Abstract
VLDL is produced by the liver. Its major protein is apoB100. Docosahexaenoic acid (DHA), a dietary polyunsaturated fatty acid (PUFA), reduces VLDL levels and is used therapeutically for hypertriglyceridemia. In model systems, DHA lowers VLDL secretion by inducing presecretory apoB100 degradation, a process dependent on PUFA-derived lipid peroxides. We hypothesized that superoxide (SO) was a major participant in DHA-induced apoB100 degradation, given its promotion of lipid peroxidation. SO levels in a model of VLDL metabolism, rat hepatoma McArdle cells, were either decreased by a mimetic of superoxide dismutase 1 (SOD1) or by overexpressing SOD1 or increased by SOD1 siRNA. ApoB100 recovery was assessed by immunoprecipitation, SO by 2-hydroxyethidine, and lipid peroxides by thiobarbituric acid reactive substances. The SOD1 mimetic or SOD1 overexpression reduced SO and inhibited apoB100 degradation in DHA-treated cells by up to 100%. Surprisingly, silencing SOD1 did not increase DHA-induced degradation, although levels of SO were higher (+44%); those of lipid peroxides were similar, and their reduction by α-tocopherol decreased degradation by 50%. SO is required for lipid peroxidation in DHA-induced apoB100 degradation, but it is the peroxide level that has a tighter relationship to the level of degradation and the regulation of VLDL production.—Andreo, U., Elkind, J., Blachford, C., Cederbaum, A. I., Fisher, E. A. Role of superoxide radical anion in the mechanism of apoB100 degradation induced by DHA in hepatic cells.
Keywords: polyunsaturated fatty acids, lipid peroxides, VLDL
Apolipoprotein B100 (apoB100) is essential for the formation of atherogenic lipoproteins and is a major risk factor for coronary artery disease (1). Dietary (n-3) fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and other polyunsaturated fatty acids (PUFAs) are known to reduce plasma levels of VLDL-triglycerides and VLDL-apoB100 (2–4) in vitro and in vivo. We have previously shown that PUFAs decrease the secretion of apoB100 by stimulating its degradation in a post-endoplasmic reticulum process that requires lipid peroxidation (5). One model for this is the association of lipid peroxides with apoB100 during VLDL assembly in the secretory pathway, leading to apoB100 aggregation and its targeting to autophagy (6).
The amount of lipid peroxides increases when hepatic cells are incubated with PUFAs as a result of the attack on the double bonds by reactive oxygen species (ROS). Many ROS species, in turn, are generated by the action of superoxide (SO). SO can be converted into hydroxyl radicals that initiate the formation of lipid radicals (L·). These lipid radicals, in turn, may then form lipid peroxy radicals (LOO·), which can attack a PUFA to form a lipid peroxide (LOOH). As the lipid peroxides degenerate, reactive aldehydes, particularly malondialdehyde (MDA), are produced, which can cross-link proteins and promote their aggregation and autophagic degradation (7).
Based on a study that showed the hepatic activity of a major scavenger of SO, SO dismutase 1 (SOD1; ref. 8), was negatively correlated with hepatic apoB100 secretion under basal (non-PUFA-induced) conditions, we hypothesized that SO may be an especially critical factor in the PUFA-induced lipid peroxidation that increases apoB100 degradation over that in the basal state. The main experimental approach to test this hypothesis was to over- and underexpress SOD1 activity in the presence and absence of DHA and included analytical measurements of the levels of SO and lipid peroxides.
MATERIALS AND METHODS
Animals and cell culture
Mice deficient in superoxide dismutase 1 (SOD1−/−) on a C57BL/6J background and wild-type (WT) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Adenoviruses Ad-LacZ and Ad-SOD1 were purchased from Vector Biolabs (Burlingame, CA, USA), amplified in HEK293 cells, and purified following a 2-round CsCl purification as described by Perez and Cederbaum (9).
Mac Ardle (McA) rat hepatoma cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM (Cellgro, Manassas, VA, USA) supplemented with 10% FBS (Gemini, Alachua, FL, USA), 10% horse serum (Sigma-Aldrich, St. Louis, MO, USA), 50 μg/ml l-glutamine, and 50 μg/ml penicillin/streptomycin at 37°C in 5% CO2. Cells were grown to 70% confluency before the experiment proceeded.
Immunofluorescence
McA cells were infected with an SOD1 adenovirus (Ad-SOD1) or an adenovirus expressing β-galactosidase (Ad-LacZ) for 48 h at 25 multiplicity of infection (MOI). Cells were then fixed using a 4% paraformaldehyde solution and permeabilized with 0.1% Triton. Immunofluorescence was performed using an anti-SOD1 mouse monoclonal antibody (Sc-17767; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and an Alexa 488-conjugated secondary antibody. Epifluorescent images were visualized using an Axiovert 200M inverted microscope (Zeiss, Oberkochen, Germany) equipped with an AxioCamMRc camera (Zeiss) and AxioVision 4.6 software (Zeiss).
Pulse-chase experiments
McA cells were pretreated with 0.1 mM BSA alone or 0.5 mM DHA complexed with BSA (5:1 M ratio; referred to as simply DHA henceforth) in the presence or absence of manganese (III) tetrakis (4-benzoic acid)porphyrin chloride (MnTBAP; 50 μM; A.G. Scientific, San Diego, CA, USA; refs. 10–12) for 1 h. Cells were then pulse labeled with 240 μCi [35S]-Met/Cys (Perkin-Elmer, Wellesley, MA, USA) for 15 min, after which the medium was collected at 20 and 90 min in the continued presence of BSA or DHA. At the end of the chase, cells and conditioned medium were collected. [35S]-labeled apoB100 from cell lysate and conditioned medium was immunoprecipitated, separated on a 4% SDS-PAGE gel, and then quantified using fluorography and densitometry. Total apoB100 protein recovery was normalized to total labeled protein precipitated with trichloroacetic acid (TCA) and represented by histogram.
For SOD1-overexpression experiments, McA cells were infected with Ad-SOD1 or Ad-LacZ for 48 h at 25 MOI, and then pulse-chase experiments were performed as described above. Efficiency of SOD1 overexpression was assessed by Western blot using an anti-SOD1 antibody (574597; Calbiochem, San Diego, CA, USA), and its activity was measured according to the protocol described by Perez and Cederbaum (9).
For siRNA experiments, cells were treated for 48 h with SiGenome pool (Dharmacon, Lafayette, CO, USA) and Dhamafect transfection reagent 4 (Dharmacon) according to the manufacturer's protocol. Pulse-chase experiments were performed as described above. Efficiency of SOD1 knockdown was assessed by Western blot using an anti-SOD1 antibody (574597; Calbiochem).
SO measurement by 2-hydroxyethidium detection
McA cells were incubated for 2 h in medium containing 1% FBS and then washed twice with PBS and incubated for 30 min in HBSS containing 1% BSA and 20 μM dihydroethidium (Invitrogen, Carlsbad, CA, USA). Cells were then washed twice with PBS and fast-frozen in liquid nitrogen. SO content correlated with the amount of 2-hydroxyethidium formed and was quantified by HPLC purification as described previously(13). All measurements were normalized to total protein concentration as determined using the Bradford method (14).
Lipid peroxidation assay
The content of lipid peroxides in cultured cell lysates was determined by the classical method of measuring thiobarbituric acid reactive substances (TBARS). McA cells were incubated with 0.1 mM BSA alone or 0.5 mM DHA complexed with BSA (5:1 M ratio) followed by 3 h incubation in phenol red-free medium. Cells were then washed twice with cold butylated hydroxytoluene (BHT) containing PBS and resuspended in PBS-BHT 15% (TCA), 0.375% thiobarbituric acid, and 0.25 N HCl and then incubated for 15 min at 100°C. Following a 5 min centrifugation at 10,000 g, the spectral absorbance of the supernatant was measured at 532 nm. The concentration of MDA equivalents was calculated using an extinction coefficient of 1.56 × 105 M−1 · cm−1 and normalized to total protein concentration as determined using the Lowry method (15).
Statistical analyses
All experiments were conducted in triplicate wells at least 2 times. Data are typically expressed as means ± se. Statistical differences were analyzed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Data in which 2 groups were compared were analyzed using an unpaired t test. Data in which >2 groups were compared were analyzed by ANOVA. Values of P <0.05 were considered significant.
RESULTS
To investigate the role of SO in DHA-induced apoB100 degradation, we first used the SOD1 mimetic, MnTBAP, in pulse-chase experiments in the McA rat hepatoma cell line. MnTBAP has previously been characterized as a potent inhibitor of lipid peroxidation (10–12). As shown in Fig. 1A, B, treatment with DHA alone caused a decrease in apoB100 recovery, as previously shown (5, 16). Pretreatment with MnTBAP decreased apoB100 degradation when cells were incubated with DHA. ApoB100 recovery was significantly increased by MnTBAP to almost 2-fold that in cells incubated with DHA alone. This protective effect of MnTBAP was correlated with a decrease in lipid peroxidation, as measured by TBARS (Fig. 1C).
Figure 1.
Scavenging superoxide inhibits apoB100 degradation induced by DHA and decreases the level of lipid peroxides. McA cells were treated with BSA or DHA/BSA complexes (DHA) in the presence (+) or absence (−) of the SO scavenger MnTBAP. ApoB100 recovery from cell lysate and medium samples was determined in a pulse-chase protocol to assess degradation of newly synthesized protein (see Materials and Methods). A) Representative fluorogram of secreted apoB100 at 90 min of chase. Doublets in this and other fluorograms represent apoB100 and the apoB95 forms that are secreted by rat hepatic cells. apoB95 form is derived from the apoB100 by proteolytic processing just before secretion (26), and its abundance was included in the recovery data for apoB100. B) Fluorograms were scanned in a densitometer; quantitative apoB100 recovery data are displayed. Histogram represents mean ± se (n=3) apoB100 recovery of 3 independent experiments. **P < 0.01. C) Lipid peroxidation was measured at different time points in the presence of DHA with (▴) or without (■) MnTBAP and is expressed as a fold increase of the initial amount of MDA measured at t = 0 in cells untreated. Data represent 3 independent experiments.
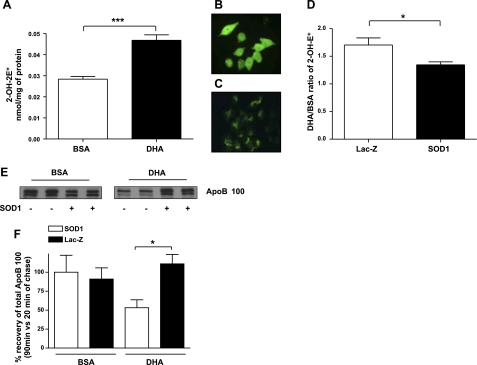
As noted earlier, SO is known to be a potent inducer of lipid peroxidation. This is most likely mediated via hydroxyl radicals produced by an iron-catalyzed Haber-Weiss type of reaction. DHA-induced degradation of apoB100 was previously shown to be blunted by the iron chelator desferrioxamine (5), which suggests a direct connection between SO and the DHA effect. Thus, we determined the relationship between DHA-induced apoB100 degradation and the level of 2-hydroxyethidium, which is produced from the reaction of SO with hydroethidine and is an accurate marker used to detect SO formation in cellular systems (13). As shown in Fig. 2A, cells incubated with DHA contain an almost 2-fold higher level of SO compared with BSA treated cells, raising the possibility that this increase was a causal factor in the results in Fig. 1.
Figure 2.
SOD1 overexpression reduces the induction by DHA of SO formation and apoB100 degradation. McA cells were treated for 2 h with BSA or DHA. A) Quantity of 2-OH-E+, representative of SO content, was measured by HPLC. Data represent quantities of 2-OH-E+ of 3 independent experiments (n=3). B) Overexpression of SOD1 in McA cells was achieved by infection with Ad-SOD1, and immunofluorescence was used to confirm SOD1 overexpression. C) Similar to B but with control (Ad-Lac-Z-infected) cells. D) Histogram comparing the ratio of 2-OH-E+ levels in McA cells treated with DHA to those treated with BSA in cells infected with either control Ad-LacZ or Ad-SOD1. Data represent quantities of 2-OH-E+ of 2 independent experiments (n=3). E) Representative fluorogram of apoB100 recovery at 90 min of chase in cells treated with BSA or DHA and infected for 48h with Ad-LacZ or Ad-SOD1. F) Histogram of apoB100 recovery at 90 min of chase (n=3) from 2 independent experiments. Values are means ± se (n=3). *P < 0.05; ***P < 0.001.
To test the specific role of SO in DHA-induced apoB100 degradation, the antioxidant enzyme SOD1 was overexpressed (Fig. 2B, C), using an adenoviral approach (9). The overexpression of SOD1 was confirmed by Western blot and by enzymatic activity assay as described previously in Perez and Cederbaum (ref. 9; data not shown). As summarized in Fig. 2D, the increased expression of SOD1 resulted in a reduction in SO (as measured by 2-hydroxyethidium content) in McA cells incubated with DHA relative to cells incubated with BSA (control condition). The functional significance of this was revealed in pulse-chase studies, which demonstrated that the overexpression of SOD1 was able to effectively decrease apoB100 degradation and significantly restore apoB100 recovery in DHA-treated McA cells (Fig. 2E, F).
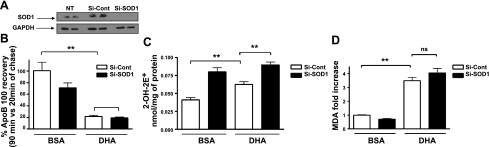
To further explore the relationship between SO and apoB100 metabolism, we used siRNA to decrease SOD1 expression in McA cells. We confirmed by Western blot that SOD1 was down-regulated by >90% at the protein level (Fig. 3A). As expected from the genetic studies in SOD1-deficient mice (8), under basal conditions (i.e., without DHA), the suppression of SOD1 induced apoB100 degradation (Fig. 3B; compare bars 1 and 2), again suggesting a key role for SO in this process. ApoB100 degradation was significantly higher in McA cells incubated with DHA (Fig. 3B; compare bars 1 and 3), but there was no additional degradation with SOD1 suppression (Fig. 3B; compare bars 3 and 4).
Figure 3.
SOD1 deficiency increases the SO level but does not further enhance apoB100 degradation induced by DHA. A) SDS-PAGE was performed on cell lysates from McA cells treated with control siRNA control (Si-Cont), SOD1-siRNA (Si-SOD1), and nontreated cells (NT). Western blots (representative blot is shown) were probed for SOD1 and GAPDH as a loading control. B) Histogram showing total apoB100 recovery after 90 min of chase in presence of BSA or DHA in McA cells treated for 48 h with Si-SOD1 or Si-Cont. C) Histogram showing quantity of 2-OH-E+ in McA cells treated for 48h with Si-SOD1 or Si-Cont and then treated with BSA or DHA. D) Lipid peroxidation was measured in the siRNA-treated cell after 3 h in the presence of BSA or DHA. Data are expressed as fold increases of the amount of MDA measured in Si-Cont-treated cells. Data represent means ± se (n=3) from 2 independent experiments. ns, nonsignificant (P>0.05). **P < 0.01.
We next examined the relationship between SO and the results in Fig. 3B by using the 2-hydroxyethidium assay on cells treated identically. As shown in Fig. 3C, there were significant increases of SO in SOD1 suppressed cells (bars 2 and 4) compared with control treated with BSA or with DHA (bars 1 and 3). These results suggested that SO was not the sole determinant of apoB100 degradation. For example, the level of SO was higher in bar 2 vs. 3 of Fig. 3C, yet the recovery of apoB100 was much higher in bar 2 vs. 3 of Fig. 3B.
We hypothesized that the main role of SO in apoB100 degradation is not to directly promote the degradation of apoB100 but rather to initiate the chain of events, e.g., hydroxyl radical formation, that results in the formation of lipid peroxides, the levels of which were inversely correlated with apoB100 recovery in rat hepatic cells incubated with a variety of PUFAs, including DHA (5). Thus, we assayed the levels of TBARS (to gauge lipid peroxidation) in McA cells incubated with either BSA or DHA and treated with control or SOD1 siRNA. As shown in Fig. 3D, the data support our hypothesis in that the level of lipid peroxides was significantly higher in the presence of DHA (bars 1 and 2 vs. 3 and 4) but not significantly different in cells incubated with DHA and treated with control or SOD1 siRNA (bar 3 vs. 4). The results also suggest that there were enough lipid peroxides produced even with SOD1 sufficiency in DHA-incubated cells to promote maximal effects on apoB100 degradation (compare bars 3 and 4 in Fig. 3D to the same bars in Fig. 3B).
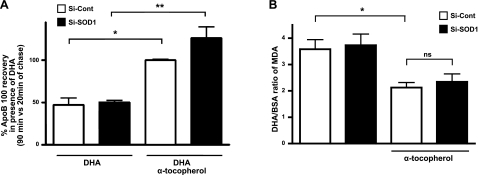
To extend these results, we reasoned that if it were the lipid peroxides that were the proximal ROS molecules required for the effects of DHA on apoB100 degradation, then pretreatment of McA cells with α-tocopherol, an antioxidant that prevents lipid peroxidation, but not SO production, would be effective in restoring apoB100 recovery independent of SOD1 status. Indeed, as shown in Fig. 4A, cotreatment with DHA and α-tocopherol reduced apoB100 degradation as efficiently in SOD1-suppressed McA cells (solid bars) as in control cells (open bars). These changes were associated with reduced lipid peroxides (as measured by TBARS) in the cells treated with α-tocopherol (Fig. 4B). Interestingly, the data in Fig. 4C show that α-tocopherol could also lower the DHA-mediated increase in the cellular level of SO, suggesting that peroxidized DHA may be a source of SO, similar to what was shown for linoleic acid (17).
Figure 4.
Inhibition of lipid peroxidation restores apoB100 recovery, even under low SOD1 expression levels. A) McA cells were treated for 48 h with SOD1 siRNA (Si-SOD1) or control siRNA (Si-Cont) and then treated with or without α-tocopherol (120 μM) in the presence of DHA. Histogram shows total apoB100 recovery after 90 min of chase from a representative experiment (n=2). B) Lipid peroxidation was measured in the siRNA-treated cell after 3 h in the presence or absence of α-tocopherol (120 μM) with BSA or DHA. Data are expressed as a ratio of the quantity of MDA in DHA vs. BSA treated cells. Data are from a representative experiment (n=3). Values are means ± SE. *P < 0.05; **P < 0.01.
DISCUSSION
It has long been known that diets rich in PUFAs reduce the plasma levels of VLDL triglycerides. One class of PUFAs, the omega-3 fatty acids enriched in marine oils (EPA and DHA), is particularly potent in this regard, and is used therapeutically in patients with hypertriglyceridemia, and more generally, as a dietary supplement, in patients at risk for coronary artery disease (e.g., see American Heart Association recommendations; ref. 18). We have previously shown that a cellular basis for the triglyceride-lowering effects of EPA and DHA is the stimulation of hepatic apoB100 degradation by post-endoplasmic reticulum presecretory proteolysis (PERPP; ref. 19), a process subsequently shown to be associated with the generation of lipid peroxides (5) and autophagy (20). In the present studies, we have focused on the role of SO in DHA-stimulated apoB100 degradation. The major rationale for this was 2-fold, one being that DHA and other PUFAs can induce production of SO from microsomal enzymes such as cyp2e1 (21, 22) and the other that SO is well known to attack PUFAs and promote lipid peroxidation. The catabolism of SO is regulated by cytosolic SOD1 and mitochondrial SOD2. Most relevant to the present studies is the report that SOD1, and not SOD2, negatively regulated apoB100 secretion in the livers of mice fed high-fat diets (8), presumably by decreasing cellular levels of SO and lipid peroxides, though this was not directly investigated.
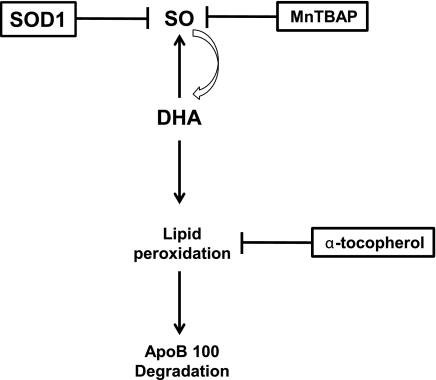
In the present study, there were two major findings: the overexpression of SOD1 or the provision of the SOD1-mimic MnTBAP reduced cellular levels of SO, SOs, and apoB100 degradation in DHA-treated cells; and, in contrast, the suppression of SOD1 and consequent elevation of SO did not further decrease apoB100 recovery in DHA-treated cells, although it did lower recovery in BSA-treated cells. Thus, it appeared that once there is sufficient SO to attack DHA, the level of lipid peroxides that promote apoB100 degradation appeared to be primarily determined by the supply of the fatty acid. The relationships among SO, lipid peroxidation, and apoB100 degradation are schematized in Fig. 5.
Figure 5.
Interrelationships among SO, lipid peroxides and apoB100 degradation. Reduction of SO levels by SOD1 or MnTBAP pointed to a key role of SO in DHA-induced apoB100 degradation. SO promotes DHA peroxidation, and this peroxidation, in turn, can contribute to more SO production. The proximal mediator of the apoB100 degradation process appears to be lipid peroxidation, based on the finding that α-tocopherol blocks the formation of lipid peroxides without affecting the SO content and still inhibits apoB100 degradation.
Note that while MnTBAP may not be specific for only reacting with SO, the experiments with SOD1 overexpression and silencing definitively support the important role of SO displayed in Fig. 5 in the potentiation of lipid peroxidation and, thereby, apoB100 degradation. It is not likely that H2O2 (e.g., derived from SO) plays a critical role in DHA-induced apoB100 degradation because we previously reported that catalase at a concentration sufficient to deplete liver cells of H2O2 had no effect on apoB100 recovery from DHA-treated cells (ref. 5 and unpublished results). In addition, we have previously shown that microsomal lipid peroxidation is independent of H2O2 and not affected by catalase or by glutathione-glutathione peroxidase (23, 24).
How do lipid peroxides promote PERPP of apoB100? Mentioned above are our previous studies demonstrating autophagy as the proteolytic process. Independent of the specific scenario, what is probably common to all of them is the attack on apoB100 by lipid peroxides that results in intra- and intermolecular cross-linking and, ultimately, the aggregation of damaged apoB100. This model is based on our specific finding in DHA-treated hepatic cells MDA + apoB100 (MDA is a lipid peroxide breakdown product that cross-links proteins) that is aggregated (20), and the general finding that these types of modifications are classic factors targeting proteins to autophagy (20). What is less certain is whether the attack on apoB100 occurs from its association with membranes in the secretory pathway (e.g., ref. 25) or from its association with lipids as part of lipoprotein assembly. It is well known that DHA becomes rapidly incorporated into membrane phospholipids, and SO frequently attacks PUFAs in membranes. Alternatively, DHA could be attacked by SO before or after its incorporation into the phospholipids or lipid esters associated with apoB100 during the VLDL assembly process.
While there are several sources of SO production by cells, the ability of cytochrome P450 enzymes to produce SO in the endoplasmic reticulum is of interest in view of their proximity to sites of lipoprotein assembly and the aforementioned reports that DHA and other PUFAs can induce production of SO from cyp2e1 (21, 22). In addition, some SO may be generated from peroxidized DHA, given that the increase in SO produced in response to DHA was blunted by α-tocopherol (Fig. 4C), which reduces lipid peroxidation. These results suggest the cyclic relationship indicated in Fig. 5 in which SO promotes lipid peroxidation and lipid peroxidation increases SO production.
In summary, these studies are important in elucidating the complex interrelationships among SO, lipid peroxides, and apoB100 degradation. Notably, the studies in which increased SOD1-like or authentic SOD1 activity substantially reversed or even eliminated the prodegradative effects of DHA demonstrate that of the multiple ROS-generating systems in the cell, those producing cytosolic SO are particularly important. This is not contradicted by the SOD1-suppression studies, which indicate that when there is sufficient SO, the degradative effect of DHA is determined by the abundance of fatty acids that can be attacked to form lipid peroxides. Overall, the results provide important insights into the mechanistic basis for the clinical lowering of plasma VLDL triglyceride levels by fish oils.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant HL-58541 (to E.A.F.), American Heart Association postdoctoral fellowship 0825883D (to U.A.), and NIH grants AA-017425 and AA-018790 (to A.I.C.). The authors thank Dr Jacek Zielonka for the 2-hydroxyethidium measurements that were performed at the Free Radical Research Center (Medical College of Wisconsin, Milwaukee, WI, USA), Lisa Grauer (New York University) for help in the manuscript preparation, and Dr. Charles (Liang) Guo (New York University) for helpful discussions.
REFERENCES
- 1. Ginsberg H. N., Fisher E. A. (2009) The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J. Lipid Res. 50(Suppl.), S162–S166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris W. S., Miller M., Tighe A. P., Davidson M. H., Schaefer E. J. (2008) Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 197, 12–24 [DOI] [PubMed] [Google Scholar]
- 3. Harris W. S., Bulchandani D. (2006) Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 17, 387–393 [DOI] [PubMed] [Google Scholar]
- 4. Tabas I., Williams K. J., Boren J. (2007) Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116, 1832–1844 [DOI] [PubMed] [Google Scholar]
- 5. Pan M., Cederbaum A. I., Zhang Y. L., Ginsberg H. N., Williams K. J., Fisher E. A. (2004) Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Invest. 113, 1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher E. A., Williams K. J. (2008) Autophagy of an oxidized, aggregated protein beyond the ER: a pathway for remarkably late-stage quality control. Autophagy 4, 721–723 [DOI] [PubMed] [Google Scholar]
- 7. Smith C., Marks A., Lieberman M. (2005) Oxygen toxicity and free radical injury. In: Marks' Basic Medical Biochemistry p. 439–457, Lippincott Williams & Wilkins, Baltimore, MD, USA [Google Scholar]
- 8. Uchiyama S., Shimizu T., Shirasawa T. (2006) CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J. Biol. Chem. 281, 31713–31719 [DOI] [PubMed] [Google Scholar]
- 9. Perez M. J., Cederbaum A. I. (2003) Adenovirus-mediated expression of Cu/Zn- or Mn-superoxide dismutase protects against CYP2E1-dependent toxicity. Hepatology 38, 1146–1158 [DOI] [PubMed] [Google Scholar]
- 10. Day B. J., Batinic-Haberle I., Crapo J. D. (1999) Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med. 26, 730–736 [DOI] [PubMed] [Google Scholar]
- 11. Trova M. P., Gauuan P. J., Pechulis A. D., Bubb S. M., Bocckino S. B., Crapo J. D., Day B. J. (2003) Superoxide dismutase mimetics. Part 2: synthesis and structure-activity relationship of glyoxylate- and glyoxamide-derived metalloporphyrins. Bioorg. Med. Chem. 11, 2695–2707 [DOI] [PubMed] [Google Scholar]
- 12. Gauuan P. J., Trova M. P., Gregor-Boros L., Bocckino S. B., Crapo J. D., Day B. J. (2002) Superoxide dismutase mimetics: synthesis and structure-activity relationship study of MnTBAP analogues. Bioorg. Med. Chem. 10, 3013–3021 [DOI] [PubMed] [Google Scholar]
- 13. Zielonka J., Vasquez-Vivar J., Kalyanaraman B. (2008) Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 3, 8–21 [DOI] [PubMed] [Google Scholar]
- 14. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 15. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 16. Wang H., Yao Z., Fisher E. A. (1994) The effects of n-3 fatty acids on the secretion of carboxyl-terminally truncated forms of human apoprotein B. J. Biol. Chem. 269, 18514–18520 [PubMed] [Google Scholar]
- 17. Fruebis J., Parthasarathy S., Steinberg D. (1992) Evidence for a concerted reaction between lipid hydroperoxides and polypeptides. Proc. Natl. Acad. Sci. U. S. A. 89, 10588–10592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kris-Etherton P. M., Harris W. S., Appel L. J. (2003) Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 23, 151–152 [DOI] [PubMed] [Google Scholar]
- 19. Fisher E. A., Pan M., Chen X., Wu X., Wang H., Jamil H., Sparks J. D., Williams K. J. (2001) The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 276, 27855–27863 [DOI] [PubMed] [Google Scholar]
- 20. Pan M., Maitin V., Parathath S., Andreo U., Lin S. X., St Germain C., Yao Z., Maxfield F. R., Williams K. J., Fisher E. A. (2008) Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc. Natl. Acad. Sci. U. S. A. 105, 5862–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caro A. A., Cederbaum A. I. (2001) Synergistic toxicity of iron and arachidonic acid in HepG2 cells overexpressing CYP2E1. Mol. Pharmacol. 60, 742–752 [PubMed] [Google Scholar]
- 22. Lu Y., Cederbaum A. I. (2008) CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 44, 723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puntarulo S., Cederbaum A. I. (1989) Interactions between paraquat and ferric complexes in the microsomal generation of oxygen radicals. Biochem. Pharmacol. 38, 2911–2918 [DOI] [PubMed] [Google Scholar]
- 24. Beloqui O., Cederbaum A. I. (1986) Prevention of microsomal production of hydroxyl radicals, but not lipid peroxidation, by the glutathione-glutathione peroxidase system. Biochem. Pharmacol. 35, 2663–2669 [DOI] [PubMed] [Google Scholar]
- 25. Tran K., Sun F., Cui Z., Thorne-Tjomsland G., St Germain C., Lapierre L. R., McLeod R. S., Jamieson J. C., Yao Z. (2006) Attenuated secretion of very low density lipoproteins from McA-RH7777 cells treated with eicosapentaenoic acid is associated with impaired utilization of triacylglycerol synthesized via phospholipid remodeling. Biochim. Biophys. Acta 1761, 463–473 [DOI] [PubMed] [Google Scholar]
- 26. Reuben M. A., Svenson K. L., Doolittle M. H., Johnson D. F., Lusis A. J., Elovson J. (1988) Biosynthetic relationships between three rat apolipoprotein B peptides. J. Lipid Res. 29, 1337–1347 [PubMed] [Google Scholar]