonsils) followed by reentry into the vascular circulation via the thoracic duct of the lymphatic system. Distinctly, monocytes constitutively emigrate from the circulation into the peripheral tissues where they differentiate into antigen presenting cells (APCs; e.g., macrophages and dendritic cells). Dendritic cells undergo upregulatable trafficking into the blind-ending lymphatic vessels of the tissue (i.e., the afferent lymphatics) and then move to lymph nodes where they interact with lymphocytes. In cases of infection, APCs bearing pathogen-derived antigen activate expansion of antigen-specific lymphocytes followed by their differentiation into effector/memory lymphocytes. These enter the circulation and then join innate immune cells (e.g., granulocytes) in homing and migrating into the infected/inflamed peripheral tissues. Each of these trafficking steps (i.e., movement into or out of the tissue) requires a diapedesis event.
It is clear, even from the oversimplified summary presented above, that individual diapedesis events may occur in vastly different settings (e.g., tissues, endothelial and leukocyte subtypes and migration/activation stimuli) with highly varied immdediate purposes. Thus, a true understanding of diapedesis mechanisms requires detailed analysis in situ. In this section we review the extensive in vivo/in situ observations of trans-cellular diapedesis that have been made over the past 50 years for both constitutive and inflammation-specific trafficking events in a range of anatomic loci (Table 1). Many of the reported in vivo observations of diapedesis utilize single-section transmission EM (TEM). It is important to recognize that though such observations are often interpreted as support for the use of either a “para-cellular” or “trans-cellular” pathway, taken alone these are essentially ambiguous. Critically, however, many studies have also been conducted with scanning EM (SEM), serial-section TEM or serial-section confocal fluorescence microscopy in the presence of junctional markers and thereby provide reasonably conclusive, and often unequivocal, assessment of route of diapedesis. In Table 1 and throughout the text we have highlighted such methodological distinctions and generally tried to emphasize the most conclusive evidence for trans-cellular diapedesis. In addition, we point out that EM assessment of leukocyte types is based on morphology and authors therefore have tended to use more general descriptive terminology (e.g. ‘agranular leukocytes’, ‘mononuclear inflammatory cells’, ‘polymorphonuclear cells’). Throughout the text and in Table 1 we adhere to these original designations.
Table 1.
In vivo and in vitro observations of trans-cellular diapedesis
| Setting | Species | Tissue1 | Leukocyte | Migratory/Inflammatory Stimuli2 | Method3 | Observed Frequency of Trans-cellular Diapedesis | Mode of Trans-cellular Diapedesis4 | Peri-Junctional5 | Podosome Like Structure6 | Year | Reference7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone Marrow | Guinea Pig | BM | Lymphocytes, Granulocytes, Reticulocytes | Untreated | Serial TEM | Predominant | P | + | + | 1971 | (28)* |
| Rat, Mouse, Guinea Pig | BM | Lymphocytes, Granulocytes, Reticulocytes | Untreated | Serial TEM | Predominant | P | + | 1972 | (30)* | ||
| Rat | BM | Lymphocytes, Megakaryocytes | Untreated | SEM, TEM | Predominant | P | 1976 | (34)* | |||
| Rat | BM | Lymphocytes, Granulocytes, Reticolcytes, Megakaryocytes | Untreated | SEM, TEM | Predominant | P | + | 1976 | (31)* | ||
| Rat | BM | Granulocytes, Reticolcytes | Untreated | SEM, TEM | Predominant | P | + | 1978 | (29)* | ||
| Rat | BM | Lymphocytes, Eosinophils | Untreated | TEM, IFM | Present | P | + | + | 1984 | (32) | |
| Guinea Pig | BM | Eosinophils | IL-5, Eotaxin | TEM | Predominant | P | 1998 | (33) | |||
| Thymus | Guinea pig | Thymus | Thymocytes | Untreated | TEM | Present | 1967 | (35) | |||
| Rat | Thymus | Lymphocytes | Untreated | SEM, TEM | Present | P | + | 1986 | (36)* | ||
| Secondary Lymphoid Organs (HEV) | Rat | LN (c,m,p) | Lymphocytes | (-/+) Staph aureus | Serial TEM | Predominant | E | 1964 | (20)* | ||
| Rat | LN (c,m,p,pa) | Lymphocytes | Untreated | Serial TEM | Predominant | P/E | + | 1975 | (41)* | ||
| Mouse, Rat, Hampster, Guinea Pig | LN (m, pa, a) | Lymphocytes | Untreated | SEM, TEM | Predominant | P | 1979 | (42)* | |||
| Mouse | LN (m) | Lymphocytes | Untreated | SEM, TEM | Predominant | P | + | 1981 | (43)* | ||
| Mouse | PP | Lymphocytes | Untreated | SEM | 37% | P | 1983 | (46)* | |||
| Rat | LN (m), PP | Lymphocytes | Untreated | SEM, Serial TEM | Predominant | P | + | 1986 | (44)* | ||
| Human | Tonsil | Lymphocytes | Tonsillitis | TEM | Present | E | 2002 | (47) | |||
| Guinea Pig | PP | Lymphocytes | Untreated, Intestinal Irritant | Serial TEM | Predominant | P | + | + | 2008 | (45)* | |
| Lymphatics | Rat | LN-Lymphatic Sinusoid | Lymphocytes, Macrophage | Untreated | SEM, Serial TEM | Predominant | P | + | 1980 | (52)* | |
| Chicken | LN-Lymphatic Sinusoid | Lymphocytes | Untreated | TEM | Predominant | P | + | 1984 | (53) | ||
| Rat | ALPA (si) | Macrophage | Untreated | Serial TEM | Predominant | P | + | 1990 | (54)* | ||
| Rat | Lacteals | Macrophage, Lymphocytes | Untreated | Serial TEM | Predominant | P | + | 1990 | (55)* | ||
| Rat | ALPA (si), ALPA (ub) | Lymphocytes, PMN | Untreated, lymphatic stasis, prolonged fast | Serial TEM | Predominant | P | + | 1990 | (56)* | ||
| Rabbit | ALPA (tm) | Lymphocytes | Untreated | Serial TEM | Predominant | P | + | 1998 | (57)* | ||
| Rabbit | PP-ALPA | Lymphocytes | Untreated | Serial TEM | Predominant | P | + | 2000 | (58)* | ||
| Mouse | ALV, TAAL | Lymphocytes, Tumor Cells | Untreated, Tumor | Serial TEM | Predominant | P | + | 2007 | (59)* | ||
| Peripheral Inflammation | Rat | Mesentary | Neutrophils, Eosinophils, Monocytes | Mechanical Trauma | TEM | Present | P | + | + | 1960 | (16) |
| Dog | Pancreas | Leukocytes | Ischemia | TEM | Present | E | 1960 | (17) | |||
| Dog | Pancreas | Leukocytes | Ischemia | TEM | Present | E | + | 1961 | (18) | ||
| Human | Skin | Neutrophils | C5a, NAP, IL-1, LTB4 | TEM | 100% | E | 1989 | (62) | |||
| Mouse | Liver, Lung, Spleen, Kidney, Heart | Lymphocytes | IL-2 | TEM | Present | P | + | 1991 | (63) | ||
| Guinea pig | Skin | Neutrophils, Eosinophils | fMLP | Serial TEM | 91% | P | + | + | 1998 | (64)* | |
| Mouse | Skin | Neutrophil | fMLP | SEM, TEM | 83% | P | 1999 | (65)* | |||
| Mouse | Cremaster | Neutrophils | MIP-2 | In Situ IFM | 14–61% | ND | 2006 | (7)* | |||
| Mouse | Cremaster | Neutrophils | MIP-2 | TEM, In Situ IFM | 20–70% | P/E | 2008 | (66)* | |||
| Blood Brain Barrier | Rat | BBB | Lymphocytes | EAN | TEM | Predominant | E | + | 1968 | (39) | |
| Rabbit | BBB | Agranular Leukocytes | Post-operative Trauma | TEM | Predominant | P | + | 1973 | (77) | ||
| Rat | BBB | Agranular Leukocytes | Thalamus Degeration | TEM | Predominant | E | + | 1974 | (78) | ||
| Cat | BBB | Neutrophils | alpha-bungaro toxin | SEM, TEM | Predominant | P/E | + | + | 1985 | (67)* | |
| Mouse | BBB | Inflammatory Cells | EAE | SEM, TEM | Predominant | P | + | + | 1989 | (68)* | |
| Mouse | BBB | Lymphocytes | EAE | TEM | Predominant | P | + | + | 1990 | (75) | |
| Mouse | BBB | Inflammatory Cells | EAE | SEM, TEM, HVEM | Predominant | P | + | + | 1991 | (69)* | |
| Mouse | BBB | Mononuclear Leukocytes | EAE | Serial TEM | 100% | P | + | + | 2005 | (70)* | |
| Blood Retinal Barrier | Rat | BRB | Lymphocytes | EAU | SEM/Serial TEM | Predominant | P | + | + | 1994 | (71)* |
| Rat | BRB | Macrophages, Granulocytes, Lymphocytes | IL-1beta | TEM | Predominant | P/E | + | + | 1997 | (80) | |
| In Vitro | Human | BBB | T lymphocytes | TNF-alpha | TEM | Present | P | + | 1999 | (83) | |
| Human | HUVEC | Monocytes | TNF-alpha, MCP-1 | IFM | 7% | P | + | 2004 | (85)* | ||
| Human | HUVEC | Neutrophils | TNF-alpha, PAF | IFM | 5% | P | + | 2004 | (85)* | ||
| Human | HUVEC | T Lymphocytes | TNF-alpha, SDF-1 | IFM | 11% | P | + | 2004 | (85)* | ||
| Human | HUVEC | Neutrophils | TNF-alpha | TEM, PCM | 5% | P | + | 2004 | (86)* | ||
| Human | HCAEC | Monocytes | IL-1beta | IFM | 15–37% | P | 2005 | (88)* | |||
| Human | HDMVEC | T lymphocytes | TNF-alpha | IFM | 31% | P | + | 2005 | (90)* | ||
| Human | HUVEC | T lymphocytes | TNF-alpha | IFM, TEM | 9% | P | + | 2005 | (90)* | ||
| Human | Lymphatic | T lymphocytes | TNF-alpha | IFM | 31% | P | + | 2005 | (90)* | ||
| Human | iHUVEC | Neutrophils | TNF-alpha | IFM | 5–60% | P | 2005 | (87)* | |||
| Human | HUVEC | T lymphocytes | TNF-alpha | IFM | 10% | P | + | + | 2007 | (91)* | |
| Human | HDMVEC | T lymphocytes | TNF-alpha | IFM, TEM | 30% | P | + | + | 2007 | (91)* | |
| Human | HLMVEC | T lymphocytes | TNF-alpha | IFM, TEM | 30% | P | + | + | 2007 | (91)* | |
| Human | Lymphatic | T lymphocytes | TNF-alpha | IFM | 30% | P | + | + | 2007 | (91)* | |
| Human | HUVEC, bend.3 | Neutrophils | TNF-alpha | AFM | Present | P | + | 2008 | (92)* |
a, axillary; ALPA, absorbing lymphatic peripheral apparatus; ALV, absorbing lymphatic vessel; BBB, blood brain barrier; BM, bone marrow; BRB, blood retinal barrier; c, cervical; LN, lymph node; p, popliteal; pa, para-aortic; m, mesenteric; PP, Peyer’s patch; si, small intestine; TAAL, tumor-associated absorbing lymphatic; tb, tibial popliteal; tm, tunica mucosa; ub, urinary bladder.
EAE, experimental autoimmune encephalomyelitis; EAN, experimental autoimmune neuritis; EAU, experimental autoimmine uveoretinitis; fMLP, formyl-met-leu-phe; IL, interleukin; LTB4, leukotriene B4; MCP-1, monocyte chemoattractant protein-1; MIP-2, macrophage inflammatory protine-2; NAP, neutrophil activating peptide; PAF, platelet activating factor; SDF-1, stromal-derived factor-1; TNF, tumor necrosis factor.
AFM, atomic force micrscopy; HVEM, high voltage electron microscopy; IFM, immuno-fluorescence microscopy; PCM, phase-contrast microscopy; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
E, emperiopolesis mode of trans-cellular diapedesis; P, pore-spanning mode of trans-cellular diapedesis.
Trans-cellular migration events observe to take place in close proximity to intact inter-endothelial junctions (i.e., ‘peri-junctionally’) are denoted.
Studies demonstrating leukocyte protrusions morphologically similar to podosomes are denoted.
Studies providing at least ‘reasonably conclusive’ or unequivocal demonstration of trans-cellular diapedesis (via either SEM, serial-section TEM or serial-section confocal IFM in the presence of junctional markers) are denoted with an asterisk.
3.1. Intravasation from the bone marrow and thymus
The bone marrow parenchyma is the primary locus for hematopoiesis. Thus, essentially all leukocytes, as well as red blood cells, platelets and certain stem cells, begin their life cycle by migrating across the bone marrow endothelium in order to enter the circulation. As a consequence, this vascular bed is subject to high steady-state levels of diapedesis. Although originally thought to have constitutive pores, the bone marrow endothelium is now recognized to be continuous and generally rather attenuated, particularly near the intercellular junctions (28).
Perhaps more so than any other tissue, EM studies of diapedesis during leukocyte intravasation in bone marrow are remarkably consistent and provide largely conclusive evidence for the predominant use of the trans-cellular pathway (28–34) (Table 1). Here, leukocytes are seen to span across trans-endothelial pores (Figure 1), with various amounts of their cell bodies protruding into the vascular lumen (presumably reflecting their stage in the progression of intravasation). The majority of such investigations find that lymphocyte, granulocyte and eosinophil trans-cellular diapedesis pores are not randomly distributed, but tend to form predominantly in close juxtaposition to intact intercellular junctions (i.e., ‘peri-junctionally’), where the endothelium is thinnest (often only 100–200 nm thick) (28–30, 32). Quantitative analysis showed that, on average, pores formed within 1.4 microns of intact junctions, but many times could be seen by serial-section TEM within 100 nm of them (29, 30). Interestingly, one of these studies demonstrated the presence of actin-rich protrusive structures in lymphocytes and eosinophils, termed ‘podosomes’, which were suggested to facilitate trans-cellular pore formation (32), as discussed below (Section 5.1.).
In addition to blood leukocytes, many studies have concomitantly characterized reticulocytes in the process of intravasation and megakaryocytes forming long processes that span across the bone marrow endothelium (which may be important for release of platelets into the blood stream) (28–31, 34). Such events were shown conclusively to occur through peri-junctional trans-cellular pores similar to those seen with leukocytes (28–31, 34).
Physiologically, a variety of blood and stem cell types are known to home to and re-enter the bone marrow via extravasation. However, we are not aware of any studies that directly characterize the route of extravasation across bone marrow endothelium. Importantly, as many authors acknowledge, direction of diapedesis is never explicit in fixed sample studies. Direction, rather, is inferred from expected overall behaviors in specific settings. Thus, while the majority of flux across the bone marrow represents intravasation, concomitant extravasation may be present in any given vessel, though not necessarily identified as such.
Unlike innate immune cells and B lymphocytes, T lymphocytes first enter the blood stream as progenitor cells and immediately home to and enter the thymus in order to undergo maturation before, once again, entering the circulation. Trafficking at this locus is relatively poorly characterized. However, two studies provide support for trans-cellular diapedesis during the intravasation phase of thymus trafficking (35, 36). The study by Ushiki et. al., provides compelling SEM, coupled with TEM, demonstrating lymphocytes emanating into the lumen of post-capillary venules of the thymus trans-cellularly at relatively central, peri-nuclear locations (36).
3.2. Secondary lymphoid organs: HEV and lymphatics
Naive lymphocytes specific for a particular antigen are very rare and a successful immune response depends on these lymphocytes finding their antigen on an APC (e.g., dendritic cell, macrophage and B cell). This is impossible to accomplish in the vast space of the peripheral tissue compartment. SLO provide a highly concentrated and organized nexus for information exchange between naive/memory lymphocytes and APCs (37). Entry into SLO occurs at two locations: Most lymphocytes enter SLO from the vascular circulation by extravasation across the HEV, whereas most APCs enter by intravasating from the tissue by crossing the afferent lymphatics and then flow into the SLO via lymph circulation. These processes occur constitutively during immune surveillance, but become further upregulated, for example, during infection.
HEV are distinct in that they have a thick cuboidal, rather than the more typical squamous, endothelial phenotype and that they constitutively express adhesion molecules (e.g., PNAd, ICAM-1, ICAM-2 and MAdCAM-1) and chemokines (e.g., CCL19 and CCL21) necessary for recruitment of naive/memory lymphocytes (38). The first studies to investigate extravasation across HEV were done in rat cervical, mesenteric and popliteal lymph nodes in the absence and presence of a Staphylococcus areaus infection (20). Through serial-section TEM, predominantly trans-cellular diapedesis was observed (20). However, in contrast to the common appearance in bone marrow of leukocytes spanning across trans-endothelial pores, lymphocytes migrating across HEV often appeared to be largely, if not completely, engulfed in an individual endothelial cell (20). This particular mode of trans-cellular diapedesis has been referred to as ‘emperiopolesis’ (Greek: em = inside, peri = around, polemai = to wander about) (39, 40). A series of subsequent studies in cervical, mesenteric, popliteal, para-aortic, axillary and tibial-popliteal lymph nodes in a range of animal models (rat, mouse, hamster, guinea pig and chicken) reported similar predominance for trans-cellular diapedesis across HEV by both pore-spanning and emperiopolesis modes (41–44).
A variety of studies have also observed either exclusive trans-cellular (44, 45) or concomitant para- and trans-cellular diapedesis (46) across the HEV of Peyer’s patches (SLO associated with the small intestine). The most recent of these studies, conducted in guinea pig in the absence and presence of intestinal inflammation, demonstrated (via complete ultra-thin serial-section TEM and three-dimensional modeling) unequivocal diapedesis through trans-cellular pores formed in extremely close proximity to intact inter-endothelial junctions (45).
One study has demonstrated the presence of both para- and trans-cellular diapedesis across HEV of human tonsils removed from tonsillitis patients (47).
Though clear evidence exists for trans-cellular diapedesis across HEV, such evidence often appears concomitantly with evidence for para-cellular migration in the same sample. In addition, many studies, not reviewed here, support exclusive use of para-cellular pathways across HEV. No obvious correlations are apparent between specific settings (e.g., SLO, species, inflammatory stimulus) and observed route preference. Thus, the relative role of para- and trans-cellular pathways seems particularly unclear and controversial for HEV. One potential contributing factor to this controversy is the technical challenge presented by the unique thickness of HEV, which during diapedesis leads to exceptionally large and complex three-dimensional endothelial-leukocyte contact surfaces.
The afferent lymphatics represent the main entry point into SLO for APCs, such as dendritic cells. Endothelium in the afferent lymphatics has been generally thought of as having rather poorly organized junctions that facilitate passive movement of fluid and tissue leukocytes. In fact, recent studies have shown that these vessels have continuous adhesion structures bordering their entire periphery, though partitioned into discrete VE-cadherin- or PECAM-1-rich zones (15). Moreover, leukocyte intravasation at this locus has been demonstrated to be an active chemokine- and adhesion molecule-dependent process (48–50). To our knowledge, route of diapedesis in afferent lymphatics has only been assessed in two studies, which, through rather limited single-section TEM, suggested para-cellular diapedesis for “dendritic cells” (51) and “leukocytes” (15). Clearly a much more extensive analysis is required and the roles for para- and trans-cellular pathways remain open questions in this setting (50).
In contrast to the afferent lymphatics, a variety of studies have examined migration of lymphocytes, macrophage, neutrophils and tumor cells across lymphatic endothelium in other structures, such as the lymphatic sinuses of the lymph node (52, 53) and various absorbing peripheral lymphatic vessels, under both normal and inflammatory conditions (54–59). In these studies, largely through serial-section TEM and three-dimensional modeling, diapedesis was found to be predominantly trans-cellular. One of these studies specifically noted vesicular structures, which resemble caveolea and vesiculo-vacuolar organelles (VVO), enriched in the endothelial cells at sites of lymphocyte protrusion that were speculated to play roles in pore formation (53), as discussed below (Section 5.2.).
3.3. Peripheral tissue inflammation
The topic of peripheral inflammation is, indeed, a broad one. Any tissue can become inflamed and the resident vascular beds may exhibit extensive phenotypic and functional diversity (11, 12). Moreover, inflammatory stimuli can be diverse and complex, including infection (by an extremely wide array of pathogens), tissue trauma, ischemia, thrombotic events, presence of allergens or underlying disease and often combinations thereof. Thematically, some aspects of inflammatory responses seem to be broadly relevant, such as the central role for inflammatory cytokines in stimulating endothelial cell activation and upregulated expression of cell surface adhesion molecules (e.g., E-selectin, ICAM-1, VCAM-1 and MAdCAM-1). Endothelial cells also upregulate expression of chemoattractants, such as MCP-1, or take up tissue chemoattractants and present them luminally on their glycocalyx (60). Collectively, these changes result in local recruitment of leukocytes into the tissues. However, the details of the specific adhesion molecules and chemoattractants expressed by the endothelium may vary greatly with tissue and inflammatory stimulus thereby causing recruitment of distinct leukocyte subtypes (61). As discussed in Section 6, such factors may influence the route of diapedesis used and it is therefore difficult to draw generalizations from the diverse studies described below.
The very first studies to directly examine route of extravasation during inflammation (or any setting) provided modest support for a trans-cellular pathway (16–19). Marchesi and Florey initially studied acute inflammation and neutrophil infiltration into rat mesentery in response to mechanical trauma (16). Single-section TEM analysis, focused on the initial stages of neutrophil transmigration, suggested predominant use of a para-cellular pathway. However, potential for trans-cellular diapedesis was suggested by the observation of neutrophil “pseudopods” that protruded deeply into cytoplasm of the endothelium and forced the endothelial apical and basal plasma membranes in close opposition as though poised for pore formation (16, 19). Simultaneously, through investigation of an ischemic model of acute inflammation in dog pancreas, Williams and Grisham also found support for the co-existence of both para- and trans-cellular diapedesis (17, 18). Using single-section TEM, a fraction of the migrating neutrophils were seen to be either partially or completely enveloped by the endothelium (i.e., undergoing emperiopolesis). Importantly, in all of these early studies it was recognized that the only definitive way to determine the route of transmigration with TEM would be to conduct serial sectioning to properly assess the relative location of the endothelial junctions with respect to the site of transmigration.
While many subsequent studies suggested exclusively para-cellular diapedesis (not reviewed here) others provided further support for trans-cellular diapedesis in a range of inflammatory settings. Neutrophil recruitment in human skin was investigated by topical application of complement split products (i.e., C5a), neutrophil activating peptide, interleukin (IL)-1alpha, and leukotriene B4. Punch biopsies from volunteers analyzed by single-section TEM suggested exclusive trans-cellular diapedesis through an emperiopolesis mode (62). Quantitatively similar results were found for lymphocytes migrating in liver, lung, spleen, kidney and heart in an IL-2-induced model of systemic vascular leak syndrome in mice (63).
Dvorak and co-workers provided the first unequivocal demonstration of inflammatory trans-cellular diapedesis via ultra-thin serial-section TEM studies of leukocyte recruitment in response intradermal injection of the bacterial derived chemoattractant formyl-met-leu-phe (fMLP) in guinea pigs (64). It was found that out of 11 complete serial-section sets/reconstructions, 10 (91%) followed an unambiguous trans-cellular diapedesis route. A similar study using fMLP injected into the skin of mouse lips provided compelling SEM and TEM that also demonstrated predominant trans-cellular diapedesis (20 out of 24; 83%) (65). A common criticism of these studies is the suggestion that the observed trans-cellular diapedesis was a result of the supra-physiologic levels of fMLP used. This suggestion would imply that the strength of migratory/inflammatory stimuli is a key modulator of route of diapedesis, a possibility that remains to be directly assessed.
Very recently, Kubes and co-workers have documented unambiguous trans-cellular diapedesis events in situ/in vivo for the first time using serial-section confocal fluorescence microscopy (7, 66). In this way, approximately15% of neutrophils could be seen transmigrating (in macrophage-inflammatory protein-2-stimulated mouse creamaster muscle post-capillary venules) at sites distant from the fluorescently tagged PECAM-1-rich junctions and, thus, could be conclusively assessed as trans-cellular (7, 66). This value increased to ~70% in neutrophils lacking expression of the integrin Mac-1 (7, 66). In either case, the remainder of diapedesis events that occurred close to the PECAM-1-rich junctions were inferred to represent para-cellular migration. However, the optimal resolution of light microscopy (approximately 200 nm) may be inadequate to assess many trans-cellular diapedesis events that occur (as determined by TEM) within a few hundred nanometers of intact junctions (20, 28–30, 43–45, 52, 54–59, 64, 67–71). Thus, the degree to which the observed peri-junctional events represent true para-cellular migration or trans-cellular migration close to the junctions remains unclear. Despite this limitation, the important advance of being able to observe unambiguous trans-cellular diapedesis in vivo via a fluorescence approach (which is of much higher throughput compared to EM) will no doubt be critical to extending our understanding of the physiologic roles and mechanisms for this pathway.
3.4. Blood-brain and blood-retinal barriers during inflammatory pathology
The central nervous system (CNS) is an immune privileged compartment in which leukocyte trafficking is tightly limited by the specialized endothelial blood brain barrier (BBB). Hallmarks of the BBB include especially well formed inter-endothelial tight junction zones and low levels of caveolae and pinocytotic vesicles which limit para- and trans-cellular permeability, respectively (72). During CNS inflammatory pathology, trafficking of leukocytes across the BBB may become significantly upregulated. As discussed below, many studies investigating mechanisms of such CNS trafficking find a predominant role for trans-cellular diapedesis pathways.
One of the most clinically important conditions for aberrant leukocyte trafficking to the CNS is multiple sclerosis (MS). The recent clinical success of a therapeutic agent that seems to act largely by blocking such trafficking (i.e., the alpha4 integrin function-blocking antibody Natalizumab) underscores the central importance of diapedesis across BBB in MS (73). An early study of transmigration in an animal model of MS (i.e., experimental autoimmune encephalomyelitis (EAE)), suggested, through relatively limited single-section TEM observations in rat, that mononuclear leukocytes might cross the BBB para-cellularly (74). Extensive, subsequent studies of murine EAE using TEM, SEM and high-voltage EM (HVEM) strongly supported trans-cellular diapedesis of mononuclear inflammatory cells via pores juxtaposed to intact tight junctions (68, 69, 75). Very recently, Engelhardt and co-workers have provided unequivocal demonstration of exclusively trans-cellular diapedesis in mouse BBB venules inflamed by EAE via complete TEM serial-sectioning encompassing entire transmigration sites (70). Morphologically, these studies showed endothelial cells ‘embracing’ (but never fully enveloping) lymphocytes as they protruded micro-scale ‘process’ across peri-junctional trans-endothelial pores. Importantly, these authors emphasize the distinct diapedesis kinetics in EAE compared to most other settings (i.e., hours instead of minutes (76)), which may have significant mechanistic implications.
Diapedesis across BBB has also been studied in a variety of other inflammatory settings. In models of acute inflammatory autoimmune neuritis (experimental allergenic neuritis) in rats (39), post-operative neural degeneration in rabbits (77) and corticectomy-induced thalamic nerve degeneration in rats (78), mononuclear leukocytes were observed to migrate across BBB exclusively via trans-cellular pathways. Moreover, in an acute inflammatory meningitis model in cats (induced with alpha-bungarotoxin), predominant trans-cellular migration of polymorphonuclear leukocytes was seen across the BBB via both pore-spanning and emperiopolesis modes (67).
The blood-retinal barrier (BRB) is a specialized endothelial structure that forms a highly selective barrier between the retinal parenchyma and the blood and is functionally identical to the BBB (79). Like the CNS, levels of leukocyte trafficking in retina are only significant during inflammatory disease, such as posterior uveitis. In an experimental model of this disease (i.e., experimental autoimmune uveoretinitis; EAU) in rat, Greenwood and coworkers showed that mononuclear leukocytes crossed the BRB exclusively via trans-cellular pores (71). These observations were extended with an IL-1beta-mediated acute inflammation model in which monocytes, neutrophils and eosinophils were all seen to transmigrate across the BRB both by trans-cellular pores and apparent emperiopolesis (80). In each of these studies leukocytes were noted as initiating diapedesis by “probing” into and penetrating endothelial cells with actin-rich “pseudopodia” that formed close to, but not through intact tight junctions.
4. IN VITRO STUDIES OF TRANS-CELLULAR DIAPEDESIS
The development of techniques in the early 1970’s for isolation and culture of primary endothelial cells as in vitro models of the endothelium (81, 82) facilitated characterization of diapedesis in experimentally tractable in vitro systems. Initial investigations using single-section TEM suggested that neutrophils stimulated with prostaglandins, thromboxane or fMLP (25, 26) or unstimulated monocytes (27) crossed endothelial monolayers para-cellularly. A variety of subsequent EM and light microscopy studies largely supported these initial findings (reviewed in (2, 9, 22–24)). However, at least one of these studies, examining lymphocytes migrating across a TNF-alpha-activated human BBB model, supported (via single-section TEM) the coexistence of both para- and trans-cellular diapedesis routes in vitro (83). Moreover, based on the extensive presence of monocyte and neutrophil ‘pseudopods’ protruding into the apical endothelial cell surface independent of junctions (in the presence of fMLP or leukotriene B4), another study suggested the potential for concomitant para- and trans-cellular diapedesis in vitro (84)
Starting in 2004, an expanding group of investigators began making the first unambiguous in vitro observations of trans-cellular diapedesis largely through advanced fluorescence imaging approaches (85–92). These observations have been made with a range of leukocytes (neutrophils, monocytes and memory and effector lymphocytes) and endothelial cells (human umbilical vein endothelial cells (HUVEC), human coronary artery endothelial cells (HCAEC) human dermal (HDMVEC) and lung (HLMVECs) microvascular endothelial cells and human lymphatic endothelial cells (HLyECs)). Moreover, studies have been done in the absence and presence of diverse migratory/inflammatory stimuli (TNF-alpha, IL1–1beta, IL-8, SDF-1, fMLP and PAF) and under both static and physiologic shear flow conditions. In all cases, the observed trans-cellular migration occurred through the pore-spanning mode.
The quantitative contribution of the trans-cellular mechanism to overall diapedesis (i.e., including both trans- and para-cellular) in these in vitro studies has ranged from 5 to 60%. Interestingly, on HUVEC models (a representative macro-vascular/conduit endothelial cell type) the percentage of trans-cellular diapedesis has been observed to be only 5–10% (85–87, 90). Alternatively, studies with micro-vascular models (i.e., HDMVECs, HLMVECs), as well as HCAECs and HLyECs tended to show much higher use of trans-cellular routes (30–40%) (88, 90, 91). These results clearly demonstrate that heterogeneity exists in the propensity of different endothelial cell types to support trans-cellular diapedesis. In addition, these results point toward the predominant use of HUVEC monolayers in the early in vitro studies as a factor that may have helped to preclude initial observation of the trans-cellular route.
5. MECHANSISMS FOR TRANS-CELLULAR DIAPEDESIS
The recent in vitro investigations have begun to illuminate mechanisms for trans-cellular diapedesis. Importantly, the wealth of preexisting morphologic observations in vivo provides significant support for these mechanistic findings. As noted throughout Section 3, trans-cellular diapedesis in vivo has been observed to occur mostly through trans-endothelial pores (‘P’ in Table 1), but also via a process whereby leukocytes apparently become transiently enveloped within endothelial cells (i.e., emperiopolesis; ‘E’ in Table 1). Currently, in vitro observations and direct mechanistic investigations are limited to the pore-spanning mode, which will be the focus of the discussion below (Figure 1). However, it seems likely that the key mechanistic components important for this mode of migration (e.g., leukocyte protrusion and endothelial membrane fusion) are shared by emperiopolesis.
5.1. Leukocyte protrusion
Following initial adhesion to the endothelium, an important prerequisite to formal diapedesis is the active seeking out and identification of sites permissive to penetration. Leukocyte polarization and integrin-dependent lateral migration over the endothelial surface are critical to this process (6, 7). It has been generally interpreted that this allows leukocytes to move toward junctions for para-cellular diapedesis (6, 7). However lateral migration is also observed to precede trans-cellular diapedesis (86, 87, 90, 91) and this likely plays an analogous role in positioning leukocytes appropriately (i.e., where trans-cellular pore formation can occur most efficiently). Early TEM studies in bone marrow recognized that the peri-junctional regions are generally the thinnest and may therefore in many cases represent the path of least resistance for trans-cellular pore formation (29). In fact, the preference for trans-cellular diapedesis near the intercellular junctions has been noted in a wide range of studies that include all of the major in vivo settings characterized to date (16, 20, 28–30, 32, 43–45, 52, 54–59, 64, 67–71, 80) (Table 1). Based on this preference, it was suggested that leukocytes somehow “seek out these regions” (30). However, the basis of how leukocytes might identify and subsequently exploit sites permissive for trans-cellular diapedesis (whether peri-junctional or otherwise) was unclear.
Recent fluorescence and electron microscopy imaging studies suggest that podosome-like protrusions formed by leukocytes may be critical for this process (91). Podosomes (i.e., ‘foot-protrusions’) are actin-dependent protrusive structures (approximately 500 nm in both diameter and depth) that form on the ventral surface of highly migratory cells such as leukocytes (93). It was recently demonstrated, through fluorescence microscopy, that lymphocytes and monocytes dynamically protrude and retract (with half-lives of about 20 seconds) dozens of podosome-like protrusions as they migrate laterally over the endothelium (91) (Figure 1A). These protrusions force cognate invaginations (termed ‘podo-prints’) into the surface of the endothelium, locally displacing cytoplasm, cytoskeleton and other organelles (91). Similar dynamic lymphocyte-induced endothelial invaginations were suggested by total internal reflection fluorescence microscopy studies (90). Importantly, efficient trans-cellular diapedesis was always preceded by, and indeed required this dynamic protrusive behavior (91). Moreover, in vitro and in vivo TEM studies revealed a continuum of protrusion depths ranging from 100 nm to 2000 nm, with the longer structures (‘invasive podosomes’) often spanning nearly the entire endothelial cell thickness, placing the apical and basal membranes in close opposition (91) (Figure 1C). Ultrastructurally similar protrusions were also observed during trans-cellular diapedesis of neutrophils (86). It was therefore proposed that dynamic podosome-like protrusions serve to stochastically ‘probe’ the endothelial surface as a means of identifying locations of relatively low endothelial resistance where protrusions can then extend progressively to promote trans-cellular pore formation (91).
Whereas the recent studies have helped to develop an integrated mechanistic model for the roles of podosome-like structures in diapedesis, the basic ideas of this model had been previously suggested and supported by diverse observations. In fact, the very first description of podosomes (and coining of term) was made in the context of lymphocyte and eosinophils trans-cellular migration across bone marrow endothelium in vivo (32). Remarkably, these authors predicted that these actin-enriched structures might supply the protrusive force necessary for trans-cellular pore formation. In the following year Marchisio and coworkers performed the first detailed molecular characterization of podosomes in transformed fibroblasts (94). Shortly thereafter this group demonstrated formation of podosomes by leukocytes (natural killer cells) adhering to endothelium in vitro and suggested that these may function in endothelial penetration during diapedesis (95). Moreover, though not formally described as ‘podosomes’, many in vivo studies have demonstrated the presence of protrusive structures with clearly podosome-like morphology (variously referred to as ‘pseudopodia’, ‘processes’, ‘probing-pseudopods’, ‘microvilli-, fillipodia- and finger-like protrusions’) formed by lymphocytes, monocytes, neutrophils, eosinophils and acute myeloid leukemia tumor cells during diverse trans-cellular diapedesis events (16, 17, 28, 31, 32, 39, 41, 45, 53, 63, 64, 67–71, 75, 77, 78, 80, 96, 97) (Table 1). Such protrusions were often interpreted as “probing” the endothelium and potentially driving trans-cellular pore formation (16, 32, 39, 41, 53, 68–71, 75, 80, 96). Finally, even among the early in vitro studies that concluded predominant use of para-cellular diapedesis, podosome-like structures were prominent (25, 26, 84). Collectively, these observations demonstrate that the ability/tendency of leukocytes to protrude podosome-like structures into the endothelial surface during diapedesis is broadly relevant.
5.2. Endothelial membrane dynamics
One principal distinction of trans-cellular diapedesis is the absolute requirement for membrane fusion and fission events to accommodate pore formation and resolution, respectively. The protrusive forces supplied by ‘probing’ leukocytes (Section 5.1.) likely act to promote initial apical and basal endothelial membrane fusion during pore formation. In addition, the endothelium appears to contribute proactively to this process through regulated membrane fusion activity. Recent in vitro studies show enrichment of the caveolae marker caveolin-1, vesicles, VVO and fusogenic proteins (i.e., the SNAREs VAMP2 and 3) in endothelium at sites of podosome-like protrusion and trans-cellular pore formation (90, 91) (Figure 1A–C). Interestingly, an earlier in vivo study, involving lymphocyte trans-cellular diapedesis in lymph node lymphatic sinusoids, noted lymphocyte protrusions (with podosome-like appearance) associated with clusters of fusing vesicles in the opposed endothelium. From this, it was speculated that “the fusing of the vesicles may form a gradual trans-endothelial channel in which the pseudopod of the lymphocyte penetrates and by this mechanism the lymphocyte may cross the endothelium” (53). Several other studies have also demonstrated local enrichment and fusion of endothelial vesicles at sites of leukocyte protrusion in vivo (71, 91, 97) and in vitro (84). Efficiency of trans-cellular diapedesis was significantly reduced by siRNA knockdown of caveolin-1 in endothelium (90, 91). Similar results obtained by pretreatment of endothelium with N-ethylmaleimide (NEM; a functional perturbant of NSF) support a potential role for the NSF/SNAP/SNARE fusogenic machinery in pore formation (91), though this idea still requires further experimental support (owing to the relatively poor selectivity of NEM). Collectively, these studies suggest a model in which, as a response to interaction with leukocytes, endothelial cells might trigger SNARE complex-mediated recruitment and fusion of vesicles. This fusion may serve to increase the local plasma membrane surface area, which could facilitate progressively deeper probing of leukocyte protrusions and ultimately trans-cellular pore formation (Figure 1B and C). Vesicle fusion could also enhance trans-cellular migration efficiency by local delivery of adhesion receptors (90, 98) and chemokines (60).
After initial pore formation, phases of expansion (to a maximum of 5 microns in diameter) and contraction occur that are largely determined by the passage of the leukocyte nucleus across the pore (Figure 1D–E). Eventually, the leukocyte exits the pore completely and the pore reseals (Figure 1F). This final step presumably involves tight constriction of the pore and membrane fission to reverse the fusion between the apical and basal membranes. It has been frequently noted in diverse in vivo studies that after diapedesis no signs of the trans-cellular pore are evident indicating that the pores are transient and close rapidly (16, 29, 30, 32, 39, 63, 65). Similar observations have been made in vitro (85–88, 90, 91). Efficient pore closure/resolution, would seem to be of critical importance for the maintenance of barrier function thereby preventing excess plasma leakage and inappropriate leukocyte migration. However, the basic mechanisms and regulation for this critical step remain essentially uncharacterized.
5.3. Adhesion molecules
Studies of diapedesis mechanisms have traditionally focused on the roles of adhesion molecules. For para-cellular diapedesis, models have emerged in which homophilic, junctionally enriched, adhesion molecules (e.g., PECAM-1, JAM-1 and CD99) play central roles (22, 23). For trans-cellular diapedesis, studies have suggested roles for specific adhesion molecules, but consistent models remain to be established.
Some initial studies have suggested that the ‘junctional’ adhesion molecules that are involved in para-cellular diapedesis (i.e., PECAM-1 and JAM-1) might also be involved in trans-cellular migration (91) and this has been suggested to potentially occur through intracellular trafficking mechanisms (98). However, more extensive analysis of this possibility is still required.
Other studies have focused on roles of leukocyte integrins and their endothelial ligands in determining the route of transmigration. One in vitro study demonstrated that neutrophil trans-cellular migration was strongly favored by high endothelial ICAM-1 expression in a largely LFA-1 (and to a lesser extent Mac-1)-dependent manner (87). Alternatively, in an in vivo model, knockout of Mac-1 integrin was also found to be associated with a large increase (70% compared to 15% for wild type) in trans-cellular migration (7). In both cases, altered lateral migration was suggested to be the basis for the change in route, but through opposite mechanisms (i.e., increased (87) versus reduced (7) adhesion). Again, a clear interpretation of these findings will require further analysis.
Relatively consistent findings have been made for the roles of endothelial ICAM-1 and VCAM-1 in both traction and barrier maintenance during trans-cellular, as well as para-cellular, migration. ICAM-1 and VCAM-1 engagement by leukocyte integrins drives proactive protrusion of actin/vimentin-enriched microvilli-like protections in endothelium that partially embrace adherent and migrating leukocytes (5, 85, 89, 99, 100) (Figure 1B). These structures, termed ‘transmigratory-cups’ or ‘docking structures’ have been observed in a wide range of in vitro and in vivo settings and are thought to facilitate para- and trans-cellular protrusion across the endothelium by providing a traction scaffold oriented in the direction of diapedesis. In addition, these structures may minimize barrier disruption by enhancing the leukocyte-endothelial contact area (18, 63, 66, 67, 70, 75, 85, 89, 90, 92, 99–101). With regard to this latter function, several studies also show strong enrichment of LFA-1 and ICAM-1 and close cell-cell opposition at the trans-cellular pores themselves (85, 87, 90, 91).
6. TRANS-CELLULAR VERSUS PARA-CELLULAR
The extensive in vivo studies accumulated over the past 50 years, coupled to the emerging in vitro data, clearly establish that two diapedesis pathways co-exist, each with the propensity to contribute in quantitatively important ways to overall leukocyte trafficking. Thus, we are faced not only with the challenges of elucidating the basic mechanisms for each pathway, but also with understanding the settings where each may be most important, the relative functional roles of each and the molecular/cellular determinants driving usage of one pathway over another. The answers to these questions will be critical for a complete understanding of leukocyte trafficking, and for efforts to develop novel anti-inflammatory therapeutics that effectively target diapedesis.
6.1. Quantitative contribution of trans-cellular diapedesis
An important starting point for understanding the overall roles/functions for trans- and para-cellular migration routes is simply to quantify their relative usage in specific in vivo settings. The existing in vivo analyses of trans-cellular diapedesis (as reviewed throughout Section 3) are far from comprehensive or systematic. Nonetheless, a few consistent themes are apparent. Perhaps the most important, is the idea that overall route usage in vivo is highly heterogeneous. Some of this variability may be attributable to specific technical challenges in assessing route (e.g., imaging HEV), but may largely come from the variability in the models/settings examined. The latter may have particular relevance to extravasation associated with peripheral inflammation, an especially broad topic. However, relatively consistent evidence for predominant usage of trans-cellular diapedesis has emerged in two settings: 1.) constitutive intravasation across bone marrow endothelium and 2.) disease-related extravasation across BBB and BRB. The reason for the dominant role of the trans-cellular route in these settings is not yet clear. We might speculate that in bone marrow it serves to accommodate the high constitutive flux of leukocyte trafficking without disrupting overall endothelial integrity (see Section 6.2., below). Alternatively, in BBB/BRB trans-cellular diapedesis may be favored as a consequence of the exceptionally strong barrier provided by the tight junctions in these endothelia (See Section 6.3., below). Experimental support for role of junctional stability in determining route was provided by Sandig and coworkers who showed that an endothelial junction-destabilizing agent (i.e. IL-1beta) reduced trans-cellular diapedesis of monocytes in favor of the para-cellular route (88).
In vitro observations made in diverse, though largely inflammatory, models have also yielded highly varied results. Of course, many of the early studies concluded exclusive use of para-cellular migration routes (22, 23, 25–27). Among the recent studies reporting trans-cellular diapedesis, the quantitative contribution of trans-cellular diapedesis has ranged from 5 to 60% with the majority of observations showing ≤ 30% (85–92). It is important to note, however, that studies relying on light microscopy (as is the case for the majority of current in vitro reports), may significantly underestimate trans-cellular diapedesis. Many unambiguous trans-cellular migration events are observed by TEM to occur within 100–200 nm of intact junctions (20, 28–30, 43–45, 52, 54–59, 64, 67–71) (Figure 1), a distance that falls at or below the limit of resolution for light microscopy. Thus, many peri-junctional events, which have been scored by default as para-cellular (85, 87, 89–91) may indeed be trans-cellular. An additional consideration for quantifying the contribution of para- and trans-cellular pathways is the role of the in vitro setting it self. Broadly speaking, endothelial cells in vivo turnover roughly once per year, and are in the presence of steady state laminar shear forces, mural cells (e.g., pericytes, vascular smooth muscle cells and astrocytes) and micromolar concentrations of plasma sphingosine-1-phosphate, all conditions that promote organization of inter-endothelial junctions and barrier function (11, 12, 102–108). Each of these factors are conspicuously absent in most in vitro models, where junctions are generally much less well organized/differentiated. Thus, in vitro models may be non-physiologically skewed toward the para-cellular route as the ‘path of least resistance’ (see Section 6.3., below).
6.2. Functional role of the trans-cellular route
The question of the functional role for having both a trans-cellular and para-cellular pathway for diapedesis remains a matter of speculation at this time. One possibility may be in maintenance of overall endothelial integrity and barrier function. The primary feature that defines endothelium as an integrated organ and barrier is the intercellular junctions (13). Clearly, para-cellular migration requires local disassembly of endothelial junctional adhesion complexes. Trans-cellular diapedesis could be viewed as a means of circumventing the junctions and thereby leaving these central structural elements intact, which could be particularly important in settings of high leukocyte flux. In this regard, it is interesting to note that the setting subject to arguably the greatest steady-state levels of diapedesis (i.e. bone marrow) is also the setting where predominant use of trans-cellular diapedesis is most clearly established (28–34). In addition, closure of the transmigration passage subsequent to diapedesis must be efficient to maintain proper barrier function. It could be envisioned that this efficiency may be relatively enhanced for trans-cellular events, which depend only on a single endothelial cell compared to para-cellular events, which rely on coordinated activity of two or more endothelial cells.
6.3. Determinants of route of diapedesis
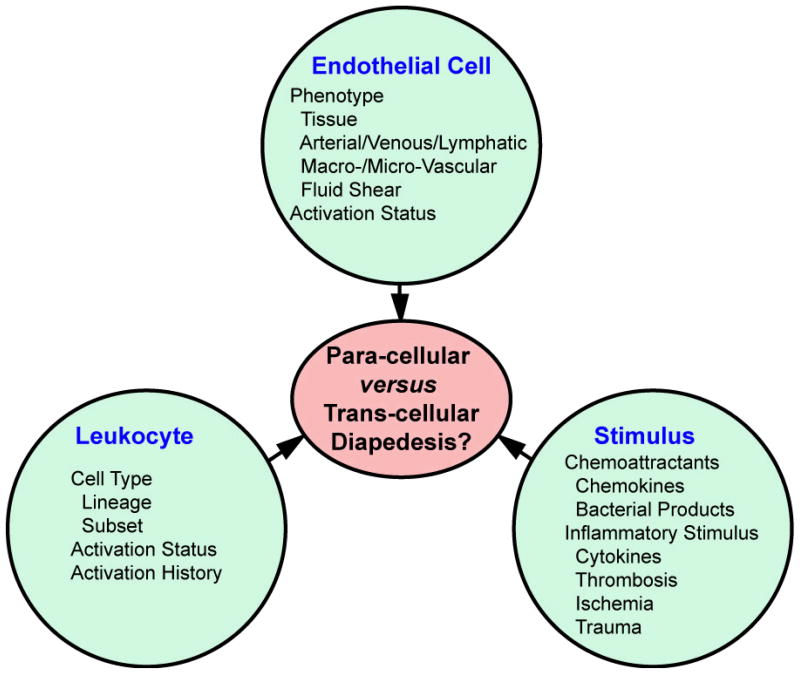
An important question for leukocyte trafficking is what determines whether a leukocyte will utilize a trans- or a para-cellular migration pathway in any given setting. As suggested in Figure 2, this will likely be established as a result of the convergence and integration of the many variables associated with three key factors: endothelium, leukocyte and migratory/inflammatory stimulus.
Figure 2.
Potential factors influencing route of diapedesis. The schematic emphasizes three main factors (endothelial cells, leukocyte and stimulus) and some of the key variables related to each of these that likely influence cell phenotype and behavior and, therefore, the route of diapedesis. Thus, route of diapedesis may be a highly context-specific issue.
It has been proposed that diapedesis occurs through the ‘path of least resistance’ (96). Simply stated, this idea suggests that the relative ease of forming a para-cellular gap versus a trans-cellular pore should be a key determinant of route utilization. De novo formation of a trans-cellular pore requires endothelial membrane bending/invagination, displacement of inter-endothelial components (e.g., cytoskeleton and other organelles) and membrane fusion. Clearly all of these activities require energy, but some loci (e.g., highly attenuated peri-junctional regions) will likely require relatively less than others. Opening of a para-cellular gap also requires energy. In general, inter-endothelial junctions in vivo tend to be highly interdigitated, involving (by cross-section) many microns of linear cell-cell contact (70, 109) and are stabilized by well-organized molecular adhesion complexes (i.e., adherens, tight and gap junctions) associated with cortical actin networks (13) (Figure 1). However, the relative degree of junctional organization and barrier function may vary greatly in many distinct settings. The mechanisms for junction disassembly during para-cellular migration are thought to include an energy-expensive process of Rho-mediated stress fiber assembly and contraction (22, 23, 110).
The path of least resistance thus relies on endothelial activity and phenotype, which in turn is determined by aspects of its specific location within the vascular tree including the tissue micro-environment, the position in the arterial, venous or lymphatic circulation and whether it is part of a macrovascular conduit structure or a microvascular structure, which is optimized for exchange of fluid and solute. Each local endothelial structure will be subject to specific micro-environmental cues including distinct laminar shear or mechanical stretch profiles, basement membrane compositions, investiture with mural cells (e.g., pericytes, vascular smooth muscle cells, and astrocytes) and exposure to inflammatory and vasoactive stimuli (thereby determining the activation status). Collectively, such factors can strongly influence endothelial junction and cytoskeleton organization, vesicle/VVO/caveolae expression, intracellular trafficking/fusion, and expression of adhesion molecules/chemoattractants. All such parameters may profoundly effect the relative patency of a para- versus trans-cellular migration pathway.
While the path of least resistance is important, the ability of leukocytes to identify and exploit this path is equally important. Thus, the specific nature of the leukocyte type, activation status and activation history (particularly important for lymphocytes), along with the specific migratory/inflammatory stimulus and endothelial adhesion molecules will collectively determine the phenotype and migratory behavior of these cells and thereby critically influence the route of diapedesis.
7. PERSPECTIVE
It has become evident through a large number of in vivo and emerging in vitro studies that two physiologically relevant pathways for leukocyte diapedesis co-exist. Contrasting the extensively studied para-cellular pathway, mechanisms for trans-cellular diapedesis are just beginning to be elucidated and include roles for podosome-like leukocyte protrusions and regulated fusogenic activity in endothelium. However, a much more detailed molecular understanding of trans-cellular pore formation and closure, as well as the determinants driving preference for trans- versus para-cellular routes is necessary. Another important goal will be to more systematically assess the relative usage of para- and trans-cellular routes in distinct tissues and settings in vivo. Such mechanistic and route usage information will be critical for a complete understanding of leukocyte trafficking and for the development of effective anti-inflammatory therapeutics that target diapedesis.
Acknowledgments
This work was supported by the NIH (T32 AI070085–01; P.T.S.) and Arthritis Foundation (C.V.C.).
Abbreviations
- APC
antigen presenting cell
- BBB
blood-brain barrier
- BRB
blood-retinal barrier
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- EAN
experimental allergic neuritis
- EAU
experimental autoimmune uveoretinitis
- fMLP
formyl-met-leu-phe
- HDMVEC
human dermal microvascular endothelial cells
- HLMVECs
human lung microvascular endothelial cells
- HUVEC
human umbilical vein endothelium
- IL
interleukin
- MS
multiple sclerosis
- SEM
scanning electron microscopy
- SLO
secondary lymphoid organ
- TEM
transmission electron microscopy
- TNF
tumor necrosis factor
- VVO
vesiculo-vacuolar organelle
References
- 1.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA., Jr Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol. 1994;125:1417–27. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–56. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 7.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–75. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvietys PR, Sandig M. Neutrophil diapedesis: paracellular or transcellular? News Physiol Sci. 2001;16:15–9. doi: 10.1152/physiologyonline.2001.16.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Muller WA. Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation. 2001;8:181–93. doi: 10.1038/sj/mn/7800078. [DOI] [PubMed] [Google Scholar]
- 10.Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–63. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 11.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 12.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–90. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 13.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 14.Pepper MS, Skobe M. Lymphatic endothelium: morphological, molecular and functional properties. J Cell Biol. 2003;163:209–13. doi: 10.1083/jcb.200308082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchesi VT, Florey HW. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960;45:343–8. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- 17.Williamson JR, Grisham JW. Leucocytic emigration from inflamed capillaries. Nature. 1960;188:1203. doi: 10.1038/1881203a0. [DOI] [PubMed] [Google Scholar]
- 18.Williamson JR, Grisham JW. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol. 1961;39:239–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesi VT. The site of leucocyte emigration during inflammation. Q J Exp Physiol Cogn Med Sci. 1961;46:115–8. doi: 10.1113/expphysiol.1961.sp001522. [DOI] [PubMed] [Google Scholar]
- 20.Marchesi VT, Gowans JL. The Migration of Lymphocytes through the Endothelium of Venules in Lymph Nodes: an Electron Microscope Study. Proc R Soc Lond B Biol Sci. 1964;159:283–90. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 21.Marchesi VT. Some Electron Microscopic Observations on Interactions between Leukocytes, Platelets, and Endothelial Cells in Acute Inflammation. Ann N Y Acad Sci. 1964;116:774–88. doi: 10.1111/j.1749-6632.1964.tb52545.x. [DOI] [PubMed] [Google Scholar]
- 22.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. Leukocyte transendothelial migration: a junctional affair. Semin Immunol. 2002;14:105–13. doi: 10.1006/smim.2001.0347. [DOI] [PubMed] [Google Scholar]
- 23.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–34. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–36. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 25.Furie MB, Naprstek BL, Silverstein SC. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J Cell Sci. 1987;88(Pt 2):161–75. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- 26.Beesley JE, Pearson JD, Hutchings A, Carleton JS, Gordon JL. Granulocyte migration through endothelium in culture. J Cell Sci. 1979;38:237–48. doi: 10.1242/jcs.38.1.237. [DOI] [PubMed] [Google Scholar]
- 27.Pawlowski NA, Kaplan G, Abraham E, Cohn ZA. The selective binding and transmigration of monocytes through the junctional complexes of human endothelium. J Exp Med. 1988;168:1865–82. doi: 10.1084/jem.168.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Bruyn PP, Michelson S, Thomas TB. The migration of blood cells of the bone marrow through the sinusoidal wall. J Morphol. 1971;133:417–37. doi: 10.1002/jmor.1051330406. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain JK, Lichtman MA. Marrow cell egress: specificity of the site of penetration into the sinus. Blood. 1978;52:959–68. [PubMed] [Google Scholar]
- 30.Campbell FR. Ultrastructural studies of transmural migration of blood cells in the bone marrow of rats, mice and guinea pigs. Am J Anat. 1972;135:521–35. doi: 10.1002/aja.1001350406. [DOI] [PubMed] [Google Scholar]
- 31.Becker RP, De Bruyn PP. The transmural passage of blood cells into myeloid sinusoids and the entry of platelets into the sinusoidal circulation; a scanning electron microscopic investigation. Am J Anat. 1976;145:183–205. doi: 10.1002/aja.1001450204. [DOI] [PubMed] [Google Scholar]
- 32.Wolosewick JJ. Distribution of actin in migrating leukocytes in vivo. Cell Tissue Res. 1984;236:517–25. doi: 10.1007/BF00217218. [DOI] [PubMed] [Google Scholar]
- 33.Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med. 1998;188:1621–32. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muto M. A scanning and transmission electron microscopic study on rat bone marrow sinuses and transmural migration of blood cells. Arch Histol Jpn. 1976;39:51–66. doi: 10.1679/aohc1950.39.51. [DOI] [PubMed] [Google Scholar]
- 35.Toro I, Olah I. Penetration of thymocytes into the blood circulation. J Ultrastruct Res. 1967;17:439–51. doi: 10.1016/s0022-5320(67)80134-5. [DOI] [PubMed] [Google Scholar]
- 36.Ushiki T. A scanning electron-microscopic study of the rat thymus with special reference to cell types and migration of lymphocytes into the general circulation. Cell Tissue Res. 1986;244:285–98. doi: 10.1007/BF00219204. [DOI] [PubMed] [Google Scholar]
- 37.Bajenoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28:346–52. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–70. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 39.Astrom KE, Webster HD, Arnason BG. The initial lesion in experimental allergic neuritis. A phase and electron microscopic study. J Exp Med. 1968;128:469–95. doi: 10.1084/jem.128.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humble JG, Jayne WH, Pulvertaft RJ. Biological interaction between lymphocytes and other cells. Br J Haematol. 1956;2:283–94. doi: 10.1111/j.1365-2141.1956.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 41.Farr AG, De Bruyn PP. The mode of lymphocyte migration through postcapillary venule endothelium in lymph node. Am J Anat. 1975;143:59–92. doi: 10.1002/aja.1001430104. [DOI] [PubMed] [Google Scholar]
- 42.Cho Y, De Bruyn PP. The endothelial structure of the postcapillary venules of the lymph node and the passage of lymphocytes across the venule wall. J Ultrastruct Res. 1979;69:13–21. doi: 10.1016/s0022-5320(79)80038-6. [DOI] [PubMed] [Google Scholar]
- 43.Cho Y, De Bruyn PP. Transcellular migration of lymphocytes through the walls of the smooth-surfaced squamous endothelial venules in the lymph node: evidence for the direct entry of lymphocytes into the blood circulation of the lymph node. J Ultrastruct Res. 1981;74:259–66. doi: 10.1016/s0022-5320(81)80117-7. [DOI] [PubMed] [Google Scholar]
- 44.Cho Y, De Bruyn PP. Internal structure of the postcapillary high-endothelial venules of rodent lymph nodes and Peyer’s patches and the transendothelial lymphocyte passage. Am J Anat. 1986;177:481–90. doi: 10.1002/aja.1001770406. [DOI] [PubMed] [Google Scholar]
- 45.Azzali G, Arcari ML, Caldara GF. The “mode” of lymphocyte extravasation through HEV of Peyer’s patches and its role in normal homing and inflammation. Microvasc Res. 2008;75:227–37. doi: 10.1016/j.mvr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi K, Schoefl GI. Blood vessels of the Peyer’s patch in the mouse: III. High-endothelium venules. Anat Rec. 1983;206:419–38. doi: 10.1002/ar.1092060408. [DOI] [PubMed] [Google Scholar]
- 47.Indrasingh I, Chandi G, Vettivel S. Route of lymphocyte migration through the high endothelial venule (HEV) in human palatine tonsil. Ann Anat. 2002;184:77–84. doi: 10.1016/S0940-9602(02)80040-1. [DOI] [PubMed] [Google Scholar]
- 48.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 49.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–77. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LA, Jackson DG. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–33. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- 51.Stoitzner P, Pfaller K, Stossel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–25. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- 52.Farr AG, Cho Y, De Bruyn PP. The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am J Anat. 1980;157:265–84. doi: 10.1002/aja.1001570304. [DOI] [PubMed] [Google Scholar]
- 53.Olah I, Glick B. Lymphocyte migration through the lymphatic sinuses of the chicken’s lymph node. Poult Sci. 1985;64:159–68. doi: 10.3382/ps.0640159. [DOI] [PubMed] [Google Scholar]
- 54.Azzali G, Gatti R, Orlandini G. Macrophage migration through the endothelium in the absorbing peripheral lymphatic vessel of the small intestine. J Submicrosc Cytol Pathol. 1990;22:273–80. [PubMed] [Google Scholar]
- 55.Azzali G. The passage of macrophage and lymphocytes from the interstitium across the lymphatic endothelium of rat lacteals. Cell Tissue Res. 1990;262:191–3. doi: 10.1007/BF00327761. [DOI] [PubMed] [Google Scholar]
- 56.Azzali G, Orlandini G, Gatti R. The migration of lymphocytes and polymorphonuclear leukocytes across the endothelial wall of the absorbing peripheral lymphatic vessel. J Submicrosc Cytol Pathol. 1990;22:543–9. [PubMed] [Google Scholar]
- 57.Azzali G. Three-dimensional and ultrastructural aspects of the lymphatic vascularization of the vermiform appendix. J Submicrosc Cytol Pathol. 1998;30:545–53. [PubMed] [Google Scholar]
- 58.Azzali G, Arcari ML. Ultrastructural and three dimensional aspects of the lymphatic vessels of the absorbing peripheral lymphatic apparatus in Peyer’s patches of the rabbit. Anat Rec. 2000;258:71–9. doi: 10.1002/(SICI)1097-0185(20000101)258:1<71::AID-AR8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Azzali G. The modality of transendothelial passage of lymphocytes and tumor cells in the absorbing lymphatic vessel. Eur J Histochem. 2007;51(Suppl 1):73–7. [PubMed] [Google Scholar]
- 60.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–60. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 61.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–43. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Schubert C, Christophers E, Swensson O, Isei T. Transendothelial cell diapedesis of neutrophils in inflamed human skin. Arch Dermatol Res. 1989;281:475–81. doi: 10.1007/BF00510083. [DOI] [PubMed] [Google Scholar]
- 63.Fujita S, Puri RK, Yu ZX, Travis WD, Ferrans VJ. An ultrastructural study of in vivo interactions between lymphocytes and endothelial cells in the pathogenesis of the vascular leak syndrome induced by interleukin-2. Cancer. 1991;68:2169–74. doi: 10.1002/1097-0142(19911115)68:10<2169::aid-cncr2820681014>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 64.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–15. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoshi O, Ushiki T. Scanning electron microscopic studies on the route of neutrophil extravasation in the mouse after exposure to the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP) Arch Histol Cytol. 1999;62:253–60. doi: 10.1679/aohc.62.253. [DOI] [PubMed] [Google Scholar]
- 66.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS ONE. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faustmann PM, Dermietzel R. Extravasation of polymorphonuclear leukocytes from the cerebral microvasculature. Inflammatory response induced by alpha-bungarotoxin. Cell Tissue Res. 1985;242:399–407. doi: 10.1007/BF00214554. [DOI] [PubMed] [Google Scholar]
- 68.Lossinsky AS, Badmajew V, Robson JA, Moretz RC, Wisniewski HM. Sites of egress of inflammatory cells and horseradish peroxidase transport across the blood-brain barrier in a murine model of chronic relapsing experimental allergic encephalomyelitis. Acta Neuropathol. 1989;78:359–71. doi: 10.1007/BF00688172. [DOI] [PubMed] [Google Scholar]
- 69.Lossinsky AS, Pluta R, Song MJ, Badmajew V, Moretz RC, Wisniewski HM. Mechanisms of inflammatory cell attachment in chronic relapsing experimental allergic encephalomyelitis: a scanning and high-voltage electron microscopic study of the injured mouse blood-brain barrier. Microvasc Res. 1991;41:299–310. doi: 10.1016/0026-2862(91)90030-f. [DOI] [PubMed] [Google Scholar]
- 70.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–90. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 71.Greenwood J, Howes R, Lightman S. The blood-retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 1994;70:39–52. [PubMed] [Google Scholar]
- 72.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 73.Engelhardt B. Immune cell entry into the central nervous system, Involvement of adhesion molecules and chemokines. J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Lampert P. Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol. 1967;9:99–126. doi: 10.1007/BF00691436. [DOI] [PubMed] [Google Scholar]
- 75.Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990;63:476–89. [PubMed] [Google Scholar]
- 76.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–65. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthews MA, Kruger L. Electron microscopy of non-neuronal cellular changes accompanying neural degeneration in thalamic nuclei of the rabbit. I. Reactive hematogenous and perivascular elements within the basal lamina. J Comp Neurol. 1973;148:285–312. doi: 10.1002/cne.901480302. [DOI] [PubMed] [Google Scholar]
- 78.Barron KD, Means ED, Feng T, Harris H. Ultrastructure of retrograde degeneration in thalamus of rat. 2. Changes in vascular elements and transvascular migration of leukocytes. Exp Mol Pathol. 1974;20:344–62. doi: 10.1016/0014-4800(74)90065-3. [DOI] [PubMed] [Google Scholar]
- 79.Lightman S, Greenwood J. Effect of lymphocytic infiltration on the blood-retinal barrier in experimental autoimmune uveoretinitis. Clin Exp Immunol. 1992;88:473–7. doi: 10.1111/j.1365-2249.1992.tb06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bamforth SD, Lightman SL, Greenwood J. Ultrastructural analysis of interleukin-1 beta-induced leukocyte recruitment to the rat retina. Invest Ophthalmol Vis Sci. 1997;38:25–35. [PubMed] [Google Scholar]
- 81.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gimbrone MA, Jr, Cotran RS, Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974;60:673–84. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong D, Prameya R, Dorovini-Zis K. In vitro adhesion and migration of T lymphocytes across monolayers of human brain microvessel endothelial cells: regulation by ICAM-1, VCAM-1, E-selectin and PECAM-1. J Neuropathol Exp Neurol. 1999;58:138–52. doi: 10.1097/00005072-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Migliorisi G, Folkes E, Pawlowski N, Cramer EB. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am J Pathol. 1987;127:157–67. [PMC free article] [PubMed] [Google Scholar]
- 85.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–88. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–91. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 87.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferreira AM, McNeil CJ, Stallaert KM, Rogers KA, Sandig M. Interleukin-1beta reduces transcellular monocyte diapedesis and compromises endothelial adherens junction integrity. Microcirculation. 2005;12:563–79. doi: 10.1080/10739680500253493. [DOI] [PubMed] [Google Scholar]
- 89.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 90.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–23. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 91.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–97. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riethmuller C, Nasdala I, Vestweber D. Nano-surgery at the leukocyte-endothelial docking site. Pflugers Arch. 2008;456:71–81. doi: 10.1007/s00424-007-0412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 94.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–57. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 95.Allavena P, Paganin C, Martin-Padura I, Peri G, Gaboli M, Dejana E, Marchisio PC, Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991;173:439–48. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19:535–64. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 97.De Bruyn PP, Cho Y, Michelson S. Endothelial attachment and plasmalemmal apposition in the transcellular movement of intravascular leukemic cells entering the myeloid parenchyma. Am J Anat. 1989;186:115–26. doi: 10.1002/aja.1001860202. [DOI] [PubMed] [Google Scholar]
- 98.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–53. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 99.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–45. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–93. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–44. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 102.Ballermann B, Ott M. Adhesion and differentiation of endothelial cells by exposure to chronic shear stress: A vascular graft model. Blood Purif. 1995;13:125–134. doi: 10.1159/000170195. [DOI] [PubMed] [Google Scholar]
- 103.Davies PF, Dewey CFJ, BSR, Gordon EJ, Gimbrone MAJ. Influence of hemodynamic forces on vascular endothelial function: in vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73:1121–1129. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kurzen H, Manns S, Dandekar G, Schmidt T, Pratzel S, Kraling BM. Tightening of endothelial cell contacts: a physiologic response to cocultures with smooth-muscle-like 10T1/2 cells. J Invest Dermatol. 2002;119:143–153. doi: 10.1046/j.1523-1747.2002.01792.x. [DOI] [PubMed] [Google Scholar]
- 105.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 106.Ott M, Olson J, Ballermann B. Chronic in vitro flow promotes ultrastructural differentiation of endothelial cells. Endothelium. 1995;3:21–30. [Google Scholar]
- 107.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 109.Feng D, Nagy JA, Dvorak HF, Dvorak AM. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microsc Res Tech. 2002;57:289–326. doi: 10.1002/jemt.10087. [DOI] [PubMed] [Google Scholar]
- 110.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–99. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]