Abstract
Polymorphous low grade adenocarcinoma (PLGA) is an uncommon tumour that affects minor salivary glands mainly. It was known to be clinically benign and histologically polymorphic; sometimes misdiagnosed as pleomorphic adenomas, monomorphic adenomas, malignant pleomorphic adenomas, adenoid cystic carcinomas and adenocarcinoma not otherwise specified. More information about PLGA is cumulating in the current literature with new evidences suggesting that the tumour may not be as indolent as it was previously thought. A thorough understanding of the clinical and histological behaviour of the lesion has serious implications in management. Here, a case of lower lip lesion with suspected lung metastasis is reported to exemplify how the clinical behaviour of the lesion may affect management.
Keywords: PLGA, Lower lip, Lung metastasis
Introduction
In 1983, Freedman and Lumerman recognized a rare, distinct, neoplastic histological entity previously misdiagnosed as pleomorphic adenomas, monomorphic adenomas, malignant pleomorphic adenomas, adenoid cystic carcinomas or adenocarcinoma not otherwise specified [1, 2]. Evans and Batsakis in 1984 [3], proposed the term Polymorphous low-grade adenocarcinoma (PLGA) to describe this tumour entity which was found mainly in the minor salivary glands. Since then, there have been several case series describing the different clinical behaviours and histological characteristics of this rare tumour. A critical look at the current literature on PLGA suggests that more knowledge is still required to facilitate prompt and proper diagnosis, and adequate treatment of the disease.
In this article, we present a concise review of the literature and a case report of PLGA; highlighting the local challenges encountered in the management.
Literature Review
Polymorphous low grade adenocarcinomas (PLGAs) are thought to be indolent tumours that affect the minor salivary glands almost exclusively [4]. The age of incidence is usually in the sixth to seventh decade of life but rare occurrences in adolescents have been reported [5–7]. There is predilection for females in the ratio of 2–4.6:1 [8]. The tumour is thought to have preference for the palate, but other intra oral sites include the upper lip, buccal mucosa, posterior tongue and retromolar trigone [2, 9]. It is reputed as the second most common malignancy of the minor salivary gland following mucoepidermoid carcinoma [9]. However, detection of PLGA in extra oral sites is increasingly being reported, thus attenuating the traditional belief that the tumour is localized exclusively to the minor salivary glands. Recently, PLGA has been reported in major salivary glands [10], the breast [11], paranasal sinuses [12], the skin and orbit [13], vulva and vagina [14]. Lung lesions have also been reported both as metastatic [4, 7] and primary lesions [5, 15]. These lesions were found to have similar histologic and immunohistochemical characteristics as those of the minor salivary gland [4, 10, 11, 14].
In spite of its locally infiltrative growth pattern and perineural and perivascular invasive characteristics, PLGA is generally known to be clinically innocuous [2, 16]. It is considered to be of low grade malignant potential in that nodal metastases are seen in only a minority, and distant spread is rare, occurring in less than 1% of cases [7, 16]. On the contrary, recent experiences have shown that the lesion is locally aggressive in a few cases [7, 16, 17]. Inadequate excision with positive margin, repeated surgery, exposure to radiation, prolonged duration and occurrence in a young patient are the various factors that have been associated with aggressive clinical progression [7, 16–18].
Histologically, PLGA is characterized by nonencapsulated, infiltrative borders of bland-looking tumour cells arranged in diverse architectural patterns. The highly variable growth patterns include tubular, solid, papillary, microcystic, cribriform, fascicular, single file, and strand-like arrangements [16, 17]. This morphologic heterogeneity is responsible for the diagnostic difficulty and confusion with other salivary gland neoplasms such as pleomorphic adenoma and adenocystic carcinoma; in which case immunohistochemical studies may be indicated [19]. An interesting observation in the recent literature is the potential for histological transformation of the lesion from a low grade to a high grade malignancy [7, 16]. A correlation between clinical progression and histologic characteristic has been documented; the papillary cystic pattern is now associated with aggressive clinical behavior [2, 16–18]. It has also been noted that this transformation phenomenon, like the clinical progression follows the course of time, and local recurrences [16].
Excision of primary lesion with wide margin produces local control in most cases of PLGA, but sometimes modified radical neck dissection is indicated [20, 21]. Recurrences after many years of local control have been known, hence prolonged and systematic follow up is recommended [18, 20, 22]. Combination of surgery and radiation has little benefit and experience with chemotherapy is very limited and controversial [20]. Some authors have noted that PLGA could be deceitfully innocuous but potentially fatal thereby suggesting the removal of the term “low grade” from the name [23]. Most patients will survive for a long time but mortality rate of up to 12.5% was documented in a series comprising forty patients followed up for 10 years [17].
PLGA was only recently distinguished, as experience with it continues to accumulate, it is becoming clear that late recurrences, metastases, and fatal outcomes, though currently infrequent, may not be as rare as previously thought.
Case Report
A 63 year old man reported at our clinic with 8 years history of lower lip swelling of insidious onset. The patient was generally healthy except for the asymptomatic lower lip swelling. The swelling was entirely in the soft tissue measuring about 5 × 3 cm and extending from the mid-line across the left corner of the mouth. The inferior limit was to a level just short of the lower border of the mandible. The overlying skin was intact, appeared normal in colour but tethered to the lesion. The swelling was firm, lobulated, not tender, and not associated with features of nerve involvement. The submandibular lymph nodes were palpable bilaterally, solitary, freely mobile, firm, not enlarged and not tender. The cervical lymph nodes were not clinically palpable.
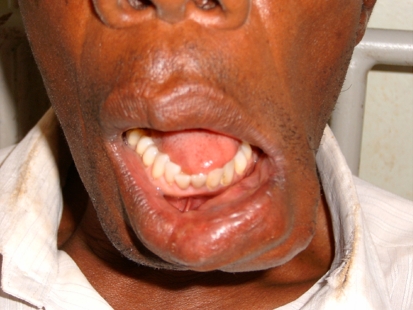
Intra-orally, the swelling was confined within the substance of the lip, extending from the midline to about 2 cm beyond the left angle of the mouth, bulging into, but not obliterating the labial sulcus (Fig. 1). The overlying mucosa was intact but adherent to the lesion. Jaw radiographs showed no bony involvement.
Fig. 1.
Lesion wholly in the substance of the lower lip, protruding into but not obliterating the labial sulcus
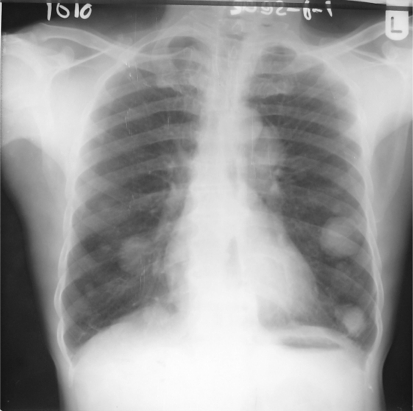
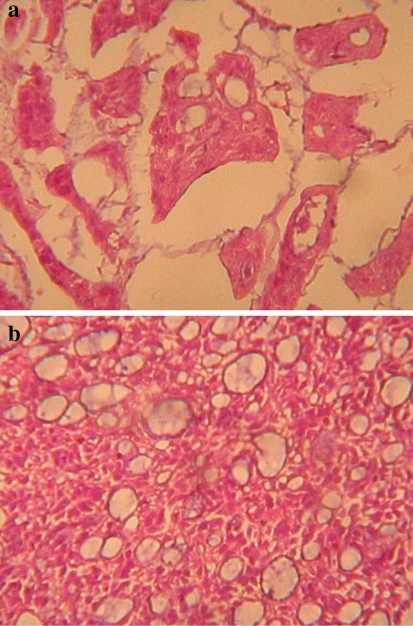
Preoperative incisional biopsy of the lip lesion was obtained and reported as PLGA. This diagnosis prompted a chest radiograph request. Thus, a chest radiograph was ordered to rule possible lung metastasis. Figure 2, the chest radiograph, showed multiple circular radio-opacities (cannon balls) of varying sizes scattered within the lung fields bilaterally. Wide local excision of the lesion with selective neck dissection and a biopsy of the chest lesion were therefore planned. Patient declined both the neck dissection and the biopsy of the chest lesions. Tumour excision alone was then done under general anaesthesia taking care to provide about 2 cm of clinically normal skin and other soft tissue circumferentially around the lesion. A frozen section was not deemed necessary as daughter cells were not expected to be present beyond the 2 cm margin around a well circumscribed lesion. The histological examination of the excised specimen confirmed the diagnosis of PLGA. The architectural pattern was typically varied, demonstrating trabecular, pseudo-cribriform and tubular differentiation, with extracellular mucin in some areas (Fig. 3a, b).
Fig. 2.
Circular cannon-ball radio-opacities of varying sizes within both lung fields
Fig. 3.
a Photomicrograph showing trabecular and tubular differentiation with abundant extracellular mucin. (H & E stain. Magnification is 2 × 220). b Photomicrograph showing the pseudo-cribriform pattern of polymorphous low-grade adenocarcinoma. (H & E stain. Magnification is 2 × 220)
The radiographic chest finding raised the suspicion of an associated lung metastases or the possibility of a synchronous primary pulmonary lesion. In search of any coexisting primary or other metachronous lesions, we performed prostate specific antigen (PSA) estimation and comprehensive abdomino-pelvic ultrasound scan (AUSS); no abnormality was detected. A minimally invasive image guided fine needle aspiration biopsy of the chest lesion was proposed but this was bluntly refused by the patient. His reason was that he had no chest or any systemic symptoms to warrant the stress and expenses of further management. Since his primary concern with the lip lesion was already addressed, he felt completely satisfied. All efforts to convince the patient to accept further investigations and possible treatment of the chest lesion failed. There was no evidence of loco-regional recurrence in the following 2 years post surgery. However, the individual sizes of the cannon balls appeared larger despite the lack of chest symptoms. After the initial 2 years, the patient stopped to attend the follow up clinics and efforts to reach him has been unsuccessful.
Discussion
PLGA is certainly a rare tumour that mostly affects minor salivary gland of the oral cavity. The palate is the preferred location while to the best of our knowledge; only one case of lower lip lesion was previously reported [19]. Distant spread to the lung is also said to be rare, although by now, two cases of microscopically confirmed secondary lung lesion [4, 7] and two cases of confirmed primary lung lesion [5, 17] have been reported.
The case here presented, typifies the characteristic benign clinical course of PLGA. The insidious growth and asymptomatic state of the disease made the patient to underestimate the potential fatality that may be associated with the tumour in the long run. The principal concern of the patient at presentation was aesthetic and functional limitation imposed on the lower lip by the tumour.
Initially, the innocuous history of the lesion did not prompt extensive systemic investigation until the incisional biopsy revealed PLGA and the chest radiograph disclosed multiple pulmonary lesions in both lung fields. Although, we did not clinically detect positive loco-cervical lymph node metastasis, Hannen et al. [4] had suggested periodic chest radiographs in patients diagnosed of PLGA. In this case, due to ethical restraints, we were unable to microscopically confirm the chest lesions as metastatic spread of PLGA from the oral lesion. Arguably, there was no other logical explanation for the chest lesions since there was no clinical, biochemical or radiological evidence of co-existing primaries elsewhere in the patient’s body.
The primary lesion in this patient exhibited the characteristic histological polymorphism known with PLGA. It is however note worthy that the papillary cystic pattern associated with clinical aggression and histological transformation to high grade malignancy was not found. Notwithstanding, the outcome of the disease in this patient remain precarious considering the established tendency to aggressive clinical behaviour with protracted clinical course. Although, local control was achieved with negative surgical margin in the patient, loco-regional recurrence after many years of primary surgery has been reported [18, 20]. This can further predispose to potentially more malignant transformation in reaction to repeated surgery [16].
The challenges encountered in the management of this case are attributable mainly to the deceptive clinical behaviour of PLGA and partly to the self pay health care system of our country. As long as he felt clinically healthy, the patient could not accept any justification for extensive investigation and treatment, including a selective neck dissection, the economic cost of the investigations and the cost and seeming inconvenience of continued clinical follow up visits.
In summary, a case of PLGA occurring in the lower lip with suspected lung metastasis has been reported. The reality of limitations to comprehensive care imposed by social, economic and ethical considerations in a developing economy is exemplified.
Acknowledgments
The authors are very grateful to Prof EEU Akang and Dr B Kolude of the departments of Pathology and Oral Pathology, respectively of the University College Hospital Ibadan for their various assistance in the management of the patient and preparation of this manuscript.
Conflict of interest The author declare that no funding was received for this work and the authors have no special interest of any sort.
References
- 1.Freedman PD, Lumerman H. Lobular carcinoma of intraoral minor salivary gland origin. Report of twelve cases. Oral Surg. 1983;56:157–165. doi: 10.1016/0030-4220(83)90282-7. [DOI] [PubMed] [Google Scholar]
- 2.Vincent SD, Hammond HL, Finkelstein MW. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1994;77:41–47. doi: 10.1016/S0030-4220(06)80105-2. [DOI] [PubMed] [Google Scholar]
- 3.Evans HL, Batsakis JG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of distinctive neoplasm. Cancer. 1984;53:935–942. doi: 10.1002/1097-0142(19840215)53:4<935::AID-CNCR2820530420>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Hannen EJ, Bulten J, Festen J, et al. Polymorphous low-grade adenocarcinoma with distant metastases and deletions on chromosome 6q23-qter and 11q23-qter: a case report. J Clin Pathol. 2000;53:942–945. doi: 10.1136/jcp.53.12.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee VK, McCaughan BC, Scolyer RA. Polymorphous low-grade adenocarcinoma in the lung: a case report. Int J Surg Pathol. 2004;12:287–292. doi: 10.1177/106689690401200313. [DOI] [PubMed] [Google Scholar]
- 6.Lengyel E, Somogyi A, Godney M, et al. Polymorphous low-grade adenocarcinoma of the nasopharynx. Case report and review of literature. Strahlenther Onkol. 2000;176:40–42. doi: 10.1007/PL00002304. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka F, Wada H, Inui K, et al. Pulmonary metastasis of polymorphous low-grade adenocarcinoma of the minor salivary gland. Thorac Cardiovasc Surg. 1995;43:178–180. doi: 10.1055/s-2007-1013795. [DOI] [PubMed] [Google Scholar]
- 8.Toida M, Shimokawa K, Makita H, et al. Intra oral minor salivary gland tumours: a clinicopathological study of 82 cases. Int J Oral Maxillofac Surg. 2005;34:528–532. doi: 10.1016/j.ijom.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323–333. doi: 10.1016/0030-4220(88)90240-X. [DOI] [PubMed] [Google Scholar]
- 10.Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofacial Surg. 2005;63:805–810. doi: 10.1016/j.joms.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Asioli S, Marucci G, Ficarra G, et al. Polymorphous adenocarcinoma of the breast. Report of three cases. Virchows Arch. 2006;448:29–34. doi: 10.1007/s00428-005-0084-2. [DOI] [PubMed] [Google Scholar]
- 12.Charous DD, Cunnane MF, Rosen MR, et al. Recurrent polymorphous low-grade adenocarcinoma manifesting as a sinonasal mass: a case report. Ear Nose Throat J. 2005;84:354–357. [PubMed] [Google Scholar]
- 13.Thomas KM, Cumberworth VL, McEwan J. Orbital and skin metastasis in polymorphous low-grade adenocarcinoma of the salivary gland. J Laryngol Otol. 1995;109:1222–1225. doi: 10.1017/s0022215100132517. [DOI] [PubMed] [Google Scholar]
- 14.Young S, Leon M, Talerman A, et al. Polymorphous low-grade adenocarcinoma of the vulva and vagina: a tumor resembling adenoid cystic carcinoma. Int J Surg Pathol. 2003;11:43–49. doi: 10.1177/106689690301100113. [DOI] [PubMed] [Google Scholar]
- 15.Kyu Do Cho, Ji Han Jung, Deog Gon Cho, et al. Primary polymorphous low grade adenocarcinoma of lung treated by sleeve bronchial resection: a case report. J Korean Med Sci. 2007;22:373–376. doi: 10.3346/jkms.2007.22.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson RH, Pereira EM, Ribeiro AC, et al. Polymorphous low grade adenocarcinoma of the salivary glands with transformation to high-grade carcinoma. Histopathology. 2002;41:250–259. doi: 10.1046/j.1365-2559.2002.01439.x. [DOI] [PubMed] [Google Scholar]
- 17.Harry L, Evans MD, Mario A, et al. Polymorphous low-grade adenocarcinoma a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24:1319–1328. doi: 10.1097/00000478-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Pelkey TJ, Mills SE. Histologic transformation of polymorphous low-grade adenocarcinoma of salivary gland. Am J Clin Pathol. 1999;111:785–791. doi: 10.1093/ajcp/111.6.785. [DOI] [PubMed] [Google Scholar]
- 19.Chaâbouni S, Ayadi L, Dhouib H, et al. Polymorphous low-grade adenocarcinoma: a palatine and a labial location. Rev Stomatol Chir Maxillofac. 2008;109:178–182. doi: 10.1016/j.stomax.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Castle JT, Thompson LD, Frommelt RA, et al. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86:207–219. doi: 10.1002/(SICI)1097-0142(19990715)86:2<207::AID-CNCR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Eugene NM, Robert LF (eds). Salivary gland disorders. Springer, Berlin
- 22.Gonzalez-Garcia R, Rodriguez-Campo FJ, et al. Polymorphous low-grade adenocarcinoma of the palate: report of cases. Auris Nasus Larynx. 2005;32:275–280. doi: 10.1016/j.anl.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Speight PM, Barrett AW. Salivary gland tumors. Oral Dis. 2002;8:229–240. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]