Abstract
Parry–Romberg syndrome or progressive hemifacial atrophy is vary rare, uncommon, degenerative, poorly understood condition characterized by a slow and progressive atrophy affecting one side of the face. The incidence and the causes of this alteration are unknown. Possible factors that are involved in the pathogenesis include disturbance of fat metabolism, trauma, viral infections, heredity, endocrine disturbances and auto-immunity. The most common complications are: trigeminal neuritis, facial paresthesia, severe headache and epilepsy. Characteristically, the atrophy progresses slowly for several years and become stable after certain time period. After stabilization of the disease multi specialty approach including physician, orthodontic treatment and reconstructive surgery with autogenous fat graft can be performed to correct the deformity. The objective of this article is to accomplish a literature review concerning general characteristics, etiology, physiopathology, differential diagnosis and treatment of progressive hemifacial atrophy.
Keywords: Progressive hemifacial atrophy, Parry–Romberg syndrome, Romberg’s Sign, En coup de sabre, Localized linear scleroderma
Introduction
Parry–Romberg syndrome was first reported by Caleb Parry in 1815 and then described by Moritz Romberg in 1846. Parry–Romberg syndrome is a condition in which there is slow and progressive shrinkage of the tissues and sometimes bones of one or occasionally both sides of the face. It is generally unilateral atrophy of facial tissues including muscles, bones and skin [9, 11]. More than an aesthetic trouble, this illness bring several functional and psychological problems, like ocular problem, migraine, epilepsy. The incidence and cause of this alteration is unknown. A cerebral disturbance on fat metabolism has been proposed as a primary cause [4], trauma, viral infections, endocrine disturbances, auto-immunity and heredity are believed to be associated to the pathogenesis of the disease [13, 18, 22].
Frequently, the onset of this syndrome occurs along first and second decades of life. The atrophy progress slowly during years and then it stabilizes at its own [13, 15, 20]. Patients who manifest atrophy in early ages, have bigger repercussions and it is directly related to growing age of patient [16].
This syndrome seems to have higher incidence in women [4, 9, 17]. The extension of the atrophy is frequently limited to one side of the face, and the ipsilateral involvement of body is rare. 5–10% of cases were described as being bilateral [14].
The most important features of this pathology are the enophthalmy, the deviation of mouth and nose towards the affected side, and unilateral exposition of teeth (when lips are involved).
Parry–Romberg Syndrome is an auto-limitable condition with no cure. Affected patients should have multidisciplinary attendance of plastic surgeon, physicians, dental surgeon, phonoaudiologists and psychologists to give a better aesthetic to patient. Nowadays, cosmetic surgeries with autogenous fat graft, injections of silicone or bovine collagen, and inorganic implants are some alternatives to correct deformities [3, 18]. Besides esthetic improvement, symptomatic treatment for neurological disorders is indicated.
Aetiology
There have been a number of theories on the etiology of Parry–Romberg Syndrome include trigemial neuritis, a chronic autoimmune neurovasculitis, a chronic infestation with a neurotropic virus (e.g. Herpes) and an increased sympathetic nerve activity triggering facial atrophy [19]. A similar condition in rats has been initiated by performing a cervical sympathectomy during the new born.
For Myelopathy—Multiple sclerosis, Vitamin B12 and Vitamin E deficiency, structural spinal cord disease, infectious myelopathy.
For Peripheral neuropathy—Vitamin B12 deficiency, Inherited demyelinating neuropathies, cerebellar dysfunction, vestibular dysfunction (peripheral), drug intoxication, alcohol, cisplatin, pyridoxine (Vitamin B12) overdose, anticonvulsant toxicity (especially phenytoin).
Signs and Symptoms (Clinical Features)
In hemi facial atrophy unilateral sunken cheek, eye and tongue is seen. Migraine or migraine like headaches, trigeminal neuralgia, seizures—starting in the opposite side of the body, alopecia, blanched hair, atrophy of fat and subcutaneous tissue on the affected side are common. Degenerative brain lesions, intracranial calcifications, Sensory impairment, excessive sweating, tear duct dysfunction may be seen [7, 9, 16, 21].
Diagnosis
Romberg’s Sign
The Romberg test is named after the nineteenth century German Ear Specialist, Moritz Heinrich Romberg (1795–1873). It is a neurological test to detect poor balance, examine proprioception also to some extent, cerebeller function. The test is performed by having the patient with feet together and eyes closed, which eliminates the visual cues that help to maintain posture. Patients with positive Romberg’s sign shows diminished proprioception begin to fall or move their feet to maintain balance. Patients with vestibular dysfunction may also exhibit positive Romberg’s sign. Romberg’s sign shows cerebellar lesion and its not seen in a normal individual.
Romberg’s sign, when positive perform other neurologic screening tests like proprioception, knee bend and to walk straight line placing heel to toe, evaluate his gait, patient’s awareness of body part position while his eyes are closed, direct the movement, repeat the movement several times, gradually increasing the speed, vibratory sense nystagmus was not observed.
Investigation
Diagnosis can be made clinically if the patient is having facial asymmetry (physical sign). MRI and CT scan should be done if the patient is having neurological symptoms [1, 6]. Patient having epilepsy should be investigated with lumber puncture and auto antibodies. Thermographic response shows that the affected part is warmer than the unaffected part [2, 10, 12].
Complications
Fits, poliosis, facial, abnormality, enophthalmos, headache, alopecia and hyperpigmentation.
Differential Diagnosis
Linear scleroderma “en coup de sabre” (or localized linear scleroderma) is a well recognized entity by dermatologists and rheumatologists. Clinically, linear scleroderma may present in childhood and it involves intense loss of subcutaneous fat with ensuing thinning and pigmentation of the skin. It is commonly seen in the paramedian forehead region. In “en coup de sabre” atrophy of underlying muscle or bone is not seen. There is prolonged nerve conduction in areas affected by scleroderma which do not exist in Romberg’s. Anti-nuclear anti-body titres are often raised with active linear scleroderma, but rarely so with Romberg’s disease.
Discussion
Progressive hemifacial atrophy is disease of unknown etiology and rare pathology. It not only involves soft tissue but sometime involve bone causing severe esthetic discrepancy. There is wasting of muscle and subcutaneous tissue. Ocular involvement is usual so enophthalmy occur due to fat loss around orbit. The eye works normally. Ear can be smaller due to atrophy. There may be localized area of alopecia. There is neurological complication including trigeminal neuralgia, epilepsy, facial paraesthesia, headache etc. [7, 9, 16]. Facial and dental midline deviation along with mouth and nose on the affected side is seen. Unilateral atrophy of muscle of the tongue and upper lip (orbicularis oris) is seen.
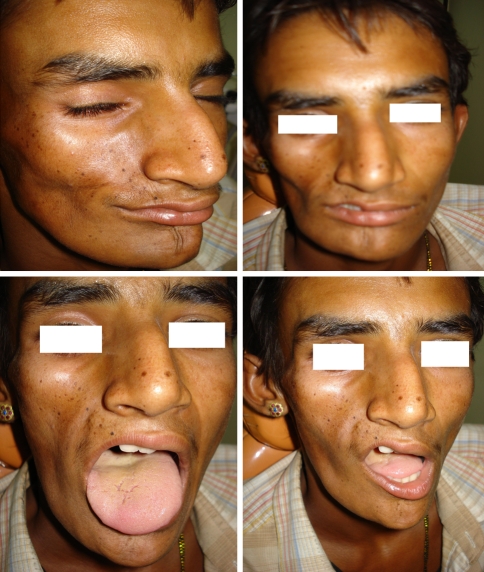
In our case (shown in photograph), atrophied right side of lip and right tongue is seen along with facial atrophy.
No radiological deficiency seen although in some cases when there is involvement of bone in dentition deficiency of root development and delayed eruption causing dental crowding, unilateral posterior crossbite [4, 5, 15]. Intraoral soft tissue and muscles of mastication is also involved but the normal function like speech, deglutition are not disturbed [13, 17] (Fig. 1).
Fig. 1.
Patient with hamifacial atrophy put in discussion at in our case (shown in photograph), atrophied right side of lip and right tongue is seen along with facial atrophy
Histologically dermis and epidermis of skin and subcutaneous tissue are atrophied [17]. There is atrophy of subcutaneous fat and degenerative changes are seen in vascular endothelium in electron microscope [17]. Treatment usually based upon, reposition of adipose tissue, autograph, silicon injection, prosthesis [3, 22]. These treatment modalities momentary resolved facial esthetic problems. Cosmetic treatment recommended when progress of the disease stops. This is the reason why our patient as not undergone any surgical treatment.
Treatment
The list of treatment mentioned in various sources for Parry–Romberg Syndrome includes the following list. Always seek professional medical advice about any treatment or change in treatment plans. Reconstructive or microvascular surgery, fat graft and dermis graft, fat cell injection (lipoinjection or lipofilling), silicone implantation, muscle graft, bone graft, orthognathic surgery, allografts, along with symptomatic and supportive therapy like assistive devices (e.g. cane, walker), surgical therapy for compressive myelopathy, spondolytic myelopathy [3, 8, 20, 22]. Supplementation of Vitamin B12, eliminate exposure to offending toxic substances, multiple sclerosis is treated with steroids, interferons, glatiramer acetate, mitoxantrone.
Prognosis
In some cases, the atrophy stops before the entire face is affected. In mild cases, the disorder usually causes no disability other than the cosmetic effects.
Recovery period for overall prognosis of Parry–Romberg syndrome is unpredictable.
References
- 1.Aher SW, Berg BO. Progressive hemifacial atrophy: report of 3 cases, including one observed over 43 years, and CT findings. Arch Neurol. 1982;39(1):44–46. doi: 10.1001/archneur.1982.00510130046011. [DOI] [PubMed] [Google Scholar]
- 2.Birdi N, Shore A, Rush P, et al. Childhood linear scleroderma: a possible role of thermography for evaluation. J Rheumatol. 1992;19(6):968–973. [PubMed] [Google Scholar]
- 3.Fuente A, Jimenez A. Latissimus dorsi free flap for restoration of facial contour defects. Ann Plast Surg. 1989;22(1):1–8. doi: 10.1097/00000637-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Finesilver B, Rosow HN. Total hemiatrophy. J Am Med Assoc. 1938;110(5):366–368. [Google Scholar]
- 5.Foster TD. The effects of hemifacial atrophy of dental growth. Br Dent J. 1979;146(5):148–150. doi: 10.1038/sj.bdj.4804213. [DOI] [PubMed] [Google Scholar]
- 6.Fry JA, Alvarellos A, Fink CW, Blaw ME, Roach ES. Intracranial findings in progressive facial hemiatrophy. J Rheumatol. 1992;19(6):956–958. [PubMed] [Google Scholar]
- 7.Gorlin RJ, Pinborg JJ, editors. Syndromes of the head and neck. New York: McGraw Hill; 1964. pp. 475–477. [Google Scholar]
- 8.Inigo F, Jimenez-Murat Y, Arroyo O, et al. Restoration of facial contour in Romberg’s disease and hemifacial microsomia: experience with 118 cases. Microsurgery. 2000;20(4):167–172. doi: 10.1002/1098-2752(2000)20:4<167::AID-MICR4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Jurkiewicz MJ, Nahai F. The use of free revascularized grafts in the amelioration of hemifacial atrophy. Plast Reconstr Surg. 1985;76(1):44–55. doi: 10.1097/00006534-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013–1020. doi: 10.1111/j.1365-2133.2006.07497.x. [DOI] [PubMed] [Google Scholar]
- 11.Lakhani PK, David TJ. Progressive hemifacial atrophy with scleroderma and ipsilateral limb wasting (Parry–Romberg syndrome) J R Soc Med. 1984;77:138–139. doi: 10.1177/014107688407700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martini G, Murray KJ, Howell KJ, et al. Juvenile-onset localized scleroderma activity detection by infrared thermography. Rheumatology. 2002;41(10):1178–1182. doi: 10.1093/rheumatology/41.10.1178. [DOI] [PubMed] [Google Scholar]
- 13.Mazzeo N, Fisher JG, Mayer MH, Mathieu GP, Mcade FGG. Progressive hemifacial atrophy (Parry–Romberg syndrome) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:30–35. doi: 10.1016/S1079-2104(05)80069-1. [DOI] [PubMed] [Google Scholar]
- 14.Miller MT, Sloane H, Goldberg MF, Grisolano J, Frenkel M, Mafee MF. Progressive hemifacial atrophy. J Pediatr Ophthamol Strabismus. 1987;24(1):27–36. doi: 10.3928/0191-3913-19870101-07. [DOI] [PubMed] [Google Scholar]
- 15.Moore MH, Wong KS, Proudman TW, David DJ. Progressive hemifacial atrophy: skeletal involvement and treatment. Br J Plast Surg. 1993;46:39–44. doi: 10.1016/0007-1226(93)90063-H. [DOI] [PubMed] [Google Scholar]
- 16.Neville BW, Damm DD, Allen CN, Bouquout JE, editors. Patologia oral e Maxilofacial. Rio de Janeiro: Guanabara Koogan; 1998. p. 35. [Google Scholar]
- 17.Pensler JM, Murphy GF, Muliken JB. Clinical and ultra-structural studies of Romberg’s hemifacial atrophy. Plast Reconstr Surg. 1990;85(5):669–676. doi: 10.1097/00006534-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro- Silva TP, Camarinha-Silva C, Limeira-Silveira CS, Ereno-Botelho PC, Rodrigues-Pinheiro MG, Viana-Pinheiro JJ. Progressive hemifacial atrophy—case report. Med Oral Patol Oral Cir Bucal. 2006;11(2):E112–E114. [PubMed] [Google Scholar]
- 19.Cory RC, Clayman DA, Faillace WJ, McKee SW, Carlos H. Gama clinical and radiologic findings in progressive facial hemiatrophy (Parry–Romberg syndrome) AJNR Am J Neuroradiol. 1997;18(4):751–757. [PMC free article] [PubMed] [Google Scholar]
- 20.Roddi R, Riggio E, Gilbert PM, Houvius SER, Vaandrager JM, Meulen JCH. Clinical evaluation of techiniques used in the surgical treatment of progressive hemifacial atrophy. J Craniomaxillofac Surg. 1994;22(1):23–32. doi: 10.1016/s1010-5182(05)80292-6. [DOI] [PubMed] [Google Scholar]
- 21.Terstegge K, Kunath B, Felber S, Speciali JG, Henkes H, Hosten N. MR of brain involvement in progressive facial hemiatrophy (Romberg disease): reconsideration of a syndrome. AJNR Am J Neuroradiol. 1994;15(1):145–150. [PMC free article] [PubMed] [Google Scholar]
- 22.Zafarulla MY. Progressive hemifacial atrophy: a case report. Br J Ophthalmol. 1985;69(7):545–547. doi: 10.1136/bjo.69.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]