Abstract
Osteoarthritis (OA) is often a consequence of excessive mechanical loading of cartilage, which produces hydrostatic stress, tensile strain, and fluid flow. Application of high fluid shear to chondrocytes recapitulates the earmarks of OA, as evidenced by the induction of cyclooxygenase-2, prostaglandins (PGs), and interleukin-6 (IL-6). Here, we delineated the signaling pathway by which high fluid shear mediates the temporal regulation of IL-6 synthesis in human chondrocytes. We determined that Toll-like receptor 4 (TLR4) and caveolin-1 are binding partners in chondrocytes. Their expression is temporally regulated by fluid shear via the sequential up-regulation of microsomal PGE synthase-1 (mPGES-1) and L-PGDS. High shear stress rapidly induces an 8-fold up-regulation of TLR4 expression via an mPGES-1-dependent pathway, whereas prolonged shear exposure concurrently down-regulates TLR4 by >4-fold and up-regulates caveolin-1 expression by > 2.5-fold in an L-PGDS-dependent manner. TLR4 and caveolin-1 exert opposing effects on the activation of ERK1/2, PI3-K and PKA signaling pathways, which, in turn, regulate the NF-κB-dependent IL-6 synthesis in a time-dependent fashion. Reconstructing the signaling network regulating shear-induced IL-6 expression in chondrocytes may provide insights for developing therapeutic strategies to combat osteoarthritis.—Wang, P., Zhu, F., Tong, Z., Konstantopoulos, K. Response of chondrocytes to shear stress: antagonistic effects of the binding partners Toll-like receptor 4 and caveolin-1.
Keywords: interleukin-6, mPGES-1, L-PGDS
Osteoarthritis (OA) is a musculoskeletal disorder characterized by the degeneration or destruction of the articular cartilage tissue that covers and protects the moving joints. The etiologies of OA include joint dysplasia, genetic and developmental joint abnormalities, aging, and joint injuries (1). Mechanical overloading of cartilage that produces hydrostatic stress, tensile strain, and fluid flow has also been implicated in the development and progression of OA (1). Indeed, prolonged application of high fluid shear to human chondrocytes in vitro recapitulates gene expression profiles associated with OA in vivo (2). Although OA is classified as a noninflammatory joint disease, prostaglandins (PGs) and cytokines are believed to play a role in the pathogenesis and progression of the disease. PGE2 and PGD2 are the major PGs synthesized by chondrocytes. The secretion of PGE2 (3), PGD2 [and its dehydration end product 15-deoxy-Δ-(12,14)-PGJ2 (15d-PGJ2); refs. 4, 5], and interleukin-6 (IL-6; ref. 6) is markedly higher in OA than healthy cartilage. PGE2 and IL-6 have been implicated in pain signaling (7), cartilage erosion (8), and inflammation associated with osteoarthritis and adjuvant-induced arthritis (3, 9). In contrast, the role of PGD2 and 15d-PGJ2 in the metabolism of articular cartilage is still a matter of debate. Although several studies support the notion that PGD2 and 15d-PGJ2 have chondroprotective effects by counteracting the IL-1β-mediated induction of matrix metalloproteinases (4, 10), others suggest that they have proapoptotic effects on chondrocytes (5, 11). PGD synthase (PGDS) and microsomal PGES-1 are responsible for the biosynthesis of PGD2 and PGE2, respectively. PGDS exists in 2 isoforms: hematopoietic (H)-type and lipocalin (L)-type PGDS. L-PGDS is the predominant isoform in human cartilage. L-PGDS (12) and mPGES-1 (13), as well as cyclooxygenase-2 (COX-2; ref. 3), are markedly up-regulated in OA relative to healthy cartilage.
We recently determined that Toll-like receptor 4 (TLR4) and caveolin-1, which are up-regulated in OA cartilage (14, 15), are positively regulated by COX-2 in sheared chondrocytes (2). Prior work suggests a role for TLR4 in the release of proinflammatory cytokines (e.g., IL-1β) and suppression of matrix synthesis by human articular chondrocytes (16). TLR4 was recently reported to interact with caveolin-1 in murine macrophage RAW264.7 cells (17). It remains to be determined whether this is the case in human chondrocytes, and whether this interaction affects the proinflammatory TLR4-mediated signaling. Although the precise role of caveolin-1 in OA has yet to be delineated, evidence suggests that caveolin-1 mediates anti-inflammatory responses in different cell types, such as macrophages (17) and stromal cells (18).
In this study, we demonstrate that TLR4 and caveolin-1 are binding partners in human chondrocytes, and their expression is regulated in a temporally distinct manner by shear stress via a COX-2-dependent mechanism. High fluid shear induces the rapid and transient expression of TLR4, which is ultimately responsible for the NF-κB-dependent IL-6 synthesis. Prolonged exposure (48 h) of chondrocytes to high shear down-regulates TLR4 and up-regulates caveolin-1 expression, resulting in diminished IL-6 mRNA and protein synthesis. The temporal regulation of TLR4 and caveolin-1 is controlled by the sequential up-regulation of mPGES-1 and L-PGDS. Taken together, these data suggest that the balance of PGE2 and PGD2 controls the synthesis of IL-6 in mechanically stimulated chondrocytes. In the resolution phase of inflammation and onset of apoptosis, the balance shifts from PGE2 and TLR4 to increased PGD2 and caveolin-1 synthesis.
MATERIALS AND METHODS
Reagents
The COX-2-selective inhibitor NS398 and the PKA inhibitor H89 were obtained from Enzo Life Sciences International Inc (Plymouth Meeting, PA, USA). The PI3-K inhibitors, LY294002 and wortmannin, were from Sigma-Aldrich (St. Louis, MO, USA). The IL-6 promoter reporter constructs pIL6-luc651 (−651/+1) and pIL6-luc651 ΔNF-κB (NF-κB site mutation) were gifts from Dr. Oliver Eickelberg (Comprehensive Pneumology Center, Institute of Lung Biology and Disease, Munich, Germany; ref. 19). The pRL-SV40 vector encoding the Renilla luciferase gene and the dual-luciferase reporter assay kit were purchased from Promega (Madison, WI, USA). The caveolin-1, TLR4, mPGES-1, and L-PGDS, EP2, EP3 cDNA plasmids were obtained from Origene Technologies (Rockville, MD, USA), and subcloned to the pCMV6-XL vector. The MEK1/2 inhibitors U0126 and PD98059, and antibodies specific for TLR4, mPGES-1, and L-PGDS were from Sigma-Aldrich. PKA C-α siRNA and antibodies specific for β-actin, caveolin-1, Akt, p-Akt (Ser473), NF-κB p65, p-p65 (Ser276), p-p65 (Ser536), CREB, p-CREB (Ser133), ERK1/2, and p-ERK1/2 (Thr 202/Tyr 204), the anti-rabbit IgG (H+L), F(ab′)2 fragment (Alexa Fluor 555 conjugate) and the anti-mouse IgG (H+L), F(ab′)2 fragment (Alexa Fluor 488 conjugate) were purchased from Cell Signaling Technology (Danvers, MA, USA). Caveolin-1, TLR4, mPGES-1, L-PGDS, and scramble siRNAs, isotype control antibodies against rabbit and mouse for cell staining, as well as a monoclonal antibody specific for IL-6, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal antibodies specific for EP2 and EP3, as well as the PGE2 and IL-6 EIA kits, were from Cayman Chemical (Ann Arbor, MI, USA), whereas the 15d-PGJ2 EIA kit was obtained from Assay Designs (Ann Arbor, MI, USA). All reagents for qRT-PCR and SDS-PAGE experiments were purchased from Bio-Rad Laboratories (Hercules, CA, USA). Reagents for EMSA were obtained from Pierce Chemical Co. (Rockford, IL, USA). All other reagents were from Invitrogen (Carlsbad, CA, USA), unless otherwise specified.
Cell culture and shear stress exposure
Human primary articular chondrocytes (Cell Applications, San Diego, CA, USA) or T/C-28a2 chondrocytic cells were grown (37°C in 5% CO2) on glass slides in F12/DMEM supplemented with 10% FBS (20–22). Before shear exposure, cells were incubated for 18 h in serum-free medium supplemented with 1% Nutridoma-SP (Roche Applied Science, Indianapolis, IN, USA), a low-serum replacement that maintains chondrocyte phenotype and establishes quiescence in the monolayer (23, 24). Cells were then subjected to a shear stress level of 20 dyn/cm2 for prescribed periods of time in medium containing 1% Nutridoma-SP, using a streamer gold flow device (Flexcell International, Hillsborough, NC, USA). In select experiments, the pharmacological agents were added to the medium at the indicated concentrations just before the onset of shear exposure.
Transient transfection and reporter gene assays
For ectopic expression of EP2, EP3, caveolin-1, TLR4, mPGES-1, or L-PGDS, T/C-28a2 chondrocytes were transfected with 1.6 μg/slide of plasmid containing cDNAs by using Lipofectamine 2000. In control experiments, cells were transfected with 1.6 μg/slide of the empty vector pCMV6-XL (OriGene Technologies, Rockville, MD, USA). In select experiments, T/C-28a2 cells were transfected with 1.6 μg/slide of the IL-6 promoter reporter construct pIL-6-luc651 or pIL6-luc651 ΔNF-κB together with the pRL-SV40 vector. In RNA interference assays, T/C-28a2 cells were transfected with 100 nM siRNA oligonucleotide sequence specific for caveolin-1, TLR4, mPGES-1, or L-PGDS. In control experiments, cells were transfected with 100 nM scramble siRNA. For EP2 or EP3 shRNA experiments, T/C-28a2 chondrocytes were transfected with 1.6 μg/slide of plasmid containing the EP2, EP3, or scramble shRNA control (25). The following oligonucleotide sequences were used to specifically target the EP3 mRNA (underscored, sense and antisense sequences in boldface, loop with linker): top strand, 5′-GATCCCCGGCCACGGCATCTCAGTCCTTCAAGAGAGGACTGAGATGCCGTGGCCTTTTTC-3′; bottom strand, 5′-TCGAGAAAAAGGCCACGGCATCTCAGTCCTCTCTTGAAGGACTGAGATGC CGTGGCCGGG-3′. The oligonucleotide sequences used for EP2 knockdown have been reported by Wang et al. (25). Top and bottom strands were annealed and subcloned into the XhoI/BglII sites of pSuper, and the resulting construct sequence was verified. Transfected cells were allowed to recover for ≥12 h in growth medium, and then incubated overnight in medium containing 1% Nutridoma-SP before their exposure to shear or static conditions. In promoter activity experiments, luciferase activities were measured by using the dual-luciferase reporter assay kit (Promega), as described previously (20, 22).
qRT-PCR
qRT-PCR assays were performed on the iCycler iQ detection system (Bio-Rad) using total RNA (Qiagen RNEasy kit; Qiagen, Valencia, CA, USA), the iScript one-step RT-PCR kit with SYBR green (Bio-Rad) and primers. The GenBank accession numbers and forward (F) and reverse (R) primers are as follows: mPGES-1 (NM_004878), F-GAAGAAGGCCTTTGCCAAC, R-GGAAGACCAGGAAGTGCATC; caveolin-1 (NM_001753), F-GGGCAACATCTAGAAGCCCAACAA, R-CTGATGCACTGAATTCCAATCAGGAA; TLR4 (NM_138554), F-TGCAATGGATCAAGGACCAGAGGC, R-GTGCTGGGACACCACAACAATCACC; ERK1 (NM_002746), F-CAACATGAAGGCCCGAAACTACC, R-TAACATCCGGTCCAGCAGGTCAAG; ERK2 (NM_002745), F-TACACCAACCTCTCGTACATCG, R-CATGTCTGAAGCGCAGTAAGATT; Akt1 (NM_001014432), F-ACCAGATGCAACCTCACTAT, R-TTAAACCTTGCTCCTCTGTC; Akt2 (NM_001626), F-AAACACAAGGAAAGGGAAC, R-CTTTGATGACAGACACCTCA; Akt3 (NM_005465), F-GCAAGTGGACGAGAATAAGT, R-CAATTTCATGCAAAAACAAA; DP1 (NM_000953), F-CTCTGCCCGTAATTTATCGC, R-CACCGGCTCCTGTACCTAAG; and DP2 (NM_004778), F-TTTCTCAACATGTTCGCCAG, R-AAGCACCAGGCAGACTTTGT.
The GenBank accession numbers and forward and reverse primers for L-PGDS, EP2, EP3, CREB, ATF4, IL-6, and GAPDH can be found in our previous publications (8, 11, 25). The specificity of primers was determined by dissociation curve and 1% agarose electrophoresis (data not shown). GAPDH was used as an internal control. Reaction mixtures were incubated at 50°C for 15 min, followed by 95°C for 5 min, and then 35 PCR cycles were performed with the following temperature profile: 95°C, 15 s; 58°C, 30 s; 68°C, 1 min; and 77°C, 20 s. Data were collected at the 77°C, 20 s step to remove possible fluorescent contribution from dimer primers (8, 11, 25). Gene expression values were normalized to GAPDH.
Western blot analysis
Human primary chondrocytes or T/C-28a2 cells, from sheared and matched static control specimens, were lysed in RIPA buffer (25 mM TrisHCl, pH 7.6; 150 mM NaCl; 1% Nonidet P-40; 1% sodium deoxycholate; and 0.1% SDS) containing a cocktail of proteinase inhibitors (Pierce Chemical). The protein content of the cell lysates was determined using bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical). Total cell lysates (4 μg) were subjected to SDS-PAGE, transferred to a membrane, and probed with a panel of specific antibodies. Each membrane was only probed using one antibody. β-Actin was used as a loading control. All Western hybridizations were performed at least in triplicate using a different cell preparation each time.
Coimmunoprecipitation
Human primary chondrocytes or T/C-28a2 cells were washed twice with ice-cold PBS(−) and homogenized in RIPA buffer with complete protease inhibitor mixture for 30 min on ice. After centrifugation for 20 min at 14,000 g at 4°C, the supernatant was collected. Protein concentrations were determined by BCA and adjusted to 1 μg/μl. A total of 0.5 ml was used for each immunoprecipitation experiment. Supernatant (10 μl) was removed and saved at 4°C until loading to the gel and used as input control. Ab or IgG control (1 μg) was added to 0.49-ml protein extracts. The mixtures were rotated at 4°C overnight. Then, 25 μl of protein G-agarose beads (Invitrogen) was added to the mixtures, and they were rotated for another 12 h at 4°C. The beads were harvested by centrifugation and washed 5 with RIPA buffer. The loading buffer was then added, and mixtures were boiled for 5 min. After centrifugation, the supernatants were subjected to immunoblot analysis.
Immunofluorescence staining
Human primary chondrocytes or T/C-28a2 cells were permeabilized with 0.1% Triton X-100 for 1 min at 4°C, fixed with 4% paraformaldehyde for 10 min at 37°C, washed with PBS(−), and incubated in buffer containing 1% BSA/PBS(+) for 10 min at room temperature. Cells were then incubated with a rabbit mAb to caveolin-1 and mouse polyclonal antibody to TLR4 for 60 min at room temperature, washed with 1% BSA/PBS(+), and incubated in buffer containing Alexa Fluor 488-labeled goat anti-rabbit IgG and Alexa Fluor 555-labeled goat anti-mouse IgG for 60 min at room temperature. Finally, the cells were washed 5× with 1% BSA/PBS(+) and observed under a confocal microscope (Zeiss 510 Meta w/ ZEN; Carl Zeiss, Oberkochen, Germany).
Preparation of cytosolic and nuclear extracts
Cytosolic and nuclear extracts were isolated using the NE-PER nuclear and cytoplasmic extraction kit (Pierce Chemical) following the manufacturer's instructions, as described previously (8, 20, 25).
Electrophoretic motility shift assay (EMSA)
A 5′-biotinylated oligonucleotide probe (5′-GGGATTTTCC-3′) was synthesized containing the NF-κB cis-element present on the IL-6 promoter. EMSAs were performed with a commercially available nonradioisotopic EMSA kit (LightShift Chemiluminescence EMSA kit; Pierce Chemical), as described previously (8, 20, 25).
Measurement of PGE2, 15d-PGJ2, and IL-6 concentration in medium
PGE2, 15d-PGJ2, and IL-6 levels in both static and sheared media were determined using the corresponding enzyme immunoassay kits, following the manufacturer's instructions. The total protein concentration in the medium was used as loading control. Data were expressed as picograms of PGE2, 15d-PGJ2, or IL-6 per microgram of total protein.
Statistics
Data represent the means ± se of ≥3 independent experiments. Statistical significance of differences between means was determined by Student's t test or 1-way ANOVA, wherever appropriate. If means were shown to be significantly different, multiple comparisons by pairs were performed by the Tukey test (24).
RESULTS
Fluid shear regulates TLR4, caveolin-1, and IL-6 synthesis in a temporally distinct manner via a COX-2-dependent pathway
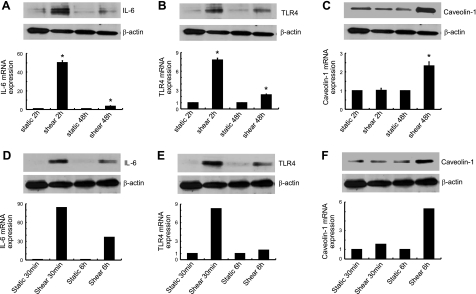
OA is often a consequence of mechanical overloading of cartilage, which produces hydrostatic stress, tensile strain, and fluid flow. Prolonged application of high fluid shear to human chondrocytes in vitro recapitulates gene expression profiles associated with OA (2), as evidenced by the up-regulation of inflammation-related genes, such as IL-6, TLR-4, and caveolin-1, and the induction of chondrocyte apoptosis (11). Continuous exposure of human T/C-28a2 chondrocytes to high shear stress (20 dyn/cm2) for 48 h induces the rapid secretion of IL-6, which reaches a plateau after 2 h of shear stimulation (Supplemental Fig. S1A). Interestingly, IL-6 mRNA and protein up-regulation reaches maximal levels after 2 h of cell stimulation with fluid shear and returns toward basal levels at 48 h (Fig. 1A and Supplemental Fig S1B). Similar observations were made using human primary articular chondrocytes (Fig. 1D and Supplemental Fig. S1C, D), thereby validating previously published data suggesting that T/C-28a2 cells represent an appropriate model for studying chondrocyte function in vitro (2, 8, 23–25). In light of the involvement of IL-6 in pain signaling associated with arthritis (7) and the role of mechanical forces in the pathogenesis and progression of OA, we aimed to delineate the signaling pathway by which fluid shear mediates the temporal regulation of IL-6 expression in human chondrocytes.
Figure 1.
Shear stress induces IL-6, TLR4, and caveolin-1 mRNA and protein expression in human chondrocytes in a temporally distinct manner. T/C-28a2 chondrocytes (A–C) or human primary articular chondrocytes (D–F) were exposed to either fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for the indicated time intervals. IL-6 (A, D), TLR4 (B, E), and caveolin-1 protein (C, F; top panels) and mRNA (bottom panels) synthesis were determined by Western blot analysis and qRT-PCR, respectively. β-Actin and GAPDH served as internal controls in immunoblotting and qRT-PCR assays, respectively. Western blots are representative of 3 independent experiments, all revealing similar results. Data shown in panels A–C represent means ± se of 3 independent qRT-PCR experiments. Data shown in panels D–F are from n = 1 experiment. *P < 0.05 vs. corresponding static controls.
We recently showed that shear stress induces IL-6 synthesis in human chondrocytes via a COX-2-dependent pathway (25). Hierarchical clustering of differentially expressed genes in T/C-28a2 chondrocytes subjected to high fluid shear in the presence or absence of the COX-2-selective inhibitor NS398 revealed that TLR4 and caveolin-1 are regulated via a COX-2-dependent mechanism (2). In view of these data and prior work showing that TLR4 activation regulates cytokine (IL-1β) synthesis in articular chondrocytes (16), we investigated the potential contributions of TLR4 and caveolin-1 to the temporal COX-2-dependent IL-6 synthesis in shear-activated chondrocytes.
Time-course experiments indicate that TLR4 mRNA (Fig. 1B and Supplemental Fig. S1E) and protein (Fig. 1B) expression reach maximal levels after 2 h of T/C-28a2 chondrocyte exposure to high fluid shear, and decline thereafter toward basal levels at 48 h. In distinct contrast, caveolin-1 expression remains at baseline levels at 2 h and progressively increases thereafter, reaching maximal levels at 48 h (Fig. 1C and Supplemental Fig. S1F). This reverse temporal regulation of TLR4 and caveolin-1 was also noted with human primary chondrocytes (Fig. 1E, F). Flow cytometry reveals the presence of both TLR4 and caveolin-1 on the surface of sheared T/C-28a2 cells and primary chondrocytes (data not shown).
TLR4 and caveolin-1 are binding partners in human chondrocytes and regulate shear-induced IL-6 synthesis
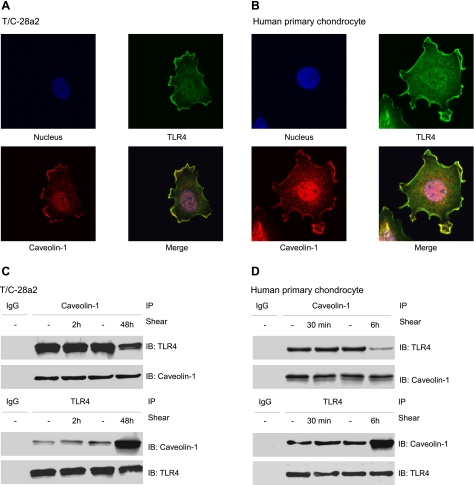
Caveolin-1, the principal structural protein of plasmalemmal caveolae, has been reported to bind TLR4 in murine peritoneal macrophages (17). Mutation studies have identified the caveolin-1 binding motif in the carboxyl-terminal intracellular domain of the mouse TLR4 gene (17). In view of our bioinformatics analysis showing that the sequence of the caveolin-1-binding motif in TLR4 is conserved between mice and humans (Supplemental Fig. S1G, H), we hypothesize that caveolin-1 binding to TLR4 in human chondrocytes regulates shear-induced IL-6 synthesis. As a first step, we demonstrate that caveolin-1 and TLR4 colocalize in the cell membrane of both T/C-28a2 cells (Fig. 2A) and human primary chondrocytes (Fig. 2B), as evidenced by double immunostaining experiments in which colocalization appears yellow in the merged images. The binding interaction between caveolin-1 and TLR4 in T/C-28a2 cells and human primary chondrocytes was confirmed by coimmunoprecipitation assays (Fig. 2C, D).
Figure 2.
TLR4 and caveolin-1 are binding partners in human chondrocytes. A, B) Static control T/C-28a2 cells (A) or human primary articular chondrocytes (B) were immunostained with anti-TLR4 (green) and anti-caveolin-1 (red) antibodies, and analyzed by confocal microscopy. Colocalization of TLR4 and caveolin-1 appears yellow in the merged images. C, D) Cell lysates from T/C-28a2 cells (C) or human primary articular chondrocytes (D) that had been subjected to either fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for the indicated time intervals were subjected to immunoprecipitation (IP) with an anti-caveolin-1 antibody and immunobloting (IB) with an anti-TLR4 antibody or vice versa. TLR4 or caveolin-1 protein was probed with an anti-TLR4 or an anti-caveolin-1 antibody, respectively. Western blots are representative of 3 independent experiments, all revealing similar results.
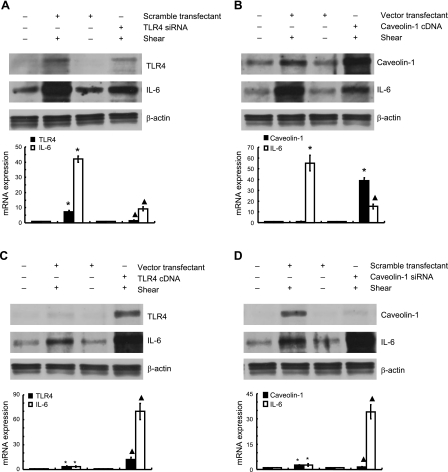
We next aimed to delineate the potential contributions of TLR4 and caveolin-1 to shear-induced IL-6 synthesis in human T/C-28a2 chondrocytes. In light of our data showing that application of high fluid shear to T/C-28a2 cells for 2 h induces maximal TLR4 synthesis without affecting the caveolin-1 mRNA and protein basal levels (Fig. 1), experiments were performed using cells transfected with either an siRNA oligonucleotide sequence specific for TLR4 or a plasmid containing the cDNA of caveolin-1. The efficacy of TLR4 knockdown and caveolin-1 overexpression is demonstrated at both the transcriptional and translational levels using appropriate controls (Fig. 3A, B). These genetic interventions result in a pronounced inhibition of IL-6 mRNA and protein synthesis in shear-activated chondrocytes (Fig. 3A, B). In view of the reverse pattern of TLR4 and caveolin-1 expression in T/C-28a2 chondrocytes subjected to high shear stress for 48 h relative to that for 2 h, experiments were carried out using cells transfected with a plasmid containing the cDNA of TLR4 or a siRNA oligonucleotide specific for caveolin-1. As shown in Fig. 3C, ectopic expression of TLR4 results in markedly increased IL-6 mRNA and protein expression in T/C-28a2 chondrocytes exposed to high shear stress for 48 h relative to appropriate controls. Similarly, knockdown of caveolin-1 increases shear-induced IL-6 synthesis at long shear exposure times (Fig. 3D). Taken together, our data illustrate the antagonistic effects of the two binding partners, caveolin-1, and TLR4, which play a pivotal role in the temporal regulation of IL-6 expression in sheared chondrocytes.
Figure 3.
TLR4 and caveolin-1 exert antagonistic effects on shear-induced IL-6 synthesis in human chondrocytes. T/C-28a2 chondrocytes were subjected to shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 2 h (A, B) or 48 h (C, D). In select experiments, T/C-28a2 chondrocytes were transfected with either a siRNA oligonucleotide sequence specific for TLR4 (A) or caveolin-1 (D) or scrambled siRNA control before their exposure to fluid shear. In separate experiments, cells were transfected with either a plasmid containing the cDNA of caveolin-1 (B) or TLR4 (C) or an empty vector (control) prior to shear exposure. TLR4 (A, C) or caveolin-1 (B, D) and IL-6 protein (top panels) and mRNA expression (bottom panels) were determined by Western blot analysis and qRT-PCR, respectively. β-Actin and GAPDH served as internal controls in immunoblotting and qRT-PCR assays, respectively. Western blots are representative of 3 independent experiments, all revealing similar results. Data represent means ± se of 3 independent qRT-PCR experiments. *P < 0.05 vs. static control; ▴P < 0.05 vs. shear alone.
High fluid shear regulates mPGES-1 and L-PGDS in a temporally distinct manner, which, in turn, modulate the TLR4/caveolin-1-mediated IL-6 synthesis in sheared chondrocytes
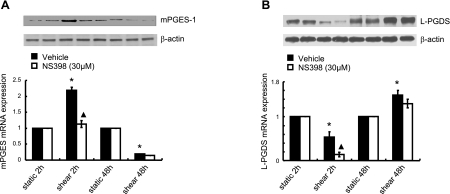
In view of our recent observations showing that shear stress induces IL-6 expression in human chondrocytes in a COX-2-dependent manner (25), we determined the potential contributions to this pathway of the two major COX-2-derived PGs synthesized by human chondrocytes: PGE2 and PGD2. Because mPGES-1 and L-PGDS are responsible for the biosynthesis of PGE2 and PGD2, respectively, in chondrocytes, we evaluated the temporal pattern of their regulation in response to fluid shear. As shown in Fig. 4A and Supplemental Fig. S2A, application of high fluid shear to human T/C-28a2 chondrocytes induces a COX-2-dependent increase in the mPGES-1 mRNA and protein expression levels at 2 h, which then decline below the basal levels at 48 h. In contrast, the L-PGDS mRNA and protein expression are below the static control levels after 2 h of shear stimulation and reach maximal levels at the 48 h time point (Fig. 4B and Supplemental Fig. S2B). In agreement with previously published data (11, 20), high fluid shear induces the release of PGE2 and PGD2 (and its dehydration product 15d-PGJ2) by human chondrocytes (Supplemental Fig. S2C, D).
Figure 4.
Shear stress modulates the expression of mPGES-1 and L-PGDS in a temporally distinct manner. T/C-28a2 chondrocytes were subjected to fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for the indicated time intervals in the absence or presence of the COX-2 inhibitor, NS-398 (30 μM). mPGES-1 (A) and L-PGDS protein (B; top panels) and mRNA expression (bottom panels) were determined by Western blot analysis and qRT-PCR, respectively. β-Actin and GAPDH served as internal controls in immunoblotting and qRT-PCR assays, respectively. Western blots are representative of 3 independent experiments, all revealing similar results. *P < 0.05 vs. static control; ▴P < 0.05 vs. shear alone.
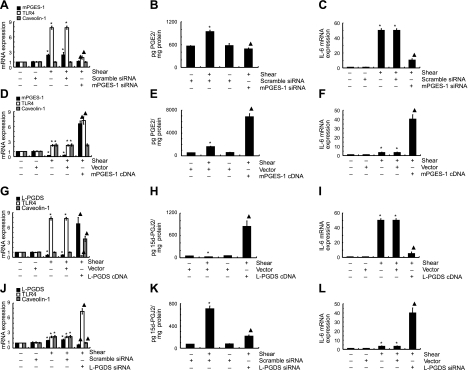
We next wished to dissect the potential roles of mPGES-1 and L-PGDS in the regulation of TLR4/caveolin-1-mediated IL-6 expression in human T/C-28a2 chondrocytes subjected to high shear stress for 2 and 48 h. In light of the temporally distinct pattern of mPGES-1 regulation in sheared chondrocytes (Fig. 4A), experiments were performed using T/C-28a2 cells transfected with either an siRNA oligonucleotide sequence specific for mPGES-1 or a plasmid containing the cDNA of mPGES-1 before their exposure to high fluid shear for 2 or 48 h, respectively. The efficacy of these genetic interventions is verified at the mRNA (Figs. 5A, D) and protein (Figs. 5B, E and 6A, C) levels. mPGES-1 knockdown inhibits the shear-induced TLR4 up-regulation at 2 h without affecting the caveolin-1 basal expression levels (Fig. 5A) and dramatically attenuates IL-6 synthesis (Fig. 5C). On the other hand, ectopic expression of mPGES-1 is effective in augmenting TLR4 mRNA synthesis at 48 h without modulating caveolin-1 expression relative to shear controls (Fig. 5D). mPGES-1 overexpression also leads to a pronounced increase in IL-6 mRNA synthesis at the 48-h time point (Fig. 5F). Taken together, these data suggest that exposure of human chondrocytes to high fluid shear for 2 h induces a COX-2-dependent mPGES-1 up-regulation, which regulates the expression of TLR4, which, in turn, controls IL-6 synthesis.
Figure 5.
mPGES-1 and L-PGDS regulate the biosynthesis of PGE2 and 15d-PGJ2, respectively, which, in turn, modulate TLR4, caveolin-1, and IL-6 expression in human chondrocytes. T/C-28a2 chondrocytes were subjected to fluid shear stress (20 dyn/cm2) for 2 h (A–C, G–I) or 48 h (D–F, J–L). In select experiments, cells were transfected with an siRNA oligonucleotide sequence specific for mPGES-1 (A–C) or L-PGDS (J–L) or a plasmid containing the cDNA of mPGES-1 (D–F) or L-PGDS (G–I) before exposure to a shear stress level of 20 dyn/cm2 for indicated time intervals. mRNA levels were determined by qRT-PCR. GAPDH served as internal control. PGE2 (B, E) or 15d-PGJ2 (H, K) production was monitored by a PGE2 or 15d-PGJ2 enzyme immunoassay kit, respectively. Data represent means ± se of ≥3 independent experiments. *P < 0.05 vs. scrambled siRNA control or empty vector, wherever appropriate; ▴P < 0.05 vs. shear alone or shear with scrambled siRNA control or empty vector control.
Figure 6.
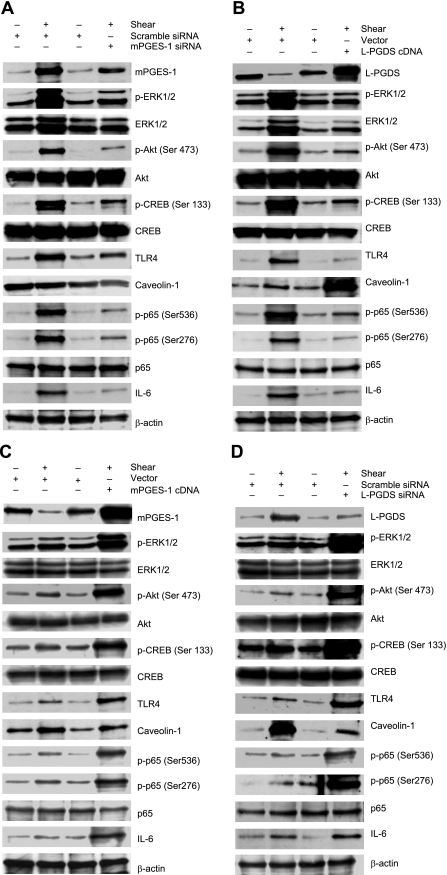
mPGES-1 and L-PGDS regulate the phosphorylation of ERK1/2, Akt, CREB, and NF-κB p65 subunit in shear-activated chondrocytes. T/C-28a2 chondrocytes were subjected to fluid shear stress (20 dyn/cm2) for 2 h (A, B) or 48 h (C, D). In select experiments, cells were transfected with an siRNA oligonucleotide sequence specific for mPGES-1 (A) or L-PGDS (D) or a plasmid containing the cDNA of mPGES-1 (C) or L-PGDS (B) before exposure to a shear stress level of 20 dyn/cm2 for indicated time intervals. Phosphorylated ERK1/2 (Thr 202/Tyr 204), Akt (Ser473), CREB (Ser133), and p65 (Ser536 and Ser276) are shown by immunoblotting using specific Abs. Equal loading in each lane is ensured by the similar intensities of total Akt, CREB, p65, and β-actin. TLR4 and caveolin-1 protein levels were also probed with an anti-TLR4 and an anti-caveolin-1 antibody, respectively. Western blots are representative of 3 independent experiments, all revealing similar results.
Similar experiments were carried out using T/C-28a2 chondrocytes transfected with either a plasmid containing the cDNA of L-PGDS or a siRNA oligonucleotide sequence specific for L-PGDS prior to the application of high shear stress for 2 or 48 h, respectively. The efficacy of L-PGDS overexpression and knockdown was validated at the transcriptional (Fig. 5G, J) and protein (Figs. 5H, K and 6B, D) levels. Interestingly, ectopic expression of L-PGDS concurrently abolishes the shear-induced TLR4 up-regulation and induces caveolin-1 expression (Fig. 5G), while diminishing IL-6 synthesis (Fig. 5I) after 2 h of shear stimulation. L-PGDS knockdown results in the simultaneous up-regulation of TLR4 expression and inhibition of caveolin-1 synthesis (Fig. 5J) at 48 h, which is accompanied by a pronounced increase in IL-6 mRNA expression (Fig. 5L). Cumulatively, these data indicate that prolonged (48 h) exposure of human chondrocytes to high shear stress induces L-PGDS synthesis, which concurrently and distinctly modulates the expression of TLR4 and caveolin-1, which, in turn, regulate IL-6 expression.
Antagonistic effects of mPGES-1 and L-PGDS, as well as TLR4 and caveolin-1, on IL-6 synthesis are exerted via the differential regulation of PI3-K, PKA, and ERK1/2 signaling pathways
In view of our recent data showing the involvement of PI3-K and PKA in the induction of IL-6 synthesis after chondrocyte exposure to high shear stress for 2 h (25), we hypothesized that the decreased IL-6 mRNA and protein levels detected after prolonged (48 h) vs. short (2 h) shear stimulation are due to diminished PI3-K and PKA activation. In addition, phosphorylation of CREB1 and Akt correlates with the activity of PKA and PI3K, respectively (26, 27). Indeed, continuous application of high fluid shear to T/C-28a2 chondrocytes results in a pronounced increase of the phosphorylation levels of Akt at Ser473 and CREB at Ser133 at 2 h (Fig. 6A), which is negated at 48 h (Fig. 6C), whereas the total Akt and CREB levels remain unaffected by shear stimulation at all time points (Fig. 6A, C). Selective knockdown of mPGES-1 (Fig. 6A) or its downstream target TLR4 (Supplemental Fig. S3A) diminishes the shear-induced Akt and CREB phosphorylation and IL-6 synthesis relative to scramble control specimens at the 2-h time point. Similar inhibitory effects were noted after 2 h of shear activation by ectopic expression of L-PGDS (Fig. 6B) or caveolin-1 (Supplemental Fig. S3B). In contrast, mPGES-1 (Fig. 6C) or TLR4 (Supplemental Fig. S3C) overexpression enhances the Akt and CREB phosphorylation, as well as IL-6 mRNA levels, relative to appropriate controls in chondrocytes subjected to shear for 48 h. Selective L-PGDS (Fig. 6D) or caveolin-1 (Supplemental Fig. S3D) knockdown exerted similar effects.
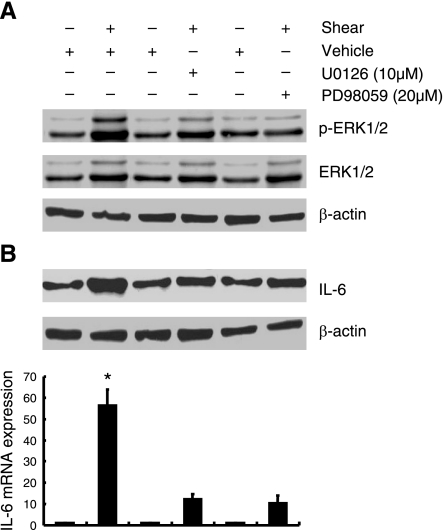
In light of prior work implicating ERK1/2 as a downstream target of PGE2 in myocytes (28), we determined the effects of fluid shear on ERK1/2 activation and its role in IL-6 synthesis in human chondrocytes. As shown in Fig. 6, the ERK1/2 phosphorylation was markedly induced after 2 h of T/C-28a2 chondrocyte exposure to high fluid shear, and declines toward baseline levels at the 48-h time point. mPGES-1 and TLR4 expression correlates positively with ERK1/2 activation, whereas L-PGDS and caveolin-1 inhibit ERK1/2 phosphorylation in human T/C-28a2 chondrocytes (Fig. 6 and Supplemental Fig. S3). To evaluate the potential involvement of ERK1/2 in the regulation of shear-induced IL-6 mRNA and protein synthesis, T/C-28a2 chondrocytes were treated with a selective MEK1/2 inhibitor, U0126, or PD98059. Both of these pharmacological agents drastically inhibit ERK1/2 phosphorylation (Fig. 7A) and IL-6 synthesis (Fig. 7B) in sheared chondrocytes.
Figure 7.
ERK1/2 regulates shear-induced IL-6 expression in human chondrocytes. T/C-28a2 chondrocytes were subjected to fluid shear stress (20 dyn/cm2) for 2 h in the presence or absence of a MEK1/2 inhibitor (U0126; 10 μM) or PD98095 (20 μM). A) Effect of MEK1/2 inhibitors on shear-induced phosphorylation of ERK1/2 (Thr202/Tyr204) is shown by immunoblotting using specific Abs. B) IL-6 protein and mRNA synthesis were determined by Western blot analysis and qRT-PCR, respectively. GAPDH and β-actin served as internal controls in immunoblotting and qRT-PCR assays, respectively. Western blots are representative of 3 independent experiments, all revealing similar results. Data represent means ± se of ≥3 independent qRT-PCR experiments. *P < 0.05 vs. pharmacological treatments. ▴P < 0.05 vs. shear alone.
mPGES-1 and L-PGDS, as well as TLR4 and caveolin-1, exert antagonist effects on NF-κB activation
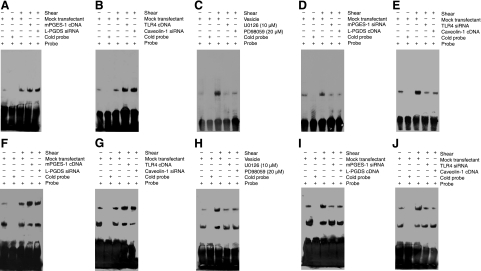
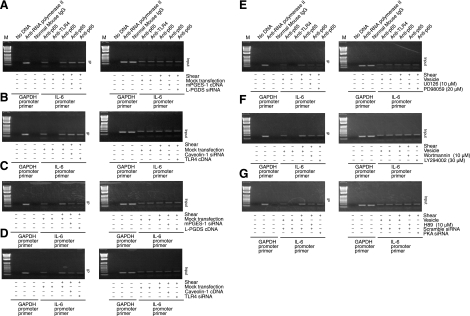
We recently showed that exposure of human chondrocytes to high shear for 2 h transactivates the NF-κB p65 subunit via phosphorylation at Ser276 and Ser536, mediated via PKA and PI3-K, respectively, and that binding of p65 to the IL-6 promoter elicits IL-6 mRNA synthesis (25). Herein, we confirm these data and further report that mPGES-1/TLR4-dependent ERK1/2 activation phosphorylates p65 at both Ser276 and Ser536 (Supplemental Fig. S4C). Moreover, prolonged (48 h) application of high fluid shear to T/C-28a2 chondrocytes diminishes p65 phosphorylation at both sites toward basal (static control) levels (Fig. 6). The decrease in p65 phosphorylation and IL-6 mRNA synthesis noted after 48 vs. 2 h of shear stimulation is reversed by ectopic expression of mPGES-1 (Fig. 6C) or TLR4 (Supplemental Fig. S3C) or knockdown of L-PGDS (Fig. 6D) or caveolin-1 (Supplemental Fig. S3D). These genetic interventions were also effective in augmenting the levels of the NF-κB gel shift and supershift detected after human T/C-28a2 chondrocyte stimulation with high shear stress for 48 h (Fig. 8). Along these lines, the shear-induced transactivation of p65 and its binding to IL-6 promoter observed after 2 h of cell stimulation is inhibited by overexpression of L-PGDS or caveolin-1 or knockdown of mPGES-1 or TLR4 or blockade of ERK1/2 activity (Figs. 6 and 8). These data were further confirmed using chromatin immunoprecipitation assays (Fig. 9).
Figure 8.
Fluid shear induces the binding of the NF-κB p65 subunit to the IL-6 promoter in human chondrocytes. T/C-28a2 chondrocytes were subjected to fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h (A, B, F, G) or 2 h (C–E, H–J). In select experiments, T/C-28a2 cells were transfected with the indicated siRNAs or cDNA constructs before exposure to shear stress. In separate experiments, T/C-28a2 cells were subjected to fluid shear stress (20 dyn/cm2) for 2 h in the presence or absence of a MEK1/2 inhibitor [U0126 (10 μM) or PD98095 (20 μM)]. A–E) Nuclear extracts were then isolated, and NF-κB-specific DNA-protein complex formation was determined by gel shift. F–J) Supershift assays using an anti-p65 Ab were carried out as outlined in Materials and Methods. Results of a competition experiment using 200-fold unlabeled NF-κB oligonucleotide (cold probe) are shown. Gels are representative of 3 independent experiments, all revealing similar results.
Figure 9.
Fluid shear induces the binding of the NF-κB p65 subunit to the IL-6 promoter in human chondrocytes. T/C-28a2 cells were subjected to fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h (A, B) or 2 h (C–G). Cells were transfected with the indicated siRNAs or cDNA constructs or treated with prescribed pharmacological inhibitors before exposure to shear stress. Cross-linked chromatin was immunoprecipitated using an antip65 antibody (left panel). In ChIP assays, the anti-RNA polymerase II antibody was used as a positive control, whereas the normal mouse IgG and anti-TLR4 antibodies were used as negative controls. DNA purified from both the immunoprecipitated (IP) and preimmune (input) specimens were subjected to PCR amplification using primers for the GAPDH (control) and p65 promoter genes. All experiments are representative of 3 independent experiments, all revealing similar results.
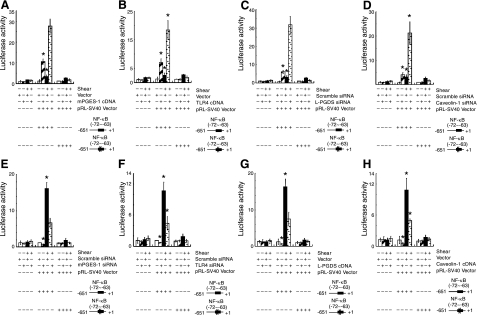
We also examined the temporal effects of shear stress on IL-6 promoter activity in T/C-28a2 chondrocytes transiently transfected with a construct encompassing the 5′-flanking region of the human IL-6 gene from −651 to +1 bp (−651/+1) cloned into a promoterless luciferase expression vector (19). The IL-6 promoter activity was rapidly induced after 2 h of shear stimulation, and decreases thereafter toward basal levels (Fig. 10). Overexpression of mPGES-1 or TLR4, or knockdown of L-PGDS or caveolin-1 increases IL-6 promoter activity relative to appropriate shear and static control specimens at 48 h. This increase is mediated via an NF-κB-dependent mechanism, as evidenced by the introduction of a mutation into the NF-κB binding site (−72/−63).
Figure 10.
Fluid shear increases the IL-6 promoter activity in human chondrocytes. T/C-28a2 cells were subjected to fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 48 h (A–D) or 2 h (E–H). T/C-28a2 cells were transfected with the indicated siRNAs or cDNA constructs along with the IL-6 promoter reporter construct pIL-6-luc651 or pIL6-luc651 ΔNF-κB before exposure to shear stress as described in Materials and Methods. Luciferase activities were measured by using the dual-luciferase reporter assay kit and normalized to sea pansy luciferase activity of cotransfected pRL-SV40. Data represent means ± se of 3 independent experiments. *P < 0.05 vs. pIL6-luc651 ΔNF-κB and vector control; ▴P < 0.05 vs. shear stress.
DISCUSSION
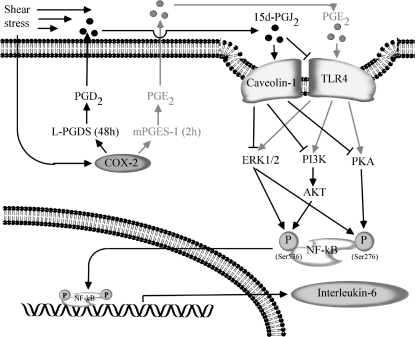
The major findings of this study are as follows: high fluid shear induces the sequential expression of mPGES-1 and L-PGDS in human chondrocytes; exposure of chondrocytes to high shear stress elicits the rapid up-regulation of TLR4 via an mPGES-1/COX-2-dependent pathway; the subsequent overexpression of L-PGDS in sheared chondrocytes is responsible for the simultaneous down-regulation of TLR4 and up-regulation of caveolin-1 at later time points; TLR4 and caveolin-1 are binding partners in human chondrocytes and exert antagonistic effects on shear-induced IL-6 synthesis; and TLR4 and caveolin-1 regulate the activity of ERK1/2, PI3-K, and PKA pathways, which, in turn, modulate the NF-κB-dependent temporal IL-6 expression in shear-activated chondrocytes (Fig. 11).
Figure 11.
Proposed cascade of signaling events regulating IL-6 synthesis in human chondrocytes stimulated with high shear stress. Elevated levels of fluid shear (20 dyn/cm2) induce COX-2 expression, which is responsible for the sequential mPGES-1 (2 h) and L-PGDS (48 h) up-regulation. mPGES-1 and L-PGDS regulate the biosynthesis and release of PGE2 and 15d-PGJ2, respectively. PGE2 stimulates TLR4 expression at short shear exposure times (2 h), whereas 15d-PGJ2 concurrently suppresses TLR4 expression and induces caveolin-1 expression at long shear exposure times (48 h). Activation of TLR4 at short shear exposure times stimulates the activity of ERK1/2, PI3K/Akt, and PKA/CREB pathways, which are inhibited by caveolin-1 overexpression at long shear exposure times. PI3-K/Akt and PKA phosphorylate the NF-κB p65 subunit at Ser536 and Ser276, respectively, leading to NF-κB activation. ERK1/2 activation phosphorylates the NF-κB p65 subunit at both Ser536 and Ser276 sites. Binding of NF-κB p65 subunit to IL-6 promoter induces IL-6 synthesis in human T/C28a2 chondrocytes.
IL-6 expression is tightly regulated under physiological conditions. In response to an inflammatory insult, IL-6 levels increase in vivo and then decline to basal levels on resolution of the insult. The sequential induction of mPGES-1 and L-PGDS in sheared T/C-28a2 chondrocytes is responsible for the temporal regulation of COX-2-dependent IL-6 synthesis, which reaches maximal levels at 2 h and then returns toward baseline levels at 48 h. Interestingly, Gilroy et al. (29) reported that COX-2-dependent metabolism shifts from PGE2 to PGD2 biosynthesis in the resolution phase of carrageenin-induced inflammation in rats. Along these lines, the sequential induction of mPGES-1 and H-PGDS (but not L-PGDS) mediates the resolution of endotoxin-induced inflammation in mouse heart (30).
In view of the critical involvement of L-PGDS/PGD2 in the regulation of IL-6 synthesis in sheared chondrocytes, we examined the potential contribution of D prostanoid receptor (DP)1 and DP2 to this process. Given that the temporal patterns of DP1/DP2 and L-PGDS mRNA synthesis are reverse in shear-activated T/C-28a2 cells (Supplemental Fig. S2E, F), we speculate that DP1 and DP2 receptors are not critical to the propagation of PGD2/15d-PGJ2 signals in sheared chondrocytes. This is substantiated by prior observations showing that PGD2/15d-PGJ2-mediated apoptosis in human T/C-28a2 cells proceeds via a DP2-independent pathway (11).
We recently reported that shear-induced COX-2-derived PGE2 signals via up-regulation of E prostanoid (EP)2 and down-regulation of EP3 receptors in human T/C-28a2 cells to raise intracellular cAMP and activate PKA and PI3-K pathways (25). In light of our observations showing that PGE2 stimulates TLR4 synthesis in sheared T/C-28a2 cells, we examined the potential interaction of TLR4 with EP2/EP3 receptors. Coimmunoprecipitation assays reveal the lack of a physical interaction between TLR4 and EP2 or EP3 (data not shown). Furthermore, selective knockdown or overexpression of TLR4 or caveolin-1 fails to modulate the mRNA levels of EP2 or EP3 in T/C-28a2 cells (data not shown). Similarly, genetic manipulation of EP2 or EP3 expression does not impair the expression levels of TLR4 or caveolin-1 (data not shown). Taken together, these data suggest the absence of crosstalk between TLR4/caveolin-1 and EP2/EP3.
TLR4 and caveolin-1, which are up-regulated in OA cartilage (14, 15), are positively regulated by COX-2 in shear-activated chondrocytes (2). Prior work has established the proinflammatory potential of TLR4, which regulates, among others, IL-6 synthesis, in diverse cell types, such as human macrophages (31) and bladder epithelial cells (32, 33). In agreement with these previous studies, we demonstrate that TLR4 mediates the rapid induction of IL-6 in sheared chondrocytes. Specifically, we show that TLR4 activates ERK1/2, PI3-K, and PKA signaling pathways in parallel, which, in turn, regulate NF-κB-dependent IL-6 production. In concert with our observations, Qian et al. (32) found that TLR4 activation induces IL-6 expression in bladder cancer cells via an ERK-dependent pathway. In contrast to our recent data (25), these authors (32) also reported that inhibition of PI3-K activity enhances IL-6 synthesis in bladder cancer cells. Although Song et al. (33) found that NF-κB is involved in TLR4-mediated IL-6 synthesis in bladder epithelial cells after prolonged (>6 h) microbial stimulation, they reported the key role of cAMP response element-binding (CREB) protein in the rapid IL-6 induction. This is in contrast to our previous observations showing that CREB1 and/or ATF4 binding is dispensable for IL-6 synthesis in PGE2 or shear-primed chondrocytes (8, 25). The aforementioned discrepancies may reflect the different species involved in these studies.
Prior work has shown that lipopolysaccharide stimulation induces membrane translocation of TLR4 to caveolar fraction in human aortic endothelial cells (34). More recently, it is a putative caveolin-1 binding motif(838FIQSRWCIF846), which, in turn, diminishes NF-κB-dependent IL-6 synthesis in murine macrophage cells (17). Bioinformatics analysis reveals that this motif is preserved in the mouse and human species. We further report for the first time that TLR4 and caveolin-1 interact in human primary articular chondrocytes and T/C-28a2 cells.
Numerous reports suggest an anti-inflammatory role for caveolin-1 in diverse cell types (17, 18). For instance, mouse RAW264.7 macrophage cells stably transfected with caveolin-1 exhibit a markedly reduced proinflammatory cytokine production (e.g., IL-6), which is accompanied by increased release of anti-inflammatory cytokines (e.g., IL-10) (35). It is believed that the anti-inflammatory effects of caveolin-1 are mediated by its binding and inactivation of ERK1/2, PI3-K and PKA (36, 37). This is in line with our observations revealing that caveolin-1 diminishes the TLR4-dependent ERK1/2, PI3-K and PKA activation, and the downstream NF-κB-mediated IL-6 synthesis. It is noteworthy that ERK1/2, PI3-K, and PKA act in parallel to transactivate NF-κB in sheared chondrocytes. No crosstalk was detected among the ERK1/2, PI3-K, and PKA pathways, as determined by treating T/C-28a2 cells with specific inhibitors (data not shown), except for the fact that H89, a PKA inhibitor, stimulates Akt phosphorylation, which is in agreement with previously published data (8, 25). We recently showed that PI3-K and PKA transactivate the NF-κB p65 subunit via phosphorylation at Ser536 and Ser276, respectively (25). We report herein that mPGES-1/TLR4-dependent ERK1/2 activation phosphorylates p65 at both sites without interfering with PI3-K and PKA activity (data not shown).
Although caveolin-1 is overexpressed in OA cartilage (14), its precise functional role in OA remains to be delineated. Prior work has established the anti-inflammatory potential of caveolin-1 in macrophages (17). Here, we demonstrate that L-PGDS-dependent caveolin-1 up-regulation diminishes the shear-induced TLR4-dependent IL-6 synthesis, implying a possible anti-inflammatory/chondroprotective role for caveolin-1. However, previous studies have also shown that caveolin-1 up-regulation induces p53 activation and premature chondrocyte senescence, which is reversed by suppression of caveolin-1 induction (14, 38). We have recently reported that L-PGDS up-regulation in shear-activated chondrocytes targets p53, which controls the transcription of p53 effectors, such as FAS and Bax, involved in chondrocyte apoptosis (11). Thus, we hypothesize that shear-induced caveolin-1 up-regulation mediated via an L-PGDS-dependent pathway diminishes cytokine synthesis and contributes to chondrocyte senescence and cell death. It is noteworthy that exposure of chondrocytes to fluid shear recapitulates the earmarks of OA, as evidenced by the release of proinflammatory cytokines, such as IL-6, detected at the early stages of OA (or short shear exposure times in vitro) and chondrocyte apoptosis observed in advanced stages of OA in vivo (or prolonged shear exposure times in vitro) (39).
In summary, we demonstrate that high fluid shear regulates the temporal IL-6 synthesis in human chondrocytes via a COX-2-dependent mechanism. The sequential induction of mPGES-1 and L-PGDS by high shear stress shifts TLR4-dependent inflammation to caveolin-1-mediated resolution of inflammation and chondrocyte senescence. Reconstructing the signaling pathway regulating the catabolic responses of chondrocytes induced by excessive shear stress may identify additional potential therapeutic targets for controlling OA pathogenesis and/or progression.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant RO1 AR053358.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Buckwalter J. A., Martin J. A., Brown T. D. (2006) Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43, 603–609 [PubMed] [Google Scholar]
- 2. Zhu F., Wang P., Lee N. H., Goldring M. B., Konstantopoulos K. (2010) Prolonged application of high fluid shear to chondrocytes recapitulates gene expression profiles associated with osteoarthritis. PLoS One 5, e15174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amin A. R., Attur M., Patel R. N., Thakker G. D., Marshall P. J., Rediske J., Stuchin S. A., Patel I. R., Abramson S. B. (1997) Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J. Clin. Invest. 99, 1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zayed N., Afif H., Chabane N., Mfuna-Endam L., Benderdour M., Martel-Pelletier J., Pelletier J.-P., Motiani R. K., Trebak M., Duval N., Fahmi H. (2008) Inhibition of interleukin-1β–induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum. 58, 3530–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shan Z. Z., Masuko-Hongo K., Dai S. M., Nakamura H., Kato T., Nishioka K. (2004) A potential role of 15-deoxy-delta(12,14)-prostaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J. Biol. Chem. 279, 37939–37950 [DOI] [PubMed] [Google Scholar]
- 6. Mohtai M., Gupta M. K., Donlon B., Ellison B., Cooke J., Gibbons G., Schurman D. J., Smith R. L. (1996) Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J. Orthop. Res. 14, 67–73 [DOI] [PubMed] [Google Scholar]
- 7. Brenn D., Richter F., Schaible H. G. (2007) Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 56, 351–359 [DOI] [PubMed] [Google Scholar]
- 8. Wang P., Zhu F., Konstantopoulos K. (2010) Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-κB activation. Am. J. Physiol. Cell Physiol. 298, C1445–C1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Portanova J. P., Zhang Y., Anderson G. D., Hauser S. D., Masferrer J. L., Seibert K., Gregory S. A., Isakson P. C. (1996) Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 184, 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fahmi H., Di Battista J. A., Pelletier J. P., Mineau F., Ranger P., Martel-Pelletier J. (2001) Peroxisome proliferator–activated receptor gamma activators inhibit interleukin-1β-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 44, 595–607 [DOI] [PubMed] [Google Scholar]
- 11. Zhu F., Wang P., Kontrogianni-Konstantopoulos A., Konstantopoulos K. (2010) Prostaglandin (PG)D(2) and 15-deoxy-Delta(12,14)-PGJ(2), but not PGE(2), mediate shear-induced chondrocyte apoptosis via protein kinase A-dependent regulation of polo-like kinases. Cell Death Differ. 17, 1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zayed N., Li X., Chabane N., Benderdour M., Martel-Pelletier J., Pelletier J.-P., Duval N., Fahmi H. (2008) Increased expression of lipocalin-type prostaglandin D2 synthase in osteoarthritic cartilage. Arthritis Res. Ther. 10, R146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X., Afif H., Cheng S., Martel-Pelletier J., Pelletier J. P., Ranger P., Fahmi H. (2005) Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J. Rheumatol. 32, 887–895 [PubMed] [Google Scholar]
- 14. Dai S. M., Shan Z. Z., Nakamura H., Masuko-Hongo K., Kato T., Nishioka K., Yudoh K. (2006) Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 54, 818–831 [DOI] [PubMed] [Google Scholar]
- 15. Karlsson C., Dehne T., Lindahl A., Brittberg M., Pruss A., Sittinger M., Ringe J. (2010) Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage 18, 581–592 [DOI] [PubMed] [Google Scholar]
- 16. Bobacz K., Sunk I. G., Hofstaetter J. G., Amoyo L., Toma C. D., Akira S., Weichhart T., Saemann M., Smolen J. S. (2007) Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of Toll-like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 56, 1880–1893 [DOI] [PubMed] [Google Scholar]
- 17. Wang X. M., Kim H. P., Nakahira K., Ryter S. W., Choi A. M. (2009) The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. 182, 3809–3818 [DOI] [PubMed] [Google Scholar]
- 18. Pavlides S., Tsirigos A., Vera I., Flomenberg N., Frank P. G., Casimiro M. C., Wang C., Fortina P., Addya S., Pestell R. G., Martinez-Outschoorn U. E., Sotgia F., Lisanti M. P. (2010) Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: A transcriptional informatics analysis with validation. Cell Cycle 9, 2201–2219 [DOI] [PubMed] [Google Scholar]
- 19. Eickelberg O., Pansky A., Mussmann R., Bihl M., Tamm M., Hildebrand P., Perruchoud A. P., Roth M. (1999) Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J. Biol. Chem. 274, 12933–12938 [DOI] [PubMed] [Google Scholar]
- 20. Healy Z. R., Lee N. H., Gao X., Goldring M. B., Talalay P., Kensler T. W., Konstantopoulos K. (2005) Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 102, 14010–14015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abulencia J. P., Gaspard R., Healy Z. R., Gaarde W. A., Quackenbush J., Konstantopoulos K. (2003) Shear-induced cyclooxygenase-2 via a JNK2/c-Jun-dependent pathway regulates prostaglandin receptor expression in chondrocytic cells. J. Biol. Chem. 278, 28388–28394 [DOI] [PubMed] [Google Scholar]
- 22. Healy Z. R., Zhu F., Stull J. D., Konstantopoulos K. (2008) Elucidation of the signaling network of COX-2 induction in sheared chondrocytes: COX-2 is induced via a Rac/MEKK1/MKK7/JNK2/c-Jun-C/EBPbeta-dependent pathway. Am. J. Physiol. Cell Physiol. 294, C1146–C1157 [DOI] [PubMed] [Google Scholar]
- 23. Goldring M. B. (2004) Culture of immortalized chondrocytes and their use as models of chondrocyte function. Methods Mol. Med. 100, 37–52 [DOI] [PubMed] [Google Scholar]
- 24. Goldring M. B., Birkhead J. R., Suen L. F., Yamin R., Mizuno S., Glowacki J., Arbiser J. L., Apperley J. F. (1994) Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J. Clin. Invest. 94, 2307–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang P., Zhu F., Lee N. H., Konstantopoulos K. (2010) Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-κB activation. J. Biol. Chem. 285, 24793–24804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bockmann S., Nebe B. (2003) The in vitro effects of H-89, a specific inhibitor of protein kinase A, in the human colonic carcinoma cell line Caco-2. Eur. J. Cancer Prev. 12, 469–478 [DOI] [PubMed] [Google Scholar]
- 27. Starkman B. G., Cravero J. D., Delcarlo M., Loeser R. F. (2005) IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem. J. 389, 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He Q., Harding P., LaPointe M. C. (2010) PKA, Rap1, ERK1/2, and p90RSK mediate PGE2 and EP4 signaling in neonatal ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 298, H136–H143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. (1999) Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 [DOI] [PubMed] [Google Scholar]
- 30. Schuligoi R., Grill M., Heinemann A., Peskar B. A., Amann R. (2005) Sequential induction of prostaglandin E and D synthases in inflammation. Biochem. Biophys. Res. Commun. 335, 684–689 [DOI] [PubMed] [Google Scholar]
- 31. Palmer C. D., Mutch B. E., Workman S., McDaid J. P., Horwood N. J., Foxwell B. M. (2008) Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NF-κB activity. Blood 111, 1781–1788 [DOI] [PubMed] [Google Scholar]
- 32. Qian Y., Deng J., Xie H., Geng L., Zhou L., Wang Y., Yin S., Feng X., Zheng S. (2009) Regulation of TLR4-induced IL-6 response in bladder cancer cells by opposing actions of MAPK and PI3K signaling. J. Cancer Res. Clin. Oncol. 135, 379–386 [DOI] [PubMed] [Google Scholar]
- 33. Song J., Duncan M. J., Li G., Chan C., Grady R., Stapleton A., Abraham S. N. (2007) A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 3, e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walton K. A., Cole A. L., Yeh M., Subbanagounder G., Krutzik S. R., Modlin R. L., Lucas R. M., Nakai J., Smart E. J., Vora D. K., Berliner J. A. (2003) Specific phospholipid oxidation products inhibit ligand activation of Toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 23, 1197–1203 [DOI] [PubMed] [Google Scholar]
- 35. Wang X. M., Kim H. P., Song R., Choi A. M. (2006) Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Respir. Cell Mol. Biol. 34, 434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muller G., Frick W. (1999) Signalling via caveolin: involvement in the cross-talk between phosphoinositolglycans and insulin. Cell. Mol. Life Sci. 56, 945–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Razani B., Lisanti M. P. (2001) Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am. J. Physiol. Cell Physiol. 281, C1241–C1250 [DOI] [PubMed] [Google Scholar]
- 38. Yudoh K., Shi Y., Karasawa R. (2009) Angiogenic growth factors inhibit chondrocyte ageing in osteoarthritis: potential involvement of catabolic stress-induced overexpression of caveolin-1 in cellular ageing. Int. J. Rheum. Dis. 12, 90–99 [DOI] [PubMed] [Google Scholar]
- 39. Pelletier J. P., Martel-Pelletier J., Abramson S. B. (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 44, 1237–1247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.