Abstract
Cytochrome P450 (CYP) epoxygenases CYP2C8 and CYP2J2 generate epoxyeicosatrienoic acids (EETs) from arachidonic acid. Mice with expression of CYP2J2 in cardiomyocytes (αMHC-CYP2J2 Tr) or treated with synthetic EETs have increased functional recovery after ischemia/reperfusion (I/R); however, no studies have examined the role of cardiomyocyte- vs. endothelial-derived EETs or compared the effects of different CYP epoxygenase isoforms in the ischemic heart. We generated transgenic mice with increased endothelial EET biosynthesis (Tie2-CYP2C8 Tr and Tie2-CYP2J2 Tr) or EET hydrolysis (Tie2-sEH Tr). Compared to wild-type (WT), αMHC-CYP2J2 Tr hearts showed increased recovery of left ventricular developed pressure (LVDP) and decreased infarct size after I/R. In contrast, LVDP recovery and infarct size were unchanged in Tie2-CYP2J2 Tr and Tie2-sEH Tr hearts. Surprisingly, compared to WT, Tie2-CYP2C8 Tr hearts had significantly reduced LVDP recovery (from 21 to 14%) and increased infarct size after I/R (from 51 to 61%). Tie2-CYP2C8 Tr hearts also exhibited increased reactive oxygen species (ROS) generation, dihydroxyoctadecenoic acid (DiHOME) formation, and coronary resistance after I/R. ROS scavengers and CYP2C8 inhibition reversed the detrimental effects of CYP2C8 expression in Tie2-CYP2C8 Tr hearts. Treatment of WT hearts with 250 nM 9,10-DiHOME decreased LVDP recovery compared to vehicle (16 vs. 31%, respectively) and increased coronary resistance after I/R. These data demonstrate that increased ROS generation and enhanced DiHOME synthesis by endothelial CYP2C8 impair functional recovery and mask the beneficial effects of increased EET production following I/R.—Edin, M. L., Wang, Z. J., Bradbury, J. A., Graves, J. P., Lih, F. B., DeGraff, L. M., Foley, J. F., Torphy, R., Ronnekleiv, O. K., Tomer, K. B., Lee, C. R., Zeldin, D. C. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart.
Keywords: CYP2J2, EETs, reactive oxygen species, leukotoxin, Langendorff
Cytochrome p450 (CYP) epoxygenases, such as those from the CYP2C and CYP2J subfamilies, catalyze the NADPH-dependent oxidation of arachidonic acid to 4 regioisomeric epoxyeicosatrienoic acids (EETs; 5,6-, 8,9-, 11,12-, and 14,15-EET). EETs are synthesized in both hepatic and extrahepatic tissues and possess potent biological activities. For example, EETs induce vasodilation by activating vascular smooth muscle large-conductance, Ca2+-activated K+ channels (1, 2). EETs are mitogenic, antiapoptotic, and proangiogenic toward vascular endothelial cells through mechanisms that involve VEGF, EGF, PPAR, and MAPK pathway activation (3–5). EETs are also anti-inflammatory in endothelial cells through inhibition of NF-kB (6, 7). In addition, EETs regulate renal sodium absorption and activate L-type Ca2+ channels to induce inotropic effects in the heart (8, 9). These effects of EETs are largely diminished by EET hydrolysis to less active dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH or Ephx2) (10–12).
Numerous studies suggest beneficial effects of EETs on cardiac recovery following ischemia/reperfusion (I/R). Expression of CYP2J2 in cardiomyocytes leads to improved functional recovery and reduced infarct size after ischemia (13). Exogenous EET treatment, pharmacologic sEH inhibition, and targeted Ephx2 gene disruption also have cardioprotective effects, which are linked to activation of multiple signaling pathways. For example, EETs directly bind to and activate ATP-sensitive K+ channels (KATP) and activate MAPK, PI3K, PKA, and GSK-3β signaling pathways (14). EETs help preserve mitochondrial integrity and membrane potential during I/R (15). Evidence also indicates that EETs act through either direct or indirect mechanisms to activate G-protein-coupled-receptors (16, 17). P450-epoxygenases also generate epoxides of ω-3 fatty acids, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which have potent vasodilatory and cardioprotective effects (18). Ephx2-deficient mice were found to have enhanced b-type natriuretic peptide (BNP) signaling, and cardioprotection in Ephx2-null or EET-treated hearts can be blocked by BNP antagonists (19). Similarly, EETs have been shown to release Met-enkephalin, which can bind δ-opioid receptors to reduce infarct size after I/R in rat heart (20).
In addition to animal models that show potent vasodilatory, anti-inflammatory, and cardioprotective effects of EETs, epidemiological studies in humans suggest a link between CYP epoxygenase and/or EPHX2 genetic variation and altered cardiovascular disease risk. For example, carriers of the CYP2J2*7 (G-50T) polymorphism have decreased CYP2J2 expression and EET production and higher risk of coronary artery disease (21, 22). Likewise, the K55R polymorphism in EPHX2 causes increased sEH activity and EET hydrolysis and is associated with higher risk of developing coronary artery disease (23). In contrast, studies evaluating associations between functionally relevant CYP2C8 polymorphisms and cardiovascular disease risk outcomes are conflicting (22, 24, 25).
Some studies suggest potential detrimental effects of CYP activity in the heart. Indeed, inhibition of CYP2C by sulfaphenazole reduces infarct size after I/R in isolated rat hearts (26). In addition to generating EETs, CYP2Cs can generate large amounts of reactive oxygen species (ROS), which can induce mitochondrial dysfunction in cardiomyocytes and vasoconstriction in coronary arteries (27–29). CYP2C epoxidation of linoleic acid also generates leukotoxins or epoxyoctadecaenoic acids (EpOMEs), which are converted by sEH to toxic compounds called leukotoxin diols or dihydroxyoctadecaenoic acids (DiHOMEs) (30). Leukotoxins and leukotoxin diols have been reported to be cytotoxic (31, 32), vasoconstrictive (33), and cardiodepressive (34). Thus, it is unclear whether enhanced CYP2C activity during I/R is beneficial via increased EET production, or detrimental via increased production of ROS and DiHOMES.
Investigation of these issues through generation of tissue-specific CYP knockouts would be ideal, but prohibitive, as the mouse has 15 CYP2C and 8 CYP2J isoforms with overlapping metabolic profiles and tissue distributions (35). To begin to address these questions, we generated mice with Tie2-promoter-driven endothelial expression of human CYP2C8, human CYP2J2, and human sEH (Tie2-CYP2C8 Tr, Tie2-CYP2J2 Tr and Tie2-sEH Tr). We recently reported that Tie2-CYP2C8 Tr and Tie2-CYP2J2 Tr mice have increased endothelial-derived EETs, increased vasodilation in response to acetylcholine, and lower blood pressure following induction of hypertension (36). Thus, we hypothesized that mice with increased endothelial EETs (Tie2-CYP2C8 Tr and Tie2-CYP2J2 Tr) would have improved functional recovery after I/R, either through enhanced coronary vasodilation and improved perfusion of the heart or through protective effects of EETs on adjacent cardiomyocytes. Likewise, we hypothesized that mice with decreased endothelial EETs (Tie2-sEH Tr) would have reduced functional recovery after I/R. We compared hearts from these transgenic mice to wild-type (WT) control mice and mice with cardiomyocyte-specific expression of CYP2J2 (αMHC-CYP2J2 Tr) using the Langendorff isolated heart perfusion method. We found that endothelial-derived EETs do not offer protection against cardiac ischemia. Indeed, transgenic mice with endothelial CYP2J2 or sEH expression do not exhibit altered functional recovery after ischemia. In contrast, transgenic mice with myocardial CYP2J2 expression have improved functional recovery after ischemia (previously shown; ref. 13) without effects on vascular function. Surprisingly, the Tie2-CYP2C8 Tr hearts had significantly worse functional recovery and increased infarct size after I/R via a mechanism that involves increased production of ROS and leukotoxin diols.
MATERIALS AND METHODS
Animals
Mice with the cardiomyocyte-specific expression of CYP2J2 (αMHC-CYP2J2 Tr) on a C57BL/6 (area R16) genetic background (Charles River, Wilmington, MA, USA) have been described previously (13). Mice with endothelial expression of CYP2C8 (Tie2-CYP2J8 Tr) and CYP2J2 (Tie2-CYP2J2 Tr) on C57BL/6 (C57BL/6Crl-R16) genetic background have been described recently (36). Transgenic mice that express sEH in endothelial cells (Tie2-sEH Tr) were developed on a C57BL/6 genetic background and characterized as part of this study. The human sEH cDNA (NM001979) was amplified with primers (Fwd: 5′-TAAGCTTGGCTGCAGACCCGCCGCCATGACG-3′, Rev: 5′-TAGCGGCCGCTCTACATCTTTGAGACCACC-3′) and subcloned downstream of the murine Tie2 promoter and upstream of the Tie2 full enhancer sequences (generously provided by Dr. Tom Sato, University of Texas Southwestern Medical Center, Dallas, TX, USA) as described previously (36, 37). Linearized constructs were excised from the vector backbone, agarose gel purified, microinjected into pronuclei of single cell C57BL/6J mouse embryos, and implanted into pseudopregnant mice. Hemizygous founder pups were identified via PCR genotyping of genomic DNA. All procedures and experiments used male and female mice aged 3–6 mo, weighing 25–35 g, in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the National Institute for Environmental Health Sciences Animal Care and Use Committee. All experiments included ≥3 independent founder lines of each genotype; results from the independent founder lines were pooled, since no significant differences were observed in the experimental outcomes by founder line. WT littermates were used as controls.
Real-time PCR
Expression of human sEH in mouse tissues was confirmed after RNA purification from mouse organs with the RNAeasy Mini kit (Qiagen, Valencia, CA, USA), conversion to cDNA with High Capacity cDNA Archive kit, (Applied Biosystems, Foster City, CA, USA) and real-time PCR using TaqMan probes to murine GAPDH (Mm 99999915_g1) and human EPHX2 (Hs 00157403_m1) (Applied Biosystems).
Immunohistochemical analysis
Wild-type (WT) and transgenic mouse tissues were prepared and stained as described previously (36). Briefly, whole heart tissues were fixed in 10% neutral buffered formalin and embedded in paraffin, and serial sections were immunohistochemically stained. Tie2-CYP2J2 Tr heart sections were incubated with anti-CYP2J2pep1 (38) or normal rabbit serum (1:50 dilution); Tie2-CYP2C8 Tr heart sections were incubated with anti-CYP2C8 (a generous gift from Dr. Joyce Goldstein, U.S. National Institutes of Health/National Institute for Environmental Health Sciences, Research Triangle Park, NC, USA) or normal rabbit serum (1:100 dilution); and Tie2-sEH Tr heart sections were incubated with anti-human sEH (a generous gift from Dr. Bruce Hammock, University of California, Davis, CA, USA) or normal rabbit serum (1:500 dilution). All sections were incubated with donkey anti-rabbit secondary antibody (1:500 dilution; Vector Laboratories, Burlingame, CA, USA) and visualized using liquid 3,3-diaminobenzidine solution (Dako, Carpinteria, CA, USA). Slides were counterstained with Harris hematoxylin (Harelco, Gibbston, NJ, USA), and image capture using ImageScope software (Aperio Technologies, Vista, CA, USA) was performed as described by the supplier.
In situ hybridization
Fragments of the human CYP2J2 and CYP2C8 cDNAs were PCR amplified with specific forward (CYP2J2: 5′-ACCGAGACAACTTGGACAAC-3′; CYP2C8: 5′-AATCCTCGGGACTTTATCG-3′) and reverse primers (CYP2J2: 5′-ATGCCCGCTTTCCTATTGAG-3′; CYP2C8: 5′-GGACAAGGTCACTGTATCTC-3′) (36). The resulting 406-bp (CYP2J2) and 314-bp (CYP2C8) products were then subcloned into the intermediate pCR2.1-TOPO vector using the TOPO TA cloning system (Invitrogen, Carlsbad, CA, USA) per the manufacturer's instructions. Hearts were excised, sliced into 3 coronal blocks, fixed in 4% paraformaldehyde in Sorensen buffer (0.03 M, pH 7.4), rinsed overnight in 20% buffered-sucrose solution (pH 7.4), embedded in O.C.T. (Sakura Finetek, Torrance, CA, USA), and frozen in isopentane at −55°C. Sections (20 μm) were cut on a cryostat, thaw-mounted onto Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA), and stored at −80°C. Slides were postfixed in 4% paraformaldehyde in Sorensen's phosphate buffer (0.03 M, pH 7.4) for 20 min, rinsed, and treated with proteinase K (1.0 μg/ml) for 4 min at room temperature.
Perfused tissue was treated with proteinase K (1.0 or 1.6 μg/ml) for 12 to 15 min. Sections were then treated with 0.1 M triethanolamine for 3 min, followed by 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min. Sections were prehybridized for 1 h at 58°C with hybridization buffer [50% formamide; 1× Denhardt's solution; 10% dextran sulfate; 100 μM dithiothreitol (DTT); 200 mM sodium chloride; 10 mM Tris-HCl, pH 8.0; 1 mM EDTA, pH 8.0; and 125 μg/ml tRNA] and then rinsed in 2× SSC buffer. The 35S-labeled antisense and sense riboprobes were heat denatured, diluted with hybridization buffer, and used at a final concentration of 2 × 104 cpm/μl. Subsequently, the sections were covered with glass coverslips, sealed, and hybridized in a moist chamber for 18 h at 58°C. After hybridization, the slides were washed in 2× SSC buffer at 58°C 4 times, reacted with RNase (20 μg/ml) for 30 min at 37°C, and washed in decreasing concentrations of SSC (2×, 1×, 0.5×, 0.25×) at 58°C with 3 changes of each solution. Finally, the slides were washed in 0.1× SSC with 1 mM DTT for 45 min at 58°C. The slides were dehydrated in 50, 80, and 90% ethanol with 300 mM ammonium acetate followed by 100% ethanol for 2 min; placed side by side on a flat surface together with autoradiographic 14C microscales; and exposed to Hyperfilm-βmax (Amersham Biosciences, Little Chalfont, UK) for 6 d at 4°C. Images were captured and processed exactly as described previously (36).
Isolated perfused hearts
Hearts were perfused in the Langendorff mode as described previously (13). Hearts were perfused with modified Krebs–Henseleit buffer (120 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 1.20 mM MgSO4, 11 mM glucose, and 1.8 mM CaCl2) bubbled with 95% air and 5% CO2 for a 40-min stabilization period, and subjected to 20 min of global no-flow ischemia, followed by 40 min of reperfusion. A balloon connected to a pressure transducer was inserted into the left ventricle to monitor cardiac function. For some experiments, hearts were stabilized for 20 min, then perfused with 4 mM N-acetyl-cysteine (NAC; Sigma, St. Louis, MO, USA), 50 U/ml superoxide dismutase (SOD; Sigma), 250 nM 9,10-DiHOME (Cayman Chemical, Ann Arbor, MI, USA), 100 μM trimethoprim (Sigma) or vehicle (water, water, ethanol, and DMSO, respectively) prior to 20 min of ischemia and 40 min of reperfusion. Recovery of contractile function was measured as left ventricular developed pressure (LVDP) at the end of reperfusion, expressed as a percentage of preischemic LVDP. Rate pressure product (RPP) was calculated as LVDP × heart rate.
TTC staining
To determine infarct size, hearts were reperfused for a total of 2 h after ischemia, incubated with a 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma) dissolved in modified Krebs–Henseleit buffer at 37°C for 10 min, then fixed in formalin and cut into thin cross-sectional slices. Images were scanned and analyzed using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA, USA). The area of infarction was quantified by a threshold measurement of stained (red, live tissue) and unstained (white, necrotic) regions as described previously (13).
Coronary resistance determination
A separate group of cannulated and perfused hearts (without balloon catheter insertion) was used to evaluate coronary resistance. After 40 min of stabilization and 20 min of ischemia (or mock ischemia), heart perfusate volume was determined at each minute. Coronary resistance (cmH2O·g·min·ml−1) was calculated from constant pressure (100 cmH2O), flow rate (ml/min), and heart weight (g) measurements, as described previously (39).
LC/MS/MS oxylipid analysis
Plasma oxylipid levels were determined by liquid chromatography, tandem mass spectroscopy (LC/MS/MS) as described previously (36). Perfusates collected from the first 20 min of reperfusion were pooled, spiked with 30 ng PGE2-d4, 10,11-DiHN, and 10,11-EpHep (Cayman) as internal standards, mixed with 0.1 vol of 1% acetic acid in 50% methanol, and extracted by serial passage through Oasis HLB C18 3ml columns (Waters, Milford, MA, USA). Columns were washed twice with 0.1% acetic acid in 5% methanol, and eluted with methanol into glass tubes containing 6 μl of 30% glycerol in methanol. The methanol was then evaporated under a stream of nitrogen gas, and the dried tubes were frozen and stored at −80°C until analysis. Online liquid chromatography of extracted samples was performed with an Agilent 1200 Series capillary HPLC (Agilent Technologies, Santa Clara, CA, USA). Separations were achieved using a Phenomenex Luna C18(2) column (5 μm, 150×1 mm; Phenomenex, Torrance, CA, USA), which was held at 40°C. Mobile phase A was 85:15:0.1 water:acetonitrile:acetic acid. Mobile phase B was 70:30:0.1 acetonitrile:methanol:acetic acid. Gradient elution was used; mobile phase percentage B and flow rate were varied as follows: 0% B at 87.5 μl/min at 0 min, ramp from 0 to 2 min to 25% B at 87.5 μl/min, ramp from 2 to 5 min to 50% B at 60 μl/min, ramp from 5 to 23 min to 92.5% B at 60 μl/min, ramp from 23 to 23.5 min to 100% B at 87.5 μl/min, hold from 23.5 to 29 min at 100% B, ramp from 29 to 29.6 min down to 0% B, and hold 0% B to 33 min. Samples were solvated in 100 μl of 40% ethanol. The injection volume was 20 μl. Samples were analyzed in triplicate. Negative ion electrospray ionization tandem mass spectrometry was used for detection. Analyses were performed on an MDS Sciex API 3000 equipped with a TurboIonSpray source (Applied Biosystems). Turbo desolvation gas was heated to 275°C at a flow rate of 5.75 L/min.
Statistical analysis
Values are expressed as means ± se. Two-tailed analyses were performed using ANOVA, repeated measures ANOVA and/or Student's t test using Systat software (Systat Inc., Chicago, IL, USA). Values of P < 0.05 were considered significantly different.
RESULTS
Characterization of transgenic mouse lines
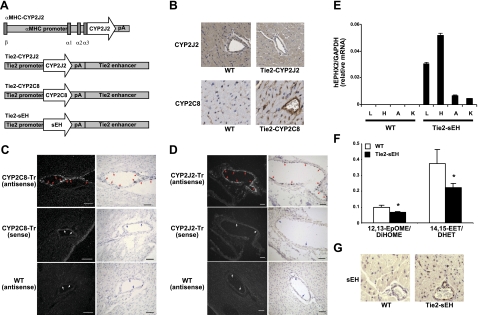
To investigate the differential roles of endothelial- vs. cardiomyocyte-derived EETs, we used several different transgenic mouse lines maintained on a C57BL6 genetic background, as shown in Fig. 1A. The αMHC-CYP2J2 Tr mice have cardiomyocyte-specific expression of CYP2J2, increased EET production, and improved cardiac functional recovery after I/R (13). The Tie2-CYP2J2 Tr mice and Tie2-CYP2C8 Tr mice have endothelial expression of CYP2J2 and CYP2C8, respectively, similar increases (∼30%) in circulating and endothelial levels of EETs, increased vasodilation in isolated vessels, and lower blood pressure following induction of hypertension (36). Immunohistochemical staining and in situ hybridization confirm the endothelial expression of human CYP2J2 and CYP2C8 in heart vessels (Fig. 1B, C). The Tie2-sEH Tr mice have expression of human sEH in several vascular tissues, lower plasma arachidonic acid and linoleic acid epoxide:diol ratios (12,13-EpOME:DiHOME and 14,15-EET:DHET), and endothelial expression of sEH in the heart by immunohistochemistry (Fig. 1E–G).
Figure 1.
Initial characterization of transgenic mice used in this study. A) Schematic representation of the constructs used to develop transgenic mice. The αMHC-CYP2J2 construct contains the αMHC promoter, CYP2J2 cDNA, and poly(A) sequence. The Tie2-CYP2J2, Tie2-CYP2C8, and Tie2-sEH constructs contain the identical Tie2 proximal promoter (2.1 kb), poly(A) sequence, and Tie2 enhancer (10 kb) surrounding human CYP2J2, CYP2C8 or sEH cDNA, respectively. B) Immunohistochemical staining showing endothelial expression of CYP2J2 and CYP2C8 in WT and transgenic hearts as indicated. C, D) In situ hybridization of CYP2C8 (C) and CYP2J2 (D). E) Real-time PCR of reverse-transcribed RNA prepared from various tissues from WT and Tie2-sEH transgenic mice. L, lung; H, heart; A, aorta; K, kidney. F) LC/MS/MS analysis of WT and Tie2-sEH plasma showing ratios of 12,13-EpOME:DiHOME and 14,15-EET:DHET. G) Immunohistochemical staining showing endothelial expression of sEH in Tie2-sEH transgenic hearts as indicated. Images are representative of ≥3 mice/group. Values are means ± se; n = 4 mice/group for E, 9–11 mice/group for F. *P < 0.05 vs. WT.
Cardiac functional recovery following I/R
Cardiac functional recovery in each of the 4 transgenic mouse lines and WT littermate controls was assessed in Langendorff isolated-perfused hearts during 40 min of baseline equilibration, 20 min of global ischemia and 40 min of reperfusion (Fig. 2A). Baseline characteristics, including heart rate, LVDP, RPP, dP/dtmax, dP/dtmin, time to ischemic contracture (TIC), and maximal ischemic contracture (Cmax) are shown in Table 1. No significant differences were found in baseline cardiac parameters between WT and any of the 4 transgenic lines. TIC was significantly shorter in αMHC-CYP2J2 Tr hearts compared to WT, as described previously (13).
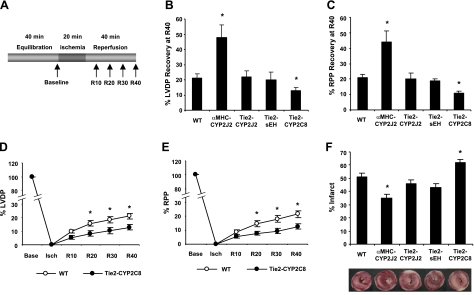
Figure 2.
Cardiac recovery in WT and transgenic hearts after global ischemia. A) Schematic representation of the Langendorff heart perfusion experiments. Hearts were allowed to equilibrate for 40 min, subjected to 20 min of global, no-flow ischemia, and then reperfused for 40 min. B) Percentage LVDP recovery at 40 min of reperfusion (R40) in WT and transgenic hearts. Data show improved recovery in αMHC-2J2 Tr hearts, no effect on recovery in Tie2-CYP2J2 Tr and Tie2-sEH Tr hearts, and diminished recovery in Tie2-CYP2C8 Tr hearts relative to WT. C) Percentage RPP recovery at R40 in WT and transgenic hearts. Data are similar to those shown in panel B. D) Time course of LVDP recovery in Tie2-CYP2C8 Tr hearts. E) Time course of RPP recovery in Tie2-CYP2C8 Tr hearts. F) Percentage infarct size and representative TTC stained images of WT and transgenic hearts. Values are means ± sem; n = 11–53 for B–E; n = 6–11 for F. *P < 0.05 vs. WT.
Table 1.
Cardiac parameters in WT and transgenic mice
| Parameter | WT | αMHC-CYP2J2 | Tie2-CYP2J2 | Tie2-CYP2C8 | Tie2-sEH |
|---|---|---|---|---|---|
| n | 53 | 11 | 17 | 23 | 11 |
| Heart rate (beats/min) | 352 ± 7 | 366 ± 11 | 372 ± 9 | 378 ± 10 | 345 ± 15 |
| LVDP (cmH2O) | 110 ± 2 | 122 ± 6 | 112 ± 3 | 111 ± 3 | 111 ± 5 |
| dP/dtmax (cmH2O/s) | 5601 ± 152 | 5590 ± 323 | 5475 ± 211 | 5461 ± 288 | 4821 ± 170 |
| dP/dtmin (cmH2O/s) | 3172 ± 107 | 3414 ± 235 | 3258 ± 104 | 3039 ± 159 | 3115 ± 180 |
| RPP (cmH2O/min) | 38011 ± 1044 | 44738 ± 2417 | 41482 ± 1456 | 41942 ± 1584 | 38540 ± 945 |
| TIC (min) | 6.73 ± 0.42 | 4.35 ± 0.9* | 6.73 ± 0.42 | 6.13 ± 0.42 | 6.28 ± 0.78 |
| Cmax (cmH2O) | 77 ± 4 | 98 ± 15 | 75 ± 5 | 71 ± 7 | 72 ± 7 |
Heart rate, LVDP, RPP, dP/dtmax and dP/dtmin were as measured at the end of 40 min of baseline perfusion. Time to ischemic contracture (TIC) and maximum contracture (Cmax) were measured during 20 min global, no-flow ischemia.
P < 0.05 vs. WT.
Similar to previous observations (13), cardiomyocyte-specific expression of CYP2J2 resulted in a significant increase in both LVDP and RPP recovery after I/R (Fig. 2B, C). In contrast, endothelial expression of CYP2J2 did not improve functional recovery significantly after I/R. Similarly, endothelial expression of sEH had little effect on functional recovery after I/R. These data suggest that, in contrast to the beneficial effects of cardiomyocyte-derived EETs or omega-3 epoxides, endothelial-derived epoxides have little effect on cardiac functional recovery after I/R. Surprisingly, endothelial expression of CYP2C8 resulted in significantly reduced functional recovery after I/R (Fig. 2B, C). Tie2-CYP2C8 Tr hearts showed significantly reduced LVDP and RPP beginning at 20 min of reperfusion and extending throughout the recovery period (Fig. 2D, E). Consistent with the changes in cardiac functional recovery, TTC staining revealed significantly reduced infarct size in αMHC-CYP2J2 Tr hearts, significantly increased infarct size in Tie2-CYP2C8 Tr hearts, and no significant change in infarct size in Tie2-CYP2J2 Tr and Tie2-sEH Tr hearts (Fig. 2F). Thus, endothelial expression of CYP2C8 has a detrimental effect in the heart, resulting in a 30% decrease in functional recovery and a 20% increase in infarct size compared to WT. Recovery of function in both WT and Tie2-CYP2C8 Tr hearts was improved by preconditioning (Supplemental Fig. S1); however, preconditioning did not completely abolish the differences between WT and Tie2-CYP2C8 Tr hearts.
Eicosanoid analysis of heart perfusates
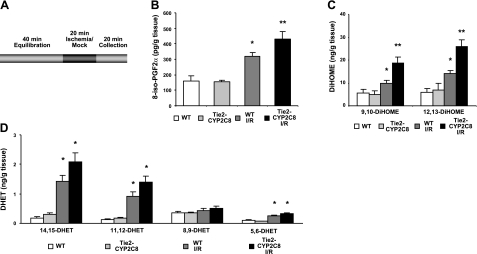
To determine whether eicosanoid levels were altered after I/R in Tie2-CYP2C8 Tr hearts, perfusates from the first 20 min of reperfusion after ischemia or mock ischemia were pooled, extracted, and assayed by LC-MS/MS (Fig. 3A). 8-Iso-PGF2α, a lipid produced under conditions of elevated ROS, is both a surrogate marker for ROS production and a potent coronary vasoconstrictor (40). Figure 3B shows that 8-isoPGF2α levels were similarly low in WT and Tie2-CYP2C8 Tr heart perfusates under basal conditions. WT hearts showed a significant increase in 8-isoPGF2α after I/R, an effect that was accentuated in Tie2-CYP2C8 Tr hearts (Fig. 3A). Levels of 9,10- and 12,13-DiHOMEs were also low in heart perfusates of WT and Tie2-CYP2C8 Tr mice under basal conditions (Fig. 3C). As in the case of 8-isoPGF2α, levels of both DiHOME regioisomers were increased to a significantly greater degree in Tie2-CYP2C8 Tr hearts than in WT hearts after I/R (Fig. 3C). In contrast, Tie2-CYP2J2 Tr hearts showed no differences in 8-isoPGF2α or DiHOMEs compared to WT (Supplemental Fig. S2). EET levels were below LC/MS/MS detection limits in heart perfusates under our experimental conditions. Levels of 14,15- and 11,12-DHET in heart perfusates were low under basal conditions and increased after I/R (Fig. 3D). There was a trend (P<0.1) toward increased 14,15- and 11,12-DHET in Tie2-CYP2C8 Tr heart perfusates compared to WT. Levels of 8,9-DHET were not increased significantly after I/R, and neither 5,6- nor 8,9-DHET was increased in Tie2-CYP2C8 Tr perfusates compared to WT. This finding is consistent with previous reports showing that CYP2C8 generates only 11,12- and 14,15-EETs (41, 42). Several other lipid analytes measured were found to be elevated after I/R in WT and Tie-CYP2C8 Tr heart perfusates. Prostaglandin (PG) D2, PGE2, PGF2α, 6-keto-PGF1α (stable prostacyclin metabolite), thromboxane B2, 5-, 8-, 11-, 12-, and 15-hydroxyeicosatetraenoic acid (HETE), and 9- and 13- hydroxyoctadecadienoic acid (HODE) were all increased after I/R; however, none were significantly different in Tie2-CYP2C8 Tr compared to WT heart perfusates (Supplemental Fig. S3). 19-HETE and 20-HETE levels were below the limits of detection. Together, this data demonstrates that the reduced cardiac functional recovery and increased infarction in Tie2-CYP2C8 Tr mice after I/R are accompanied by increased cardiac perfusate 8-isoPGF2α and DiHOME levels, whereas levels of other lipid metabolites in cardiac perfusates are not different from those in WT mice.
Figure 3.
LC/MS/MS analysis of lipids released in the first 20 min of reperfusion in WT and Tie2-CYP2C8 Tr hearts. A) Schematic of perfusate collection experiments. Hearts were allowed to equilibrate for 40 min, subjected to 20 min of mock ischemia or global, no-flow ischemia, and then reperfused. Heart perfusates for the first 20 min of reperfusion were pooled for lipid analysis. B–D) 8-Iso-PGF2α levels (B), DiHOME levels (C), and DHET levels (D) in heart perfusates were measured following ischemia or mock ischemia in WT and Tie2-CYP2C8 Tr mice. Values reflect picograms lipid per gram wet heart weight. Values are means ± se; n = 6 for nonischemic groups, n = 21–22 for I/R groups. *P < 0.05 vs. nonischemic WT; **P < 0.05 vs. WT I/R.
Coronary resistance in Tie2-CYP2C8 hearts
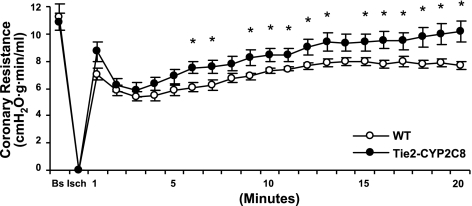
Flow rates were measured at baseline and throughout the first 20 min of reperfusion in both WT and Tie2-CYP2C8 Tr hearts. WT and Tie2-CYP2C8 hearts had similar flow rates at baseline (1.28±0.10 vs. 1.29±0.10 ml/min, respectively). Similarly, calculated coronary resistance was not altered in Tie2-CYP2C8 Tr hearts relative to WT prior to ischemia. However, Tie2-CYP2C8 Tr hearts showed consistently and significantly higher coronary resistance from 9 to 20 min of postischemic reperfusion (Fig. 4). These data demonstrate that endothelial CYP2C8 expression is accompanied by increased coronary resistance in mice while CYP2J2 expression resulted in no change in coronary resistance (Supplemental Fig. S2).
Figure 4.
Coronary resistance in WT and Tie2-CYP2C8 Tr hearts before and after I/R. WT and Tie2-CYP2C8 Tr hearts were equilibrated for 40 min (baseline), then subjected to 20 min global, no-flow ischemia, and 20 min reperfusion. Flow rates were measured for the first 20 min of reperfusion; coronary resistance is expressed as cmH2O · g · min · ml−1, as described in Materials and Methods. Values are means ± se, n = 19–21/group. *P < 0.05 vs. WT.
Role of CYP2C8, ROS and DiHOMEs in reduced recovery of Tie2-CYP2C8 hearts
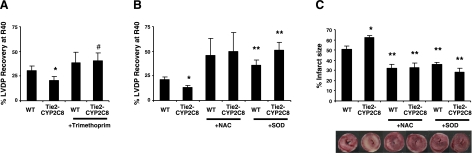
To support the hypothesis that differences in functional recovery in Tie2-CYP2C8 Tr hearts were due to CYP2C8 enzymatic function and not to compensatory changes, we treated hearts with the CYP2C8-selective inhibitor trimethoprim. Trimethoprim selectively inhibits CYP2C8, but not other human P450s at concentrations up to 100 μM (43). Trimethoprim may be less selective in mice, as it has not been tested against the murine P450s. Tie2-CYP2C8 Tr functional recovery remained significantly worse than WT in vehicle (DMSO)-treated hearts (P<0.05; Fig. 5A). Trimethoprim treatment had little effect on functional recovery in WT hearts (P=0.59) but led to significantly improved functional recovery in Tie2-CYP2C8 Tr hearts relative to vehicle (P<0.05). Thus, trimethoprim abolished the differences in functional recovery between Tie2-CYP2C8 Tr and WT mice.
Figure 5.
Effects of CYP2C8 and ROS inhibition on LVDP recovery in Tie2-CYP2C8 Tr and WT hearts. A) LVDP recovery at R40 after treatment of WT and Tie2-CYP2C8 hearts with vehicle (0.05% DMSO) or trimethoprim (100 μM). B) LVDP recovery at R40 after treatment of WT and Tie2-CYP2C8 hearts with NAC (4 μM), or SOD (50 U/ml). C) Percentage infarct size and representative TIC stained images in WT and Tie2-CYP2C8 Tr hearts after treatment with NAC or SOD. Both NAC and SOD significantly increase LVDP recovery, reduce infarct size, and abolish the differences between WT and Tie2-CYP2C8 Tr hearts. Values are means ± se, n = 6–12/group. *P < 0.05 vs. WT; **P < 0.05 vs. untreated of same genotype; #P < 0.05 vs. Tie2-CYP2C8 Tr vehicle treated.
To determine the role of increased ROS in the decreased functional recovery and increased infarction in Tie2-CYP2C8 Tr hearts after I/R, hearts were studied after treatment with the ROS scavenger NAC and the antioxidant enzyme SOD. Treatment with either NAC or SOD led to improved functional recovery and reduced infarction after I/R in WT mice (Fig. 5B, C). Notably, both NAC and SOD also abolished the differences in functional recovery and infarct size between Tie2-CYP2C8 Tr and WT mice. This finding suggests that increased ROS played a significant role in diminished recovery and increased infarction after I/R in Tie2-CYP2C8 Tr hearts.
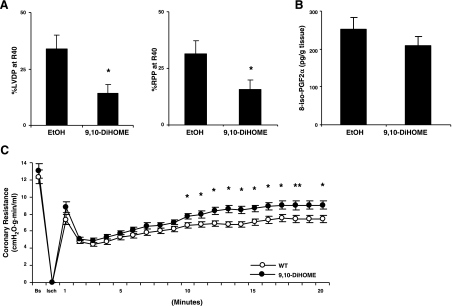
DiHOMEs have been reported to have several effects that could impair heart function, including increased ROS generation, cytotoxicity, impaired muscle contraction, and vasoconstriction (31–34). To determine the potential role of DiHOMEs in the decreased functional recovery and increased infarction in Tie2-CYP2C8 Tr hearts after I/R, WT hearts were studied after treatment with physiologically relevant concentrations (250 nM) of 9,10-DiHOME from 10 min prior to ischemia to the end of reperfusion. DiHOME treatment had no significant effect on baseline flow rates, coronary resistance, heart rate, LVDP, or RPP (data not shown). Notably, DiHOME treatment significantly impaired recovery of heart function after I/R (Fig. 6A). This impairment was not associated with increased ROS production as evidenced by unchanged levels of 8-isoPGF2α in heart perfusate (P=0.3; Fig. 6B). Likewise, DiHOME treatment was not accompanied by changes in levels of other lipid metabolites in heart perfusate (Supplemental Fig. S4). However, DiHOME treatment did increase coronary resistance from 10–20 min after reperfusion (Fig. 6C). Thus, increased production of DiHOMEs may contribute to the decreased functional recovery and increased coronary resistance in Tie2-CYP2C8 Tr hearts after I/R through a mechanism that does not involve increased ROS or changes in levels of other lipid metabolites.
Figure 6.
Effects of 9,10-DiHOME treatment on LVDP recovery, 8-iso-PGF2α levels and coronary resistance in WT hearts. WT hearts were isolated and perfused exactly as shown in Fig. 1A. Starting 10 min prior to ischemia and throughout recovery, hearts were treated with either vehicle (100% EtOH) or 250 nM 9,10-DiHOME. A) Treatment of WT hearts with 9,10-DiHOME results in reduced LVDP and RPP recovery of LVDP at R40 compared to vehicle; n = 9–10/group. B) Treatment of WT hearts with 9,10-DiHOME did not result in increased generation of 8-iso-PGF2α in heart perfusates compared to vehicle; n = 15/group. C) Treatment of WT hearts with 9,10-DiHOME results in increased coronary resistance compared to vehicle; n = 21/group. Values are means ± se. *P < 0.05 vs. vehicle.
DISCUSSION
CYP epoxygenases generate EETs, which have potent cardioprotective effects in the heart (13, 44). However, CYPs also generate many other fatty acid epoxides of poorly understood function. This study examined the role of endothelial vs. cardiomyocyte CYP epoxygenases in a mouse model of cardiac I/R. While cardiomyocyte expression of CYP2J2 led to improved functional recovery and decreased infarct size after ischemia, endothelial expression of CYP2J2 failed to improve these outcomes. Similarly, endothelial expression of sEH, the enzyme that is responsible for EET hydrolysis, had no effect on postischemic functional recovery or myocardial infarction. Thus, in contrast to cardiomyocyte-derived EETs, endothelial-derived EETs appear to have little or no effect on these outcomes in the postischemic heart.
Several factors may account for the striking differences in recovery of function and myocardial infarction between endothelial and cardiomyocyte expression of CYP2J2. This condition is likely to be an EET dosage effect. The αMHC promoter which was used to drive cardiomyocyte expression of CYP2J2 leads to higher cardiac DHET levels than the Tie2 promoter, which was used to drive endothelial expression of CYP2J2 (Supplemental Fig. S2). This finding may be related to the abundance of cardiomyocytes relative to endothelial cells in the heart, differences in promoter strength, or differences in arachidonic acid availability between cardiomyocytes and endothelial cells. However, both transgenic mice exhibit increased EET biosynthesis, albeit in a cell-specific fashion. Isolated endothelial cells from Tie2-CYP2J2 Tr mice generate twice as many EETs compared to WT (36), while cardiomyocytes from αMHC-CYP2J2 Tr mice generate ∼75% more EETs than WT (13). Plasma from Tie2-CYP2J2 Tr mice contains ∼40% more EETs than WT (36), whereas plasma levels are unchanged from WT in αMHC-CYP2J2 Tr mice (unpublished results).
EET compartmentalization could also be an issue. Endothelial-derived EETs may be hydrolyzed rapidly to less active DHETs by endothelial sEH, or they may not diffuse sufficiently to adjacent cardiomyocytes where they can affect ion channels, kinases, or mitochondrial function. Synthetic EET treatment can improve cardiac functional recovery in vivo (45) or ex vivo (44), with effects on cardiomyocyte channels and signaling. Thus, EETs have been shown to diffuse through endothelial cells and vessel walls to affect cardiomyocytes. However, the concentrations used in those studies (0.128 mg/kg, and 1 μM) are supraphysiologic and are well above those achieved in this study. Lastly, while Tie2 promoter-driven CYP2J2 expression is sufficient to enhance vasodilatory responses in isolated afferent arterioles and lower systemic blood pressure (36), endothelial CYP2J2 expression does not induce coronary vasodilation (Supplemental Fig. S2D) or affect cardiac recovery or myocardial infarction after I/R in isolated hearts. Conversely, while αMHC-CYP2J2 Tr hearts generated significantly more EETs than WT, this was not sufficient to induce coronary vasodilation. Significant vasodilation does occur early during reperfusion. The effects of additional EETs may have been masked by larger vasodilation induced by nitric oxide formation or cyclooxygenase metabolites.
In contrast to Tie2-CYP2J2 Tr hearts, Tie2-CYP2C8 Tr hearts showed significantly increased infarct size and decreased functional recovery after I/R. Differences can be found between the CYP2C8 and CYP2J2 enzymes, which may explain why CYP2C8 expression was detrimental whereas CYP2J2 expression was not. First, CYP2J2 has a low propensity for ROS generation (46), whereas CYP2Cs, including CYP2C8, have a high propensity for ROS production during metabolism (27). ROS production may be associated with cardiodepression and increased myocardial injury/infarction (28). Second, the two CYP epoxygenases differ in their selectivity for arachidonic acid vs. linoleic acid as substrates. CYP2J2 prefers arachidonic acid over linoleic acid (catalytic turnover: 160 pmol/nmol P450/min vs. 110 pmol/nmol P450/min, respectively; ref. 47), whereas CYP2C8 prefers linoleic acid over arachidonic acid (catalytic turnover: 300 pmol/nmol P450/min vs. 100 pmol/nmol P450/min, respectively; ref. 48). Thus, CYP2J2 is more likely to metabolize arachidonic acid to cardioprotective EETs, whereas CYP2C8 is more likely to metabolize linoleic acid to EpOMEs, which on conversion by sEH to DiHOMEs, have cytotoxic and cardiodepressive effects (33, 34, 49).
Tie2-CYP2C8 Tr hearts showed significantly increased production of 8-isoPGF2α, a marker of ROS generation. ROS is particularly damaging to the heart, as it causes oxidative modification of mitochondrial proteins, lipids and DNA, which results in mitochondrial dysfunction and further ROS production (28). ROS also reacts with nitric oxide to form peroxynitrite, which reduces nitric oxide availability, causes additional lipid peroxidation, and reduces functional recovery after ischemia (50, 51). Consistent with increased ROS production, Tie2-CYP2C8 Tr hearts also showed increased coronary resistance in the first 20 min after ischemia. Notably, treatment with NAC or SOD increased cardiac functional recovery and decreased infarct size after ischemia and abolished the differences between Tie2-CYP2C8 Tr and WT hearts. These data are consistent with previous reports where the CYP2C-selective inhibitor sulfaphenazole reduced ROS production, vascular dysfunction, and infarct size in rat hearts after ischemia (26, 29). Thus, CYP2C8-mediated increased endothelial ROS generation likely played a significant role in the decreased functional recovery after ischemia in Tie2-CYP2C8 Tr hearts.
In addition to increased ROS production, we observed significant elevation of several lipids, including prostaglandins and HETEs in WT heart perfusates after ischemia. However, the only lipids that were further increased in Tie2-CYP2C8 Tr hearts compared to WT were 9,10- and 12,13-DiHOME. At high levels (>10 μM), the EpOMEs or DiHOMEs have been reported to have a variety of effects, including cytotoxicity to renal tubules (31), stimulation of ROS production (32), increased contractility in rat hearts (52), cardiodepression in dogs (34), inhibition of papillary muscle contraction, and vasoconstriction of isolated arteries (33). To examine the role of DiHOMEs on functional outcomes after I/R, we treated WT hearts with physiologically relevant concentrations (250 nM) of 9,10-DiHOME. This dose is much lower than that used in previous experiments (31–34) but is similar to that which can be found during sepsis (53) or in burn victims (54). While we observed no significant changes in coronary resistance, heart rate, or LVDP prior to ischemia, 9,10-DiHOME significantly reduced functional recovery after ischemia. 9,10-DiHOME did not elicit this effect through increased ROS formation or changes in other lipid metabolites. 9,10-DiHOME did significantly increase coronary resistance after ischemia, an effect was slightly less pronounced than that seen in Tie2-CYP2C8 Tr hearts. No previous reports have investigated the role of DiHOMEs during cardiac I/R. The effects of DiHOMEs on coronary resistance and cardiac function may be synergistic with those of ROS on the postischemic heart.
Our data may offer mechanistic insight into the epidemiological studies, which have examined associations between genetic variation in CYP2J2 and CYP2C8 and cardiovascular disease risk in humans (55). The CYP2J2*7 polymorphism abolishes an SP1 promoter binding site and reduces CYP2J2 expression and activity. This polymorphism has been associated with higher risk of adverse cardiovascular events including myocardial infarction (21, 24). In contrast, CYP2C8 genetic variations leading to reduced catalytic efficiency of the enzyme have not been associated with adverse cardiovascular outcomes in multiple studies (22, 24, 25). Thus, while CYP2J2 may lead to cardiovascular protection via enhanced cardiac EET production, CYP2C8 may also produce deleterious factors, including ROS and DiHOMEs, which can offset these potential benefits. It should be noted that our study examined the effects of increased CYP epoxygenase expression/activity in mice, whereas the human polymorphism studies examined cardiovascular disease risk in individuals with lower CYP epoxygenase expression/activity. In addition, previous human studies have examined the role of CYP2C8 in the incidence of myocardial infarction, whereas our mouse studies suggest increased severity of myocardial infarction in those with increased CYP2C8 activity. Notably, our studies suggest the possibility that selective CYP2C8 inhibitors may represent a novel therapeutic approach to patients with ischemic heart disease. Indeed, selective CYP2C inhibition would be predicted to reduce both ROS generation and DiHOME formation, and thus protect hearts against the functional consequences of global ischemia. sEH inhibition, which has also been previously shown to protect hearts against I/R injury (44, 56), may act both by elevating cardiac EET levels and by suppressing conversion of cardiac EpOMEs to toxic DiHOMEs during I/R.
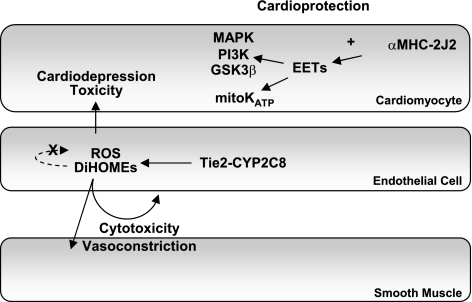
In summmary, our data show that CYP2J2 expression in cardiomyocytes is protective against I/R injury, whereas CYP2J2 expression in endothelial cells and increased endothelial expression of sEH have negligible effects. Endothelial CYP2C8 expression increases ROS generation and DiHOME formation, which in turn increase coronary vasoconstriction, reduce left ventricular functional recovery and increase infarct size after I/R. The detrimental effects of CYP2C8 expression appear to outweigh the potential benefits of increased EET biosynthesis in this model. Our work further defines the role of endothelial vs. cardiomyocyte-derived EETs in cardioprotection following I/R and begins to elucidate the complex mechanisms through which CYP2C expression may have conflicting effects in the ischemic myocardium (Fig. 7).
Figure 7.
Schematic diagram summarizing the effects of cardiomyocyte expression of CYP2J2 and endothelial expression of CYP2J2, CYP2C8, and sEH in the ischemic heart. Expression of CYP2J2 in cardiomyocytes (αMHC-CYP2J2 Tr) leads to cardioprotection via EET effects on MAPK, PI3K, GSK-3β and KATP channels, as described previously (13). In contrast, endothelial expression of CYP2J2 or sEH (Tie2-CYP2J2 Tr and Tie2-sEH Tr) does not lead to cardioprotection. Endothelial expression of CYP2C8 (Tie2-CYP2C8 Tr) leads to increased ROS and DiHOME production, which leads to reduced recovery after ischemia and increased infarction via enhanced coronary vasoconstriction and/or cardiodepressive mechanisms.
Supplementary Material
Acknowledgments
The authors thank Dr. Bruce Hammock (University of California, Davis, CA, USA) for providing the sEH antibody, Dr. Joyce Goldstein [U.S. National Institutes of Health (NIH)/National Institute for Environmental Health Sciences (NIEHS), Research Triangle Park, NC, USA] for providing the CYP2C8 antibody, and Dr. Tom Sato (University of Texas Southwestern Medical Center, Dallas, TX, USA) for providing the Tie2 promoter construct. The authors also thank staff at Xenogen Biosciences (Cranberry, NJ, USA) for assistance with pronuclear injections and staff in the NIEHS Laboratory of Experimental Pathology (Natasha Clayton and Tiwanda Masinde) for assistance with immunohistochemistry.
This work was supported with funds from the Intramural Research Program of the NIH, NIEHS, to K.B.T. (Z01 ES050167), D.C.Z. (Z01 ES025034), and C.R.L. (R01 GM088199).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Campbell W. B., Gebremedhin D., Pratt P. F., Harder D. R. (1996) Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 78, 415–423 [DOI] [PubMed] [Google Scholar]
- 2. Li P. L., Campbell W. B. (1997) Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ. Res. 80, 877–884 [DOI] [PubMed] [Google Scholar]
- 3. Michaelis U. R., Fisslthaler B., Barbosa-Sicard E., Falck J. R., Fleming I., Busse R. (2005) Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J. Cell Sci. 118, 5489–5498 [DOI] [PubMed] [Google Scholar]
- 4. Wang Y., Wei X., Xiao X., Hui R., Card J. W., Carey M. A., Wang D. W., Zeldin D. C. (2005) Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J. Pharmacol. Exp. Ther. 314, 522–532 [DOI] [PubMed] [Google Scholar]
- 5. Liu Y., Zhang Y., Schmelzer K., Lee T. S., Fang X., Zhu Y., Spector A. A., Gill S., Morisseau C., Hammock B. D., Shyy J. Y. (2005) The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. U. S. A. 102, 16747–16752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng Y., Edin M. L., Theken K. N., Schuck R. N., Flake G. P., Kannon M. A., Degraff L. M., Lih F. B., Foley J., Bradbury J. A., Graves J. P., Tomer K. B., Falck J. R., Zeldin D. C., Lee C. R. (2011) Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 25, 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285, 1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Satoh T., Cohen H. T., Katz A. I. (1993) Intracellular signaling in the regulation of renal Na-K-ATPase. II. Role of eicosanoids. J. Clin. Invest. 91, 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J., Capdevila J. H., Zeldin D. C., Rosenberg R. L. (1999) Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol. Pharmacol. 55, 288–295 [DOI] [PubMed] [Google Scholar]
- 10. Spector A. A., Norris A. W. (2007) Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. 292, C996–1012 [DOI] [PubMed] [Google Scholar]
- 11. Imig J. D., Hammock B. D. (2009) Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. 8, 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gross G. J., Nithipatikom K. (2009) Soluble epoxide hydrolase: a new target for cardioprotection. Curr. Opin. Investig. Drugs 10, 253–258 [PMC free article] [PubMed] [Google Scholar]
- 13. Seubert J., Yang B., Bradbury J. A., Graves J., Degraff L. M., Gabel S., Gooch R., Foley J., Newman J., Mao L., Rockman H. A., Hammock B. D., Murphy E., Zeldin D. C. (2004) Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circulation Res. 95, 506–514 [DOI] [PubMed] [Google Scholar]
- 14. Seubert J. M., Zeldin D. C., Nithipatikom K., Gross G. J. (2007) Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 82, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katragadda D., Batchu S. N., Cho W. J., Chaudhary K. R., Falck J. R., Seubert J. M. (2009) Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J. Mol. Cell. Cardiol. 46, 867–875 [DOI] [PubMed] [Google Scholar]
- 16. Wong P. Y., Lai P. S., Shen S. Y., Belosludtsev Y. Y., Falck J. R. (1997) Post-receptor signal transduction and regulation of 14(R),15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J. Lipid Mediat. Cell Signal. 16, 155–169 [DOI] [PubMed] [Google Scholar]
- 17. Chen J. K., Capdevila J., Harris R. C. (2002) Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 99, 6029–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konkel A., Schunck W. H. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta 1814, 210–222 [DOI] [PubMed] [Google Scholar]
- 19. Chaudhary K. R., Batchu S. N., Das D., Suresh M. R., Falck J. R., Graves J. P., Zeldin D. C., Seubert J. M. (2009) Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated improved post-ischaemic recovery of heart contractile function. Cardiovasc. Res. 83, 362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gross G. J., Baker J. E., Hsu A., Wu H. E., Falck J. R., Nithipatikom K. (2010) Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 298, H2201–H2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spiecker M., Darius H., Hankeln T., Soufi M., Sattler A. M., Schaefer J. R., Node K., Borgel J., Mugge A., Lindpaintner K., Huesing A., Maisch B., Zeldin D. C., Liao J. K. (2004) Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation 110, 2132–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee C. R., North K. E., Bray M. S., Couper D. J., Heiss G., Zeldin D. C. (2007) CYP2J2 and CYP2C8 polymorphisms and coronary heart disease risk: the Atherosclerosis Risk in Communities (ARIC) study. Pharmacogen. Genomics 17, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee C. R., North K. E., Bray M. S., Fornage M., Seubert J. M., Newman J. W., Hammock B. D., Couper D. J., Heiss G., Zeldin D. C. (2006) Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Hum. Mol. Genet. 15, 1640–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marciante K. D., Totah R. A., Heckbert S. R., Smith N. L., Lemaitre R. N., Lumley T., Rice K. M., Hindorff L. A., Bis J. C., Hartman B., Psaty B. M. (2008) Common variation in cytochrome P450 epoxygenase genes and the risk of incident nonfatal myocardial infarction and ischemic stroke. Pharmacogenet. Genom. 18, 535–543 [DOI] [PubMed] [Google Scholar]
- 25. Yasar U., Bennet A. M., Eliasson E., Lundgren S., Wiman B., De Faire U., Rane A. (2003) Allelic variants of cytochromes P450 2C modify the risk for acute myocardial infarction. Pharmacogenetics 13, 715–720 [DOI] [PubMed] [Google Scholar]
- 26. Granville D. J., Tashakkor B., Takeuchi C., Gustafsson A. B., Huang C., Sayen M. R., Wentworth P., Jr., Yeager M., Gottlieb R. A. (2004) Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc. Natl. Acad. Sci. U. S. A. 101, 1321–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleming I., Michaelis U. R., Bredenkotter D., Fisslthaler B., Dehghani F., Brandes R. P., Busse R. (2001) Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ. Res. 88, 44–51 [DOI] [PubMed] [Google Scholar]
- 28. Gustafsson A. B., Gottlieb R. A. (2008) Heart mitochondria: gates of life and death. Cardiovasc. Res. 77, 334–343 [DOI] [PubMed] [Google Scholar]
- 29. Hunter A. L., Bai N., Laher I., Granville D. J. (2005) Cytochrome p450 2C inhibition reduces post-ischemic vascular dysfunction. Vasc. Pharmacol. 43, 213–219 [DOI] [PubMed] [Google Scholar]
- 30. Greene J. F., Newman J. W., Williamson K. C., Hammock B. D. (2000) Toxicity of epoxy fatty acids and related compounds to cells expressing human soluble epoxide hydrolase. Chem. Res. Toxicol. 13, 217–226 [DOI] [PubMed] [Google Scholar]
- 31. Moran J. H., Weise R., Schnellmann R. G., Freeman J. P., Grant D. F. (1997) Cytotoxicity of linoleic acid diols to renal proximal tubular cells. Toxicol. Appl. Pharmacol. 146, 53–59 [DOI] [PubMed] [Google Scholar]
- 32. Viswanathan S., Hammock B. D., Newman J. W., Meerarani P., Toborek M., Hennig B. (2003) Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J. Am. Coll. Nutr. 22, 502–510 [DOI] [PubMed] [Google Scholar]
- 33. Siegfried M. R., Aoki N., Lefer A. M., Elisseou E. M., Zipkin R. E. (1990) Direct cardiovascular actions of two metabolites of linoleic acid. Life Sci. 46, 427–433 [DOI] [PubMed] [Google Scholar]
- 34. Sugiyama S., Hayakawa M., Nagai S., Ajioka M., Ozawa T. (1987) Leukotoxin, 9, 10-epoxy-12-octadecenoate, causes cardiac failure in dogs. Life Sci. 40, 225–231 [DOI] [PubMed] [Google Scholar]
- 35. Nelson D. R., Zeldin D. C., Hoffman S. M., Maltais L. J., Wain H. M., Nebert D. W. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14, 1–18 [DOI] [PubMed] [Google Scholar]
- 36. Lee C. R., Imig J. D., Edin M. L., Foley J., Degraff L. M., Bradbury J. A., Graves J. P., Lih F. B., Clark J., Myers P., Perrow A. L., Lepp A. N., Kannon M. A., Ronnekleiv O. K., Alkayed N. J., Falck J. R., Tomer K. B., Zeldin D. C. (2010) Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 24, 3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schlaeger T. M., Bartunkova S., Lawitts J. A., Teichmann G., Risau W., Deutsch U., Sato T. N. (1997) Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 94, 3058–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King L. M., Ma J., Srettabunjong S., Graves J., Bradbury J. A., Li L., Spiecker M., Liao J. K., Mohrenweiser H., Zeldin D. C. (2002) Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol. Pharmacol. 61, 840–852 [DOI] [PubMed] [Google Scholar]
- 39. Kusmic C., Lazzerini G., Coceani F., Barsacchi R., L'Abbate A., Sambuceti G. (2006) Paradoxical coronary microcirculatory constriction during ischemia: a synergic function for nitric oxide and endothelin. Am. J. Physiol. Heart Circ. Physiol. 291, H1814–H1821 [DOI] [PubMed] [Google Scholar]
- 40. Mobert J., Becker B. F., Zahler S., Gerlach E. (1997) Hemodynamic effects of isoprostanes (8-iso-prostaglandin F2alpha and E2) in isolated guinea pig hearts. J. Cardiovasc. Pharmacol. 29, 789–794 [DOI] [PubMed] [Google Scholar]
- 41. Daikh B. E., Lasker J. M., Raucy J. L., Koop D. R. (1994) Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J. Pharmacol. Exp. Therapeut. 271, 1427–1433 [PubMed] [Google Scholar]
- 42. Zeldin D. C., DuBois R. N., Falck J. R., Capdevila J. H. (1995) Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 322, 76–86 [DOI] [PubMed] [Google Scholar]
- 43. Wen X., Wang J. S., Backman J. T., Laitila J., Neuvonen P. J. (2002) Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab. Dispos. 30, 631–635 [DOI] [PubMed] [Google Scholar]
- 44. Seubert J. M., Sinal C. J., Graves J., DeGraff L. M., Bradbury J. A., Lee C. R., Goralski K., Carey M. A., Luria A., Newman J. W., Hammock B. D., Falck J. R., Roberts H., Rockman H. A., Murphy E., Zeldin D. C. (2006) Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ. Res. 99, 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nithipatikom K., Moore J. M., Isbell M. A., Falck J. R., Gross G. J. (2006) Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 291, H537–H542 [DOI] [PubMed] [Google Scholar]
- 46. Yang B., Graham L., Dikalov S., Mason R. P., Falck J. R., Liao J. K., Zeldin D. C. (2001) Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol. Pharmacol. 60, 310–320 [DOI] [PubMed] [Google Scholar]
- 47. King L. M., Gainer J. V., David G. L., Dai D., Goldstein J. A., Brown N. J., Zeldin D. C. (2005) Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet. Genom. 15, 7–13 [DOI] [PubMed] [Google Scholar]
- 48. Bylund J., Ericsson J., Oliw E. H. (1998) Analysis of cytochrome P450 metabolites of arachidonic and linoleic acids by liquid chromatography-mass spectrometry with ion trap MS. Anal. Biochem. 265, 55–68 [DOI] [PubMed] [Google Scholar]
- 49. Fukushima A., Hayakawa M., Sugiyama S., Ajioka M., Ito T., Satake T., Ozawa T. (1988) Cardiovascular effects of leukotoxin (9, 10-epoxy-12-octadecenoate) and free fatty acids in dogs. Cardiovasc. Res. 22, 213–218 [DOI] [PubMed] [Google Scholar]
- 50. Poyton R. O., Ball K. A., Castello P. R. (2009) Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metabol. 20, 332–340 [DOI] [PubMed] [Google Scholar]
- 51. Kojda G., Harrison D. (1999) Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 43, 562–571 [DOI] [PubMed] [Google Scholar]
- 52. Mitchell L. A., Grant D. F., Melchert R. B., Petty N. M., Kennedy R. H. (2002) Linoleic acid metabolites act to increase contractility in isolated rat heart. Cardiovasc. Toxicol. 2, 219–230 [DOI] [PubMed] [Google Scholar]
- 53. Hanaki Y., Kamiya H., Ohno M., Hayakawa M., Sugiyama S., Ozawa T. (1991) Leukotoxin, 9, 10-epoxy-12-octadecenoate: a possible responsible factor in circulatory shock and disseminated intravascular coagulation. Jpn. J. Med. 30, 224–228 [DOI] [PubMed] [Google Scholar]
- 54. Kosaka K., Suzuki K., Hayakawa M., Sugiyama S., Ozawa T. (1994) Leukotoxin, a linoleate epoxide: its implication in the late death of patients with extensive burns. Mol. Cell. Biochem. 139, 141–148 [DOI] [PubMed] [Google Scholar]
- 55. Theken K. N., Lee C. R. (2007) Genetic variation in the cytochrome P450 epoxygenase pathway and cardiovascular disease risk. Pharmacogenomics 8, 1369–1383 [DOI] [PubMed] [Google Scholar]
- 56. Chaudhary K. R., Abukhashim M., Hwang S. H., Hammock B. D., Seubert J. M. (2010) Inhibition of soluble epoxide hydrolase by trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is protective against ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. 55, 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.