Abstract
N-Acetyl-d-neuraminic acid (NeuNAc) aldolase is an important enzyme for the metabolic engineering of cell surface NeuNAc using chemically modified d-mannosamines. To explore the optimal substrates for this application, eight N-acyl derivatives of d-mannosamine were prepared, and their accessibility to NeuNAc aldolase was investigated quantitatively. The N-propionyl-, N-butanoyl-, N-iso-butanoyl-, N-pivaloyl- and N-phenylacetyl-d-mannosamines proved to be as good substrates as, or even better than, the natural N-acetyl-d-mannosamine, while the N-trifluoropropionyl and benzoyl derivatives were poor. It was proposed that the electronic effects might have a significant influence on the enzymatic aldol condensation reaction of d-mannosamine derivatives, with electron-deficient acyl groups having a negative impact. The results suggest that N-propionyl-, N-butanoyl-, N-iso-butanoyl- and N-phenylacetyl-d-mannosamines may be employed to bioengineer NeuNAc on cells.
Keywords: N-acyl-d-mannosamine, N-acetyl-d-mannosamine, N-acetyl-d-neuraminic acid, sialic acid, aldolase
1. Introduction
As the terminal and thereby the most exposed sugar unit of a majority of oligosaccharides of natural glycoproteins and glycolipids on cell surfaces, N-acetyl-d-neuraminic acid (NeuNAc, 1, also known as N-acetylsialic acid) plays a critical role in various biological events.1–3 For example, the overexpression of NeuNAc is closely correlated with many phenotypes of tumors, so sialylated carbohydrate antigens on tumor cells are important molecular templates and targets for cancer vaccine design and other biological investigations.4–10
The biosynthesis of NeuNAc, and therefore sialoglycoconjugates, utilizes N-acetyl-d-mannosamine (ManNAc, 2) as a key substrate.11 It is well recognized that this biosynthetic pathway can tolerate unnatural substrates, especially ManNAc derivatives with modified N-acyl groups, and the phenomenon was extensively exploited by Reutter12–14 and Bertozzi15–17 and their coworkers in the bioengineering of NeuNAc on cell surfaces. Based upon these pioneering works, we recently developed a new strategy to overcome the problem of immunotolerance to tumor-associated carbohydrate antigens (TACAs) for cancer vaccine development.18 The underlining principle is that after a cancer patient or an animal is immunized with a synthetic, artificial TACA bearing modified sialic acid(s) to establish a specific immune response, the patient or animal is treated with a correspondingly modified biosynthetic precursor of sialic acid to initiate the expression of the artificial TACA on tumor cells. The pre-stimulated immune system will then wipe out the marked tumors.
Our preliminary studies utilizing N-propionyl-d-mannosamine (ManNPr 3) as the glycoengineering precursor and polysialic acid on cancer cells as the target antigen18 gave encouraging results with regard to the glycoengineering and selective immunotargeting of cancer. However, the initial design was not necessarily the optimal one, because more distinctive modifications of NeuNAc may produce more effective vaccines and make the glycoengineered tumors more distinguishable. In this context, the cavity is that the modified d-mannosamine as precursor must be available to the biosynthetic pathways of sialic acid and sialoglycoconjugates.
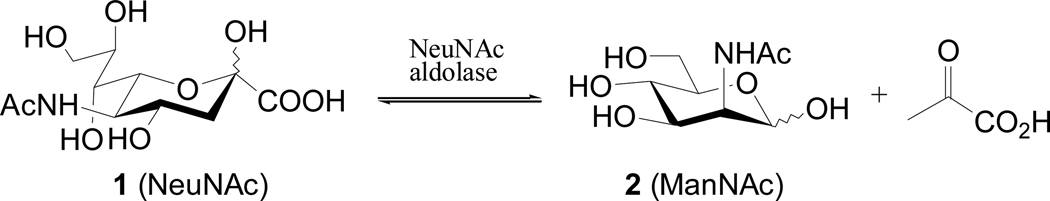
Recently Bertozzi and her coworkers19 identified that the bottleneck for the biosynthetic machinery of sialic acid to use N-acyl-d-mannosamines was its 6-O-phosophrylation, the first biosynthetic step, while the enzymes involved in the remaining steps were more permissive. The efficient incorporation of various N-acyl-d-mannosamines into cell surface sialoglycoconjugates12,19 suggests that the modified d-mannosamine precursors can somehow bypass the substrate-specific step. It was proposed that the conversion of unnatural d-mannosamines to sialic acids took advantage of NeuNAc aldolase,19 an enzyme that usually catalyzes the degeneration of sialic acid (Scheme 1). However, since this reaction is completely reversible, NeuNAc aldolase is also involved in the biosynthesis of NeuNAc.20 Moreover, it has been observed that NeuNAc aldolase can tolerate a variety of modifications of d-mannosamine. Thus, NeuNAc aldolase has been used to synthesize a variety of NeuNAc derivatives or analogs by enzymatic method.19–27
Scheme 1.
NeuNAc aldolase-catalyzed reaction
To find out the optical substrates for the bioengineering of NeuNAc on tumor cells, and other cells as well, by utilizing the permissibility of NeuNAc aldolase, it is necessary to understand the reactions of various N-acyl-d-mannosamines with the enzyme. For this purpose, the accessibility of a number of N-acyl derivatives of d-mannosamine to NeuNAc aldolase was investigated.
2. Results and Discussion
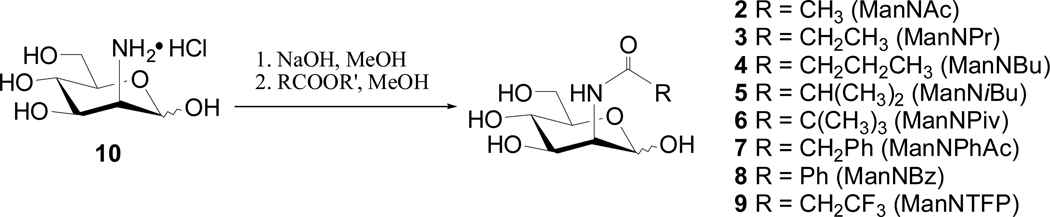
Eight N-acyl-d-mannosamines, including N-acetyl (ManNAc, 2), N-propionyl (ManNPr, 3), N-butanoyl (ManNBu, 4), N-iso-butanoyl (ManNiBu, 5), N-pivaloyl (ManNPiv, 6), N-phenylacetyl (ManNPhAc, 7), N-benzoyl (ManNBz, 8) and N-trifluoropropionyl (ManNTFP, 9) derivatives, were prepared from commercial d-mannosamine (10) by reaction with the corresponding acyl anhydrides or, for ManNTFP, by reaction with 1-hydroxybenzotriazole (HOBt) ester of trifloropropionic acid in methanol (Scheme 2). The expected products were obtained in 68–75% yields after silica gel column chromatography and subsequent recrystallization from mixtures of ethanol and ethyl acetate.
Scheme 2.
Preparation of N-acyl-D-mannosamines (2–9)
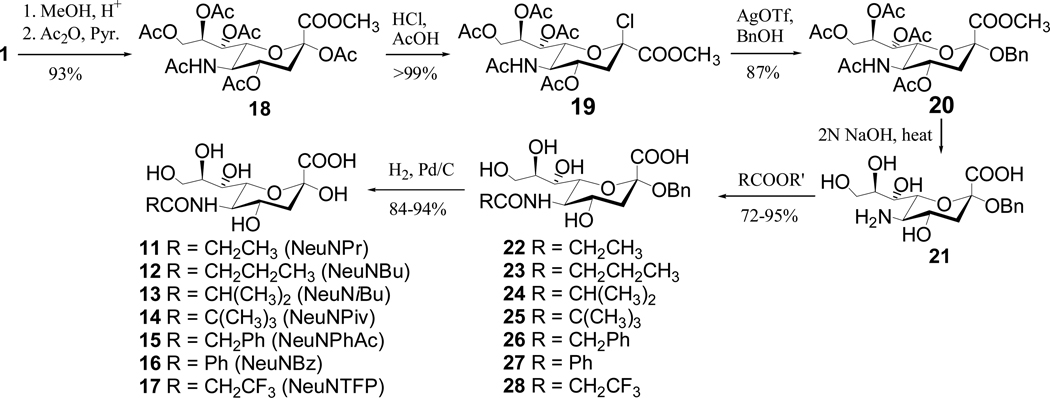
The standard samples of N-acylsialic acids (11–17) were prepared according to Scheme 3. Direct hydrolysis of NeuNAc under either acidic or basic conditions caused its decomposition probably due to the involvement of its keto α-hydrogens in the open chain form. Therefore, NeuNAc (1) was first converted into the benzyl glycoside 2028–30 that could be hydrolyzed under basic conditions smoothly to afford 21. Selective acylation of the free amino group of 21 using various acyl anhydrides or the active ester of trifluoropropionic acid was followed by catalytic debenzylation to give N-acylsialic acids 11–18. All synthetic intermediates and final products in Schemes 2 and 3 were positively identified by NMR and MS.
Scheme 3.
Preparation of N-acyl-d-neuraminic acids (11–17)
Commercial NeuNAc aldolase purchased from CALBIOCHEM® was utilized to investigate the enzymatic accessibility of N-acyl-d-mannosamines. The reactions were carried out at 37 °C in a buffer (pH 7.4, I = 0.1) containing 0.05 M potassium phosphate and 7.5% (v/v) dithiothreitol.23,24 Pyruvic acid was used in large excess (10 equivalents) and its concentration could be considered as a constant during the treatment of reaction kinetics. The sialic acid products of the reactions were assayed with the modified Svennerholm (resorcinol) method.31
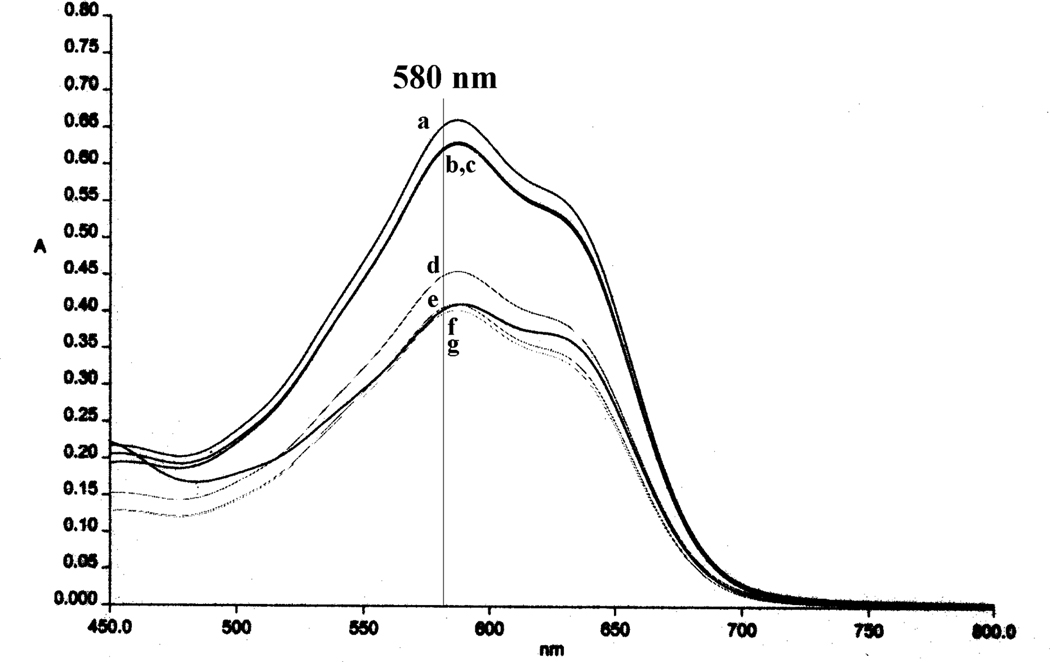
The UV-visible absorption spectra of the Svennerholm reaction products of N-acylsialic acids 1 and 11–17 were analyzed first. As shown in Figure 1, although they had nearly identical λmax (587 nm), their molar extinction coefficients (ε) were different. Since the structure of Svennerholm product is not well defined,31 how the acyl groups affected the ε was not clear. Nevertheless, it seemed that bulky (Piv) and electron-deficient (Bz, PhAc and TFP) acyl groups caused the decline of ε values of the Svennerholm reaction products. Moreover, the product of NeuNBz showed a much stronger absorption band at ca. 425 nm than all other derivatives. Due to the differences of the UV-visible spectra, the individual calibration curves of 1 and 11–17 with regard to their absorbance at 580 nm were obtained using the sialic acid concentrations ranging from 0 to 0.2 mM, all of which gave excellent linear correlations. It was also proved that N-acyl-d-mannosamines, pyruvic acid or their mixture had no influence on the sialic acid analysis under the Svennerholm conditions. Therefore, the method could be safely used to assay the enzymatic reactions.
Figure 1.
UV-visible spetra of the Svennerholm reaction products of: a) NeuNAc, b) NeuNPr, c) NeuNBu and NeuNiBu, d) NeuNPhAc, e) NeuNTFP, f) NeuNPiv, and g) NeuNBz
Next, we established the suitable concentrations of N-acyl-d-mannosamine (5–50 mM) and aldolase (0.9 unit/mL) for the convenient and accurate analysis of sialic acid products by Svennerholm method. The transformation of N-acyl-d-mannosamines to N-acylsialic aicds was thus studied with 18.2 mM of N-acyl-d-mannosamine, 0.9 unit/mL of aldolase and 182 mM of pyruvic acid.
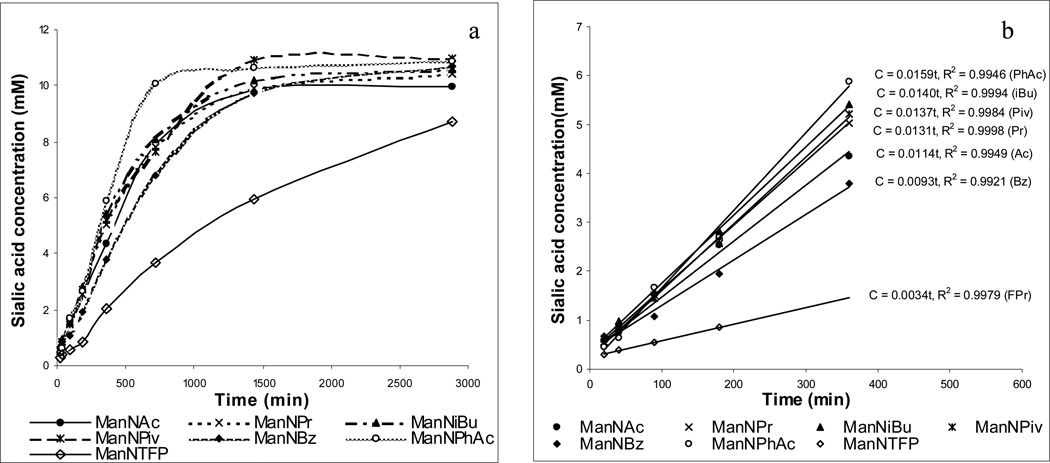
For the reaction analysis, exact aliquots of the reaction mixtures were withdrawn at various time points and examined by Svennerholm method.31 The results are summarized in Figures 2a and 2b. The reactions had two phases (Figure 2a). During the first 5 hours, they exhibited the pseudo-zero order kinetics (Figure 2b). Thereafter, most reactions leveled off and eventually reached a plateau within 24 hours. The reaction of ManNTFP was the slowest of all 8 derivatives tested, but it reached the similar plateau ca. 50 hours later.
Figure 2.
The reaction kinetics of N-acyl-d-mannosamines (18.2 mM) with pyruvic acid (182 mM) and NeuNAc aldolase (0.9 unit/mL): 37 °C, 0.05 M potassium phosphate buffer (pH 7.4, I = 0.1) containing 7.5% (v/v) dithiothreitol.
It is well established that aldolase-catalyzed reactions are reversible [eq. (1)],21,22 and the reaction rate can then be expressed by eq. (2).32 At the initial stage, the concentration of NeuNR was rather low, so the reverse reaction (k−1[NeuNR][Enz]) could be neglected and eq. (2) can be simplified to eq. (3). Meanwhile, pyruvic acid was used in large excess and the enzyme remained unchanged. Thus, the concentrations of enzyme [Enz] and pyruvic acid [PA] could also be taken as constants. Although the substrate concentration [ManNR] changed with the proceeding of reactions, at the initial stage this change was relatively small, and [ManNR] could be considered as a consistent. Eq. (3) can thus be simplified to eq. (4) and (5), showing the zero order kinectics.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
On the other hand, with the accumulation of NeuNR, the term k−1[NeuNR][Enz] of eq. (2) would become more and more significant, while the consumption of ManNR reduced k1[ManNR][PA][Enz]. As a result, the reactions slowed down and gradually reached the equilibrium (the plateau).
The initial reaction rates of various N-acyl-d-mannosamine derivatives were different (Figure 2b), even though their conversion rates at the equilibriums were similar (69% ± 1.8%). Compared to ManNAc (k' = 0.0114), the reactions of ManNPr (k' = 0.0131), ManNiBu (k' = 0.0140), ManNPiv (k' = 0.0137) and ManNPhAc (k' = 0.0159) were slightly faster, but the reactions of ManNBz (k' = 0.0093) and ManNTFP (k' = 0.0034) were obviously slower. Since 5–7 that bear rather bulky N-acyl groups such as iBu, Piv and PhAc were as good substrates as ManNAc, it seems that the steric effect is not critical for determining the enzymatic accessibility of these substrates. On the contrary, ManNTFP and ManNBz with strongly electron-deficient acyl groups were poor substrates, which may indicate that the electronic effect probably has a significant influence on the accessibility of N-acyl-d-mannosamines to the aldolase and the highly electron-deficient acyl groups retard the reaction. However, ManNPhAc with a mildly electron-deficient acyl group is a good substrate, which suggests that, besides electronic effect, other factors such as hydrophobic interactions may also affect the substrate-aldolase binding.
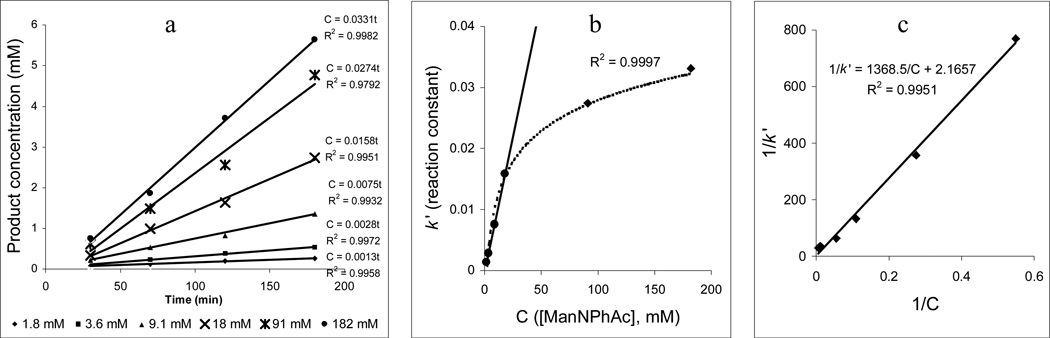
ManNPhAc was especially interesting to us, as it was the best substrate for NeuNAc aldolase and thus possibly the most efficient precursor for engineering cell surface sialic acid. Meanwhile, since a phenylacetyl group is distinctly different from an acetyl group, the phenylacetyl derivatives may form effective vaccines. The reaction of ManNPhAc was then studied in detail. As shown in Figure 3a, with pyruvic acid in excess, the reaction rate increased with the concentration of ManNPhAc from 1.82 to 182 mM. At low concentrations, the reaction rate (k') had a linear correlation with [ManNPhAc] (Figure 2b), which can be explained by eq. (3). However, the reaction rate leveled off at higher [ManNPhAc]. The results fit Lineweaver-Burk plot (Figure 2c).32 Because cellular levels of mannosamine are usually much lower than the tested concentrations, the NeuNAc aldolase-catalyzed reaction rate will probably observe a linear correlation with the concentration of ManNPhAc in the biological system. Therefore, any measures that can improve the cellular concentrations of ManNPhAc would be able to increase the biosynthesis of PhAc-modified sialic acid by cells and thereby the efficiency of cell surface sialic acid engineering.
Figure 3.
The impact of ManNPhAc concentrations (1.8, 3.6, 9.1, 18, 91 and 182 mM) on its reaction with pyruvic acid (182 mM) and NeuNAc aldolase (0.9 unit/mL): 37 °C, 0.05 M potassium phosphate buffer (pH 7.4, I = 0.1) containing 7.5% (v/v) dithiothreitol.
In conclusion, a number of N-acyl derivatives of d-mannosamine were prepared and their accessibility to NeuNAc aldolase was studied. It was demonstrated that NeuNAc aldolase could tolerate a range of N-modifications of D-mannosamine and, as the substrates of NeuNAc aldolase, ManNPr, ManNBu, ManNiBu, ManNPiv and ManNPhAc were as good as or even better than the natural ManNAc. These results suggest that ManNPr, ManNBu, ManNiBu, ManNPiv and ManNPhAc may be used as effective biosynthetic precursors to engineer cell surface sialic acid. Coupled with the fact that artificially modified sialooligosaccharides are highly immunogenic,33–35 these results should be valuable for the development of new cancer immunotherapies. Among the potentially useful biosynthetic precursors identified, ManNiBu and ManNPhAc are especially interesting to us, because our initial studies also indicated that N-iBu and N-PhAc modified sialic acids could induce robust IgG antibodies desirable for cancer immunotherapy.36 Therefore, we are currently investigate in detail the bioavailability of ManNiBu and ManNPhAc to cells, as well as their efficiency to glycoengineer tumors in vitro and in vivo.
3. Experimental
3.1. General methods
NMR spectra were recorded on a Gemini-300 FT NMR spectrometer. Proton chemical shifts are reported in ppm (δ) downfield from tetramethylsilane (TMS). Coupling constants (J) are reported in hertz (Hz). The fast atom bombardment mass spectra (FABMS) were obtained with a Kratos MS-25RFA spectrometer. Optical rotations were determined with a Perkin-Elmer 241 polarimeter, and thin layer chromatography (TLC) was performed on silica gel GF254 plates with detection by charring with phosphomolibdic acid-EtOH or 5% H2SO4-EtOH. Anhydrous solvents and other reagents were purchased from venders and used without further purification.
3.2. N-Acyl derivatives (2–8) of d-mannosamine
To the solution of d-mannosamine•HCl (300 mg, 1.39 mmol) in 5 mL MeOH and 0.5 mL of 3 N NaOH was added 1.0 mL of an acyl anhydride dropwise in an ice-water bath. After the reaction finished within 3 h as indicated by TLC, the mixture was neutralized with 2 N HCl and then condensed under reduced pressure. The residue was purified by silica gel column chromatography (eluent: MeOH/CH2Cl2) to afford N-acylmannosamine that was further purified by passing through a Biogel P2 column using distillated water as eluent. The fractions containing the expected product were combined, freeze-dried and finally recrystallized from ethyl alcohol and ethyl acetate to give a white crystalline product.
N-Acetyl-d-mannosamine (2)
210 mg (70%); [α]D20 +11 (c 1.0, H2O) (lit.37 + 9.7); 1H NMR (D2O, 300 MHz): (α-isomer) δ 5.10 (d, 1 H, J1,2 1.3 Hz, H-1), 4.30 (dd, 1 H, J2,3 4.6 Hz, H-2), 4.02(dd, 1 H, J3,4 9.7 Hz, H-3), 3.81-3.83 (m, 2 H, H-6,6’), 3.80 (m, 1 H, H-5), 3.59 (dd, 1 H, J4,5 9.0 Hz, H-4), 2.03 (s, 3 H, Ac); (β-isomer) δ 5.00 (d, 1 H, J1,2 1.6 Hz, H-1), 4.42 (dd, 1 H, J2,3 4.4 Hz, H-2), 3.81 (m, 1 H, H-3), 3.80-3.85 (m, 2 H, H-6,6’), 3.50 (dd, 1 H, J 9.9, 9.6 Hz, H-4), 3.41-3.43 (m, 1 H, H-5), 2.07 (s, 3 H, Ac); FABMS: m/z 204.0 [M + H+ - H2O], 221.1 [M+], 222.1 [M + H+], 244.1 [M + Na+]; HRFABMS: Calcd for C8H15NO6 [M + H+] m/z 222.0972, found 222.1016.
N-Propionyl-d-mannosamine (3).38
215 mg (68%); [α]D20 +7.0 (c 1.0, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 5.08 (d, 1 H, J1,2 1.6 Hz, H-1), 4.29 (dd, 1 H, J2,3 4.8 Hz, H-2), 4.02 (dd, 1 H, J3,4 9.7 Hz, H-3), 3.75-3.86 (m, 3 H, H-5,6,6’), 3.58 (t, 1 H, J 9.5, H-4), 2.29 (q, 2 H, J 7.6 Hz, Pr), 1.07 (t, 3 H, J 7.6 Hz, Pr); (β-isomer) δ 4.99 (d, 1 H, J1,2 1.7 Hz, H-1), 4.42 (dd, 1 H, J2,3 4.4 Hz, H-2), 3.75-3.86 (m, 3 H, H-3,6,6’), 3.48 (t, 1 H, J 9.5, H-4), 3.33-3.43 (m, 1 H, H-5), 2.33 (q, 2 H, J 7.7 Hz, Pr), 1.09 (t, 3 H, J 7.7 Hz, Pr); FABMS: m/z 218.1 [M + H+ - H2O], 236.1 [M + H+], 258.1 [M + Na+]; HRFABMS: Calcd for C9H17NO6 [M + H+] m/z 236.1129, found 236.1133.
N-Butanoyl-d-mannosamine (4).39
264 mg (75%); [α]D20 +9.0 (c 1.0, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 5.07 (d, 1 H, J1,2 1.3 Hz, H-1), 4.28 (dd, 1 H, J2,3 4.8 Hz, H-2), 4.02 (dd, 1 H, J3,4 9.7 Hz, H-3), 3.75-3.84 (m, 3 H, H-5,6,6’), 3.58 (dd, 1 H, J4,5 10.8 Hz, H-4), 2.22 (t, 2 H, J 7.6 Hz, Bu), 1.47-1.60 (m, 2 H, Bu), 0.86 (t, 3 H, J 7.5 Hz, Bu); (β-isomer) δ 5.0 (d, 1 H, J1,2 1.7 Hz, H-1), 4.41 (d, 1 H, J2,3 4.3 Hz, H-2), 3.74-3.84 (m, 3 H, H-3,6,6’), 3.48 (dd, 1 H, J 9.8, 9.3 Hz, H-4), 3.32-3.45 (m, 1 H, H-5), 2.25 (t, 2 H, J 7.3 Hz, Bu), 1.47-1.68 (m, 2 H, Bu), 0.89 (t, 3 H, J 7.4 Hz, Bu); FABMS: m/z 232.2 [M + H+ - H2O], 250.3 [M + H+]; HRFABMS: Calcd for C10H19NO6 [M + H+] m/z 250.1285, found 250.1293.
N-iso-Butanoyl-d-mannosamine (5)
252 mg (73%); [α]D20 +10 (c 1.0, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 5.11 (d, 1 H, J1,2 1.2 Hz, H-1), 4.32 (dd, 1 H, J2,3 4.7 Hz, H-2), 4.07 (dd, 1 H, J3,4 9.7 Hz, H-3), 3.77-3.92 (m, 3 H, H-5,6,6’), 3.63(t, 1 H, J 9.6 Hz, H-4), 2.54-2.70 (m, 1 H, i-Bu), 1.10 (d, 6 H, J 6.9 Hz, i-Bu); (β-isomer) δ 5.04 (d, 1 H, J1,2 1.3 Hz, H-1), 4.45 (dd, 1 H, J2,3 3.9 Hz, H-2), 3.77-3.92 (m, 3 H, H-3,6,6’), 3.52 (dd, 1 H, J 9.9, 9.6 Hz, H-4), 3.38-3.44 (m, 1 H, H-5), 2.54-2.70 (m, 1 H, i-Bu), 1.11 (d, 6 H, J 6.9 Hz, i-Bu); FABMS: m/z 250.1 [M + H+], 272.1 [M + Na+], 232.1 [M + H+ - H2O]; HRFABMS: Calcd for C10H19NO6 [M + H+] m/z 250.1285, found 250.1273. Anal. Calcd for (C10H18NO6) C, 48.19; H, 7.68; N, 5.62; Found: C, 47.79; H, 7.73; N, 5.44.
N-Pivaloyl-d-mannosamine (6)
273 mg (76%); [α]D20 +10 (c 1.0, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 5.13 (s, 1 H, H-1), 4.33(dd, 1 H, J1,2 1.6 Hz, J2,3 4.6 Hz, H-2), 4.09 (dd, 1 H, J3,4 9.6 Hz, H-3), 3.80-3.92 (m, 3 H, H-5,6,6’),3.63 (t, 1 H, J 9.1 Hz, H-4), 1.21 (s, 9 H, Me); (β-isomer) δ 5.08 (d, 1 H, J1,2 1.6 Hz, H-1), 4.47 (dd, 1 H, J2,3 4.1 Hz, H-2), 3.8-3.9 (m, 3 H, H-5,6,6’), 3.47 (t, 1 H, J 9.4 Hz, H-4), 3.40-3.46 (m, 1 H, H-5),1.23 (s, 9 H, Me); FABMS: m/z 246.1 [M + H+ - H2O], 264.1 [M + H+]; HRFABMS: calcd. for C11H21NO6 [M + H+] m/z 264.1442, found 264.1455. Anal. Calcd for (C11H20NO6) C, 50.18; H, 8.04; N, 5.32; Found: C, 50.39; H, 8.12: N, 5.22.
N-Phenylacetyl-d-mannosamine (7)
300 mg (73%); [α]D20 −10 (c 1.0, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 7.33-7.45 (m, 5 H, H-Ar), 5.11 (d, 1 H, J1,2 1.5 Hz, H-1), 4.33 (dd, 1 H, J2,3 4.7 Hz, H-2), 4.08 (dd, 1 H, J3,4 9.9 Hz, H-3), 3.80-3.87 (m, 3 H, H-5,6,6’), 3.63 (dd, 1 H, J4,5 9.5, Hz, H-4), 3.69, 3.65 (2 d, 2 H, J 15.0 Hz, Bn); (β-isomer) δ 7.33-7.45 (m, 5 H, H-Ar), 5.04 (d, 1 H, J1,2 1.5 Hz, H-1), 4.46 (dd, 1 H, J2,3 4.4 Hz, H-2), 3.80-3.87 (m, 2 H, H-3,6), 3.77 (dd, 1 H, J 12.0, 5.4 Hz, H-6’), 3.75 (s, 2 H, Bn), 3.51 (dd, 1 H, J 9.7, 9.5 Hz, H-4), 3.40 (ddd, 1 H, J 9.7, 4.8, 2.3 Hz, H-5); FABMS: m/z 280.1 [M + H+ - H2O], 298.1 [M + H+], 320.1 [M + Na+], 336.1 [M + K+]; HRFABMS: Calcd for C14H19NO6 [M + H+] m/z 298.1285, found 298.1348. Anal. Calcd for (C14H18NO6) C, 56.56; H, 6.44; N, 4.71; Found: C, 56.16; H, 6.39: N, 4.46.
N-Benzoyl-d-mannosamine (8)
300 mg (74%); [α]D20 −17 (c 1.0, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 7.5-7.8 (m, 5 H, H-Ar), 5.26 (s, 1 H, J1,2 1.1 Hz, H-1), 4.58 (dd, 1 H, J2,3 4.7 Hz, H-2), 4.17 (dd, 1 H, J3,4 9.8 Hz, H-3), 3.8-3.9 (m, 3 H, H-5,6,6’), 3.65-3.78 (t, 1 H, J2,3 9.7 Hz, H-4); (β-isomer) δ 7.5-7.8 (m, 5 H, H-Ar), 5.14 (s, 1 H, H-1), 4.72-4.73 (m, 1 H, H-2), 3.8-3.9 (m, 3 H, H-3,6,6’), 3.64 (t, 1 H, J 9.8 Hz, H-4), 3.44-3.50 (m, 1 H, H-5); FABMS: m/z 266.3 [M + H+ - H2O], 284.3 [M + H+]; HRFABMS: Calcd for C13H17NO6 [M + H+] m/z 284.1129, found 284.1131.
3.3. N-Trifluoropropionyl-d-mannosamine (9)
To the solution of 1-hydroxybenzotriazole (297 mg) and N,N’-dicyclohexylcarbodiimide (454 mg) in 5 mL of DMF was added 194 µL of 3,3,3-trifluoropropionic acid (2.2 mmol). After the reaction mixture was stirred at 0°C for 1 h and at rt for additional 3 h, d-mannosamine•HCl (300 mg, 1.39 mmol) was added. The reaction mixture was stirred overnight and was then filtered off. The filtrate was condensed under reduced pressure and the residue was purified by silica gel column chromatography (eluent: MeOH/CH2Cl2) to afford 9 that was further purified by a Biogel P2 column and finally recrystallized from ethyl alcohol and ethyl acetate to give the white crystalline 9 (329mg, 82%). [α]D20 +3.0 (c 0.5, H2O); 1H NMR (D2O, 300 MHz): (α-isomer) δ 5.11 (d, 1 H, J1,2 1.6 Hz, H-1), 4.35 (dd, 1 H, J2,3 4.6 Hz, H-2), 4.05 (dd, 1 H, J3,4 9.7 Hz, H-3), 3.78-3.86 (m, 3 H, H-5,6,6’), 3.58 (dd, 1 H, J 9.9, 9.7 Hz, H-4), 3.32 (q, 2 H, JHF 10.7, CH2CF3); (β-isomer) δ 5.02 (d, 1 H, J1,2 1.6 Hz, H-1), 4.48 (dd, 1 H, J2,3 4.4 Hz, H-2), 3.78-3.86 (m, 3 H, H-3,6,6’), 3.48 (t, 1 H, J 9.9 Hz, H-4), 3.38-3.42 (m, 1 H, H-5), 3.36 (q, 2 H, JHF 10.7, CH2CF3); FABMS: m/z 272.3 [M + H+ - H2O], 290.1 [M + H+], 312.1 [M + Na+]; HRFABMS: Calcd for C9H14NO6F3 [M + H+] m/z 290.0846, found 290.0856. Anal. Calcd for (C9H13NO6F3) C, 37.38; H, 4.88: N, 4.84; Found: C, 37.01; H, 4.70: N, 5.09.
3.4 Benzyl glycoside of d-Neuraminic acid (21)
After the solution of compound 2030 (2.4 g, 4.1 mmol) was refluxed in 2 N NaOH (50 mL) for 6 h and cooled to rt, concentrated HCl was added to neutralize it to pH 6. The mixture was then condensed under reduced pressure, and the residue was extracted with MeOH to remove NaCl. The extracts were combined, concentrated and purified by column chromatography (MeOH and EtOAc 1:6) to give 21 (1.25 g, 85%). [α]D20 −40 (c 0.5, H2O); Rf = 0.59 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): δ 7.45(m, 5 H, H-Ar), 4.70, 4.53 (2 d, 2 H, J 10.9 Hz, CH2Ph), 4.15 (dd, 1 H, J 10.2, 1.9 Hz, H-6), 3.81-3.94 (m, 4 H), 3.76 (dd, 1 H, J 12.6, 6.1 Hz, H-9), 3.31 (t, 1 H, J 10.3 Hz, H-5), 2.85 (dd, 1 H, J, 12.4, 4.6 Hz, H-3e), 1.79 (t, 1 H, J 12.4 Hz, H-3a).
3.5. Benzyl glycosides of N-Acyl-d-neuraminic acid (22–27)
To a solution of 21 (150 mg, 0.42 mmol) in MeOH (4 mL) was added an anhydride (1.26 mmol) at 0 °C slowly. The mixture was stirred at 0 °C for 1 h and then at rt until TLC showed that the reaction had completed. Workup as shown in Section 3.2 and column chromatography (MeOH and EtOAc 1:4 to 1:1) of the product afforded 22–27.
Benzyl glycoside of N-propionyl-d-neuraminic acid (22)
165 mg (95%); Rf = 0.59 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): δ 7.40 (m, 5 H, H-Ar), 4.80, 4.52 (2 d, 2 H, J 11.3 Hz, Bn), 3.96-3.60 (m, 6 H), 3.58 (d, 1 H, J 9.0 Hz, H-7), 2.88 (dd, 1 H, J 4.4 Hz, 12.2 Hz, H-3e), 2.34 (m, 2 H, COCH2), 1.71 (t, 1 H, J 11.8 Hz, H-3a), 1.10 (t, 3 H, J 7.5 Hz, CH3); HRFABMS: Calcd for C19H27NO9 [M + Na+] m/z 436.1584, Found 436.1584.
Benzyl glycoside of N-butanoyl-d-neuraminic acid (23)
167 mg (93%); Rf = 0.65 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): δ 7.40 (m, 5 H, H-Ar), 4.77, 4.53 (2 d, 2 H, J 11.3 Hz, Bn), 3.96-3.60 (m, 7 H), 2.80 (dd, 1 H, J 4.4 Hz, 13.2 Hz, H-3e), 2.30 (t, 2 H, J 7.2 Hz, COCH2), 1.72 (t, 1 H, J 11.8 Hz, H-3a), 1.64 (q, 2 H, J 7.2 Hz, CH3CH2), 0.94 (t, 3 H, J 7.2 Hz, CH3); HRFABMS: Calcd for C20H29NO9 [M + Na+] m/z 450.1740, Found 450.1740.
Benzyl glycoside of N-iso-butanoyl-d-neuraminic acid (24)
151 mg (84%); Rf = 0.80 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): δ7.40 (s, 5 H, H-Ar), 4.75, 4.52 (2 d, 2 H, J 10.8, CH2Ph), 3.89-3.55 (m, 6 H), 3.56 (d, 1 H, J 8.6 Hz, H-7), 2.81 (dd, 1 H, J 4.4, 13.2 Hz, H-3e), 2.55 (m, 1 H, COCH), 1.71 (t, 1 H, J 12.8, H-3a), 1.15 (2 d, 6 H, J 7.0 Hz, 2 CH3); HRFABMS: Calcd for C20H29NO9 [M + Na+] m/z 450.1740, Found 450.1747.
Benzyl glycoside of N-trimethyl-d-neuraminic acid (25)
176 mg (95%); Rf = 0.64 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 200 MHz): δ7.37 (m, 5 H, H-Ar), 4.70, 4.48 (2 d, 2 H, J 10.8, CH2Ph), 4.05 (d, 1 H, J 10.3, H-6), 3.85-3.73 (m, 4 H), 3.64-3.53 (m, 1 H), 3.47 (m, 1 H), 2.82 (dd, 1 H, J 3.6, 12.4 Hz, H-3e), 1.64 (t, 1 H, J 12.1 Hz, H-3a), 1.14 (s, 9 H, 3 CH3); HRFABMS: Calcd for C19H27NO9 [M + Na+] m/z 436.1584, Found 436.1584.
Benzyl glycoside of N-phenylacetyl-d-neuraminic acid (26)
181 mg (91%); Rf = 0.78 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 200 MHz): δ7.3-7.5 (m, 10 H, H-Ar), 4.67, 4.52 (2 d, 2 H, J 9.5 Hz, CH2Ph), 3.75-3.68 (m, 6 H), 3.60 (s, 2 H, CH2Ph), 3.49 (dd, 1 H, J 12.3, 7.1 Hz, H-9), 2.74 (dd, 1 H, J 4.0, 12.4 Hz, H-3e), 1.64 (t, 1 H, J 12.2 Hz, H-3a); HRFABMS: Calcd for C24H29NO9 [M + Na+] m/z 498.1740, Found 498.1740.
Benzyl glycoside of N-benzoyl-d-neuraminic acid (27)
174 mg (90%); Rf = 0.59 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): δ 8.01-7.43 (m, 10 H, H-Ar), 4.70, 4.54 (2 d, 2 H, J 10.0 Hz, CH2Ph), 4.05 (d, 1 H, J 10.3, H-6), 4.2-3.6 (m, 6 H), 2.83 (dd, 1 H, J 4.8, 12.5 Hz, H-3e), 1.77 (t, 1 H, J 12.3 Hz, H-3a); HRFABMS: Calcd for C23H27NO9 [M + Na+] m/z 484.1584, Found 498.1570.
3.6. Benzyl glycoside of N-trifluoropropionyl-d-neuraminic acid (28)
To the solution of 1-hydroxybenzotriazole (149 mg) and N,N’-dicyclohexylcarbodiimide (227 mg) in 5 mL of DMF was added 97 µL of 3,3,3-triflouropropionic acid (1.1 mmol). After the reaction mixture was stirred at 0°C for 1 h and then at rt for 3 h, 21 (150 mg, 0.42 mmol) was added to it. The reaction mixture was stirred overnight and then filtered off. The filtrate was condensed under reduced pressure and the residue was purified by silica gel column chromatography to afford 28 (140 mg, 72%). Rf = 0.79 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): δ 7.40 (br, 5 H, H-Ar), 4.74, 4.54 (2 d, 2 H, J 10.2 Hz, CH2Ph), 3.30 (q, 2 H, J 10.9 Hz, CH2CF3), 2.79 (dd, 1 H, J 12.5, 4.6 Hz, H-3e), 1.86 (t, 1 H, J 12.5 Hz, H-3a); HRFABMS: Calcd for C19H24F3NO9 m/z [M + Na+] 490.1301, Found 490.1354.
3.7. N-Acyl-d-neuraminic acids 11–17
A mixture of 22–28 (0.1 mmol) and 10% palladium on carbon (0.01 mol) in MeOH (5 mL) was stirred vigorously at rt under a hydrogen atmosphere until TLC showed the disappearance of the starting material. The catalyst was filtered off and the filtrate was condensed under reduced pressure. The residue was purified by silica gel column chromatography (eluent: MeOH, EtOAc and AcOH, 20:80:1 to 50:50:1) to give 11–17 that were further purified by a Biogel P-2 column using distilled water as eluent and finally recrystallized from EtOH and EtOAc (1:2).
N-Propionyl-d-neuraminic acid (11)
30 mg (94%); [α]D20 −21 (c 1.0, H2O); Rf = 0.28 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): (β-isomer) δ 4.07-3.98 (m, 3 H), 3.85-3.83 (m, 2 H), 3.66 (dd, 1 H, J 12.5, 7.2 Hz, H-9), 3.59 (d, 1 H, J 8.1 Hz, H-7), 2.34 (q, 2 H, J 7.9 Hz, COCH2), 2.26 (dd, 1 H, J 4.2, 13.0 Hz, H-3e), 1.86 (t, 1 H, J 12.4 Hz, H-3a), 1.15 (t, 3 H, J 7.6 Hz,CH3). HRFABMS: Calcd for C12H21NO9 m/z [M + Na+] 346.1114, Found 346.1117.
N-Butanoyl-d-neuraminic acid (12)
30 mg (90%); [α]D20 −27 (c 1.0, H2O); Rf = 0.31 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): (β-isomer) δ 4.02-3.98 (m, 2 H), 3.95 (d, 1 H, J 9.6 Hz, H-9), 3.85-3.74 (m, 2 H), 3.61 (dd, 1 H, J 12.0, 6.4 Hz, H-9’), 3.55 (d, 1 H, J 9.0 Hz, H-7), 2.29 (t, 2 H, J 7.2 Hz, COCH2), 2.26 (dd, 1 H, J 4.2, 13.0 Hz, H-3e), 1.86 (t, 1 H, J 12.4 Hz, H-3a), 1.63 (q, 2 H, J 7.2 Hz, CH3CH2), 0.95 (t, 3 H, J 7.2 Hz, CH3). HRFABMS: Calcd for C13H23NO9 [M + Na+] m/z 360.1271, Found 360.1247.
N-iso-Butanoyl-d-neuraminic acid (13)
31 mg (93%); [α]D20 −28 (c 1.0, H2O); Rf = 0.38 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): (β-isomer) δ 4.08-3.78 (m, 4 H), 3.62 (dd, 1 H, J 12.9, 6.6 Hz, H-9), 3.54 (d, 1 H, J 8.8 Hz, H-7), 2.58 (m, 1 H, COCH), 2.26 (dd, 1 H, J 5.2, 12.8 Hz, H-3e), 1.85 (t, 1 H, J 12.5 Hz, H-3a), 1.15 (2d, 2×3 H, J 7.0 Hz, 2 CH3). HRFABMS: Calcd for C13H23NO9 [M − H + 2Na]+ m/z 382.1089, Found 382.1084.
N-Trimethylacetyl-d-neuraminic acid (14)
32 mg (93%); [α]D20 −31 (c 1.0, H2O); Rf = 0.40 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 600 MHz): (β-isomer) δ 4.08-3.78 (m, 4 H), 3.60 (dd, 1 H, J 12.0, 6.0 Hz, H-9), 3.45 (d, 1 H, J 9.0 Hz, H-7), 2.25 (dd, 1 H, J 4.8, 12.6 Hz, H-3e), 1.84 (t, 1 H, J 12.6 Hz, H-3a), 1.06 (s, 9 H, 3 CH3). HRFABMS: Calcd for C14H25NO9 [M + Na+] m/z 374.1427, Found 374.1482.
N-Phenylacetyl-d-neuraminic acid (15)
35mg (91%); [α]D20 −14 (c 1.0, H2O); Rf = 0.40 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300MHz): (β-isomer) δ 7.42 (m, 5 H, H-Ar), 4.08-3.96 (m, 3 H), 3.77 (m, 1 H), 3.71-3.66 (m, 1 H), 3.68 (s, 2 H, CH2Ph), 3.54 (dd, 1 H, J 11.7, 6.4 Hz, H-9), 3.49 (d, 1 H, J 7.8 Hz, H-7), 2.32 (dd, 1 H, J 4.9, 13.1 Hz, H-3e), 1.86 (t, 1 H, J 13.0 Hz, H-3a). HRFABMS: Calcd for C17H23NO9 [M + Na+] m/z 408.1270, Found 408.1271.
N-Benzoyl-d-neuraminic acid (16)
31 mg (84%); [α]D20 −15 (c 1.0, H2O); Rf = 0.39 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): (β-isomer) δ 7.64-7.43 (m, 5 H, H-Ar), 4.15 (s, 3 H), 3.83-3.73 (m, 2 H), 3.65-3.50 (m, 2 H), 2.26 (dd, 1 H, J 4.2, 12.5 Hz, H-3e), 1.86 (t, 1 H, J 11.6 Hz, H-3a). HRFABMS: Calcd for C16H21NO9 [M + Na+] m/z 394.1114, Found 394.1140.
N-Trifluoropropionyl-d-neuraminic acid (17)
34 mg (90%); [α]D20 −16 (c 1.0, H2O); Rf = 0.43 (eluent: BuOH, HOAc, EtOAc and H2O 1:1:1:1); 1H NMR (D2O, 300 MHz): (β-isomer) δ 4.13-4.03 (m, 3 H), 3.86-3.78 (m, 2 H), 3.60 (dd, 1 H, J 11.4, 6.6 Hz, H-9), 3.55 (d, 1 H, J 9.0 Hz, H-7), 3.35 (q, 2 H, J 10.5, CF3CH2), 2.32 (dd, 1 H, J 4.6, 13.2 Hz, H-3e), 1.86(t, 1 H, J 13.2 Hz, H-3a). HRFABMS: Calcd for C12H18F3NO9 [M + H+] m/z 378.1012, Found 378.1013.
3.8. General procedure for the enzymatic reactions
To a 1.5 mL vial was added ManNR (7.27 µmol) in 130 µL of 0.05 M potassium phosphate buffer (pH 7.4, I=0.1), 72.73 µmol pyruvic acid in 100 µL buffer, 2.0 µmol dithiothreitol in 30 µL buffer, and 140 µL diluted NeuNAc aldolase solution (0.35 U). The reaction was kept at 37°C with occasional shaking. At the time point of 20, 40, 90, 180, 360, 720 and 1440 min, 40 µL aliquots were taken out and put on dry ice for 30 minutes. After that, the samples were directly added into resorcinol reagent and analyzed by the modified Svennerholm method described below.
3.9. Modified Svennerholm method31 for the analysis of sialic acid products
An aliquot (40 µL) of the enzymatic reaction mixture was added to a 1.5 mL solution of the resorcinol reagent (0.2% w.t. resorcinol, 80% v/v conc. HCl and 0.25% v/v 0.1M CuSO4, in H2O). After the mixture was heated in a boiling water bath for 30 min and cooled to rt, to it was added 3 mL of the extraction solvent (butyl acetate and 1-butanol 85:15). The mixture was vigorously shaken on a vortex mixer, and after the mixture was settled down, the two layers were separated carefully. The absorbance of the organic layer at 580 nm was measured with a UV-visible spectrophotometer using a 1.0 cm quartz cuvette using the extraction solvent as blank. The calibration curves of all sialic acid derivatives were obtained by the same measurement.
Supplementary Material
Acknowledgements
We thank the financial support for this work by a research grant from NIH/NCI (1R01 CA95142) and a seed grant from the Ohio Cancer Research Associates. MS measurements were performed by Mr. J. Faulk.
References
- 1.Rosenberg AE. Biology of the Sialic Acid. New York: Plenum Press; 1995. [Google Scholar]
- 2.Reutter W, Stasche R, Stehling P, Baum O. In: The biology of sialic acids: insights into their structure, metabolism and function in particular during viral infection. Gabius H-J, Gabius S, editors. Weinheim: Chapman & Hall; 1997. pp. 245–259. [Google Scholar]
- 3.Varki A. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 4.Schauer R. Adv. Exp. Med. Biol. 1988;228:47–72. doi: 10.1007/978-1-4613-1663-3_2. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S, Zhang Y. Chem. Biol. 1997;3:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 6.Kobata A. In: Cancer cells and metastasis. Montreuil J, Vliegenthart JFG, Schachter H, editors. Amsterdam: Elsevier; 1996. pp. 183–241. [Google Scholar]
- 7.Livingston PO. Curr. Opin. Immunol. 1992;4:624–629. doi: 10.1016/0952-7915(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 8.Ragupathi G. Cancer Immunol. Immunother. 1998;46:82–87. [Google Scholar]
- 9.Livingston PO. Immunol. Rev. 1995;145:147–156. doi: 10.1111/j.1600-065x.1995.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 10.Danishefsky SJ, Allen JR. Angew. Chem. Int. Ed. 2000;39:837–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Comb DG, Roseman S. J. Am. Chem. Soc. 1958;80:497–499. [Google Scholar]
- 12.Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Glycobiology. 2001;11:11R–18R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- 13.Kayser H, Geilen CC, Paul C, Zeitler R, Reutter W. FEBS. 1992;301:137–140. doi: 10.1016/0014-5793(92)81233-c. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt C, Stehling P, Schnitzer J, Reutter W, Horstkorte R. J. Biol. Chem. 1998;273:19146–19152. doi: 10.1074/jbc.273.30.19146. [DOI] [PubMed] [Google Scholar]
- 15.Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. J. Biol. Chem. 1998;273:31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- 16.Mahal KL, Yarema KJ, Bertozzi CR. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 17.Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. J. Biol. Chem. 2000;275:32832–32836. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]
- 20.Fitz W, Schwark J-R, Wong C-H. J. Org. Chem. 1995;60:3663–3670. [Google Scholar]
- 21.Gijsen HJM, Qiao L, Fitz W, Wong C-H. Chem. Rev. 1996;96:443–473. doi: 10.1021/cr950031q. [DOI] [PubMed] [Google Scholar]
- 22.Wymer N, Toone EJ. Curr. Opin. Chem. Biol. 2000;4:110–119. doi: 10.1016/s1367-5931(99)00061-7. [DOI] [PubMed] [Google Scholar]
- 23.Fitz W, Wong C-H. J. Org. Chem. 1994;59:8279–8280. [Google Scholar]
- 24.Ichikawa Y, Liu JLC, Shen GJ, Wong C-H. J. Am. Chem. Soc. 1991;113:6300–6302. [Google Scholar]
- 25.Lin C-C, Lin C-H, Wong C-H. Tetrahedron Lett. 1997;38:2649–2652. [Google Scholar]
- 26.Sparks MA, Williams KW, Lukacs C, Schrell A, Priebe G, Spaltenstein A, Whitesides GM. Tetrahedron. 1993;49:1–12. [Google Scholar]
- 27.Halcomb RL, Fitz W, Wong C-H. Tetrahedron: Asymmetry. 1994;5:2437–2442. [Google Scholar]
- 28.Roy R, Laferriere C. Can. J. Chem. 1990;68:2045–2054. [Google Scholar]
- 29.Byramova NE, Tuzikov AB, Bovin NV. Carbohydr. Res. 1992;237:161–175. [Google Scholar]
- 30.Brossmer R, Gross HJ. Methods Enzymol. 1994;247:153–176. doi: 10.1016/s0076-6879(94)47013-8. [DOI] [PubMed] [Google Scholar]
- 31.Svennerholm L. Biochim. Biophys. Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 32.Marangoni AG. Enzyme kinetics: A modern approach. Hoboken, N.J: Wiley-Interscience; 2002. [Google Scholar]
- 33.Ritter G, Boosfeld E, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Immunobiology. 1990;182:32. doi: 10.1016/S0171-2985(11)80581-4. [DOI] [PubMed] [Google Scholar]
- 34.Ritter G, Boosfeld E, Asluri S, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Int. J. Cancer. 1991;48:379–385. doi: 10.1002/ijc.2910480312. [DOI] [PubMed] [Google Scholar]
- 35.Jennings H. Int. J. Infect. Dis. 1997;1:158–164. [Google Scholar]
- 36.Chefalo P, Pan Y, Nagy N, Harding C, Guo Z. 2004 submitted. [Google Scholar]
- 37.Spivak CT, Roseman S. J. Am. Chem. Soc. 1959;81:2403–2404. [Google Scholar]
- 38.Keppler OT, Stehling P, Herrmann M, Kayser H, Grunow D, Reutter W, Pawlita M. J. Biol. Chem. 1995;270:1308–1314. doi: 10.1074/jbc.270.3.1308. [DOI] [PubMed] [Google Scholar]
- 39.Charter NW, Mahal LK, Koshland DEJ, Bertozzi CR. J. Biol. Chem. 2002;277:9255–9261. doi: 10.1074/jbc.M111619200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.