Abstract
Liver biopsies are performed for both focal and nonfocal lesions (parenchymal). In our center, majority of liver biopsies are performed for parenchymal liver disease. Parenchymal liver biopsy plays a key role in the diagnosis of various diffuse liver dysfunctions. Results of the biopsy help grade the disease, facilitating prognostication, which helps in planning specific treatment strategies. Imaging guidance is gaining wide acceptance as the standard procedure. Ultrasound (US) guidance is currently considered the most cost-effective and safe way to perform parenchymal liver biopsies. Radiologists worldwide and particularly in the United States are increasingly performing this procedure. Radiologists performing biopsies generally use the cutting needle. Different needle sizes, techniques and preference for biopsy of the right or left lobe have been described. We attribute these preferences to prior training and individual radiologist's comfort level. We describe the algorithm followed at our institution for performing percutaneous US-guided parenchymal liver biopsy. While clinical societies have recommended a minimum of 40 liver biopsies as a requirement for proficiency of clinicians, specific to radiology trainees/fellows interested in pursuing a career in intervention, we feel a total of 20 liver biopsies (includes assisted and independently performed biopsies under supervision) should be adequate training.
Keywords: Liver biopsy, percutaneous, ultrasound guidance
INTRODUCTION

The primary indication for parenchymal liver biopsy has traditionally been for the diagnosis of hepatic disease. This has expanded to include in addition to diagnosis the assessment of the extent of fibrosis and diagnosis, but also describe the extent of fibrosis and inflammation, that helps stage and prognosticate liver disease and assists in clinical management decisions. While serological tests for fibrosis (fibro test) and non-invasive tests (elastography) are available, these have not been uniformly standardized nor are widely available.[1] Histology based on liver biopsy continues to be the most acceptable test. Performance of the procedure varies amongst institutions and no universal guidelines exist. Worldwide nearly 50% of liver biopsies are still performed without imaging guidance. The use of imaging guidance is likely to reduce complications and increase the likelihood of specimen adequacy and procedural success. Nearly 50% of liver biopsies in the United States are performed by radiologists.[2] While ultrasound (US) guidance is the favored modality, transjugular, computerized tomography (CT), laparoscopic and endoscopic ultrasound (EUS) have all been used. In this article, we essentially focus on the performance of the procedure by the commonest modality: US guidance. We retrospectively reviewed our US-guided parenchymal liver biopsy data in adult patients over a 3-year period (July 01, 2007 to June 30, 2010). Necessary permission from institutional review board was obtained.
INDICATIONS
A large number of parenchymal liver biopsies are still performed with the primary intent of diagnosing specific hepatic diseases, including acute and chronic hepatitis, hepatic steatosis, disorders of cholestasis, infection and granulomatous disease, infiltrative liver disease, and hepatic storage disorders [Table 1]. The ability of pathologists to grade hepatic fibrosis has led to the use of parenchymal biopsy as a tool to offer prognostic information in a host of conditions including cirrhosis, hepatitis, non-alcoholic fatty liver disease (NAFLD), and primary biliary cirrhosis (PBC).[1,2] In patients with portal hypertension, the extent of fibrosis has been linked to overall prognosis. Biopsy may also demonstrate the presence of fibrotic regression in some cases. Liver biopsy is also useful for evaluation of abnormal liver function and rejection, following orthotopic liver transplant. The data obtained from parenchymal biopsy are also used to develop specific treatment regimens for diseases such as hemachromatosis, PBC, and Wilson's disease. Biopsy result may affect decisions regarding the initiation of treatment in chronic hepatitis, allow for the assessment of response to therapy, and assist in the prediction of relapse in autoimmune hepatitis (AIH).
Table 1.
Indications for parenchymal (nonfocal) liver biopsy

PRE-BIOPSY WORK UP
Pre-procedural evaluation of potential biopsy patients should include consideration of the indication for the procedure, alternative methods of answering the clinical question, if possible, the relevant medical history, and any prior imaging studies. Ideally, the patient should attend a pre-biopsy clinic visit for discussion of procedural details, potential complications, and preparation. The patient may be counseled in this regard by the ordering physician or the radiologist performing the procedure. The discussion should include specifics to mitigate patient anxiety, including: who will perform the procedure, when the procedure will be performed, when the results will be available, whether or not sedation will be administered, and how long the patient might be absent from work, school, or other activities.
The pre-procedure evaluation should include a review of the patient's medications and instructions to withhold medications as necessary, most importantly any anticoagulants. The data supporting recommendations to discontinue anticoagulants prior to the procedure are limited.[2–4] However, the liver is a highly vascular organ and the procedure is not considered risk free. Antiplatelet medications such as aspirin and Plavix should be discontinued 7–10 days prior to the procedure. They may be resumed 48–72 hours post procedure. Warfarin should be discontinued 5–7 days prior to the procedure and it may be resumed the day following the procedure. Heparin should be withheld 6–12 hours prior to the procedure. Therapy must be individualized for specific patients and bridging anticoagulation may be considered in patients with coronary stents, prosthetic valves, etc. Consultation with the patient's cardiologist may be helpful in these situations. Coagulation abnormalities, if any, are corrected prior to the procedure. Low platelet counts are treated with platelet replacement. An elevated International Normalized Ratio (INR) is corrected with Fresh Frozen Plasma (FFP) administration. Chronic renal failure patients and those on hemodialysis may benefit from oral desmopressin.
As with medication discontinuation, there is limited evidence supporting routine pre-procedural laboratory studies.[5] It is reasonable to obtain a Complete Blood Count (CBC) with platelet count, Prothrombin Time (PT), INR, and Activated Partial Thromboplastin Time (APTT). A relatively mild degree of disordered coagulation is considered a relative contraindication to the procedure, as are ascites, morbid obesity, known focal hepatic lesions or vascular anomalies, and hydatid cysts. A significant, uncorrected coagulopathy is an absolute contraindication. Other absolute contraindications include an uncooperative patient, intrahepatic abscess or hepatic bed infection, and extrahepatic biliary obstruction.
Prior to the procedure, a 4-hour fast (more so when conscious sedation is being considered) is common practice, although not well supported by direct evidence. Fasting will result in distension of gall bladder with a small risk of gall bladder perforation. On the other hand, encouraging a light breakfast may ensure a contracted gall bladder, but increase the portal blood flow with increased risk of bleeding complications.[2] Before initiating the procedure, an intravenous line should be started and the patient placed on continuous hemodynamic monitoring. The procedure room should be a spacious US suite, large enough to accommodate the monitor, adequate resuscitation equipment including a crash cart and multiple personnel (radiologist, US technician, radiology nurse, and the resident/fellow where applicable). Informed consent obtained prior to the procedure should include discussion of infection, bleeding including fatal hemorrhage, non-target visceral injury, vascular injury (pseudoaneurysm, fistula), biliary injury (including biloma, peritonitis), non-diagnostic specimen, and sedation reaction. At our institution, percutaneous parenchymal biopsy is an outpatient procedure performed under moderate sedation. Patients must have a full history and physical examination within 30 days; otherwise, it is performed by the radiologist prior to the procedure [Joint Commission on Accreditation of Healthcare Organizations’ (JCAHO) guidelines]. Patients are instructed to discontinue anticoagulants as outlined earlier, fast on the day of the procedure, and take their other usual medications with sips of water. Patients are admitted to the subacute care unit (SACU) prior to the procedure and an intravenous line is started. Blood is drawn for PT, INR, APTT, and platelets stat in SACU unless on file within the past 30 days [Table 2, for normal values]. Blood group typing and tests for bleeding and clotting time are not insisted on. Informed consent, pre-procedural assessment and physical exam are completed at the SACU by the performing radiologist.
Table 2.
Acceptable laboratory values at our institution

THE PROCEDURE
When imaging guidance is employed, it can take one of two forms: US-guided “marking” in which a mark is made upon the skin during US examination for a biopsy to be performed later without imaging guidance or real-time US guidance.
Multiple studies have compared the safety and efficacy of US-guided percutaneous biopsy versus blind puncture. There is no definitive randomized trial and it is unknown if such a study will be performed given the existing data and the increasing use of US guidance. Although opportunities for further study exist, the available data regarding US guidance show a decreased complication rate, decreased number of passes required, decreased pain and pain-related morbidity, decreased chance of requiring repeat procedure, superior quality of specimens obtained and marginally increased cost, when compared to the blind technique.[1,2,6–12] Studies have also shown that the use of US guidance is cost-effective when specimen adequacy and incidence of complications are considered.[6–9] Histological analyses of samples obtained via blind approach indicated biopsy of renal tissue, pericolic fat, and myenteric plexus.[6] While the most frequent major complication of liver biopsy is hemorrhage, death from biliary injury including gallbladder perforation with the blind technique has also been reported.[13]
The patient is positioned supine, with the hands comfortably resting behind the head and hemodynamic monitoring in place [Figure 1]. This also ensures that the site of the pulse oximeter placement and intravenous (IV) line being monitored by the nurse are away from the radiologist's operative field. For performance of right-sided biopsies, the patient may be turned slightly to the right with a wedge behind the patient's back for comfort. A preliminary US scan is performed to identify the target and mark the skin. The preliminary scan also ensures that no major vessels, dilated biliary channels or gall bladder are in the path of the biopsy needle. After this, a safety pause is performed according to the hospital guidelines, with the entire team verifying the correct patient, the correct site, and the correct procedure is being performed. Before the procedure is started, breathing instructions are practiced with the patient. Right-sided liver biopsies are performed with the breath held in expiration. This will minimize risk of injury to the pleura or lung. The skin site is prepped and draped to ensure asepsis [Figures 2 and 3]. At our institute, the sterile biopsy procedure pack by Medline (Medline Industries Inc., Mundelein, IL, USA) is routinely used. The local area is anesthetized with a local anesthetic, lidocaine 1% (App Pharmaceuticals LLC, Schaumburg, IL, USA), generally buffered with sodium bicarbonate (Hospira Inc., Lake Forest, IL, USA) with a 1 in 20 dilution. Under US guidance, the deep soft tissues and liver pericapsular areas are also infiltrated with the local anesthetic [Figures 4–6]. The anesthetic may be directed at the capsular surface while the patient is holding his/her breath. Pericapsular anesthesia should not be performed while the patient is breathing to avoid the capsule being dragged against the needle tip.
Figure 1.
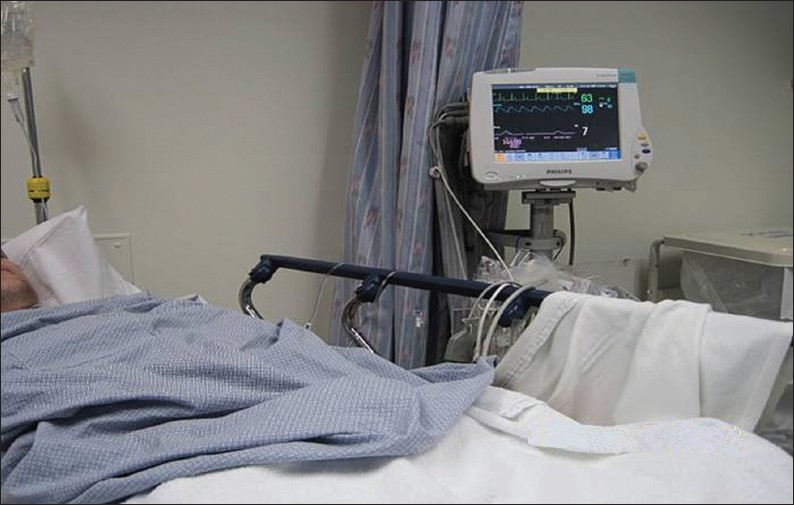
The patient is under constant monitoring with evaluation of the heart rate, blood pressure, and oxygen saturations.
Figure 2.
The skin site is prepped and draped in a sterile fashion.
Figure 3.
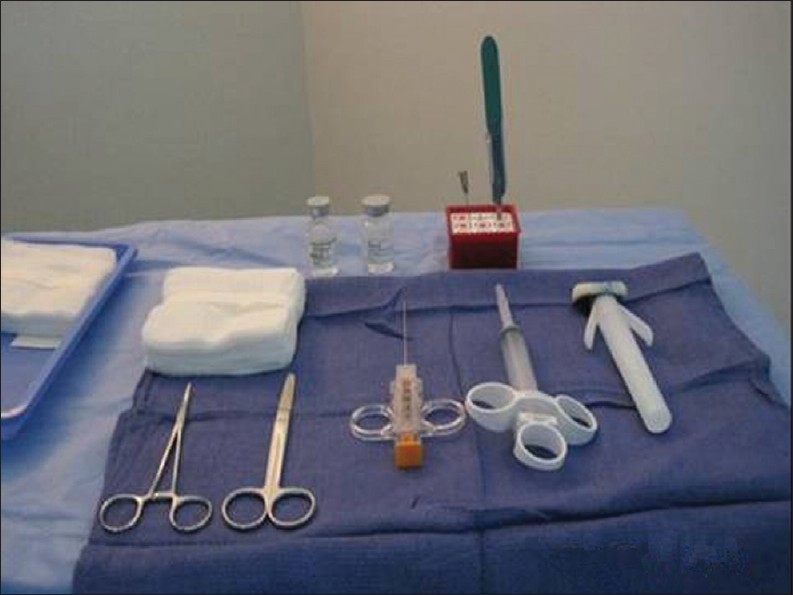
Sterile biopsy procedure pack including the biopsy needle, local anesthetic, and 4 × 4 gauzes.
Figure 4.
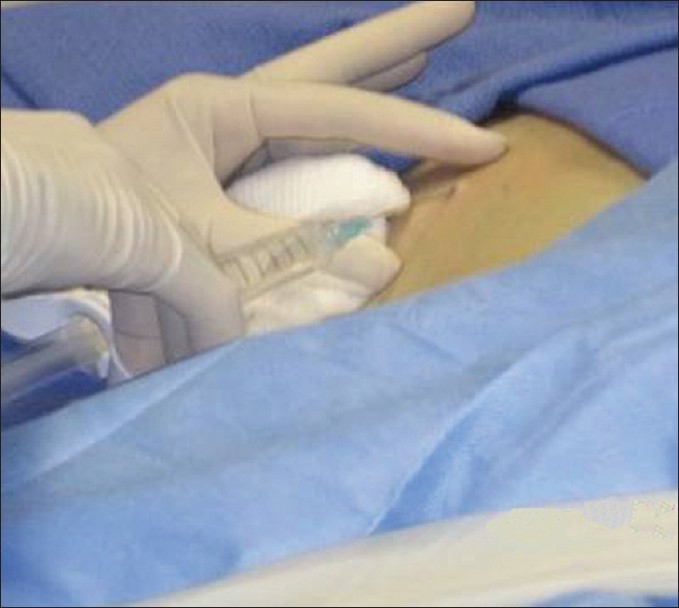
The local area is anesthetized with a local anesthetic buffered with sodium bicarbonate in a 1 in 20 dilution.
Figures 5 and 6.
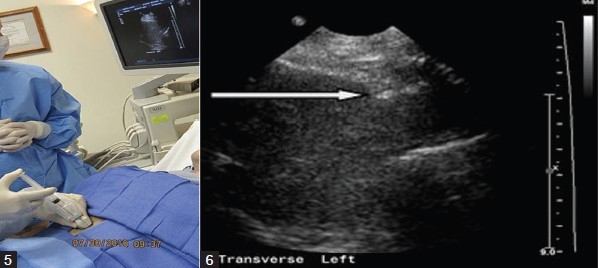
Under ultrasound guidance, the deep soft tissues and liver pericapsular areas are also infiltrated with local anesthetic.
During the time of biopsy, the patient is instructed to hold his/her breath in the same manner as during local anesthetic infiltration. Under US guidance, observe as the needle tip crosses the capsule prior to deploying the cutting device. In patients who have difficulty complying with breath holding, they may be allowed to breathe once the needle tip is deep into the liver capsular surface. The cutting needle is then fired with US documentation of the site [Figures 7–9]. Ensuring that the tip of the needle is deep into the capsule prior to firing reduces the number of passes required to obtain an adequate specimen. At our institute, most radiologists prefer using 18-G cutting Temno needle (Cardinal Health, McGaw Park, IL, USA).
Figures 7-9.
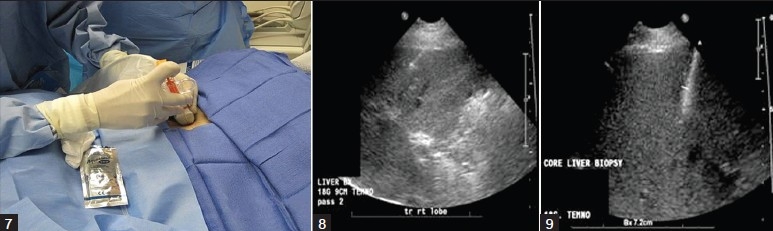
After the patient is instructed to hold his/her breath, under ultrasound guidance, observe as the needle tip crosses the capsule prior to deploying the cutting device.
Most of these procedures have been performed using the free hand technique and monitoring the needle position during the entire course of the biopsy. Depending on operator preference and training, the coaxial technique and guide technique have also been used at our institute. A post-procedure scan is routinely performed to evaluate for any immediate post-biopsy bleed.
Prior to use of imaging guidance, standard liver biopsy approach was used for the transthoracic sampling of the right lobe. In this technique, the needle is directed cephalad to rib to avoid intercostal arteries, with caution to avoid a steep needle angle which might cause diaphragmatic injury or pneumothorax. The biopsy is performed with breath held in expiration, to avoid lung descent. With US guidance, both lobes of the liver may be assessed in real time and a favorable approach decided upon. Many radiologists now prefer the ease of access to left lobe via a subxiphoid/subcostal approach.[2] This technique is performed near the midline away from major vessels, in the area of the linea alba. Radiologists must maintain awareness of the position of the heart and pericardium, as well as provide the patient with appropriate breathing instructions to avoid excessive motion. Usually, this involves breath held in inspiration to bring the left lobe inferior. The needle is then directed to the right into the left lobe of the liver, with or without a transducer needle guide. There are no clear data regarding a difference in complications with regard to these approaches. The left lobe subcostal approach may necessitate steep needle angle and deeper hepatic penetration in some instances. At our institution, a review of 776 parenchymal biopsies over 3 years revealed 535 (69%) left- and 239 (31%) right-sided biopsies. In two instances, specimens were obtained from the right and left lobes.
OTHER IMAGING MODALITIES FOR PERFORMANCE OF LIVER BIOPSIES
Computed tomography
Computed tomography (CT)-guided biopsies are less frequently performed in current practice [Figure 10]. This is because it is more time-consuming, is associated with radiation exposure, ties up resources and is more expensive. CT-guided procedures are generally reserved for tissue sampling of focal hepatic lesions, not accessible by US. A parenchymal biopsy of an uninvolved site may be obtained during the same procedure in some instances.
Figure 10.
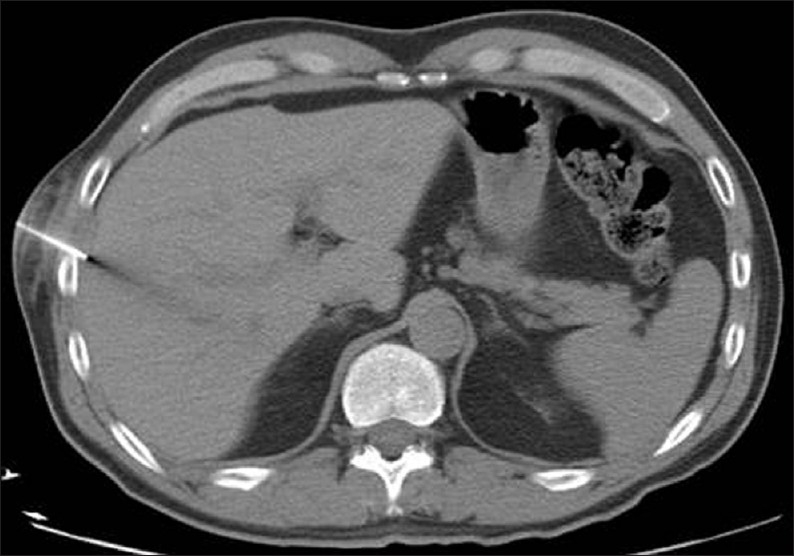
CT-guided parenchymal liver biopsy with the biopsy needle seen traversing the right liver lobe in this axial CT image.
Intravascular
Transjugular biopsy of the liver has been performed in patients with contraindications to standard percutaneous biopsy [Figure 11]. There are several considerations for a non-percutaneous approach, including the presence of ascites, known coagulopathy and a shrunken, cirrhotic liver. Evidence of increased hemorrhage risk in the setting of ascites is not strong.
Figure 11.
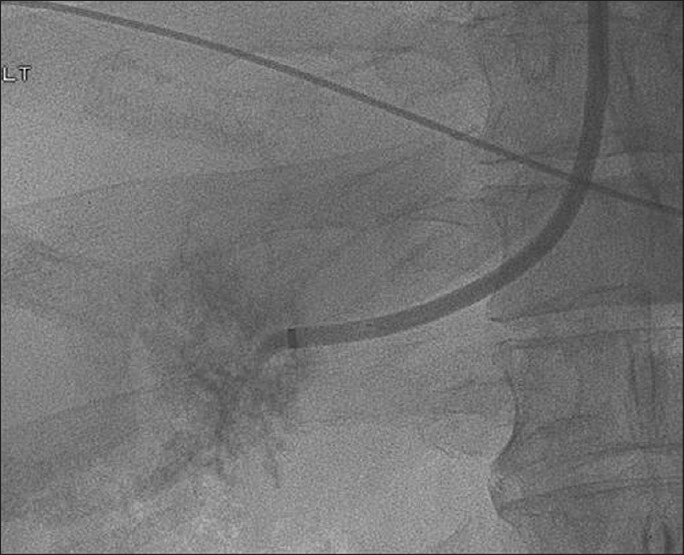
Frontal fluoroscopic image demonstrates a catheter seen within the right hepatic vein during a transjugular liver biopsy.
Endoscopic ultrasound
In a limited study from Indiana University Medical Center,[14] the endoscopic route was explored as an alternative to the traditional percutaneous or transvenous route. The tissue yield with a 19-G needle was noted to be slightly suboptimal. However, this continues to be a promising route in extremely obese patients or in patients with an unacceptably high risk of complication.
Laparoscopy
Wedge resection or biopsies with typical needle devices have been performed under direct vision at the time of laparoscopic surgery. While hemostasis and adequate tissue is always achieved, the procedure is done under general anesthesia by skilled surgeons and involves considerable additional dollar expense.
POST-BIOPSY MONITORING
Several studies indicate that most major complications are clinically evident within 2 hours post procedure.[15,16] The presence of biliary injury or bile peritonitis may be recognized during the procedure. Major hemorrhage usually presents acutely. While most major complications present acutely, delayed hemorrhage may occur and present later. In our institution, the current practice post procedure includes observation in the SACU for 4 hours with assessment of vital signs every 15 minutes for 1 hour and then every 30 minutes for 2 hours and an hour later prior to discharge. An algorithm followed at our institute for performance of parenchymal liver biopsies is outlined in Figure 12. If the patient experiences minor pain, Tylenol can be administered. Narcotic pain medications can be ordered as needed by the patient. A clear liquid diet and bed rest is also ordered in the first hour and advancing the diet and activity as tolerated. The patient is also adviced not to lift heavy weights or indulge in strenuous physical activity for 48 hours post procedure.
Figure 12.
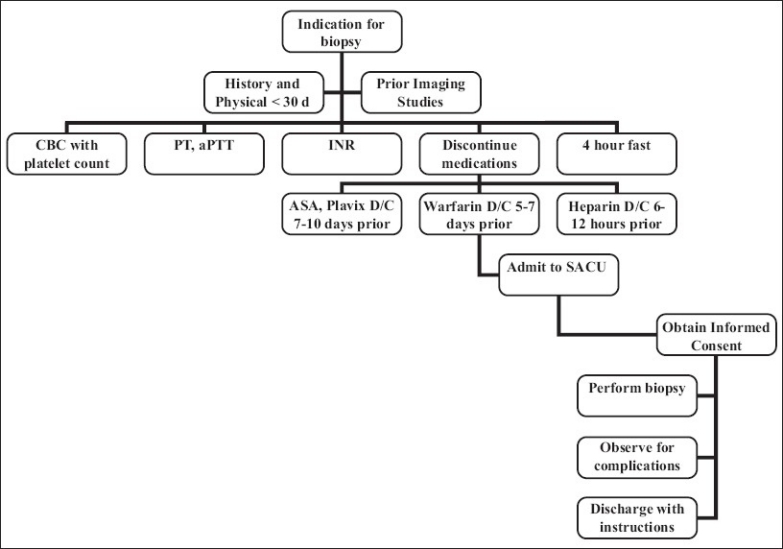
Parenchymal liver biopsy: Patient flowchart
The American College of Physicians Guidelines for outpatient procedures recommend that patients stay within 30 minutes of the medical facility for 24 hours post procedure and that a responsible adult remains with the patient for the first 24 hours after the procedure. Also, it recommends that the medical facility where the biopsy is performed should have a blood bank, laboratory, inpatient beds, and personnel available for the patient for at least 6 hours post procedure.
SPECIMEN ADEQUACY
In order to justify risks of the procedure, care must be taken to ensure specimen adequacy [Figures 13–16]. The size of specimen is extremely important for diagnosis and some pathologists recommend up to 11 complete portal triads for adequacy,[2,17,18] while others suggest a number of 6–8 for diagnostic accuracy.[15,16] The American Association for Study of Liver Diseases (AASLD) recommends a 16-gauge biopsy of 2–3 cm in length for the diagnosis, grading, and staging of diffuse, non-neoplastic parenchymaldisease.[2] Despite this recommendation, the use of 18-gauge spring-loaded cutting needles is a common practice. Larger needle sizes such as 14 G and 16 G should ensure better specimens. However, the use of larger needles and increased number of passes has also been associated with a higher complication rate. In an experimental[19] ex vivo animal organ study, curving the cutting needle resulted in a larger core specimen. While this technique may have the potential to reduce the number of passes, it has never been extrapolated to living human subjects. There are no commercially available curved cutting needles. Also, the risk of organ laceration and bleeding merits consideration.
Figure 13.
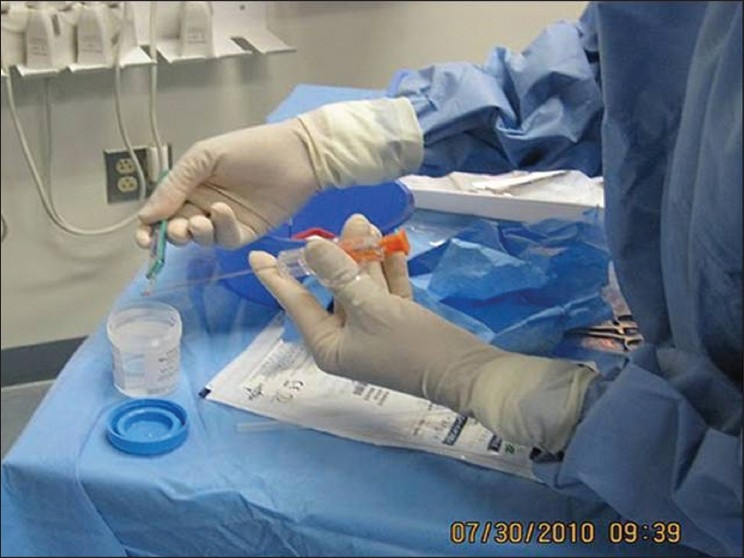
After the biopsy needle has been deployed, the specimen is placed within a sterile container filled with formalin.
Figure 16.
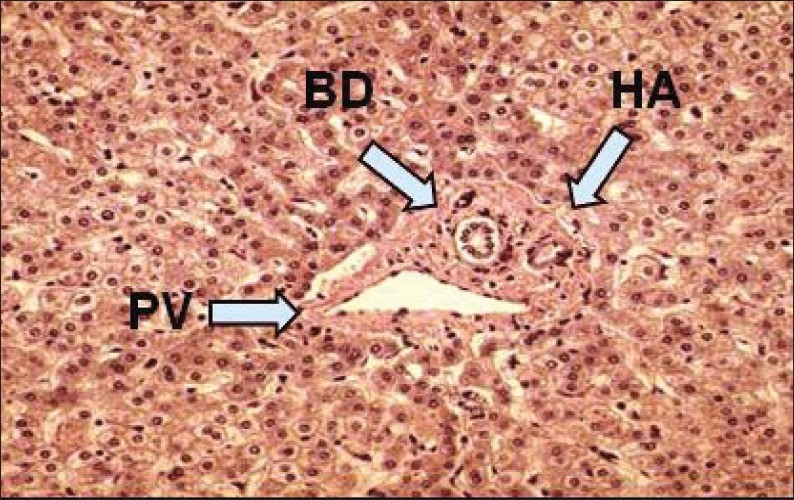
Histology of a portal triad (BD, bile duct; HA, hepatic artery; PV, portal vein).
Figure 14.
The size of the specimen is very important for diagnosis and some pathologists recommend that the specimen contain from 6 to 11 complete portal triads for specimen adequacy. This is an adequate size and appearance of a desirable specimen.
Figure 15.
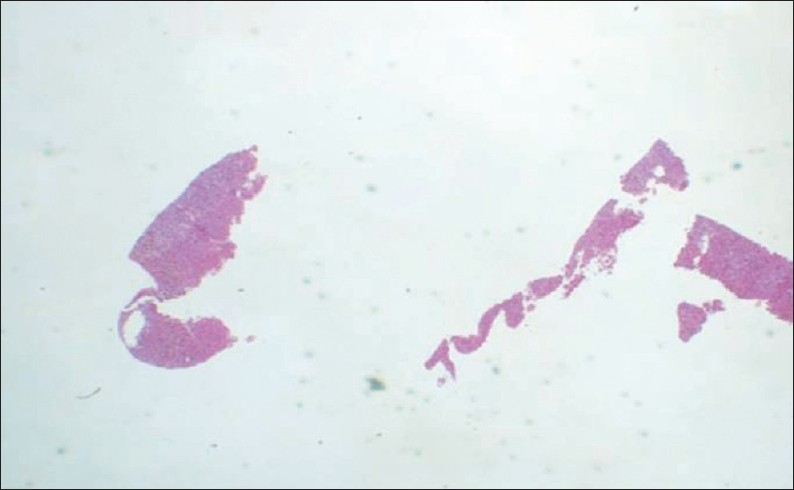
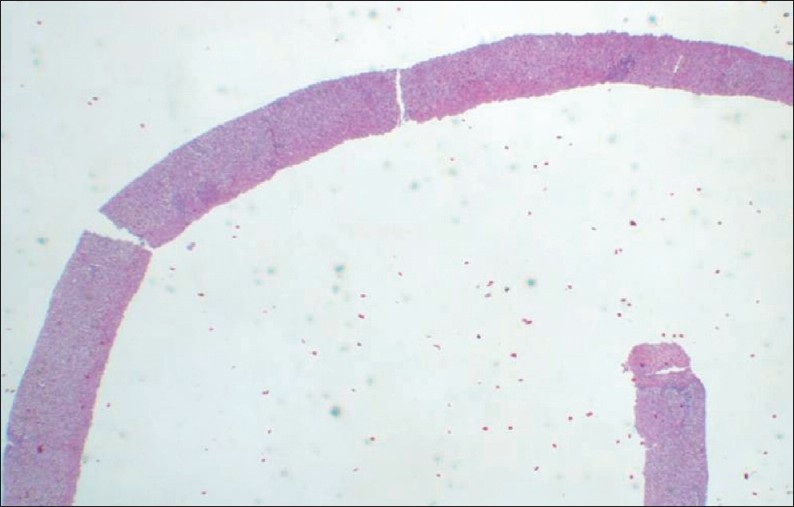
Example of a fragmented specimen.
At our institute, the preference is to use an 18-G cutting needle, but 19-G and 20-G needles have been used in a small number of cases. Though fragmentation is more common with the suction type “Menghini Needle”, cutting needle specimens also tend to fragment (personal observation). We routinely recommend the use of at least two biopsy specimens to ensure adequate tissue.
COMPLICATIONS
The use of US guidance should essentially eliminate the risk of pneumothorax, gallbladder, and other visceral injuries because of direct visualization of the structures. Pain is the commonest complication.[2,16] Though a subjective symptom, nearly up to 75% of patients express some discomfort. At our facility, any pain greater than 5/10 is initially treated with oral analgesics (Tylenol or Vicodin 325 mg/5 mg) and evaluated on an individual basis by the radiologist, who decides on subsequent management and follow-up. While most clinicians prefer to biopsy the right lobe, many radiologists prefer to biopsy the left lobe via an anterior epigastric approach. Tan et al.[20] in their study noted no difference in the pain scale irrespective of which lobe was biopsied. In view of the subjective variability in evaluation of pain and multiple operators in our study, we could not conclude about any meaningful difference in pain outcome between right and left lobe parenchymal liver biopsies.
Severe hemorrhage (necessitating transfusions, hospital admission for observation or intervention) is the most frequent major complication, with reported frequency in the literature ranging from 0.35 to 1.7%.[2,8,16] This complication is probably due to damage to smaller arteries that are generally not visible during the real-time guided procedure and is more common in patients with cirrhosis. Patients generally manifest with severe pain (>7/10 on the pain scale), lack of response to oral pain medication, hypotension, tachycardia, and a drop in hematocrit. Follow-up imaging with CT scan is the next useful step. Occasionally, an angiogram may be required to demonstrate a bleeding vessel and, if required, appropriate measures to control the bleeding. At our institution, a review of 776 procedures over 3 years performed with US guidance showed an incidence of significant hemorrhage in 6/776 (0.8%), including one death attributable to a post-procedural bleed, all following left lobe biopsies.
TRAINING
In order to become competent in the performance of percutaneous parenchymal liver biopsies, trainees observe, assist, and then perform the procedure under direct supervision with graduating levels of independence. The American College of Radiology (ACR)[21] recommends that trainees in radiology perform at least 35 supervised percutaneous image-guided biopsies during residency, without specifying target organs. At our institution, there is graduated responsibility with approximately 20 percutaneous hepatic biopsies during training considered adequate given the overlap with similar percutaneous procedures. The AASLD recommends that trainees perform 40 parenchymal liver biopsies, as well as receive training in imaging guidance and sonographic hepatic anatomy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/30/82082
REFERENCES
- 1.Sporea I, Popescu A, Sirli R. Why, who and how should perform liver biopsy in chronic liver diseases. World J Gastroenterol. 2008;14:3396–402. doi: 10.3748/wjg.14.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. AASLD position paper: Liver biopsy. Hepatology. 2009;49:1017–44. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 3.Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: A systematic review. Arch Intern Med. 2003;163:901–8. doi: 10.1001/archinte.163.8.901. [DOI] [PubMed] [Google Scholar]
- 4.Atwell TD, Smith RL, Hesley GK, Callstrom MR, Schleck CD, Harmsen WS, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194:784–9. doi: 10.2214/AJR.08.2122. [DOI] [PubMed] [Google Scholar]
- 5.Segal JB, Dzik WH. Transfusion Medicine/Hemostasis Clinical Trials Network.Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 6.Farrell RJ, Smiddy PF, Pilkington RM, Tobin AA, Mooney EE, Temperley IJ, et al. Guided versus blind liver biopsy for chronic hepatitis C: Clinical benefits and costs. J Hepatol. 1999;30:580–7. doi: 10.1016/s0168-8278(99)80187-1. [DOI] [PubMed] [Google Scholar]
- 7.Lindor KD, Bru C, Jorgensen RA, Rakela J, Bordas JM, Gross JB, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology. 1996;23:1079–83. doi: 10.1002/hep.510230522. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Teran JC, Ganiats TG, Carey WD. Ultrasound-guided liver biopsy for parenchymal liver disease: An economic analysis. Dig Dis Sci. 1998;43:46–50. doi: 10.1023/a:1018815802500. [DOI] [PubMed] [Google Scholar]
- 9.Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology. 1998;27:1220–6. doi: 10.1002/hep.510270506. [DOI] [PubMed] [Google Scholar]
- 10.Papini E, Pacella CM, Rossi Z, Bizzarri G, Fabbrini R, Nardi F, et al. A randomized trial of ultrasound-guided anterior subcostal liver biopsy versus the conventional Menghini technique. J Hepatol. 1991;13:291–7. doi: 10.1016/0168-8278(91)90071-i. [DOI] [PubMed] [Google Scholar]
- 11.Chiavaroli R, Grima PF, Calabrese P, Grima P. Routine ultrasound-guided liver biopsy versus echo-assisted procedure in viral chronic hepatitis. Radiol Med. 2008;113:992–8. doi: 10.1007/s11547-008-327-9. [DOI] [PubMed] [Google Scholar]
- 12.Flemming JA, Hurlbut DJ, Mussari B, Hookey LC. Liver biopsies for chronic hepatitis C: Should nonultrasound-guided biopsies be abandoned? Can J Gastroenterol. 2009;23:425–30. doi: 10.1155/2009/370651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med. 1993;118:96–8. doi: 10.7326/0003-4819-118-2-199301150-00003. [DOI] [PubMed] [Google Scholar]
- 14.DeWitt J, McGreevy K, Cummings O, Sherman S, LeBlanc JK, McHenry L, et al. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest Endosc. 2009;69:535–42. doi: 10.1016/j.gie.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 15.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 16.Zaman A, Ingram K, Flora KD. Diagnostic liver biopsy. [Last updated on 2009 Nov 18]. Available from: http://emedicine.medscape.com .
- 17.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: The smaller the sample, the milder the disease. J Hepatol. 2003;39:239–44. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 18.Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: A quantitative reference standard. Hepatology. 1998;28:323–31. doi: 10.1002/hep.510280206. [DOI] [PubMed] [Google Scholar]
- 19.Karam AR, Nugent W, Khan A, Desai D, Shankar S. Curved stylet core biopsy results in larger cores. AJR Am J Roentgenol. 2010;195:242–4. doi: 10.2214/AJR.09.3472. [DOI] [PubMed] [Google Scholar]
- 20.Tan KT, Rajan DK, Kachura JR, Hayeems E, Simons ME, Ho CS. Pain after percutaneous liver biopsy for diffuse hepatic disease: A randomized trial comparing subcostal and intercostals approaches. J Vasc Interv Radiol. 2005;16:1215–9. doi: 10.1097/01.RVI.0000173282.14018.79. [DOI] [PubMed] [Google Scholar]
- 21.ACR-SIR Practice guidelines for imaging guided percutaneous needle biopsies in adults. Available from: http:// www.acr.org;revised 2008 .


