Abstract
Diabetes is one of the most common chronic disorders with an increasing incidence worldwide. Technologic advances in the field of diabetes have provided new tools for clinicians to manage this challenging disease. For example, the development of continuous subcutaneous insulin infusion systems have allowed for refinement in the delivery of insulin, while continuous glucose monitors provide patients and clinicians with a better understanding of the minute to minute glucose variability, leading to the titration of insulin delivery based on this variability when applicable. Merging of these devices has resulted in sensor-augmented insulin pump therapy, which became a major building block upon which the artificial pancreas (closed-loop systems) can be developed. This article summarizes the evolution of sensor-augmented insulin pump therapy until present day and its future applications in new-generation diabetes management.
Keywords: artificial pancreas, closed-loop, continuous glucose monitors, continuous subcutaneous insulin infusion, diabetes, sensor-augmented pump therapy
Diabetes is one of the most common chronic diseases in the world, affecting 23.6 million individuals in the USA [101] with increasing incidence [1]. Individuals with diabetes suffer from high morbidity and mortality rates due to complications that could be prevented with intensive therapy [2]. Insulin remains the lifesaving treatment for Type 1 diabetes (T1D) and is also required by many patients with Type 2 diabetes to achieve optimal metabolic control. While various improvements and innovations have been made in insulin manufacturing and insulin delivery devices over the past 30 years, many patients remain unable to achieve target glucose and A1c levels, partially due to compliance issues. The culprit behind poor compliance seems to be how, when, and if insulin is administered.
Injection therapy was the mainstay of insulin delivery in T1D until the insulin pump was introduced more than 30 years ago, allowing patients to achieve more physiologic replacement of insulin through the use of a mechanical delivery system. As the brainchild of engineers and physicians, insulin pump therapy and meters for self monitoring of blood glucose (SMBG) levels that were developed around the same time were the tipping points for the incorporation of technology into diabetes care. Advances in pump technology over the years have led to the replacement of the early primitive devices with the smaller and multifunctional ‘smart’ pumps of today, and blood glucose meters may be replaced in the future by continuous glucose monitoring (CGM) systems.
Continuous glucose monitoring devices that measure interstitial fluid (IF) glucose values continuously were first introduced approximately 10 years ago. Early ‘professional’ CGM systems that were used by diabetes practices and only provided data for brief periods for retrospective analysis were quickly followed by real-time CGM (RT-CGM) devices for personal daily use by patients at home. The impact of RT-CGM on glucose control in insulin pump-treated patients, also known as sensor-augmented pump (SAP) therapy, has become an area of intense investigation. Moreover, the development of SAP platforms that literally integrate the two technologies into a single device (Figure 1) represents the first step towards the ultimate goal of developing an artificial endocrine pancreas. Such closed-loop insulin delivery systems will administer insulin through the pump controlled by an algorithm calculating the insulin dose based upon real-time glucose information from the sensor. This article will review the history of SAP therapy and discuss its impact on diabetes management at present and in the future.
Figure 1. Sensor-augmented pump therapy.
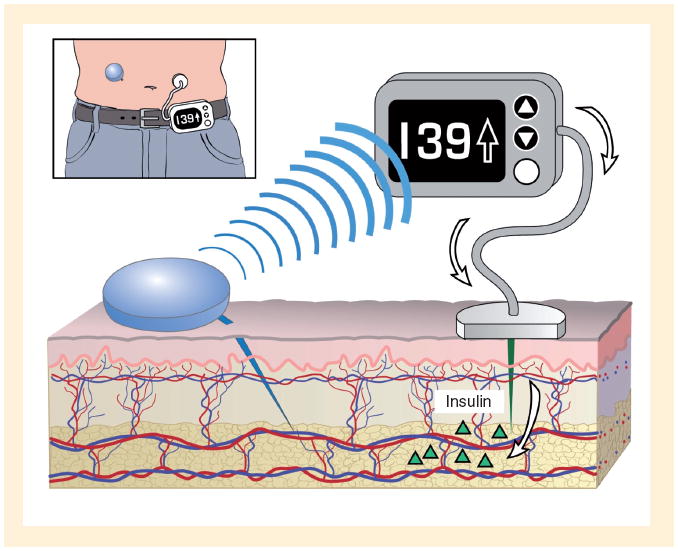
Real-time glucose sensor (in blue), sitting on the skin surface with its tip inserted in the tissue interstitial fluid, measures glucose levels. The glucose value is transmitted to the insulin pump by radiofrequency, and sensor glucose values, graphs and trend arrows are displayed on its screen. An appropriate insulin dose is calculated based on the glucose value by insulin pump software and can be administered, if approved by the user, through the pump infusion set (in gray) with its catheter in the subcutaneous tissue delivering insulin.
Insulin pumps
Insulin pump therapy had its inception in the late 1970s [3,4]. It pioneered a new strategy of providing independently adjustable boluses of rapid-acting insulin before each meal and snack in combination with a continuous basal insulin infusion to maintain glycemic control between meals and during the overnight period. Continuous subcutaneous insulin infusion was clearly superior to the once or twice a day insulin injection regimens that were the standard of diabetes care at the time. After an initial burst of enthusiasm by the medical community and medical device industry, most companies dropped out of this business when it became apparent that pump therapy was targeted to a relatively small fraction of the diabetes market at the time; namely, patients with T1D who were attempting to intensively treat their diabetes. The playing field changed dramatically in the early 1990s following the report of the results of the Diabetes Control and Complications Trial (DCCT) about the effect of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus [2].
The demonstration that most, if not all, patients with T1D should be treated intensively to delay or prevent long-term vascular complications markedly increased the pool of potential pump candidates, brought new companies into the field and lead to substantial improvements in pump capabilities. The renewed interest in pump therapy in pediatrics was particularly gratifying [5-10].
The current generation of small and reliable ‘smart pumps’ is equipped with a variety of programmable insulin delivery options, built-in dosage calculators and alarms to alert the user to malfunctions with the device. They offer the advantage of different bolus delivery modes (e.g., squarewave boluses that infuse the bolus over an extended period of time, and dual-wave boluses that deliver a fraction of the bolus immediately and the rest over an extended period of time) that can be used for different types of meals and different types of foods. The bolus history function allows clinicians to assess patient compliance to pre-meal bolus dosing; a feature that is especially useful in managing adolescents with T1D [11,12]. Insulin pump data management software programs allow the downloading and viewing of data including modal day blood glucose values, details about daily activities and personalized diet histories. Table 1 reviews the insulin pumps currently available in the USA and their key features. Innovations in the field of insulin pump therapy, like tubeless patch pumps [13], continue to reshape the day-to-day management of diabetes by our technology-driven society.
Table 1.
Pump options and features.
| Pump | Insulin reservoir capacity (units) | Minimal basal rate increments (U/h) | Minimal bolus dose increments (units) | Other features |
|---|---|---|---|---|
| Animas Ping® | 200 | 0.025 | 0.05 | Smallest pump |
| Largest display screen | ||||
| Meter-remote can wirelessly beam blood glucose and deliver insulin within 10 ft | ||||
| CalorieKing database on meter | ||||
|
| ||||
| Deltec Cozmo® | 300 | 0.05 | 0.05 | Integrated Freestyle meter |
| Enhanced Meal Maker® | ||||
| Basal rates by day of week | ||||
| Replacement of basal rate after disconnecting pump | ||||
| Production stopped 25 March 2009 – users transitioning to different pumps | ||||
|
| ||||
| Roche-Disetronic Accu-Chek® Spirit | 315 | 0.1 | 0.1 | Reversible display |
| Menu display customization option | ||||
|
| ||||
| Medtronic Paradigm® REAL-Time Revel 523/723 | 180 or 300 | 0.025 | 0.025 | Only available pump with real-time CGMS on the market |
| Optional remote control for bolus dosing | ||||
| CareLink Personal Therapy Management Tool | ||||
|
| ||||
| Insulet Omnipod® | 200 | 0.05 | 0.05 | No tubing |
| 1000 common foods in PDA | ||||
| Freestyle Meter in PDA component | ||||
|
| ||||
| Nipro Amigo® | 300 | 0.05 | 0.05 | No software to download |
| Propriety infusion set connections | ||||
CGMS: Continuous glucose monitoring system; PDA: Personal digital assistant.
Glucose sensors
The key component of currently approved CGM devices is the glucose oxidase-based, electrochemical sensor that is inserted through the skin by a needle introducer. The oxidation of interstitial glucose by the sensor generates an electrical current. Electrical current data are filtered and cleared from background noise by the transmitter and sent to the receiver. The receiver processes transmitted signals further by additional filters and unique software that account for the lag time and converts electrical current signals into glucose data. Glucose data are displayed on the receiver screen at 1–5-min intervals with symbols that represent the direction and rate of glucose change. Other data displays include graphic representations indicating the glucose pattern over varying periods of time.
There are unique challenges in measuring IF rather than blood glucose [14]. Glucose is transferred from the blood through the capillary endothelium to the IF space. The metabolic rate of cells in IF space and other factors, such as insulin, affect glucose uptake by cells, the glucose supply from the blood vessel and blood flow to the area. In addition, interstitial glucose levels are influenced by the permeability of capillaries, which can be altered by many factors, including nerve stimulation [15]. Changes in sensor glucose levels lag an average of 15 min behind changes in blood glucose levels due to the physiologic delay in transferring glucose between the blood and IF space (~2–8 min), the transit time of IF glucose through the sensor membrane (1–2 min) and signal filtering (3–12 min) [16,17]. Sensor insertion creates trauma to the tissues surrounding the sensor, and a waiting period, depending on the sensor type, is needed for the sensor signal to stabilize [18,19]. Biocompatibility problems like bio-fouling (obstruction of fluid exchange after nonspecific protein adsorption), passivation of electrodes (weakening of signal by reduction in conductivity) and degeneration can lead to changes in sensor function that contribute to drift in sensor signal over time [20,21].
The glucose sensor must be calibrated against corresponding blood glucose meter levels to ensure the continuous accuracy of sensor data. Such calibrations transform the sensor signals into matching capillary glucose levels and assumes that the plasma to IF glucose gradient remains relatively constant [22]. Recalibration at fixed intervals is required to overcome signal drift issues [15]. Calibration should take place when blood glucose levels are relatively stable; namely, the rate of change in sensor glucose values should be < ±0.5 mg/dl/min [16,23].
While the concept of CGM has been around for 50 years [24], the use of this technology did not become commercially available until the original MiniMed Continuous Glucose Monitoring System (CGMS), now CGMS Gold (Medtronic MiniMed, CA, USA) was approved in 1999. This device was similar to a Holter monitor in that data were collected for up to 3 days, after which time the data were downloaded for retrospective review of sensor glucose values [25]. An initial small-scale study from our pediatric diabetes clinic demonstrated the usefulness of the retrospective CGMS in revealing patterns of glucose excursions that were not evident with SMBG. That study showed that 56 pediatric patients with T1D who were considered ‘well controlled’ with A1c levels averaging 7.7% had peak postprandial sensor glucose levels of >300 mg/dl approximately 50% of the time and 70% of the subjects had asymptomatic hypoglycemia during the night [26].
Real-time CGM became available shortly after CGMS and it has become the most common type of device that is currently used in the clinical setting. Salient features of the US FDA-approved RT-CGM systems are shown in Table 2. As sensors are not as accurate as home glucose meters, the FDA has relegated them to being an adjunctive therapy, in that abnormal sensor glucose values should be confirmed by capillary blood glucose determinations prior to taking corrective measures [102].
Table 2.
Features of the real-time continuous glucose monitoring systems (at the time of publication†).
| Feature | Abbott Free Style Navigator® | DexCom™ SEVEN® PLUS | Medtronic MiniMed Paradigm® Real-time Revel System or Guardian® REAL-Time |
|---|---|---|---|
| Range of glucose values | 20–500 mg/dl | 40–400 mg/dl | 40–400 mg/dl |
| Update of glucose values | Every min | Every 5 min | Every 5 min |
| Sensor duration | Up to 120 h (5 days) | Up to 168 h (7 days) ‡ | Up to 72 h (3 days) ‡ |
| Sensor length, angle and gauge | 6 mm, 90°, 21 gauge | 13 mm, 45°, 26 gauge | 12 mm, 45–60°, 23 gauge |
| Transmitter size | 2.05″ × 1.23″ × 0.43″ | 1.5″ × 0.9″ × 0.4″ | 1.4″ × 1.1″ × 0.3″ |
| Number of components to wear/carry | Receiver, transmitter (home glucose meter built in to receiver) | Receiver, transmitter and home glucose meter | Receiver, transmitter and home glucose meter |
| Warm-up period before glucose readings displayed | 10 h | 2 h | 2 h |
| Required frequency of calibration | Four times at approximately 10, 12, 24 and 72 h after sensor insertion | Two times a day (every 12 h) | Two times a day (every 12 h) |
| Available alarms | High and low glucose alarms; projected high and low glucose alarms | High and low glucose alarms | High and low glucose alarms; predicted high and low glucose alarms; rate of change alarms |
| Glucose display graphs | 2-, 4-, 6-, 12- and 24-h | 1-, 3-, 6-, 12- and 24-h | 3-, 6-, 12- and 24-h |
| Trending arrows | Yes | Yes | Yes |
| Capacity to enter events | Insulin, meals, exercise, health, other | Insulin, meals, exercise and health | Insulin, meals, exercise |
| US FDA approval status | Age 18 years and older with blood sugar testing using a home glucose meter | Age 18 years and older with blood sugar testing using a home glucose meter | Age 7 years and older with blood sugar testing using a home glucose meter |
Changes and improvements to all devices are ongoing so the features may have changed since the time of publication.
Users report longer wear times.
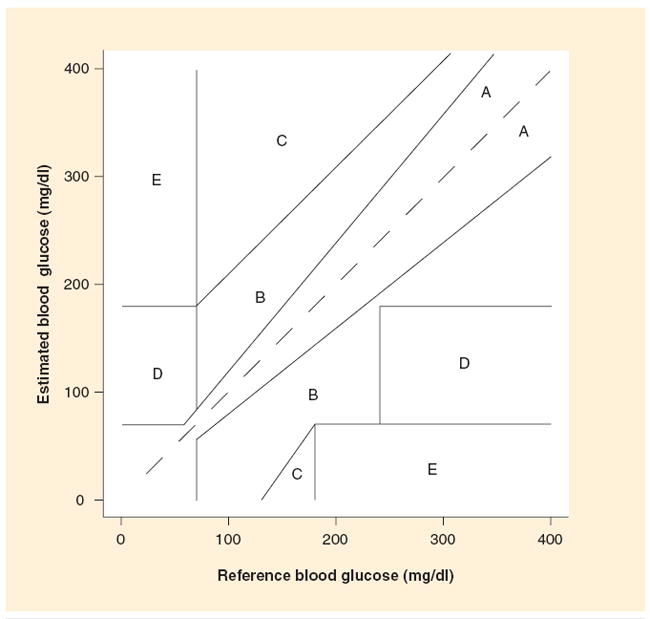
In assessing the accuracy of RT-CGM, descriptive statistics such as the mean/median relative absolute difference (RAD) are often used [27]. In calculating the RAD, the sensor value is subtracted from the reference blood glucose value. The difference is divided by the reference glucose value and expressed as a percentage. As the term is absolute, high and low sensor glucose values are weighted equally. The current generation of sensors has a median RAD of approximately 15%. However, variables such as the current blood glucose and glucose level rate of change affect the accuracy of the sensor such that the sensors appear to perform better at higher blood glucose levels and less well at hypoglycemic values and during rapid fluctuations in glucose levels. The median RAD for the Guardian® RT/Medtronic RT CGM sensor is 10.9–14% [28,29], 9–14% for the Navigator® [30-32], 12.8% for the DexCom™ SEVEN® [30] and 13.0% for the DexCom SEVEN PLUS [33]. Another method to determine sensor accuracy is the Clarke error grid analysis for glucose sensor (CG-EGA), which utilizes both blood glucose point accuracy, sensor readings versus reference blood glucose determinations, and rate and direction of change accuracy [34]. As shown in Figure 2, the CG-EGA has five zones of accuracy with zones A (clinically accurate) and zones B (benign errors) expressed as ‘clinically acceptable’ [35].
Figure 2. Clarke error grid with zones A and B representing acceptable zones.
Data management software to allow for retrospective analysis of RT-CGM data has been developed for devices currently approved by the FDA. The Navigator CoPilot [103] and the DexCom SEVEN PLUS Data Manager 3 (DM3) use software that is installed on a personal computer [104]. These programs differ from the downloading that is available from Medtronic, which is a web-based system called Carelink [105]. The three systems allow for similar reports to be generated. The Modal Day report that is often used in the clinical setting allows for overlapping of a few days of sensor data to allow for clinicians and patients to search for patterns. As demonstrated in Figure 3, it is easier to identify times of the day when the glucose is out of range and thus make changes to insulin therapy. Pie charts with estimates of the percent of time spent in range are also useful and available from downloading software. Patients find this information to be easily understood, thereby allowing for refinement of insulin delivery based on these reports. Sensor readings from one day can provide evidence of the benefits of interrupting basal delivery overnight if a low glucose threshold is reached and an alarm is not responded to, as seen in Figure 4.
Figure 3. Overlay of several days of glucose values demonstrates the need for changes in insulin therapy for carbohydrate coverage at dinner as sensor values are above the target range after dinner.
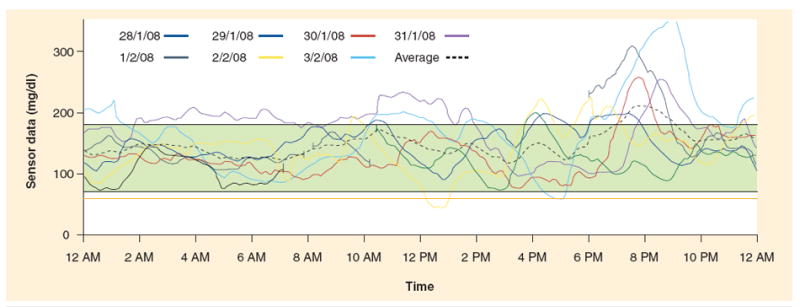
Figure 4. Sensor-augmented pump daily summary graph.
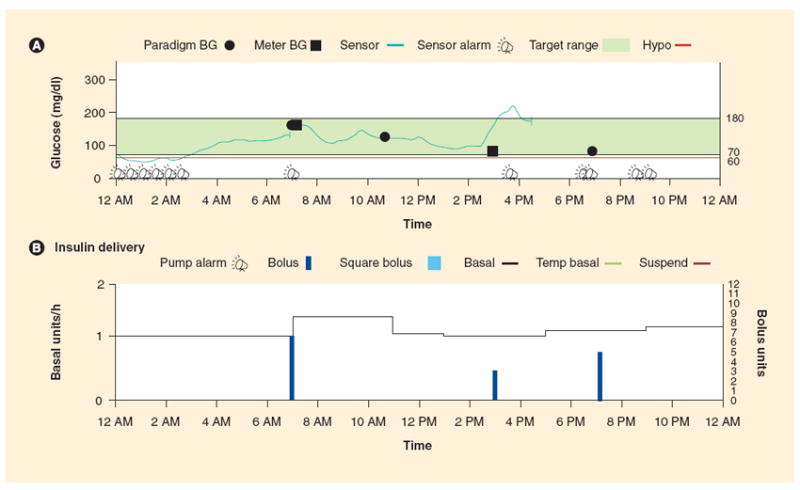
(A) A typical daily sensor-augmented pump graph report of sensor glucose values with meter glucose values recorded for calibration and as confirmatory fingerstick testing (squares). The green shaded area represents the target glucose range, and multiple low glucose alarms are noted between 12 and 3 AM with no response from the user of the continuous glucose monitoring device. (B) Basal and bolus insulin delivered during a 24 h period, including insulin delivery status at the time of hypoglycemic episodes.
BG: Blood glucose.
The Medtronic MiniMed Paradigm® REAL-Time Revel System distinguishes itself by being the only truly integrated system in which the receiver of sensor signals is contained within the insulin pump. The system uses the insulin pump screen to display sensor glucose values, graphs and trend arrows, eliminating the need for a separate receiver device. However, a partnership agreement has been signed between DexCom and Animas that will integrate DexCom’s CGM technology with Animas’ insulin pumps [106]. More recently, the Juvenile Diabetes Research Foundation (JDRF) formed a partnership with Animas to develop a first-generation closed-loop system for management of T1D that will employ the DexCom sensor [107].
Where’s the evidence: CGM versus SMBG?
Since adding RT-CGM as an adjunct to standard SMGB adds to the costs of diabetes care and the burdens of the disease on patients, families and clinicians, great effort has been expended over the past 10 years to determine the effectiveness of CGM in allowing a greater proportion of T1D patients to achieve and maintain target A1c levels without increasing the risk of severe hypoglycemia. Almost all of these studies maintained patients on their pre-study insulin regimens, either insulin pump alone or insulin pump and multiple daily injection (MDI) treatment, and there was no evidence that the method of insulin administration affected the response to CGM. Indeed, in the Sensor-Augmented Pump Therapy for A1c Reduction (STAR) 1 study that compared the clinical effectiveness of CGM versus standard glucose monitoring exclusively in patients receiving pump treatment, no differences in the reduction in HbA1c levels were observed between the two treatment groups [36].
The JDRF CGM randomized controlled trial (RCT) [37], Guard Control Study [38] and O’Connell et al. [39] demonstrated that adults with T1D had a greater reduction in A1c levels with use of RT-CGM and SMBG than with SMBG alone. In the 6-month JDRF CGM RCT in T1D patients with HbA1c ≥7.0%, 83% of adults wore their CGM devices 6–7 days a week and lowered HbA1c levels by 0.53% compared with controls. Furthermore, the improvement in A1c with CGM was not accompanied by an increase in biochemical hypoglycemia.
It has been much more difficult to demonstrate a benefit of CGM in pediatric patients with T1D. Overall, lowering of A1c levels in subjects in the JDRF CGM RCT study aged 8–17 years who were assigned to the CGM group did not differ from that in the SMBG control group [40]. Nevertheless, those who wore the CGM device 6–7 days a week during the first 6 months of the study lowered HbA1c levels by 0.8% without increasing the frequency of low sensor glucose concentrations. Moreover, the improvement in glycemic control was maintained for a full 12 months in those subjects who were able to continue the frequent use of these devices. Unfortunately only 21% of the pediatric cohort used the sensor that frequently for the full 12 months, and those that reverted to less frequent sensor use during the second 6 months lost their A1c benefits. The DirecNet GlucoWatch 2 Biographer [41], Guard Control [38] and STAR 1[36] randomized clinical trials have all demonstrated a similar CGM usage-dependent effect of lowering HbA1c in youth with T1D.
Patients with T1D who are successfully meeting treatment goals are often excluded from RCTs evaluating the efficacy of new drugs and devices because lowering A1c levels into the target range is often the primary outcome of interest. Since RT-CGM might assist patients with low A1c levels by reducing exposure to biochemical hypoglycemia, the JDRF CGM Study Group also carried out a RCT that examined the efficacy and safety of RT-CGM in adult and pediatric patients with T1D who had achieved HbA1c levels <7.0% [42]. In that study, RT-CGM use not only reduced the frequency of hypoglycemia but also helped maintain HbA1c levels <7.0% compared with SMBG in adults and children; 88% of CGM subjects maintained A1c levels under 7.0% versus only 63% of SMBG control patients.
Guidelines for utilizing CGM in an effective way for the management of diabetes are still evolving. CGM technology is new for many clinicians and patients, necessitating the need for extra training on how to respond to alarms and real-time trends to minimize over reaction and data overload. The consensus guidelines for CGM published in 2008 are a reliable source with practical recommendations for clinicians planning to implement CGM technology to their diabetes management [43]. The availability of training materials for patients and clinicians to familiarize them with new versions of this fast-developing technology will enhance their widespread use and minimize user-related mistakes.
Evidence for SAP versus MDI treatment with SMBG
A number of previous studies have compared insulin pump versus MDI therapy, as well as CGM versus SMBG. However, no study had examined whether, and to what extent, switching directly to SAP therapy might improve metabolic control in patients with T1D who were previously unable to reach glycemic targets with MDI and SMBG until recently. The STAR 3 study was undertaken to answer this question in adults and children with T1D [44]. This study is also noteworthy because it is the largest (485 subjects) and longest (12 months) randomized clinical trial involving CGM that has been completed to date. In the SAP group, pump therapy was started first and RT-CGM initiated 3–4 weeks later. The most important findings were:
A decline in mean A1c levels was clinically and statistically significantly greater in those assigned to SAP versus MDI therapy (SAP group: from 8.3 to 7.5%; MDI group: from 8.3 to 8.1%; p < 0.0001);
The improvement with SAP therapy was similar in adult and pediatric patients;
Within the pediatric age group, the improvement in A1c was similar in pre-teens (8–12 years of age) as it was in teenagers (13–18 years of age);
A greater proportion of adult and pediatric subjects in the SAP group compared with the MDI group reached A1c targets recommended by the American Diabetes Association;
Rates of severe hypoglycemia and diabetic ketoacidosis were low and did not differ between the treatment groups;
The combined benefit of SAP appears to be greater than that of pump or CGM alone.
Thus, even though it can be a daunting task to introduce both of these new technologies nearly simultaneously, there can be benefit to both pediatric and adult patients with T1D.
Another randomized clinical trial, this time from Europe, compared the glycemic control with SAP versus MDI for adult subjects with poorly controlled T1D [45]. The subjects randomized to SAP treatment lowered their mean HbA1c from 8.5 to 7.2% after only 26 weeks of SAP treatment as compared with subjects who were on MDI with an HbA1c of 8.6% at baseline and 8.5% at the end of the study. An important finding of this study was that there was no increase in episodes of hypoglycemia despite the significant HbA1c reduction for the SAP group.
SAP at onset of T1D in children with T1D
The recent Pediatric Onset Study compared SAP versus insulin pump therapy with SMBG at the onset of T1D in 160 children and adolescents aged 1–16 years [46]. This was a negative study in that A1c levels after 12 months did not differ between the two treatment groups, Once again, the lack of benefit of SAP appeared to be due to low sensor use in the SAP group.
Expert commentary & five-year view
Understanding the challenges & opportunities of SAP therapy
The SAP therapy leans on three major components by design: CGM, insulin pump and rapid-acting insulin analogs. Although the effectiveness of CGM depends on consistent and persistent use, such use has been difficult to achieve in T1D patients in routine care. To identify ways to reduce barriers to more consistent CGM use, it is important to understand how patients perceive the benefits and barriers of CGM and how these perceptions are associated with frequency of use. To answer these questions, patients with T1D in the JDRF CGM trials completed the CGM Satisfaction Scale (CGM-SAT), in which higher scores reflect greater benefits or fewer hassles with CGM use [47]. As might be expected, frequent CGM users reported greater satisfaction and had higher scores on the two subscales compared with infrequent users. However, the greatest differences between the two groups involved the hassles items. This suggests that individuals using CGM who perceive the benefits and wear the device frequently are less bothered by the hassles, while those individuals who wear the device infrequently focus more on the hassles than the benefits. The future of SAP therapy in diabetes management rests on several practicality, safety and technological issues of CGM. The lag between blood glucose and sensor readings can lead to miscalculation of insulin dose by the SAP system. These miscalculations are likely to occur at times of rapid blood glucose change. In order to address these issues, up and coming CGM technologies utilize techniques other than glucose oxidase (e.g., cell based, boronic acid matrix), better filters and enhanced software to measure and report IF glucose levels [108]. Consequently, the continuing development of sensor technology that leads to less intrusive, less painful, more accurate and easier to use systems should result in more consistent use by more patients and a greater improvement in metabolic control of T1D.
Even after more than 30 years of development, there is still room for some improvements in insulin pumps, with considerable attention now focused on patch pumps that eliminate the problems with infusion set tubing. More research is also underway to develop a combined sensor and pump infusion set; however, the effect of this combination set on sensor accuracy is not completely clear [48]. Moreover, new insulin infusion sets durable for longer periods of time will be a prerequisite since currently insulin infusion sets are replaced after 2–3 days and the sensor can be worn for up to 7 days.
The delayed peak and relatively prolonged duration of action of rapid-acting insulin analogs given subcutaneously remains a challenge to overcome, especially with respect to the development of external closed-loop insulin delivery systems that use subcutaneous insulin infusion. On average, peak insulin action is 90–130 min after bolus administration with a total duration of action that often exceeds 5 h [49,50]. β-cells release insulin to the portal system almost immediately after the elevation of glucose level above physiologic levels, therefore mimicking β-cell function necessitates high-speed insulin action to achieve such control. New insulin formulations, novel devices and infusion sets to accelerate insulin absorption have the potential to tackle these issues and transform SAP therapy [51,52].
SAP system with low glucose suspend
The first, relatively small step in the direction of a closed-loop system has already been achieved by the approval of the MiniMed Paradigm Veo System in Europe, Canada and Australia [109,110]. The low glucose suspend feature of this SAP system allows the basal rate of the patient’s pump to be suspended for up to 2 h if the patient fails to respond to the sensor’s low glucose alarm. Several lines of evidence support the efficacy and safety of this approach to preventing a potentially catastrophic hypoglycemic event, especially at night:
Window of opportunity: in a case series, Buckingham and colleagues demonstrated that low sensor glucose levels were detected 2–4 h prior to seizures in the middle of the night [53];
Efficacy and safety: several years ago our group demonstrated that interrupting the basal infusion of lispro insulin for 2 h in the middle of the night in pump patients with baseline plasma glucose levels of approximately 90 mg/dl resulted in a 15–30 mg/dl rise in plasma glucose and a trivial increase in plasma β-hydroxybutryate levels [54].
The clinical outcome from the automatic pump shut down feature in case of low IF readings is not yet known. There are clinical studies underway in the USA and Europe to investigate their effect on the prevention of hypoglycemia.
Closed-loop system: the ultimate SAP
Scientists are currently working on fully closed-loop systems that will utilize control algorithms that automatically regulate insulin infusion rates through the pump based on sensor glucose readings, thus markedly reducing the burdens on patients to make meal-to-meal and day-to-day decisions. This ‘auto-pilot’ insulin infusion system will need to be able to be easily managed by patients, protected against system errors that lead to the over-infusion of insulin, and able to adapt itself to challenges of human physiology during normal daily activities (e.g., exercise and stress) and due to changes in insulin sensitivity. While it has already been demonstrated that closed-loop systems can control blood glucose levels effectively in an inpatient setting [55,56], much work remains to be carried out to ensure the safety of these systems before they are ready for outpatient use.
Key issues.
Insulin pumps have gained new functions but they are still not able to navigate diabetes management in a fully automated fashion.
The interface of technology to diabetes management is accelerating with the sensor-augmented pump being one of the pioneers of this important shift.
This technology is still relatively new and there is a lot that needs to be investigated before its widespread implementation to diabetes management.
The evidence so far is quite promising, and the grand momentum in this field suggests that a mechanical solution to the problems in managing Type 1 diabetes through a closed-loop artificial pancreas is much closer to fruition than biological solutions through islet or β-cell transplantation.
Such closed-loop systems are within our reach provided that improvements and safeguards are built into the systems that make over-delivery of insulin due to a system malfunction extremely unlikely.
Acknowledgments
William Tamborlane is a consultant for Medtronic, Novo Nordisk, Eli Lilly, Bayer and Unomedical, and a speaker for Novo Nordisk and Eli Lilly. Stuart Weinzimer is a consultant for Medtronic, Animas, BD Medical and Bayer, and a speaker for Eli Lilly. This publication was made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood Type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–2020: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br Med J. 1978;1(6107):204–207. doi: 10.1136/bmj.1.6107.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300(11):573–578. doi: 10.1056/NEJM197903153001101. [DOI] [PubMed] [Google Scholar]
- 5.Ahern JA, Boland EA, Doane R, et al. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatr Diabetes. 2002;3(1):10–15. doi: 10.1034/j.1399-5448.2002.30103.x. [DOI] [PubMed] [Google Scholar]
- 6.Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with Type 1 diabetes. Diabetes Care. 1999;22(11):1779–1784. doi: 10.2337/diacare.22.11.1779. [DOI] [PubMed] [Google Scholar]
- 7.Maniatis AK, Klingensmith GJ, Slover RH, Mowry CJ, Chase HP. Continuous subcutaneous insulin infusion therapy for children and adolescents: an option for routine diabetes care. Pediatrics. 2001;107(2):351–356. doi: 10.1542/peds.107.2.351. [DOI] [PubMed] [Google Scholar]
- 8.Plotnick LP, Clark LM, Brancati FL, Erlinger T. Safety and effectiveness of insulin pump therapy in children and adolescents with Type 1 diabetes. Diabetes Care. 2003;26(4):1142–1146. doi: 10.2337/diacare.26.4.1142. [DOI] [PubMed] [Google Scholar]
- 9.Sulli N, Shashaj B. Continuous subcutaneous insulin infusion in children and adolescents with diabetes mellitus: decreased HbA1c with low risk of hypoglycemia. J Pediatr Endocrinol Metab. 2003;16(3):393–399. doi: 10.1515/jpem.2003.16.3.393. [DOI] [PubMed] [Google Scholar]
- 10.Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with Type 1 diabetes. J Pediatr. 2003;143(6):796–801. doi: 10.1067/S0022-3476(03)00579-1. [DOI] [PubMed] [Google Scholar]
- 11.Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3 Pt 1):e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 12.Pankowska E, Skorka A, Szypowska A, Lipka M. Memory of insulin pumps and their record as a source of information about insulin therapy in children and adolescents with Type 1 diabetes. Diabetes Technol Ther. 2005;7(2):308–314. doi: 10.1089/dia.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 13.Anhalt H, Bohannon NJ. Insulin patch pumps: their development and future in closed-loop systems. Diabetes Technol Ther. 2010;12(Suppl. 1):S51–S58. doi: 10.1089/dia.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11(Suppl. 1):S11–S16. doi: 10.1089/dia.2009.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koschinsky T, Heinemann L. Sensors for glucose monitoring: technical and clinical aspects. Diabetes Metab Res Rev. 2001;17(2):113–123. doi: 10.1002/dmrr.188. [DOI] [PubMed] [Google Scholar]
- 16.Aye T, Block J, Buckingham B. Toward closing the loop: an update on insulin pumps and continuous glucose monitoring systems. Endocrinol Metab Clin North Am. 2010;39(3):609–624. doi: 10.1016/j.ecl.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebrin K, Sheppard NF, Jr, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol. 2010;4(5):1087–1098. doi: 10.1177/193229681000400507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerritsen M, Jansen JA, Kros A, et al. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mater Res. 2001;54(1):69–75. doi: 10.1002/1097-4636(200101)54:1<69::aid-jbm8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Kyrolainen M, Rigsby P, Eddy S, Vadgama P. Bio-/haemocompatibility: implications and outcomes for sensors? Acta Anaesthesiol Scand Suppl. 1995;104:55–60. doi: 10.1111/j.1399-6576.1995.tb04255.x. [DOI] [PubMed] [Google Scholar]
- 20.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 21.Mang A, Pill J, Gretz N, et al. Biocompatibility of an electrochemical sensor for continuous glucose monitoring in subcutaneous tissue. Diabetes Technol Ther. 2005;7(1):163–173. doi: 10.1089/dia.2005.7.163. [DOI] [PubMed] [Google Scholar]
- 22.Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000;2(3):461–472. doi: 10.1089/15209150050194332. [DOI] [PubMed] [Google Scholar]
- 23.Wolpert HA. The nuts and bolts of achieving end points with real-time continuous glucose monitoring. Diabetes Care. 2008;31(Suppl. 2):S146–S149. doi: 10.2337/dc08-s238. [DOI] [PubMed] [Google Scholar]
- 24.Updike SJ, Hicks GP. The enzyme electrode. Nature. 1967;214(5092):986–988. doi: 10.1038/214986a0. [DOI] [PubMed] [Google Scholar]
- 25.Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract. 1999;46(3):183–190. doi: 10.1016/s0168-8227(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 26.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with Type 1 diabetes. Diabetes Care. 2001;24(11):1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 27.Kollman C, Wilson DM, Wysocki T, Tamborlane WV, Beck RW. Limitations of statistical measures of error in assessing the accuracy of continuous glucose sensors. Diabetes Technol Ther. 2005;7(5):665–672. doi: 10.1089/dia.2005.7.665. discussion 673–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the Guardian RT continuous glucose monitor in children with Type 1 diabetes. Diabetes Technol Ther. 2008;10(4):266–272. doi: 10.1089/dia.2007.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastrototaro J, Shin J, Marcus A, Sulur G. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with Type 1 diabetes. Diabetes Technol Ther. 2008;10(5):385–390. doi: 10.1089/dia.2007.0291. [DOI] [PubMed] [Google Scholar]
- 30.Garg SK, Smith J, Beatson C, Lopez-Baca B, Voelmle M, Gottlieb PA. Comparison of accuracy and safety of the SEVEN and the Navigator continuous glucose monitoring systems. Diabetes Technol Ther. 2009;11(2):65–72. doi: 10.1089/dia.2008.0109. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 32.Wilson DM, Beck RW, Tamborlane WV, et al. The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with Type 1 diabetes. Diabetes Care. 2007;30(1):59–64. doi: 10.2337/dc06-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey T, Zisser H, Chang A. New features and performance of a next-generation SEVEN-day continuous glucose monitoring system with short lag time. Diabetes Technol Ther. 2009;11(12):749–755. doi: 10.1089/dia.2009.0075. [DOI] [PubMed] [Google Scholar]
- 34.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 35.Clarke WL, Kovatchev B. Continuous glucose sensors: continuing questions about clinical accuracy. J Diabetes Sci Technol. 2007;1(5):669–675. doi: 10.1177/193229680700100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-totarget study. Diabetes Technol Ther. 2008;10(5):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 37.Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of Type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 38.Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with Type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in Type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 40.Chase HP, Beck RW, Xing D, et al. Continuous glucose monitoring in youth with Type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2007;12(7):507–515. doi: 10.1089/dia.2010.0021. [DOI] [PubMed] [Google Scholar]
- 41.Chase HP, Beck R, Tamborlane W, et al. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with Type 1 diabetes. Diabetes Care. 2005;28(5):1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 42.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well-controlled Type 1 diabetes. Diabetes Care. 2009;32(8):1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM) Diabetes Technol Ther. 2008;10(4):232–244. doi: 10.1089/dia.2008.0016. quiz 245-236. [DOI] [PubMed] [Google Scholar]
- 44.Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in Type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 45.Hermanides J, Norgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011 doi: 10.1111/j.1464-5491.2011.03256.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Kordonouri O, Pankowska E, Rami B, et al. Sensor-augmented pump therapy from the diagnosis of childhood Type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53(12):2487–2495. doi: 10.1007/s00125-010-1878-6. [DOI] [PubMed] [Google Scholar]
- 47.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12(9):679–684. doi: 10.1089/dia.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindpointner S, Korsatko S, Kohler G, et al. Glucose levels at the site of subcutaneous insulin administration and their relationship to plasma levels. Diabetes Care. 2010;33(4):833–838. doi: 10.2337/dc09-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swan KL, Dziura JD, Steil GM, et al. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with Type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32(2):240–244. doi: 10.2337/dc08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swan KL, Weinzimer SA, Dziura JD, et al. Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with Type 1 diabetes. Diabetes Care. 2008;31(1):44–46. doi: 10.2337/dc07-0737. [DOI] [PubMed] [Google Scholar]
- 51.Cengiz E, Tamborlane WV, Sherr J, et al. Faster is better: investigating the effect of a novel warming device on the pharmacodynamics of rapid acting insulin in youth with Type 1 diabetes (T1D) International Society of Pediatric and Adolescent Diabetes 2010 Meeting Abstract, (P170) 2010;99 [Google Scholar]
- 52.Muchmore DB, Vaughn DE. Review of the mechanism of action and clinical efficacy of recombinant human hyaluronidase coadministration with current prandial insulin formulations. J Diabetes Sci Technol. 2010;4(2):419–428. doi: 10.1177/193229681000400223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. 2008;31(11):2110–2112. doi: 10.2337/dc08-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attia N, Jones TW, Holcombe J, Tamborlane WV. Comparison of human regular and lispro insulins after interruption of continuous subcutaneous insulin infusion and in the treatment of acutely decompensated IDDM. Diabetes Care. 1998;21(5):817–821. doi: 10.2337/diacare.21.5.817. [DOI] [PubMed] [Google Scholar]
- 55.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with Type 1 diabetes: a Phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 56.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with Type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
Websites
- 101.National Diabetes Fact Sheet (2007) [March 2011]; www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
- 102.FDA summary of safety and effectiveness data for Continuous Subcutaneous Glucose Monitoring System. [June 2011]; www.fda.gov/ohrms/dockets/dockets/05m0454/05m-0454-aav0001-03-SSED-vol1.pdf.
- 103.CoPilot® for FreeStyle Navigator® System. [June 2011]; www.abbottdiabetescare.com/copilot-for-freestyle-navigator-system.html.
- 104.DexCom™ SEVEN® PLUS Software. [January 2011]; www.dexcom.com/products/dm3_software#preview.
- 105.Medtronic CareLink Therapy Management Software for Diabetes. [January 2011]; https://carelink.minimed.com.
- 106.DexCom™ Announces Joint Development Agreement with Animas Corporation. [January 2011]; http://investor.shareholder.com/dexcom/releasedetail.cfm?ReleaseID=286182.
- 107.JDRF Forms Partnership With Animas to Develop First-Generation Automated System for Managing Type 1 Diabetes. [December 2010]; www.prnewswire.com/news-releases/jdrf-forms-partnership-with-animas-to-develop-first-generation-automated-systemfor-managing-type-1-diabetes-81323767.html.
- 108.Feasibility Study to Assess the Safety and Functionality of the GluSense Continuous Glucose Monitor in Diabetic Patients. [December 2010]; http://clinicaltrials.gov/ct2/show/NCT01198678.
- 109.Paradigm® Veo™. [January 2011]; www.medtronic-diabetes.co.uk/product-information/paradigm-veo/index.html.
- 110.The MiniMed Paradigm® Veo™ System. [January 2011]; http://medtronic-diabetes.com.au/paradigm-veo.html.


