Abstract
Diffusion-Weighted Magnetic Resonance Imaging (DWI) obtains information useful in diagnosing several diseases through the measurement of random, Brownian diffusion of water molecules in tissues. This pictorial essay illustrates the main factors, i.e., ratio between the volume occupied by cells and the extracellular space, composition of the extracellular space, and temperature, that determine the rate of the water diffusion. The mechanism through which these influencing factors affect water diffusion is explained. Clinical and experimental examples, derived both from physiology and from non-human models, are described.
Keywords: Diffusion weighted imaging, MR imaging, water, MRI Physics
INTRODUCTION

Fluids in tissues flow either in an “orderly” or in a random manner. Examples of “orderly” motion are blood flowing through the vessels in the human body or solutes moving along a concentration gradient. The random motion or diffusion, known as Brownian motion, is isotropic, i.e., it has no preferred direction, and is driven by the thermal energy of molecules. Diffusion – best described by a probabilistic model in which each molecule behaves independently of the others (“random walk”) – is the basic means of transport in all biological systems and is thought to be responsible for all chemical reactions.[1]
In Diffusion-Weighted Magnetic Resonance Imaging (DWI), two short-duration gradient pulses (equal in intensity and duration, but opposite in polarity) are applied before and after the 180° refocusing pulse on a spin-echo sequence. The random-moving spins get displaced and acquire phase shifts causing a signal loss proportional to their displacement that can be both represented on an image (in gray-scale and in a color map) and quantified in mm2/sec.
Apparent diffusion coefficient (ADC) measures the rate of diffusion of water molecules within a tissue. A low value for ADC indicates that the tissue is well organized, while a high value for ADC indicates that the tissue is not well organized.
DISCUSSION
According to current understanding, DWI actually measures the signal from the extracellular diffusion of water molecules [Figure 1]. The speed of diffusion of the water molecules is influenced mainly by three main factors. These factors are (1) the ratio of the volume occupied by the cells to the volume of the extracellular space, (2) the composition of the extracellular space, and (3) temperature [Figure 2].
Figure 1.
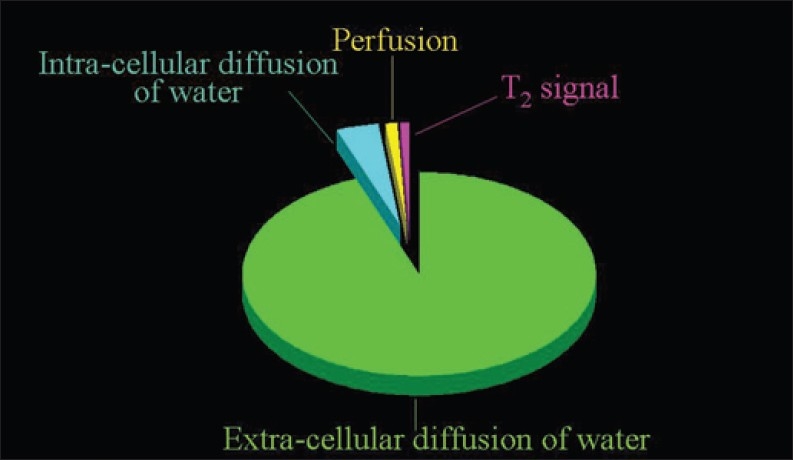
The sources of the signal measured with the Diffusion-Weighted Magnetic Resonance Imaging protocols routinely adopted. The overwhelming portion of the signal derives from the extracellular diffusion of water
Figure 2.
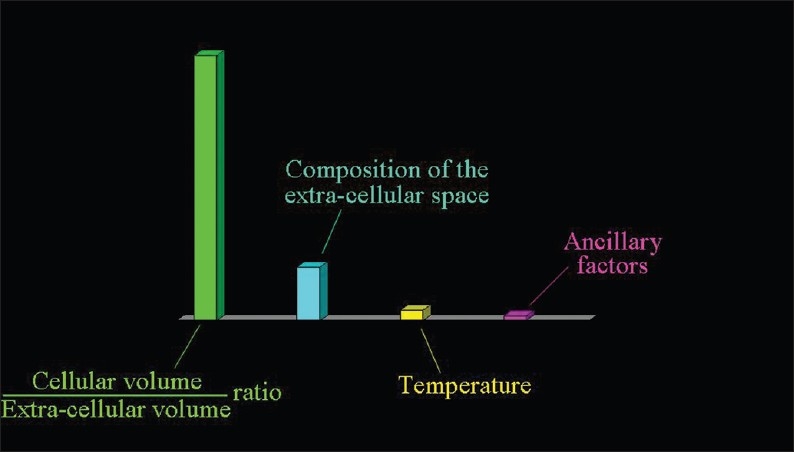
The factors determining the speed of the extracellular diffusion of water: the ratio between the volume occupied by cells and the extracellular space (the most important), the composition of the extracellular milieu, and the temperature.
Ratio of the volume occupied by the cells to the volume of the extracellular space
Ratio of the volume occupied by the cells to the volume of the extracellular space is a major factor influencing the speed of diffusion of the water molecules.
Cells restrict the free interstitial translation of water.[2,3] An inverse relationship between cellularity and ADC values has been reported in vitro as well as in healthy and diseased living tissues.[4] The higher the percentage of tissue occupied by cells the slower the extracellular motion of water molecules [Figure 3].
Figure 3.
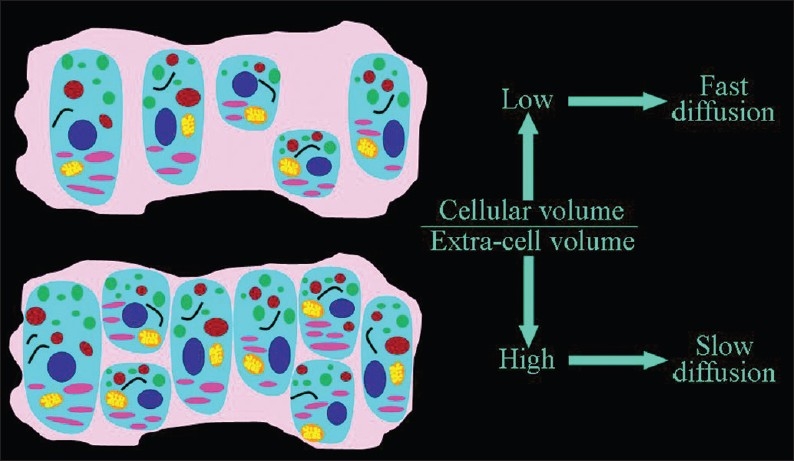
The dependence of the speed of the extracellular diffusion on the ratio between the volume occupied by cells and the extracellular space. The higher the percentage of tissue occupied by cells, the slower the extracellular motion of water molecules
Changes in the cellular/extracellular volume ratio may be caused by the variation in the number of cells, variation in the volume of the cells, and variation in the volume of extracellular space [Figure 4].
Figure 4.
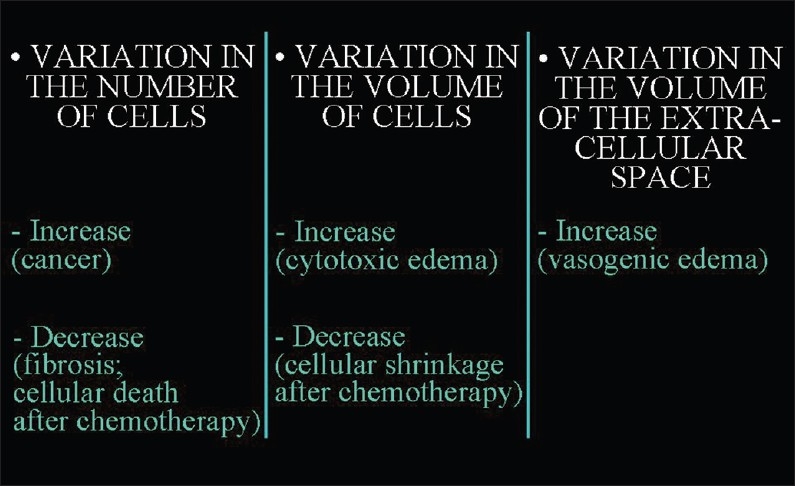
The factors that lead to changes in the ratio between the volume occupied by cells and the extracellular space.
Variation in the number of cells
When the number of cells increase, as in a cancer tissue, the ratio of (volume of cells)/(volume of extracellular space) increases and this results in decreased diffusion of extracellular water [Figure 5].
Figure 5.
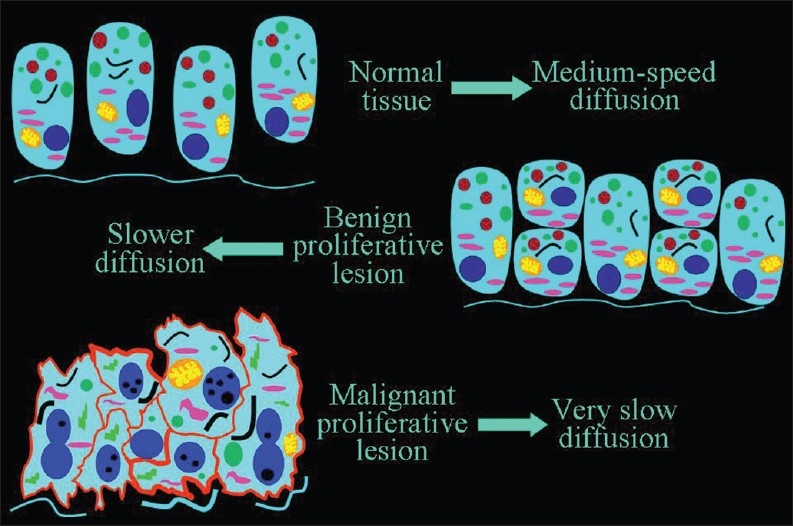
The inverse relationship of the speed of diffusion to the number of cells. A slower diffusion can be forecast in malignant than in benign proliferative lesions, and in benign lesions than in normal tissue
In recent years, a statistically significant difference in ADC values between benign and malignant proliferative lesions has been demonstrated in different organs and tissues, such as the breast [Figure 6],[5] the endometrium,[6] the lungs, the lymph nodes, the liver, the thyroid, and the kidneys. As a result, the clinical application of DWI in the detection, characterization, staging, and follow-up of malignancy is gaining recognition. DWI can be useful in the quantification of myometrial invasion by endometrial cancer, or in the identification of peritoneal spread of ovarian neoplasms,[6] and follow-up of malignancy. DWI can also be used to check for relapse after successful therapy. ADC value increases due to lower cellularity, cell membrane lysis, and permeability from toxic effects.[7] Recurring areas of low ADC indicate tumor relapse.
Figure 6.
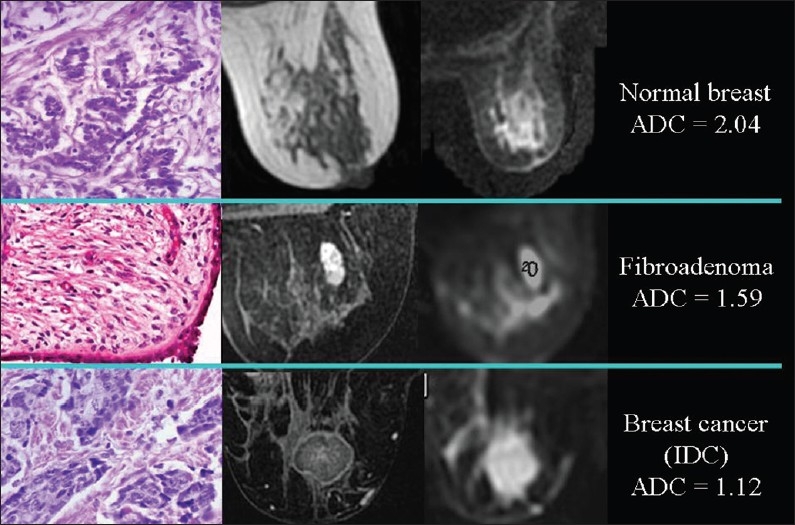
The relationship of diffusion to the number of cells. ADC values are lower, because of the higher cellularity, in proliferative lesions than in normal breast tissue; in an invasive ductal carcinoma the diffusion is slower than in a fibroadenoma
While some tumors (such as uterine and rectal) invariably exhibit a low ADC, a faster diffusion may be observed in non-hypercellular cancers (well-differentiated adenocarcinomas like HCCs), ovarian cancers with large cystic components,[6] scirrhous and mucinous adenocarcinomas of the breast.[7] On the other hand, low ADC values can be found in benign hypercellular lesions, such as breast papillomas.[7]
Lower-grade tumors have higher ADCs. However, cellularity is only one of the features used to determine tumor grade; other parameters such as nuclear atypia are not assessed with DWI. Necrosis, an indicator of poor differentiation, is a confounding factor. In necrosis, the number of cells is reduced and this results in an increase in ADC levels.
Variation in the volume of cells
When the cells swell, the volume of the cells increases leading to an increase in the ratio of the (cellular volume)/(extracellular volume) and diffusion of extracellular water becomes slower [Figure 7]. The most common cause of cellular swelling is ischemia. Ischemia impairs cell membrane function leading to increased permeability, displacement of extracellular water into the cells, and an observed decrease in ADC values [Figure 8] in the 30-50% ratio.[3,7]
Figure 7.
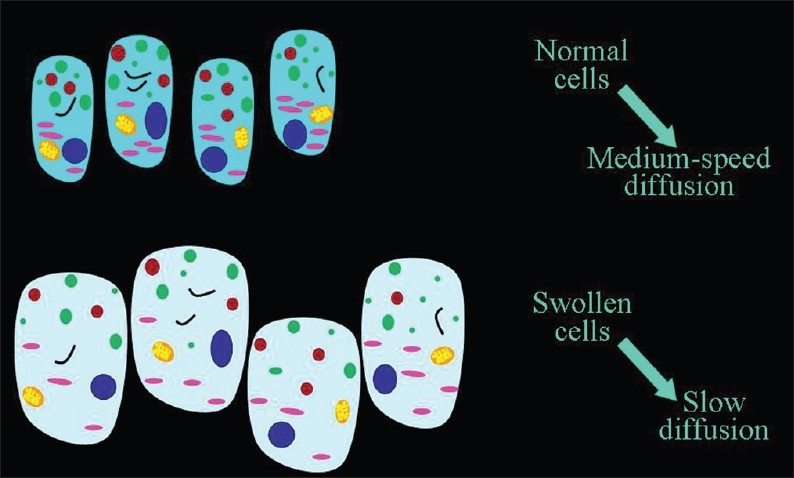
The inverse relationship of the speed of diffusion to the volume of cells
Figure 8.
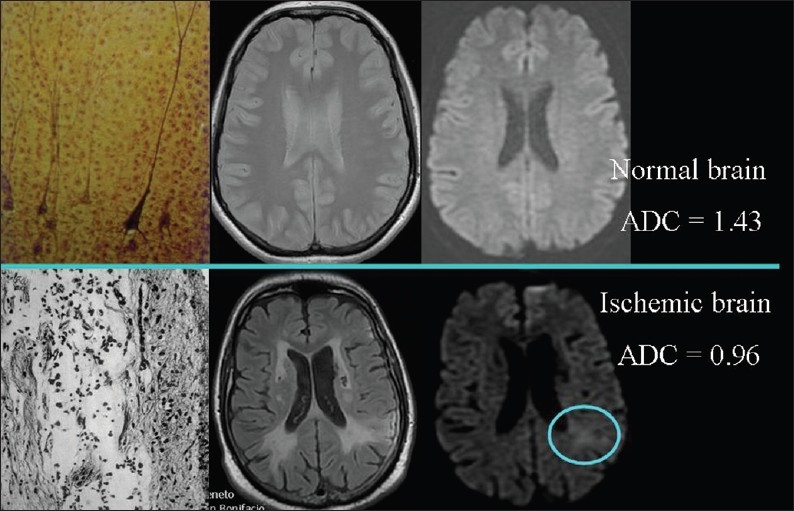
The relationship of diffusion to the volume of cells. ADC values are lower, because of cellular swelling, in areas of cerebral ischemia (light blue circle) than in normal brain
The same effect on the speed of diffusion is observed in other conditions (such as in hypoglycaemia, low interstitial osmolarity due to hyponatriemia) in which membrane depolarization makes excess extracellular water molecules move into the cells.[4,8]
Besides the historical clinical application of DWI as in the early detection of brain ischemia, the ADC variations resulting from the changes in cell volume allow the spatial delimitation of the cerebral region affected by ischemia (diffusion is faster in the adjacent edematous areas) and the monitoring of outcome. ADC accelerates with restitutio ad integrum following the increase in extracellular water content.
On the same basis, ischemic damage may be diagnosed by means of DWI in extracerebral structures, such as the renal tubular epithelium in acute renal failures due to epithelial necrosis or in the femoral head when ischemic necrosis occurs.
Variation in the volume of the extracellular space
Interstitial edema widens the extracellular space, which decreases the cellular/extracellular volume ratio and therefore accelerates diffusion [Figure 9]. Acute inflammation associated with vasogenic edema is the most common condition. High ADC values can be found in acute demyelinating plaques in patients with multiple sclerosis [Figure 10], during inflammation of joints (sacroiliitis) and muscles (polymyositis, dermatomyositis), in autoimmune pancreatitis, and in edematous muscles resulting from radiation therapy.
Figure 9.
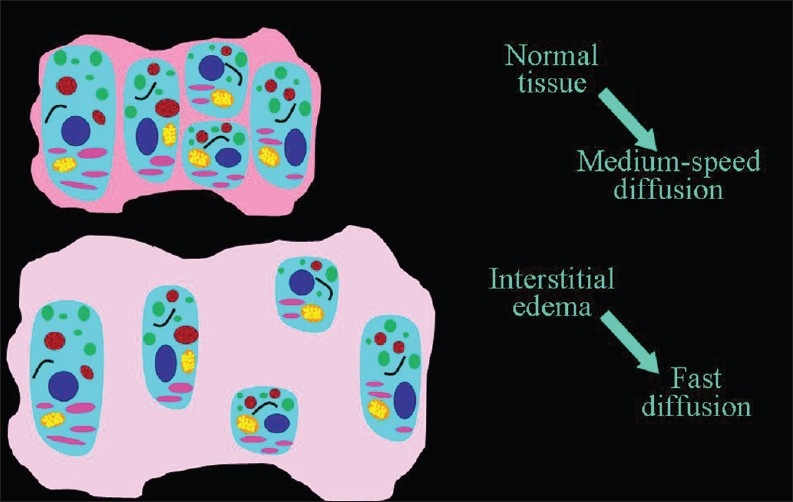
The direct relationship of the speed of diffusion to the volume of extracellular space.
Figure 10.
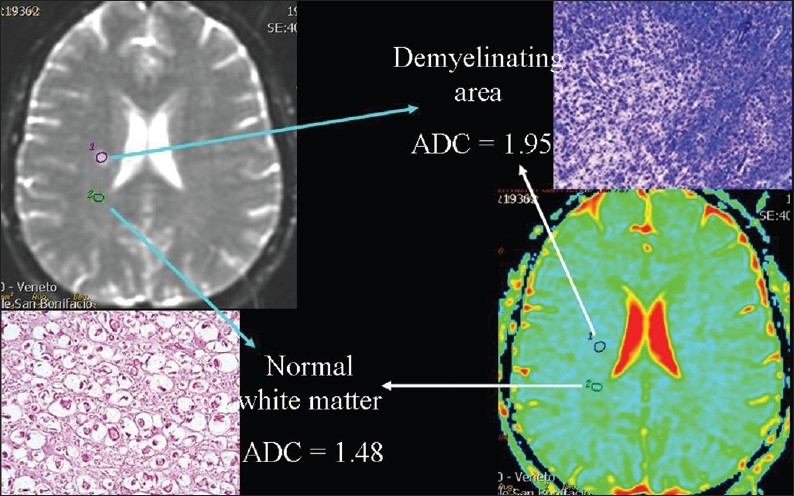
The relationship of diffusion to the volume of extra-cellular space. ADC values are higher, because of interstitial edema, in an acute demyelinating plaque than in a normal area of white matter in a patient suffering from multiple sclerosis.
A differential diagnosis may be obtained with DWI between osteoporotic (fast diffusion due to edema) and pathological (slow diffusion due to neoplastic hypercellularity) vertebral fractures.
Composition of the extracellular space
Macromolecules located in the interstitial space restrict the free movement of water[2,3] and an inverse relationship between ADC values and viscosity can be observed under several conditions. Higher the interstitial viscosity, slower is the extracellular motion of water molecules [Figure 11]. A slow diffusion due to a viscous extracellular space is measured in uterine leiomyomas [Figure 12]. In uterine leiomyomas abundant collagen deposition among smooth muscle cells makes the extra-cellular space viscous.[9] Increased extracellular viscosity is observed in endometriosic cysts due to the presence of blood. In mature cystic teratomas extracellular viscosity increases due to the presence of keratinoid substances. In liver cirrhosis, extracellular viscosity increases due to fibrosis. In abscesses, the presence of proteins and cellular debris originating from bacteria and inflammatory cells increase the viscosity, and in exudative pleural effusions viscosity of the extracellular space increases due to the high protein content.
Figure 11.
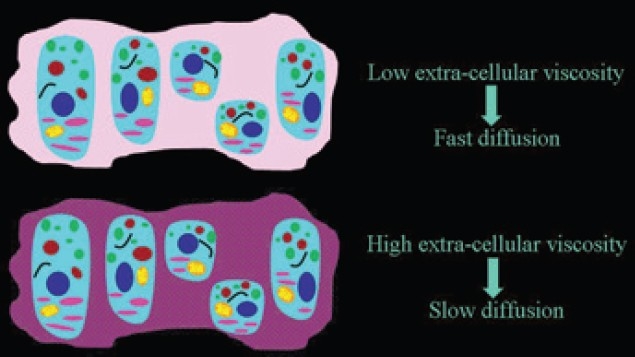
The dependence of the speed of the extracellular diffusion on the composition of the extracellular space. The higher the interstitial viscosity, slower is the extracellular motion of water molecules.
Figure 12.
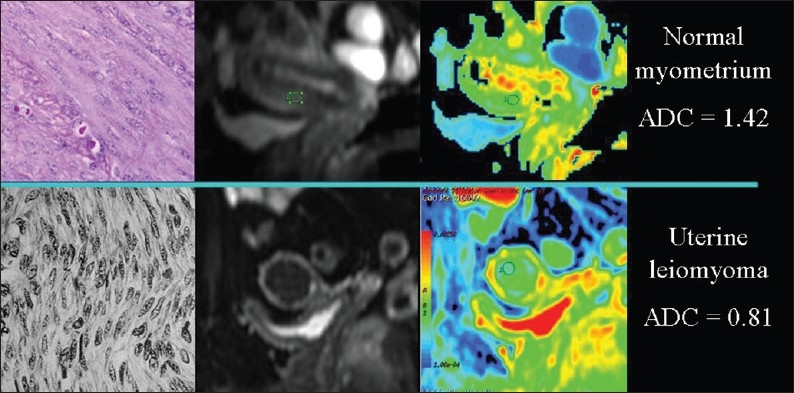
The relationship of diffusion to the composition of the extracellular space. ADC values are lower, because of collagen deposition, in a uterine leiomyoma than in the normal myometrium
Moreover, in the hematopoietic bone marrow, fatty infiltration reduces diffusion, making ADC values lower in the elderly than in the young and lower in osteoporotic than in non-osteoporotic post-menopausal women.
Although our data differ from those reported in the only published paper concerning this topic,[10] in the normal endometrium we measured higher ADC values in the menstrual phase than in the periovulatory phase [Figure 13]. We hypothesize that the extrusion of glycogen from the epithelial endometrial cells occurring in the peri-ovulatory phase may increase the extracellular viscosity, which in turn slows down diffusion.
Figure 13.
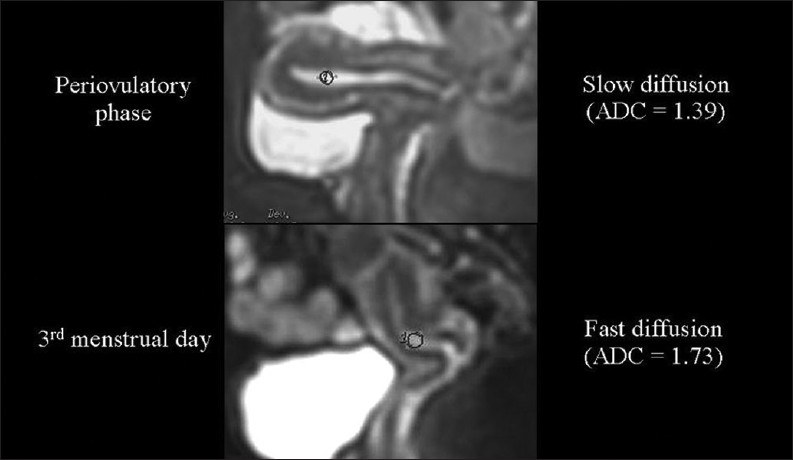
The relationship of diffusion to the composition of the extracellular space. Endometrial ADC values are lower (we hypothesize because of glycogen extrusion), during the periovulatory than during the menstrual phase.
We also observed very low ADC values in breast fat necrosis, which may reflect the obstacle to water diffusion caused by a great number of extracellular hydrophobic lipid molecules [Figure 14].[5]
Figure 14.
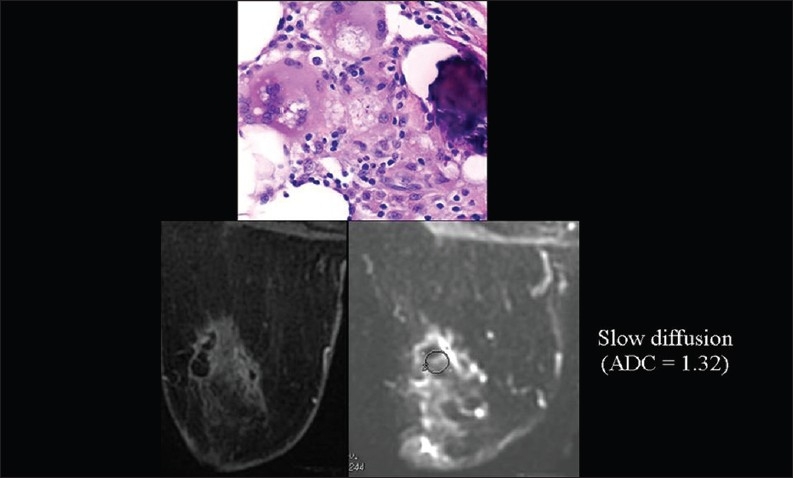
The relationship of diffusion to the composition of the extracellular space. ADC values are very low (we hypothesize because of the high amount of extracellular hydrophobic lipid molecules), mimicking cancer, in breast fat necrosis.
Hydrophobic lipid molecules make ADC values lower in an emulsion of butter in water than in pure water [Figure 15]. The same effect (lower ADC levels) can be observed in meat broth (due to presence of proteins originating from muscle tissue) when broth is compared to pure water [Figure 16]. ADC values are lower in the white of a hard-boiled egg (due to the increased viscosity following denaturation of albumin molecules in the egg white) in comparison to a raw egg [Figure 17]. A boiled potato (due to the many small saccharide molecules resulting from degradation of starch) has lower ADC values in comparison to a raw potato [Figure 18].
Figure 15.
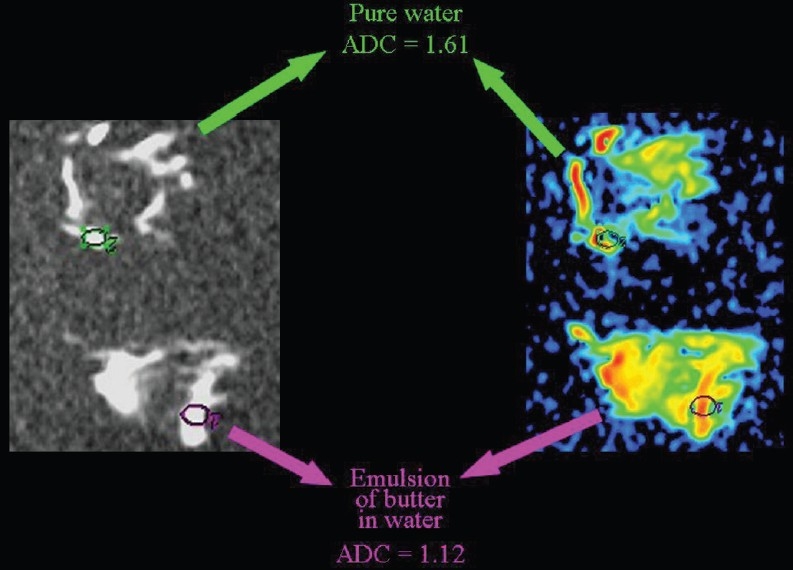
The relationship of diffusion to the composition of the extracellular space. ADC values are lower (we hypothesize because of the presence of hydrophobic lipid molecules) within an emulsion of butter in water than in pure water.
Figure 16.
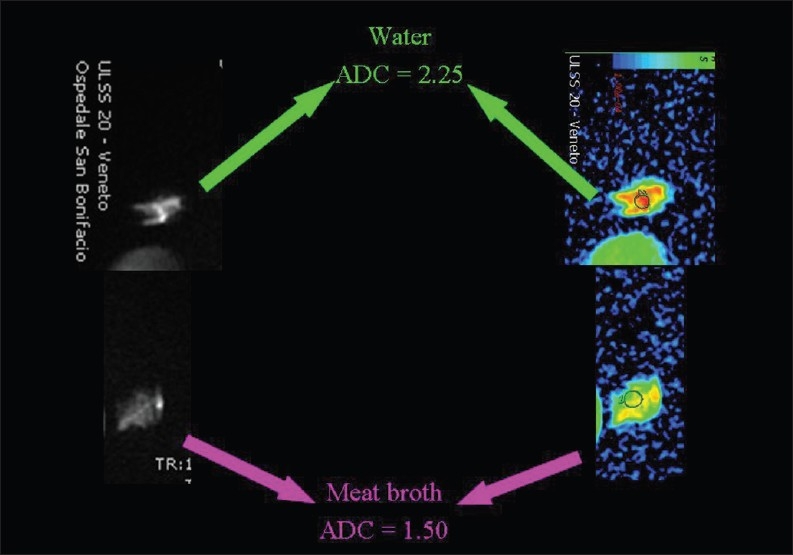
The relationship of diffusion to the composition of the extracellular space. ADC values are lower (we hypothesize because of the presence of proteins extruded from muscle tissue) within meat broth than in pure water
Figure 17.
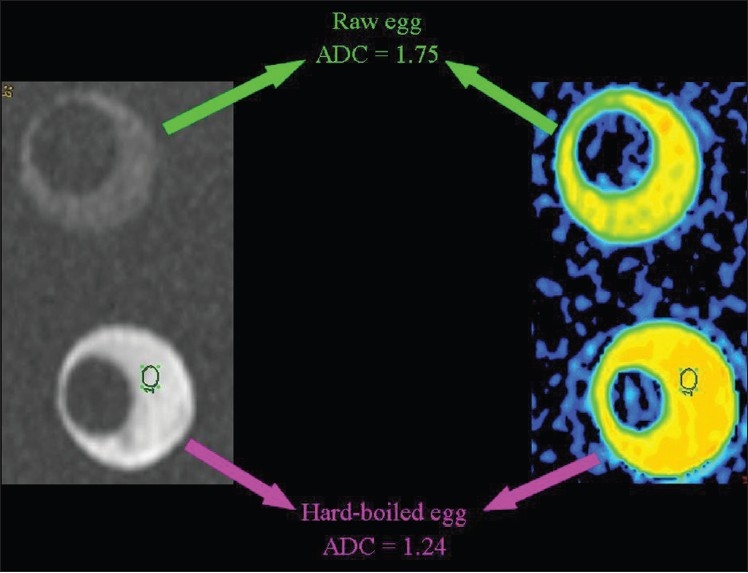
The relationship of diffusion to the composition of the extracellular space. ADC values are lower (we hypothesize because of the increased viscosity subsequent to denaturation of albumin molecules) in the white of a hard-boiled than of a raw egg.
Figure 18.
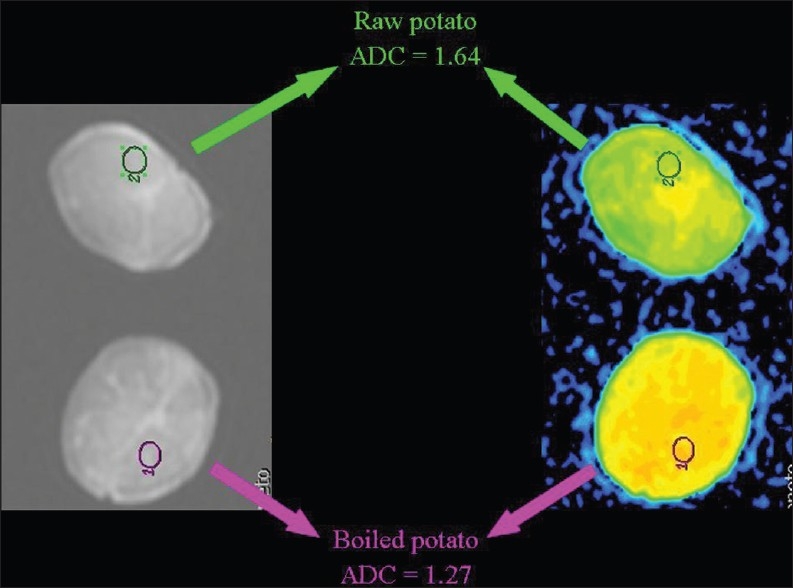
The relationship of diffusion to the composition of the extracellular space. ADC values are lower (we hypothesize because of the increased viscosity subsequent to degradation of starch to numerous small saccharide molecules) in a boiled than in a raw potato
Temperature
In as much as it is a Brownian motion driven by internal kinetic energy, diffusion has a direct relationship to temperature, which has been demonstrated in a bio-phantom containing cultured human cells [Figure 19].[4]
Figure 19.
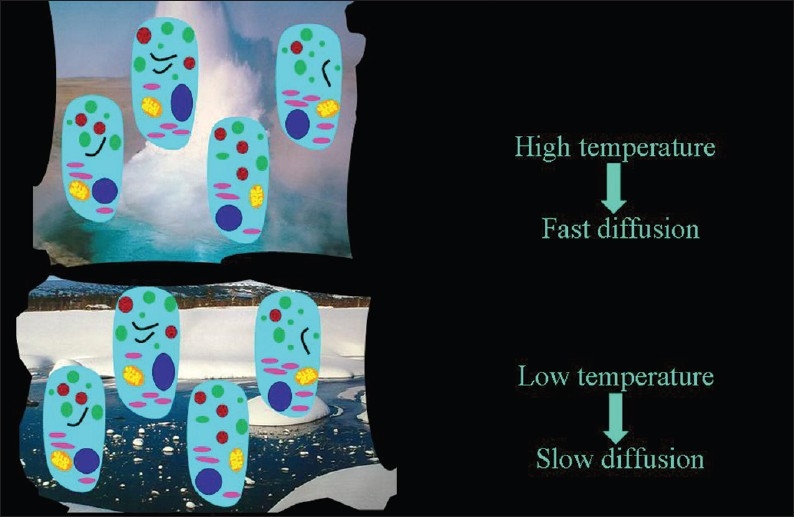
The direct relationship of the speed of diffusion to the temperature
In muscle tissue, exercise is accompanied by heat production [Figure 20]. ADC values increase during exercise [Figure 21] and decrease at rest or with experimental cooling.[11] DWI may be used to measure the temperature of cerebrospinal fluid within the lateral ventricles in patients in whom hypothermia is induced. Deactivation of the cerebral metabolism affects water diffusion.[12]
Figure 20.
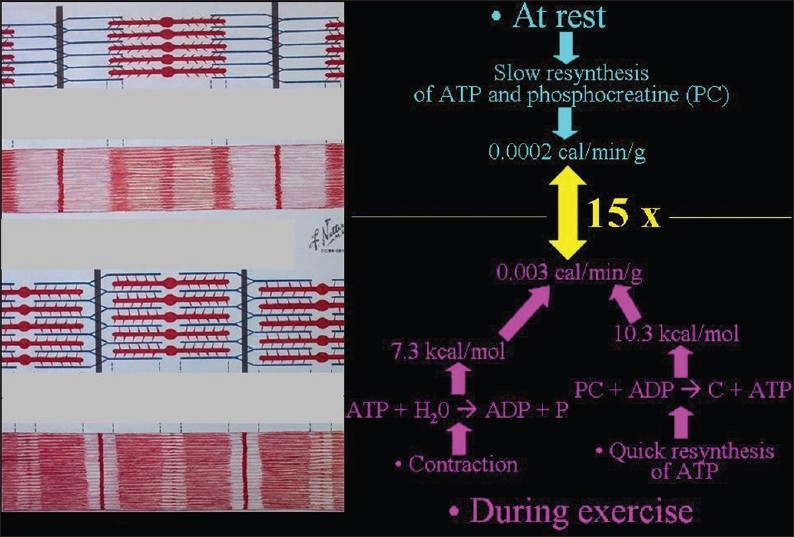
The biochemical basis of heat production in muscles during exercise
Figure 21.
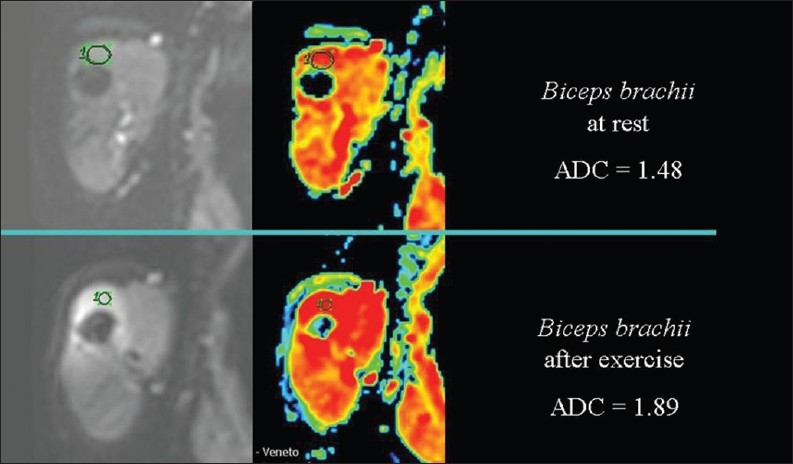
The relationship of diffusion to the temperature. ADC values are higher, because of heat production, in the arm muscle of a healthy volunteer after exercise than at rest
The influence of temperature on the speed of diffusion can be demonstrated by comparing the ADC value of water at room temperature, which is lower when compared to warm pure water [Figure 22]. The ADC values are lower in a frozen beefsteak in comparison to a beefsteak at room temperature [Figure 23].
Figure 22.
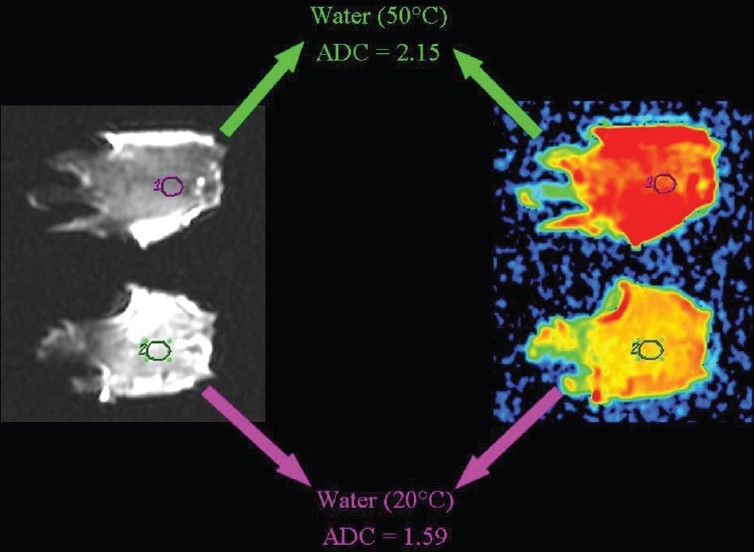
The relationship of diffusion to the temperature. ADC values are higher in warm than in room temperature pure water
Figure 23.
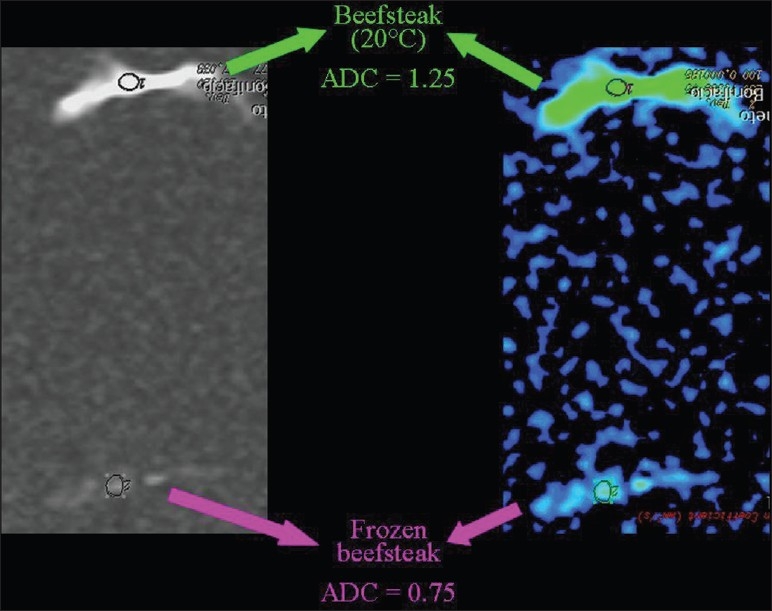
The relationship of diffusion to the temperature. ADC values are lower in a frozen than in a room temperature beefsteak
CONCLUSION
The cellular/extracellular volume ratio, the composition of the extracellular space, and temperature are the main factors influencing the speed of diffusion measured with DWI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/27/81294
REFERENCES
- 1.Price WS. Concepts Magn Reson. 1997;9:299. [Google Scholar]
- 2.Clark CA, Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med. 2000;44:852–9. doi: 10.1002/1522-2594(200012)44:6<852::aid-mrm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Harkins KD, Galons JP, Secomb TW, Trouard TP. Assessment of the effects of cellular tissue properties on ADC measurement by numerical simulation of water diffusion. Magn Reson Med. 2009;62:1414–22. doi: 10.1002/mrm.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto Y, Kuroda M, Matsuya R, Kato H, Shibuya K, Oita M, et al. In vitro experimental study of the relationship between the apparent diffusion coefficient and changes in cellularity and cell morphology. Oncol Rep. 2009;22:641–64. doi: 10.3892/or_00000484. [DOI] [PubMed] [Google Scholar]
- 5.Fornasa F, Pinali L, Gasparini A, Toniolli E, Montemezzi's S. Diffusionweighted magnetic resonance imaging in focal breast lesions: Analysis of 78 cases with pathologic correlation. Radiol Med. 2011;116:264–75. doi: 10.1007/s11547-010-0602-4. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR. Diffusion-weighted MR imaging of female pelvic tumors: A pictorial review. Radiographics. 2009;29:759–74. doi: 10.1148/rg.293085130. [DOI] [PubMed] [Google Scholar]
- 7.Bammer R, Liu C, Po J, Moseley ME. Diffusion-weighted Magnetic Resonance Imaging. In: Edelman S, Hesselink JR, Zlatkin MB, Crues JV, editors. Clinical Magnetic Resonance Imaging. 3rd ed. Philadelphia: Saunders; 2008. pp. 288–319. [Google Scholar]
- 8.Sevick RJ, Kanda F, Mintorovitch J, Arieff AI, Kucharczyk J, Tsuruda JS, et al. Cytotoxic brain edema: Assessment with diffusion-weighted MR imaging. Radiology. 1992;185:687–90. doi: 10.1148/radiology.185.3.1438745. [DOI] [PubMed] [Google Scholar]
- 9.Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A. Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol. 2009;50:340–7. doi: 10.1080/02841850902735858. [DOI] [PubMed] [Google Scholar]
- 10.Kido A, Kataoka M, Koyama T, Yamamoto A, Saga T, Togashi K. Changes in apparent diffusion coefficients in the normal uterus during different phases of the menstrual cycle. Br J Radiol. 2010;83:524–8. doi: 10.1259/bjr/11056533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagisawa O, Shimao D, Maruyama K, Nielsen M. Evaluation of exercised or cooled skeletal muscle on the basis of diffusion-weighted magnetic resonance imaging. Eur J Appl Physiol. 2009;105:723–9. doi: 10.1007/s00421-008-0954-9. [DOI] [PubMed] [Google Scholar]
- 12.Khachaturian MH, Arsenault J, Ekstrom LB, Tuch DS, Vanduffel W. Focal reversible deactivation of cerebral metabolism affects water diffusion. Magn Reson Med. 2008;60:1178–89. doi: 10.1002/mrm.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]


