Abstract
Degradation of immunoglobulins is an effective strategy of bacteria to evade the immune system. We have tested whether human IgG is a substrate for gingipain K of Porphyromonas gingivalis and found that the enzyme can hydrolyze subclass 1 and 3 of human IgG. The heavy chain of IgG1 was cleaved at a single site within the hinge region, generating Fab and Fc fragments. IgG3 was also cleaved within the heavy chain, but at several sites around the CH2 region. Investigation of the enzyme kinetics of IgG proteolysis by gingipain K, using FPLC- and isothermal titration calorimetry-based assays followed by Hill plots, revealed non-Michaelis-Menten kinetics involving a mechanism of positive cooperativity. In ex vivo studies, it was shown that gingipain K retained its IgG hydrolyzing activity in human plasma despite the high content of natural protease inhibitors; that IgG1 cleavage products were detected in gingival crevicular fluid samples from patients with severe periodontitis; and that gingipain K treatment of serum samples from patients with high antibody titers against P. gingivalis significantly hindered opsonin-dependent phagocytosis of clinical isolates of P. gingivalis by neutrophils. Altogether, these findings underline a biological function of gingipain K as an IgG protease of pathophysiological importance.—Vincents, B., Guentsch, A., Kostolowska, D., von Pawel-Rammingen, U., Eick, S., Potempa, J., Abrahamson, M. Cleavage of IgG1 and IgG3 by gingipain K from Porphyromonas gingivalis may compromise host defense in progressive periodontitis.
Keywords: cysteine proteases, cysteine peptidases, host-pathogen interactions, enzyme kinetics, immunoglobulin G, native substrates
Immunglobulins (Igs) are fundamental components of the adaptive immune response. Because of their unique domain architecture, they hold a functional versatility, allowing them to recognize foreign materials (e.g., invading microorganisms) and mediate their elimination. On one hand, the variable parts of the Fab fragments promote the specific recognition of antigen epitopes. On the other, multiple binding sites at the lower hinge region and at the Fc fragment initiate activation of the classical complement pathway and mediate interactions with Fc receptors on immune cells, including neutrophils and macrophages (1).
For a long time, proteases of pathogenic microorganisms have been recognized as important virulence factors essential for colonization and evasion of the host immune response (2). One of the best examples of such use of proteases by bacterial pathogens is proteolysis of Ig, which hinders antibody-dependent antibacterial mechanisms of the immune system. This contention is justified considering the widespread occurrence of IgA1-specific proteases of different catalytic classes among mucosal pathogens, including Streptococcus pneumoniae, Neisseria gonorrhoeae, and Haemophilus influenzae (3). The objective to evade the host antibody response should best be reached by specific proteolysis of immunoglobulins at the hinge region, leading to dissection of the antigen-recognizing Fab fragment from the effector Fc part of the molecule. Indeed, Streptococcus pyogenes secretes a specific IgG protease, IdeS, cleaving IgG at the hinge region (4). Many other pathogenic bacteria, exemplified by Staphylococcus aureus and S. pyogenes, in addition, express proteases with capacity to indiscriminantly degrade Ig of different classes (5, 6).
Porphyromonas gingivalis is an important human pathogen implicated in development of chronic periodontitis (7). This highly adapted anaerobic bacterium secretes a number of cysteine proteases, including gingipains, periodontain, PrtT protease, and Tpr protease (7), as well as several serine- and metalloproteases (8). Out of all proteolytic enzymes of P. gingivalis, the gingipains are by far the major ones secreted by this bacterium (9). Gingipains are characterized by strict substrate specificity dictated by the P1 position at the substrate cleavage site and are encoded by 3 genes (rgpA, rgpB, and kgp). The R gingipains (RgpA and RgpB) are closely related, and both hydrolyze Arg-Xaa peptide bonds, whereas gingipain K is specific for Lys-Xaa peptide bonds (10). The gingipains have been assigned to their own family of cysteine proteases, family C25. Structurally, they belong to clan CD, hence showing a distant evolutionary relationship to the caspases (family C14) and asparaginyl peptidases or legumains (family C13) (MEROPS database; ref. 11). A crystal structure has been solved for one of the R gingipains only. This revealed that the catalytic domain of gingipain resembles caspase-3 in its dimeric form (12).
The spectrum of human proteins cleaved by gingipains is broad. Apart from direct degradation of extracellular matrix proteins, antibacterial peptides, cytokines, and cell surface receptors, gingipains target several tightly regulated host systems in a biphasic manner (7, 8, 13–15). Their action is dictated by their strict specificity, to cleave substrate Arg-Xaa or Lys-Xaa peptide bonds. Thus, mimicking host proteases, R gingipains very efficiently activate coagulation and kinin generation cascades by limited proteolysis of several zymogens. They also signal via protease-activated receptors (PARs), which requires specific cleavage at Arg-Xaa peptide bonds. In addition, R gingipains at low concentration can activate the complement cascade, but at higher concentration, they destroy this important branch of innate immunity. The activity of gingipain K seems to be more destructive in many respects. Working synergistically with gingipain R, this protease contributes to P. gingivalis resistance to complement, prevents blood clotting, and provides essential nutrients (15). Pathophysiological substrates for gingipain K include cadherins at the adherence junction (16, 17), membrane TNF-α (18), interleukin-8 (19, 20), interleukin-6 receptor (21), thrombomodulin (22), complement regulatory protein CD46 (23), and osteoprotegrin (24). While most of the substrates listed above are also degraded by gingipain R, osteoprotegrin is exclusively cleaved by gingipain K, and its proteolytic destruction promotes osteoclastogenesis and alveolar bone resorption, a hallmark of periodontitis. Significantly, the recently determined concentrations of gingipains in gingival crevicular fluid (GCF; ref. 25) clearly indicate that all substrates and pathways listed above could be affected in vivo. Therefore, it is not surprising that numerous studies show that gingipains are indispensable for P. gingivalis pathogenicity, with gingipain K being a more important virulence factor than either of the R gingipains. An essential contribution of gingipain K to virulence has been verified by treatment with specific inhibitors, which significantly reduced P. gingivalis pathogenicity in murine models of infection (26, 27).
Because it has been shown that P. gingivalis can degrade Ig (28), we asked whether the gingipains are able to degrade any of the 4 subclasses of human IgG. We identified gingipain K as the protease active on IgGs with preference for subclass 1 and 3. Results of enzyme kinetic studies in vitro, investigations ex vivo in plasma, and analysis of samples from patients with periodontitis associated with P. gingivalis infections all clearly implicated gingipain K as a potent IgG protease. Highly specific and efficient hydrolysis of IgG1 at the hinge region and cleavage of IgG3 within the CH2 domain of the heavy chain by gingipain K most likely contributes significantly to bacterial evasion of the humoral immune response.
MATERIALS AND METHODS
Materials
Normal human γ globulin (Kabi Pharmacia, Uppsala, Sweden) for use as substrate in a FPLC-based protease assay was repurified as described previously (29). Gingipain K (EC 3.4.22.47), high-molecular-mass gingipain R (HRgpA), and low-molecular-mass gingipain R (RgpB) were purified from culture medium of P. gingivalis strain H66 as described previously (13, 30). IgG1, IgG2, IgG3, and IgG4 were obtained from ART (Athens, GA, USA) or Calbiochem (San Diego, CA, USA).
IgG degradation analysis
IgG1, IgG2, IgG3, and IgG4 at final concentrations of 15 μM were incubated with gingipain K, HRgpA, or RgpB (10 nM active protease) in 0.1 M Tris buffer (pH 8.0) with 1 mM EDTA and 2 mM l-cysteine at 37°C for 4 h. The reactions were terminated by Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK; Sigma-Aldrich, St. Louis, MO, USA) added to 5 mM final concentration. Time-course analyses of IgG1 and IgG3 hydrolysis by gingipain K were performed as above; samples were taken out at the specified times, and the reaction was stopped with TLCK. The samples were boiled in reducing SDS-PAGE sample buffer and resolved by SDS-PAGE using the Schägger and von Jagow system (31) or 4–12% gradient Novex gels (Invitrogen, Carlsbad, CA, USA) stained with Coomassie Brilliant Blue R-250. Prestained markers of 225, 76, 52, 38, 31, 24, 17 and 12 kDa were used (GE Healthcare, Piscataway, NJ, USA). The gels were inspected visually and analyzed densitometrically using Phoretix 1D (Nonlinear Dynamics, Newcastle on Tyne, UK) to assess the size of the individual protein bands. For N-terminal sequence analysis of IgG1- and IgG3-derived products generated by gingipain K, reaction mixtures were subjected to SDS-PAGE followed by blotting onto a PVDF membrane. Protein bands stained with Coomassie were excised and subjected to automatic Edman degradation with an Applied Biosystems 4760A gas-phase sequenator (Applied Biosystems, Inc., Foster City, CA, USA) using the program designated by the manufacturer.
FPLC assay to measure IgG-degrading activity
Enzyme kinetic parameters for gingipain K activity were assessed by a method described elsewhere (29), with some modifications. In brief, the concentration of the active enzyme was determined by active site titration with Cbz-Phe-Lys-CH2OCO-2,4,6-Me3-Ph (9). Gingipain K (1.6 μM final concentration) was added to various concentrations of repurified human IgG (4–130 μM) in 0.1 M Tris buffer (pH 8.0) with 1 mM EDTA and 2 mM l-cysteine in final reaction volumes of 25 μl. The gingipain K reactions were stopped after 8 min with TLCK at 1 mM final concentration. Hydrolysis reactions (25 μl) were applied automatically with an autosampler (ÄKTA A900; Amersham-Pharmacia Biosciences, Uppsala, Sweden) to a size-exclusion chromatography column (Superdex 75 3.2/30; Amersham-Pharmacia) controlled by a FPLC system (ÄKTA purifier) run by Unicorn 4.12, and separation of the hydrolysis products was carried out in 0.1 M Tris buffer (pH 8.0) at 0.05 ml/min. The peak heights for each reaction were measured at 1.12 ml, corresponding to elution of the Fc hydrolysis product, and background corrected due to peak tailing of uncleaved IgG. Enzymatic rates were determined by dividing the peak heights with the reaction time, giving a velocity unit of milli-absorbance units per minute. Plots of v vs. [E] and [P] vs. time from results of preliminary experiments defined the limits of linearity of the assay.
Enzyme kinetic calorimetry studies
Enzyme kinetic studies were performed using a VP-ITC microcalorimetry instrument (Microcal, Northampton, MA, USA), as described elsewhere (32–34). All reagents were exhaustively dialyzed against 0.1 M Tris buffer (pH 8.0) at 26°C, I = 154 μM adjusted with NaCl, 2 mM l-cysteine, or 1 mM DTT. Human IgG in large excess (740–904 μM; estimated by absorption at 280 nm using A280, 0.1% = 1.37) was titrated into a gingipain K solution (varying concentrations were used: 50 pM to 0.65 μM, determined by active site titration as above; ref. 9), hence creating pseudo-first-order reaction conditions. Steady-state reactions were obtained before next titration with the substrate. Volumes of 10 μl IgG were titrated into the cell under mechanical stirring (310 rpm) with varying spacing times, 80–300 s, to optimize the assay. The substrate range analyzed was 4–120 μM. ΔHapparent of the hydrolysis of human IgG by gingipain K was estimated to an average value of 6409 cal/mol from repeated ITC experiments (data not shown). All data were visually inspected, and analysis was performed using the Origin software package (provided with the Microcal instrument) with the exception that the substrate concentration after each injection was manually corrected. Because of a nonhorizontal preinjection baseline, linear best fits were calculated for the baseline (0–300 s) and the steady-state baseline 30 s before next injection, which was used to estimate dQ/dt. The velocities were estimated as dQ/dt divided by the reaction volume and ΔHapparent.
Enzyme kinetic parameters
For gingipain K, Vmax was estimated from both the velocity curve obtained from the FPLC assay and that of the ITC assay, and the results were used for Hill plots (log (v/Vmax−v) against log [S]), for [S] in the range of 4.7–28.1 μM (FPLC assay) and 22.2–68.0 (ITC assay), from which K′ values, as well as the Hill coefficient, were estimated. In brief, two straight lines were fitted to the data points of the FPLC assay and the ITC assay, respectively. The x-intercept equals log K′, and the slope of the fitted curve designates the Hill coefficient, reflecting cooperativity.
Immunoblotting of IgG in human blood
Fresh fasted human blood plasma, undiluted and diluted to 1 and 10% in D-PBS alone, or with 1 mM EDTA, or 1 mM Pefabloc (Roche Diagnostics, Mannheim, Germany), was incubated for 40 min at 37°C with gingipain K (0.39 μM final concentration) preactivated in 1 mM DTT. The hydrolysis products were separated by SDS-PAGE and subjected to Western blot analysis using a human CH2 heavy-chain-specific primary antibody (Dako, Glostrup, Denmark) and developed by ECL Plus (Amersham Biosciences, Piscataway, NJ, USA).
Sampling and analysis of crevicular fluid and analysis
Crevicular washes were obtained using a previously described method (35) from 6 patients with chronic periodontitis (mean age 59.2±7.7 yr) with average pocket depth (PD) of 5.36 ± 1.59 mm, attachment loss (CAL) of 6.32 ± 1.21 mm, and 78.6 ± 20.1% positive sites with bleeding on probing (BoP). For analysis of P. gingivalis presence, DNA was extracted from 5 μl of crevicular fluid using the High Pure PCR template preparation kit (Roche Diagnostics), according to the manufacturer's recommendations. Real-time PCR was carried out using a RotorGene 2000 (Corbett Research, Sydney, NSW, Australia). Primers specific for 16S rDNA from P. gingivalis were designed as described by Ashimoto et al. (36). PCR amplification was carried out as described earlier (30). Determination of cleaved IgG1 and IgG2 in GCF samples was performed by Western blot analysis using mouse mAb to human IgG1 and IgG2 (Zymed Labs, San Francisco, CA, USA).
Phagocytosis assay
The phagocytosis assay used has been described before (37). Briefly, after collecting venous blood samples from subjects with a known high antibody titer against P. gingivalis, polymorphonuclear neutrophils (PMNs) were isolated by using dextran sedimentation and suspended in Hanks' balanced salt solution (HBSS). The serum samples were heat inactivated to exclude an effect of the complement system on bacteria phagocytosis. One part of the serum was mixed with gingipain K (which had been preincubated in activation buffer; 0.2 M Tris, pH 7.6, with 1 mM CaCl2 and 10 mM cysteine hydrochloride), and to the other part of the serum, an equal volume of activation buffer was added. After an incubation time of 1 h at 37°C, the gingipain-treated and control sera were added to P. gingivalis M5-1-2 (a clinical isolate) for 10 min to let bacteria be opsonized. The samples were then centrifuged, and the bacterial pellet was suspended in HBSS. PMNs and bacterial suspensions were mixed to give a cellular ratio of PMNs to bacteria of 1:20. The mixture was then incubated for up to 30 min in an atmosphere of 5% CO2 at 37°C. At 5 and 30 min, the suspension was centrifuged (400 g, 4 min, 20°C). Aliquots of supernatants were plated onto Schaedler's agar to enumerate the colony-forming units as the number of viable extracellular bacteria. Bacterial suspensions in HBSS kept in the same conditions were also plated, and the number of colony-forming units was taken as 100% of P. gingivalis survival. All experiments were made at least in quadruplicate.
RESULTS
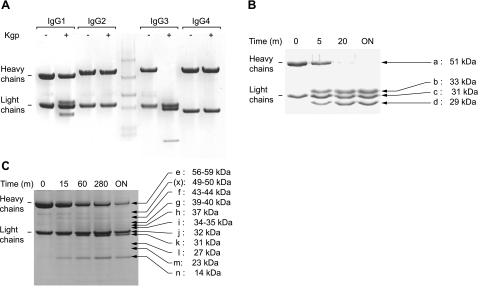
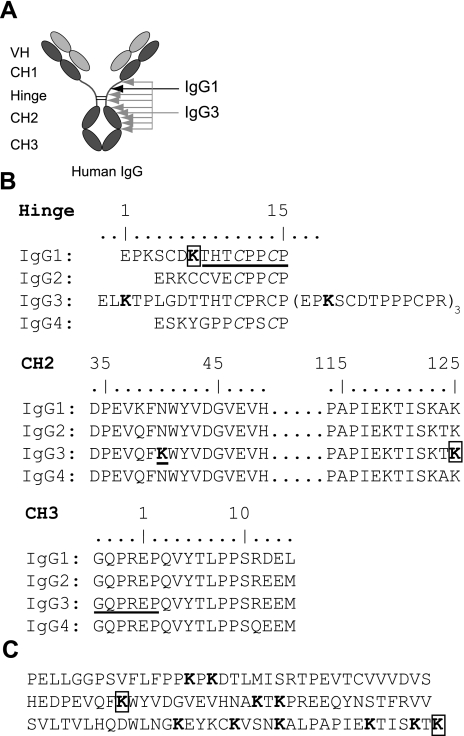
Since the ability of P. gingivalis to evade elimination by the host immune system may be partially due to degradation of Ig (28), we evaluated the role of gingipains for IgG cleavage. First, we tested whether gingipains can hydrolyze human IgG and found that under the conditions used (10 nM enzyme concentration, 4 h incubation at 37°C) only gingipain K was able to cleave this substrate. Interestingly, gingipain K hydrolyzed the heavy chains of IgG1 and IgG3, but showed no activity against IgG2 or IgG4, according to SDS-PAGE analysis (Fig. 1A). To identify any intermediate hydrolysis products, we performed a time course degradation of the two identified IgG substrates (Fig. 1B, C). IgG1 was cleaved in a single hydrolysis step of the heavy chain, generating two apparently stable hydrolysis products, seen as protein bands b and d in Fig. 1B, in addition to the light chain (band c) and uncleaved heavy chain (band a). N-terminal sequencing of the IgG1 hydrolysis products revealed a cleavage site in the hinge region after Lys6 (IMGT numbering; ref. 38), generating 2 Fab fragments and a Fc fragment of the antibody (Fig. 2). The Lys6 residue is uniquely present in the hinge region of IgG1, as apparent from alignment of sequences of the 4 IgG subclasses (Fig. 2B). Cleavage of the Lys-Thr peptide bond agrees with the subsite preference of gingipain K for a lysine residue in the P1 position (10). During the time course of IgG3 cleavage, 7 intermediate hydrolysis products were transiently observed (Fig. 1C; bands e–n), of which two were the uncleaved heavy chain and the light chain (Fig. 1C; bands e and j, respectively). Attempts to identify the cleavage sites from the hydrolysis products only succeeded for protein band n with a molecular mass of 14 kDa. The determined N-terminal sequence identified this product as the CH3 domain, released by hydrolysis after Lys125 (IMGT-numbering) and apparently fairly resistant to further proteolysis (Fig. 1A, C). Data from mass spectrometry and N-terminal sequencing of the other hydrolysis products were inconclusive (not shown). Consequently, possible cleavage sites in the CH2 region had to be assigned on the basis of molecular mass estimation from densitometric scanning of gels and sequence alignment analysis. Intriguingly, a unique lysine residue in the IgG3 heavy chain at position 40 in the CH2 region (Fig. 2B) was identified, which could well be a target for gingipain K and the primary cleavage site in IgG3. In support, a hypothetical cleavage at this point should give a heavy-chain fragment of 39–40 kDa, corresponding well to band g (Fig. 1C). As a result of the proposed primary cleavage, a series of lysine residues seen in a repetitive pattern in the degenerated CH2 region and hinge region (Fig. 2C) could be exposed and accessible to gingipain K hydrolysis, which should generate a mixture of degradation products. Experimental support for this hypothesis can be seen in bands h, i, k, l, m, and n (Fig. 1C), whereas the minor peptide fragments generated by the postulated cleavages are all <10 kDa (see Fig. 2C) and not visible in the gel.
Figure 1.
IgG subclass specificity of gingipain K. A) Human IgG subclasses 1–4 were incubated with gingipain K for 4 h at 37°C and visualized by SDS-PAGE under reducing conditions. From densitometric scanning, molecular masses of IgG heavy and light chains indicated in the figure were estimated to 51 and 31 kDa, respectively for IgG1, and 56 and 32 kDa for IgG3. Middle lane shows prestained marker proteins. B, C) The two IgG subclasses affected by gingipain K activity, IgG1 (B) and IgG3 (C), were further analyzed in time-course experiments from 5 min (m) to overnight (ON) incubation to investigate possible intermediate hydrolysis steps. Molecular masses given for indicated protein bands were estimated from densitometric scanning. Protein band denoted (x) is apparently a contaminant already present at time 0.
Figure 2.
Alignment of sequences for γ-chain regions of human IgG. To seek explanations for the subclass specificity of gingipain K, the individual domains (VH, CH1–3, and hinge) of IgG were aligned according to IMGT numbering of residues (39). Underscored residues specify N terminals obtained experimentally for gingipain K hydrolysis products (see Fig. 1). A) Schematic illustration of a human IgG molecule with its different regions and indications of cleavage sites by gingipain K in IgG1 (black arrow) and IgG3 (gray arrows; longest arrow indicates suggested primary cleavage site). B) Alignment of the hinge region of all four IgG subclasses, revealing that only IgG1 contains a lysine residue at the specific hydrolysis site, i.e., position 7 (in boldface and boxed), hence explaining the subclass specificity of gingipain K. Boldface indicates lysines that serve as putative gingipain K cleavage sites. The partial alignment of the CH2 region sequences of all four IgG subclasses shown below reveals that only IgG3 contains a lysine residue at position 40 (underscored). All four subclasses contain a lysine as the last residue of the CH2 region, after which hydrolysis also occurred in IgG3. The partial alignment of CH3 region sequences shown at the bottom displays the N-terminal sequence obtained from hydrolysis product n (Fig. 1C). C) Full sequence of the CH2 sequence of IgG3; boldface indicates lysine residues that are theoretical gingipain K cleavage sites. Putative primary cleavage site and an experimentally confirmed cleavage site, at positions 40 and 125, respectively, are boxed.
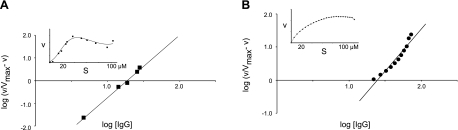
To investigate the in vitro cleavage of IgG subclass 1 and 3 by gingipain K in more detail, we estimated the enzyme kinetic parameters, Vmax and Km, for its reaction with native human IgG. To this end, we used both a discontinuous FPLC assay, where the hydrolysis products were separated from uncleaved IgG and an indirect kinetic assay based on isothermal titration calorimetry (ITC). As a substrate, we used a mixture of all IgG subclasses, reflecting the natural composition of human plasma, in which the majority is IgG1 (60–71%) and IgG3 constitutes only a minor fraction (3–7%) of the γ globulins (39). The FPLC-based gingipain K assay resulted in a velocity curve that was basically sigmoidal in shape, with a drop in velocity at elevated substrate concentration, from which Vmax was estimated to 39 mAU/min (Fig. 3A, inset). The ITC-based assay also showed a velocity curve with a decrease in velocity at elevated substrate concentrations but with no obvious sigmoidal shape (Fig. 3B, inset). From this assay, Vmax was estimated as 94 nM/s. The shapes of the individual velocity curves from the two different assays indicate that IgG hydrolysis by gingipain K does not obey Michaelis-Menten kinetics. Hence, in kinetic terms, it is incorrect to use a Km value to characterize the reaction. Therefore, we denoted a pseudo-equilibrium constant K′. Consequently, we analyzed Hill plots (log (v/Vmax−v) against log [S]) using data sets from both assays to investigate for a putative cooperativity of the gingipain K catalyzed reaction of the IgG hydrolysis, as well as to estimate the K′ value (Fig. 3). Hill plots of data from the two assays resulted in similar slopes of the curves, yielding comparable values of the Hill coefficient of 2.8 and 2.4 for the FPLC and ITC-based assays, respectively. This finding strongly indicates a positive cooperative catalytic mechanism (34). Correspondingly, the Hill plots from the FPLC and ITC-based assays allowed determination of the K′ value, at 19 μM and 24 μM. After adjustment for the content of IgG1 and IgG3 in the Ig fraction (70%) the K′ values were calculated to be 13 μM and 17 μM, respectively.
Figure 3.
Enzyme kinetic investigations of the gingipain K interaction with human IgG. Whole human IgG was used as a substrate to reflect the overall effect of gingipain K hydrolysis. Since gingipain K does not display Michaelis-Menten enzyme kinetics, it is not correct to denote the pseudoequilibrium constant Km, and it is, therefore referred to as K′. A) Hill plot of kinetic data obtained by the FPLC-based assay used to estimate K′ and to analyze for positive cooperativity. Inset: velocity curve with raw data (substrate range, 4–130 μM). B) Hill plot of kinetic data obtained by the ITC-based assay used to analyze for cooperativity and the K′ value. Inset: corresponding velocity curve (substrate range, 4–120 μM).
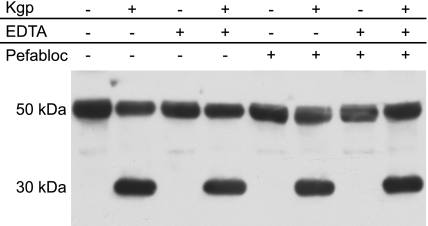
As all of the experiments above indicated a significant activity of gingipain K against IgG1 and IgG3, we sought to verify such an activity also under ex vivo conditions. We therefore tested the hydrolytic activity of gingipain K in human plasma and visualized IgG proteolysis by Western blot analysis using a CH2-specific antibody (Fig. 4). The enzyme efficiently cleaved the IgG heavy chain (∼50 kDa), releasing Fc fragments of ∼30 kDa. To eliminate the distant possibility that the IgG degradation was indirect, e.g., exerted by a gingipain-activated host metalloprotease or serine protease present in blood, we tested whether EDTA or PMSF had any effect on the observed IgG cleavage. Neither of these general inhibitors had an effect on IgG cleavage (Fig. 4). Together, these results indicate that gingipain K directly cleaves IgG in human plasma in a manner not significantly affected by the presence of other competing substrates, protease inhibitors, and other proteases in blood.
Figure 4.
Ex vivo activity of gingipain K in human plasma. Fresh human plasma (diluted to 10% in physiological buffer to allow electrophoretic analysis) was incubated with gingipain K for 40 min at 37°C. Incubations in the presence of EDTA and/or PMSF were included as indicated, to investigate for possible activation of metalloprotease or serine proteases with effect on IgG hydrolysis. Hydrolysis products were separated by SDS-PAGE under reducing conditions and visualized by Western blot analysis using an antibody directed against the CH2 region of human IgG. Hydrolyzed heavy chains are detected at 30 kDa (see Fig. 1).
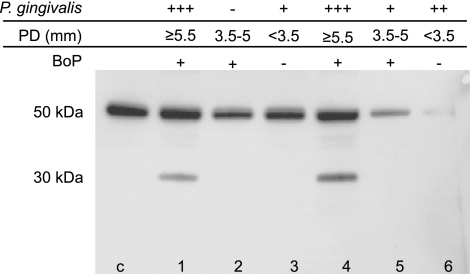
Because P. gingivalis is the major etiologic factor of chronic periodontitis, we investigated samples of GCF from patients suffering from periodontitis for the presence of IgG hydrolysis products, which may have arisen by gingipain K activity in vivo. Each periodontal site sampled for GCF was characterized with respect to the load of P. gingivalis, PD, and BoP. Western blot analysis using an antibody specific for IgG1 revealed significant amounts of IgG1 heavy-chain fragments of the molecular mass (∼30 kDa) corresponding to the Fc fragment in some GCF samples (lanes 1 and 4 in Fig. 5). The clinical sites from which these GCF specimens were sampled were characterized by severe P. gingivalis infection (>50,000 bacteria/sample), PD ≥ 5.5 mm, and BoP. In contrast, other GCF samples were devoid of the IgG degradation product, in correlation with significantly lower load of P. gingivalis and less severe clinical parameters of periodontitis at the sample site, including PD and BoP. Testing of IgG subclass 2 did not show any degradation products, and subclass 3 and 4 were inconclusive (data not shown). Collectively, the results presented above strongly suggest gingipain K involvement in IgG1 cleavage in vivo, which may coincide with progression of periodontitis caused by P. gingivalis infection.
Figure 5.
Analysis of IgG1 hydrolysis in patients suffering from periodontitis. GCF samples were collected from 6 patients with chronic periodontitis characterized with regard to PD and BoP (− or +). The samples were analyzed for the P. gingivalis load using qPCR (−, negative, <100 bacteria/sample; +, 100-1000 bacteria/sample; ++, 10,000–50,000 bacteria/sample; +++, >50,000 bacteria/sample) and subjected to SDS-PAGE followed by immunoblotting to identify possible IgG1 hydrolysis. Lane c, control containing human IgGs; lanes 1–6, samples from patients 1–6, respectively. Microbiological and clinical characteristics of each sample are outlined above the panel.
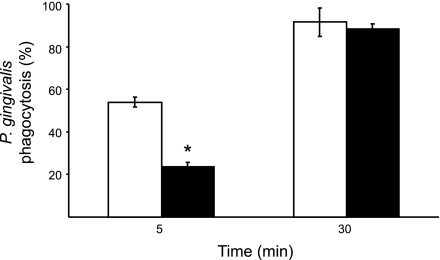
Finally, we investigated whether gingipain K can affect Ig opsonization-dependent phagocytosis and elimination of P. gingivalis by PMNs. To this end, PMNs and serum were obtained from peripheral blood of patients with periodontitis having high titers of antibodies against P. gingivalis. We found that after 5 min of incubation, bacteria opsonized with complement-inactivated serum treated with gingipain K were neutralized less efficiently (24±7% ingested) than P. gingivalis opsonized with the control serum. In the latter case, 54 ± 2% bacteria were cleared from medium (Fig. 6). After 30 min of coculturing of P. gingivalis with PMNs, the difference in bacterial elimination due to serum treatment with gingipain K disappeared, and practically all bacteria were removed from suspension by the PMNs regardless of serum treatment.
Figure 6.
Phagocytosis assay. Polymorphonuclear neutrophil (PMN) phagocytosis of the clinically separated P. gingivalis strain M5-1-2, after preincubation with complement-inactivated serum, pretreated, and not pretreated with gingipain K. The periodontitis patient serum used contained high titers of opsonizing antibodies against the bacterium. PMNs were mixed with bacteria at a 1:20 ratio for the indicated times, and remaining bacteria in the supernatants were counted. Bacteria mixed with gingipain K-treated serum (solid bars) resulted in a lower degree of phagocytosis by PMNs after 5 min as compared to the control (open bars).
DISCUSSION
IgG plays a key role in the immune response by recognizing invading pathogens and activating the classical pathway of the complement system that leads to efficient killing of invaders by neutrophils and macrophages. Seen from the perspective of the bacteria, it would be clearly beneficial to disable such an intimidating molecule. In the present work, we have shown that gingipain K is capable of compromising the effector functions of opsonizing human IgG of subclass 1 and 3. Apparently, gingipain K can recognize discrete sites in the heavy chain among the 4 IgG subclasses and cleaves after unique lysine residues in the hinge region of IgG1 and in the CH2 region of IgG3. Although IgG2 and IgG4 also contain Lys residues in their hinge regions, they are still not cleaved according to our results. The hinge region of IgG2 contains 4 disulfide bridges rather than 2 as in IgG1, which may confer more rigidity to the IgG2 hinge region. Furthermore, the hinge regions of both IgG2 and IgG4 are shorter than that in IgG1 by three residues (Fig. 2B). These subtle structural differences between the IgG subclasses likely reduce the accessibility of the lysine residues for gingipain K, clarifying the substrate discrimination. Previous reports describing the cleavage of other native, physiological substrates by gingipain K, e.g., human cystatin C, confirm a strict specificity of cleavage after lysine residues, but only when a Lys-Xaa substrate bond is exposed in a surface segment without secondary structure restrictions (40).
In attempt to understand the enzymatic properties of gingipain K as an enzyme specifically cleaving IgG1 and IgG3 and address the pathogenic significance of this activity in vivo, we used native IgG as substrate for enzyme kinetic studies. The velocity curves from two different assays displayed non-Michaelis-Menten kinetics, which indicates a nonclassical enzymatic mechanism of IgG cleavage by gingipain K. The decline in velocity of substrate hydrolysis at elevated substrate concentrations (after Vmax was reached) implies inhibition of the reaction by a substrate or a product. The same conclusion is apparent from the sigmoidal shape of the velocity curve evident in the kinetics of IgG hydrolysis determined by the FPLC-based assay. Apart from product or substrate inhibition, such shape can be due to the lack of an enzyme cofactor (41). Finally, Hill plot analysis suggests that a cooperativity-based mechanism is involved in IgG cleavage by gingipain K. This indicates involvement of multimers or exosites in the substrate binding (41, 42). It has to be kept in mind, however, that one explanation does not rule the other out, and a kinetically complex mechanism can be engaged in IgG hydrolysis by gingipain K. Clearly, characterization of this mechanism requires further detailed studies.
The Km value of an enzymatic reaction is a parameter that allows prediction of efficiency of the substrate turnover in vivo. In other words, the substrate turnover will occur with a significant rate only at substrate concentrations higher than the Km value for a given reaction (41). The concentration of IgG subclass 1 and 3 in blood is 38–83 μM (43), and the IgG concentration in GCF, the main place of gingipain K action, is variable but comparable to that in serum or a little lower; around 17 μM (44). The pseudoequilibrium constant for the reaction, K′, was here estimated to an average of 15 μM from the two independent assays. Hence, K′ corresponds well with the IgG concentration in vivo ([S]≥K′), suggesting an adjusted proteolytic system with capacity to increase its turnover in case the substrate concentration increases. Another way of regarding the catalytic efficiency is to calculate the specificity time of the reaction defined as K′/Vmax, which is estimated to 160 s from the results of the present study. This means that it would take <3 min for gingipain K to consume all of the substrate if the reaction occurs under first-order conditions (45). The first-order conditions at inflamed periodontitis sites are probably established by continuous influx of blood plasma carrying IgGs. Therefore, very efficient IgG cleavage by gingipain K may be of great importance for the bacterium to avoid opsonizing antibodies and could constitute an important pathogenic strategy to derail host antibacterial activity of joint innate (phagocytes) and acquired (antibodies) systems.
Human blood plasma and tissue secretions contain high concentrations of protease inhibitors effective against different catalytic enzyme classes, including cysteine proteases, which may protect the host against IgG-cleaving enzymes, such as gingipain K. Such proteins could interfere with a proteolytic activity derived from the pathogen and directed against IgG. From the results of our experiments, we concluded that neither endogenous inhibitors nor other proteins present in human plasma can interfere significantly with the gingipain K ability to cleave IgG. Also, on the basis of the finding that gingipains are able to activate host proteolytic systems (13), one can consider that the IgG cleavage in plasma is exerted by a host protease activated by gingipain K. This scenario is not likely to happen since the present results show that IgG cleavage in plasma is not inhibited by general metalloprotease or serine protease inhibitors, which strongly suggests that degradation is directly linked to the gingipain K activity.
IgG subclasses 1 and 3 constitute preferred targets to be neutralized by hydrolysis as a part of a defense strategy of P. gingivalis, since these two subclasses are the strongest complement activators and have the highest affinity for Fc receptors on phagocytic cells (46). Our investigation of samples from patients with severe chronic periodontitis showed a correlation between the presence of P. gingivalis and IgG1 cleavage, indicating that the reaction takes place in vivo. Interestingly, this correlation was only observed in deep pockets, at periodontal sites sensitive to BoP, which is an indicator of an ongoing inflammatory reaction. Our attempts to show also the presence of cleaved IgG3 failed most likely due to the low concentration of IgG3 in blood, being below the detection level of the applied Western blot technique. An alternative explanation may be that fragments of IgG3 generated by gingipain K was further degraded by other, host or bacterium-derived, proteases present in GCF.
IgG1 is the major IgG subclass in periodontitis (47, 48) with ability to promote phagocytosis of P. gingivalis by PMNs (49). From a pathogenesis point of view, it is important to underscore that opsonophagocytosis of P. gingivalis by PMNs is clearly hindered by gingipain K, according to our results. This effect is most likely because of degradation of IgG molecules attached to the bacteria. Although IgG3 degradation may contribute to the protective effect the specific cleavage of IgG1 at the hinge region, removing the effector Fab part of Ig, must be instrumental in prevention of P. gingivalis uptake by phagocytes. Fortunately, the clearance of bacteria by PMNs is not entirely dependent of phagocytic uptake but also on extracellular entrapment and killing of bacteria by neutrophil extracellular traps (NETs; refs. 50, 51). In our experiment, this latter strategy is likely involved in equally efficient clearance of P. gingivalis opsonized with control and gingipain K-treated serum after 30 min of incubation with PMNs. The finding that P. gingivalis can trigger NET formation (unpublished observations) lends credit to this explanation.
Patients with progressed periodontitis produce specific antibodies against the gingipains (48, 52, 53). Whether gingipain K could cleave such antigingipain K IgG clearly depends on their subclass as shown here. However, which domains and regions on the gingipain K molecule that are recognized by the antibodies may, obviously, also be important. It has been reported that isolated IgG from periodontitis patients had a low response against the catalytic domain of gingipain K (53). Hence, obstruction of its proteolytic activity is apparently not very successful, meaning that the enzyme can still protect the bacterium against opsonizing antibodies.
GCF is a very complex pathophysiological fluid since, in addition to a blood plasma infiltrate, it contains tissue exudate and bacterial products (54). Although bacteria-derived enzyme activity, including gingipain activity, can be determined in GCF (55), the potential correlation with disease status is obscured by a myriad of interactions in the complex environment of the periodontal pocket. This complexity also makes it difficult to trace evidence of a direct action of bacterial enzymes on host proteins. In this respect, identification of IgG1 fragments generated by gingipain activity opens an interesting venue for future investigations of the importance of P. gingivalis in development and progression of chronic periodontitis.
In summary, gingipain K seems to be a sophisticated virulence factor that functions in a hostile environment such as human blood and knocks down with a surgical precision host defenses dependent on opsonizing IgG. This activity is likely responsible for the notably low protective effect of the humoral response to P. gingivalis antigens in chronic periodontitis.
Acknowledgments
The authors are grateful to Dr. Peter Westh (Roskilde University, Roskilde, Denmark) for use of his ITC equipment and helpful comments. J.P. acknowledges support from Foundation for Polish Science (TEAM project DPS/424-329/10).
This study was partially supported by funding from the Ministry of Science and Higher Education (Warsaw, Poland; 1642/B/P01/2008/35), the European Commission (FP7-HEALTH-2010-261460; Gums and Joints), and the U.S. National Institutes of Health (DE 09761), and by grants from the Swedish Research Council (14800, 14767, and 09915), the Commission of the European Communities, the Crafoord and A. Österberg foundations; the Royal Physiographic Society (Lund, Sweden), the Faculty of Medicine at Lund University, and Insamlingsstiftelsen at Umeå University. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (POIG.02.01.00-12-064/08; Molecular Biotechnology for Health).
REFERENCES
- 1. Jefferies R., Lund J., Pound J. D. (1998) IgG-Fc-mediated effector functions: Molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol. Rev. 163, 59–76 [DOI] [PubMed] [Google Scholar]
- 2. Potempa J., Pike R. N. (2009) Corruption of innate immunity by bacterial proteases. J. Innate Immun. 1, 70–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kilian M., Reinholdt J., Lomholt H., Poulsen K., Frandsen E. V. (1996) Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS 104, 321–338 [DOI] [PubMed] [Google Scholar]
- 4. Von Pawel-Rammingen U., Johansson B. P., Björck L. (2002) IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21, 1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Travis J., Potempa J. (2000) Bacterial proteinases as targets for the development of second-generation antibiotics, Biochim. Biophys. Acta 1477, 35–50 [DOI] [PubMed] [Google Scholar]
- 6. von Pawel-Rammingen U., Björck L. (2003) IdeS and SpeB: Immunoglobulin-degrading cysteine proteinases of Streptococcus pyogenes. Curr. Opin. Microbiol. 6, 50–55 [DOI] [PubMed] [Google Scholar]
- 7. Potempa J., Sroka A., Imamura T., Travis J. (2003) Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr. Protein Pept. Sci. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 8. Potempa J., Banbula A., Travis J. (2000) Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol. 2000 24, 153–192 [DOI] [PubMed] [Google Scholar]
- 9. Potempa J., Pike R., Travis J. (1997) Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol. Chem. 378, 223–230 [DOI] [PubMed] [Google Scholar]
- 10. Pike R., McGraw W., Potempa J., Travis J. (1994) Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 269, 406–411 [PubMed] [Google Scholar]
- 11. Rawlings N. D., Barrett A. J., Bateman A. (2010) MEROPS: The peptidase database. Nucleic Acids Res. 38, D227–D233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eichinger A., Beisel H. G., Jacob U., Huber R., Medrano F. J., Banbula A., Potempa J., Travis J., Bode W. (1999) Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 18, 5453–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imamura T., Travis J., Potempa J. (2003) The biphasic virulence activities of gingipains: activation and inactivation of host proteins. Curr. Protein Pept. Sci. 4, 443–450 [DOI] [PubMed] [Google Scholar]
- 14. Nilsson T., Carlsson J., Sundqvist G. (1985) Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect. Immun. 50, 467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo Y., Nguyen K. A., Potempa J. (2010) Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol. 2000 54, 15–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz J., Yang Q. B., Zhang P., Potempa J., Travis J., Michalek S. M., Balkovetz D. F. (2002) Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect. Immun. 70, 2512–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheets S. M., Potempa J., Travis J., Casiano C. A., Fletcher H. M. (2005) Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect. Immun. 73, 1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mezyk-Kopec R., Bzowska M., Potempa J., Bzowska M., Jura N., Sroka A., Black R. A., Bereta J. (2005) Inactivation of membrane tumor necrosis factor alpha by gingipains from Porphyromonas gingivalis. Infect. Immun. 73, 1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mikolajczyk-Pawlinska J., Travis J., Potempa J. (1998) Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. 440, 282–286 [DOI] [PubMed] [Google Scholar]
- 20. Dias I. H., Marshall L., Lambert P. A., Chapple I. L., Matthews J. B., Griffiths H. R. (2008) Gingipains from Porphyromonas gingivalis increase the chemotactic and respiratory burst-priming properties of the 77-amino-acid interleukin-8 variant. Infect. Immun. 76, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oleksy A., Banbula A., Bugno M., Travis J., Potempa J. (2002) Proteolysis of interleukin-6 receptor (IL-6R) by Porphyromonas gingivalis cysteine proteinases (gingipains) inhibits interleukin-6-mediated cell activation. Microb. Pathog. 32, 173–181 [DOI] [PubMed] [Google Scholar]
- 22. Inomata M., Ishihara Y., Matsuyama T., Imamura T., Maruyama I., Noguchi T., Matsushita K. (2009) Degradation of vascular endothelial thrombomodulin by arginine- and lysine-specific cysteine proteases from Porphyromonas gingivalis. J. Periodontol. 80, 1511–1517 [DOI] [PubMed] [Google Scholar]
- 23. Mahtout H., Chandad F., Rojo J. M., Grenier D. (2009) Porphyromonas gingivalis mediates the shedding and proteolysis of complement regulatory protein CD46 expressed by oral epithelial cells. Oral Microbiol. Immunol. 24, 396–400 [DOI] [PubMed] [Google Scholar]
- 24. Yasuhara R., Miyamoto Y., Takami M., Imamura T., Potempa J., Yoshimura K., Kamijo R. (2009) Lysine-specific gingipain promotes lipopolysaccharide- and active-vitamin D3-induced osteoclast differentiation by degrading osteoprotegerin. Biochem. J. 419, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guentsch A., Kramesberger M., Sroka A., Glockmann E., Pfister W., Potempa J., Jentsch H., Eick S. (2011) Comparison of gingival crevicular sampling methods in patients with severe chronic periodontitis. J. Periodontol. 82, 1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curtis M. A., Aduse Opoku J., Rangarajan M., Gallagher A., Sterne J. A., Reid C. R., Evans H. E., Samuelsson B. (2002) Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect. Immun. 70, 6968–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toh E. C., Dashper S. G., Huq N. L., Attard T. J., O'Brien-Simpson N. M., Chen Y. Y., Cross K. J., Stanton D. P., Paolini R. A., Reynolds E. C. (2011) Porphyromonas gingivalis cysteine proteinase inhibition by κ-casein peptides. Antimicrob. Agents Chemother. 55, 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundqvist G., Carlsson J., Herrmann B., Tärnvik A. (1985) Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J. Med. Microbiol. 19, 85–94 [DOI] [PubMed] [Google Scholar]
- 29. Vincents B., von Pawel-Rammingen U., Björck L., Abrahamson M. (2004) Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry 43, 15540–15549 [DOI] [PubMed] [Google Scholar]
- 30. Potempa J., Mikolajczyk-Pawlinska J., Brassell D., Nelson D., Thogersen I. B., Enghild J. J., Travis J. (1998) Comparative properties of two cysteine proteinases (gingipain Rs), the products of two related but individual genes of Porphyromonas gingivalis. J. Biol. Chem. 273, 21648–21657 [DOI] [PubMed] [Google Scholar]
- 31. Schägger H., von Jagow G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 32. Todd M. J., Gomez J. (2001) Enzyme kinetics determined using calorimetry: A general assay for enzyme activity? Anal. Biochem. 296, 179–187 [DOI] [PubMed] [Google Scholar]
- 33. Willemoes M., Sigurskjold B. W. (2002) Steady-state kinetics of the glutaminase reaction of CTP synthase from Lactococcus lactis. The role of the allosteric activator GTP incoupling between glutamine hydrolysis and CTP synthesis. Eur. J. Biochem. 269, 4772–4779 [DOI] [PubMed] [Google Scholar]
- 34. Vincents B., Vindebro R., Abrahamson M., von Pawel-Rammingen U. (2008) The human protease inhibitor cystatin C is an activating cofactor for the streptococcal cysteine protease IdeS. Chem. Biol. 15, 960–968 [DOI] [PubMed] [Google Scholar]
- 35. Guentsch A., Puklo M., Preshaw P. M., Glockman E., Pfister W., Potempa J., Eick S. (2009) Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Periodontal. Res. 44, 368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashimoto A., Chen C., Bakker I., Slots J. (1996) Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 11, 266–273 [DOI] [PubMed] [Google Scholar]
- 37. Puklo M., Guentsch A., Hiemstra P., Eick S., Potempa J. (2008) Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathalicidin LL-37 in innate immune response against periodontopathogenic bacteria. Oral Microbiol. Immunol. 23, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lefranc M.P., Giudicelli V., Ginestoux C., Jabado-Michaloud J., Folch G., Bellahchene F., Wu Y., Gemrot E., Brochet X., Lane J., Regnier L., Ehrenmann F., Lefranc G., Duroux P. (2009) IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 32, D160–D164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. French M. A. (1986) Serum subclasses in normal adults. Monogr. Allergy 19, 100–107 [PubMed] [Google Scholar]
- 40. Abrahamson M., Wikström M., Potempa J., Renvert S., Hall A. (1997) Modification of cystatin C activity by bacterial proteinases and neutrophil elastase in periodontitis. Mol. Pathol. 50, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Segel I. (1975) Enzyme Kinetics, John Wiley & Sons, New York [Google Scholar]
- 42. Cornish-Bowden A., Cardenas M. L. (1987) Cooperativity in monomeric enzymes. J. Theor. Biol. 124, 1–23 [DOI] [PubMed] [Google Scholar]
- 43. Vlug A., Nieuwenhuys E. J., van Eyk R. V., Geertzen H. G., van Houte A. J. (1994) Nephelometric measurement of human IgG subclasses and their reference ranges. Ann. Biol. Clin. 52, 561–567 [PubMed] [Google Scholar]
- 44. Wilton J. M., Bampton J. L., Hurst T. J., Caves J., Powell. J.R. (1993) Interleukin-1 beta and IgG subclass concentrations in gingival crevicular fluid from patients with adult periodontitis. Arch. Oral Biol. 38, 55–60 [DOI] [PubMed] [Google Scholar]
- 45. Cornish-Bowden A. (1987) The time dimension in steady-state kinetics: a simplified representation of control coefficeints. Biochem. Educ. 15, 144–147 [Google Scholar]
- 46. Janeway C. A., Travers P. (1994) Immunobiology: The Immune System in Health and Disease. Blackwell Scientific Publications, Oxford, UK [Google Scholar]
- 47. Takeuchi Y., Aramaki M., Nagasawa T., Umeda M., Oda S., Ishikawa I. (2006) Immunoglobulin G subclass antibody profiles in Porphyromonas gingivalis-associated aggressive and chronic periodontitis patients. Oral Microbiol. Immunol. 21, 314–318 [DOI] [PubMed] [Google Scholar]
- 48. Gibson F.C., III, Savelli J., van Dyke T. E., Genco C. A. (2005) Gingipain-specific IgG in the sera of patients with periodontal disease is necessary for opsonophagocytosis of Porphyromonas gingivalis. J. Periodontol. 76, 1629–1636 [DOI] [PubMed] [Google Scholar]
- 49. Kinane D. F., Lappin D. F., Koulouri O., Buckley A. (1999) Humoral immune responses in periodontal disease may have mucosal and systemic immune features. Clin. Exp. Immunol. 115, 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Medina E. (2009) Neutrophil extracellular traps: A strategic tactic to defeat pathogens with potential consequences for the host. J. Innate Immun. 1, 176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ermert D., Urban C. F., Laube B., Goosmann C., Zychlinsky A., Brinkmann V. (2009) Mouse neutrophil extracellular traps in microbial infections. J. Innate Immun. 1, 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Brien-Simpson N. M., Black C. L., Bhogal P. S., Cleal S. M., Slakeski N., Higgins T. J., Reynolds E. C. (2000) Serum immunoglobulin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect. Immun. 68, 2704–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Inagaki S., Ishihara K., Yasaki Y., Yamada S., Okuda K. (2003) Antibody responses of periodontitis patients to gingipains of Porphyromonas gingivalis. J. Periodontol. 74, 1432–1439 [DOI] [PubMed] [Google Scholar]
- 54. Lamster I. B., Ahlo J. K. (2007) Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann. N. Y. Acad. Sci. 1098, 216–229 [DOI] [PubMed] [Google Scholar]
- 55. Eley B. M., Cox S. W. (2003) Proteolytic and hydrolytic enzymes from putative periodontal pathogens: characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontol. 2000. 31, 105–124 [DOI] [PubMed] [Google Scholar]