Abstract
A growing awareness indicates that many G-protein-coupled receptors (GPCRs) exist as homodimers, but the extent of the cooperativity across the dimer interface has been largely unexplored. Here, measurement of the dissociation kinetics of a fluorescent agonist (ABA-X-BY630) from the human A1 or A3 adenosine receptors expressed in CHO-K1 cells has provided evidence for highly cooperative interactions between protomers of the A3-receptor dimer in single living cells. In the absence of competitive ligands, the dissociation rate constants of ABA-X-BY630 from A1 and A3 receptors were 1.45 ± 0.05 and 0.57 ± 0.07 min−1, respectively. At the A3 receptor, this could be markedly increased by both orthosteric agonists and antagonists [15-, 9-, and 19-fold for xanthine amine congener (XAC), 5′-(N-ethyl carboxamido)adenosine (NECA), and adenosine, respectively] and reduced by coexpression of a nonbinding (N250A) A3-receptor mutant. The changes in ABA-X-BY630 dissociation were much lower at the A1 receptor (1.5-, 1.4-, and 1.5-fold). Analysis of the pEC50 values of XAC, NECA, and adenosine for the ABA-X-BY630-occupied A3-receptor dimer yielded values of 6.0 ± 0.1, 5.9 ± 0.1, and 5.2 ± 0.1, respectively. This study provides new insight into the spatial and temporal specificity of drug action that can be provided by allosteric modulation across a GPCR homodimeric interface.—May, L. T., Bridge, L. J., Stoddart, L. A., Briddon, S. J., Hill, S. J. Allosteric interactions across native adenosine-A3 receptor homodimers: quantification using single-cell ligand-binding kinetics.
Keywords: dissociation, cooperativity, GPCRs
G-protein-coupled receptors (GPCRs) represent a large superfamily of cell surface receptors that share many structural motifs, including an extracellular N terminus, 7 α-helical transmembrane domains, and an intracellular C terminus. Despite this common architecture, endogenous agonist/GPCR/effector complexes engender enormous diversity within cellular signaling. GPCRs are involved in the majority of physiological and pathological signal transduction mechanisms, which, in turn, has driven this receptor superfamily to be the most commonly targeted group of proteins in terms of pharmaceutical therapeutics (1). However, of the 323 nonvisual GPCRs that represent realistic drug targets, only 46 have been targeted effectively with new chemical entities, and recent GPCR drug discovery programs have had little success in expanding the repertoire of drugged GPCRs (2). This may be due, in part, to a relatively limited understanding with regard to the composition and membrane distribution of GPCRs at the cell surface.
GPCRs are highly plastic proteins that can adopt a broad spectrum of conformations. Binding of natural or synthetic ligands, in addition to interactions with intracellular and/or transmembrane signaling proteins, can enrich distinct subsets of GPCR conformations, many of which may vary in their downstream signaling profiles (3). The majority of drugs in clinical practice mediate their effects through the endogenous ligand-binding site, otherwise known as the orthosteric site. However, targeting topographically distinct allosteric binding sites is of increasing interest as allosteric modulators can provide a number of advantages, including spatiotemporal specificity and greater subtype selectivity (4–6). As allosteric binding sites include any receptor site that is topographically distinct from the endogenous ligand-binding site, interacting intracellular and transmembrane proteins can be described as endogenous GPCR allosteric modulators. Allosteric interactions can be neutral, positive, or negative and are reciprocal in nature. The cooperativity between simultaneously bound orthosteric and allosteric binding sites can be probe dependent. That is, allosteric interactions between orthosteric ligands, allosteric ligands, and/or GPCR-interacting proteins, such as the G protein or β-arrestin, can stabilize distinct subsets of GPCR conformations. The phenomenon of functional selectivity arises from the generation of discrete intracellular signaling profiles due to probe-dependent variations in the degree and/or direction of cooperativity between two GPCR binding sites (7). Therapeutically, functionally selective ligands may represent a novel method to selectively modulate a pathway of interest and as such minimize adverse effects (8, 9).
Despite intense debate, it is now generally accepted that GPCRs can form homodimers, heterodimers and/or higher-order oligomeric complexes (10–12). Nonvisual GPCRs are commonly classified into 3 families, A–C, on the basis of sequence similarity within the predicted hydrophobic transmembrane domains (13, 14). A number of elegant studies have demonstrated that family C GPCRs exist as stable and obligate dimers (15). In contrast, the extent, stability, and physiological consequence of GPCR-GPCR interactions between family A GPCRs, which include the well-characterized adrenoceptors, adenosine receptors, and muscarinic receptors, remain a subject of debate (16–18). Classical receptor theory has been built on the assumption that family A GPCRs exist and function as monomers. Supporting this assumption, recent studies using high-density lipoprotein phospholipid bilayer particles have demonstrated that monomeric rhodopsin, β2-adrenergic and μ-opioid receptors can couple to G proteins (19–21). However, over the past decade, a large body of evidence has revealed that family A GPCRs can interact with one another to form receptor dimers and/or higher-order oligomeric complexes (11, 12). As with other allosteric interactions, GPCR-GPCR interactions can selectively enrich a distinct subset of GPCR conformations. As a consequence, the intracellular signaling profile of natural or synthetic agonists may alter depending on the oligomeric state of the GPCR, which, in turn, may vary depending on the cellular context in which the receptor is expressed. Furthermore, allosteric interactions can occur across a dimer interface. As such, ligand binding to one protomer can be modulated by targeting a binding site within the second, interacting protomer. Allosterism across a GPCR complex represents an alternative therapeutic approach toward targeting currently intractable GPCRs. However, prior to becoming a realistic therapeutic avenue, a mechanistic framework is required to quantify homomeric and heteromeric allosteric interactions in terms of affinity and cooperativity.
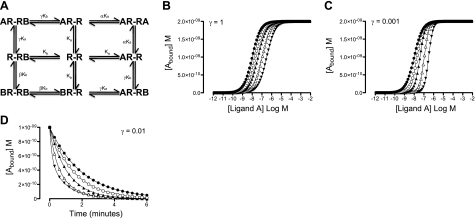
Various mathematical models have been proposed to describe GPCR dimerization (22–31). Within the simplest schematic describing receptor homodimerization, cooperative interactions in the presence of a single ligand have characteristic features when investigated over a wide concentration range (Supplemental Fig. S1A, B and ref. 25). On extension to a 2-drug model of receptor homodimerization, interactions indistinguishable from classical competition are observed under conditions of neutral cooperativity (Fig. 1A, B). Strong positive cooperativity between two ligands introduces extra inflections into equilibrium binding curves and, as such, is clearly distinguishable from that of neutral cooperativity (Supplemental Fig. S1C). In contrast, only minor changes in equilibrium binding are observed under conditions of strong negative cooperativity (Fig. 1C). In these cases, dissociation kinetic assays represent a more sensitive alternative for detecting negatively cooperative interactions (Fig. 1D).
Figure 1.
The 2-drug model of constitutive GPCR homodimerization. A) Within the 2-drug model of constitutive GPCR homodimerization, ligand A and ligand B can bind to the first and/or second protomer in the homodimer, R-R. Binding of ligand A and ligand B is defined by their affinity for the free GPCR homodimer, Ka and Kb, respectively, and the cooperativity factors α, β, and γ. B, C) Influence of γ = 1 (B) and γ = 0.001 (C) on the equilibrium binding properties A and B. D) Influence of γ = 0.01 on the dissociation of A in the presence of increasing concentrations of B. [Ligand B] = ● 0 nM, ○ 10 nM, ▴ 30 nM, ▵ 100 nM, ▾ 300 nM; ka+ = 5 × 107 M−1 s−1; ka− = 0.5 s−1; kb+ = 5 × 107 M−1 s−1; kb− = 0.5 s−1; α = 1; β = 1; Rtot = 1 × 10−9 M.
Previously, we have used dissociation kinetics in single living cells to study negatively cooperative allosteric interactions induced by allosteric ligands at the A3 adenosine receptor (A3-AR; refs. 32, 33). Under conditions of “infinite” dilution and/or isotopic dilution, ligands that compete for the same site on a monomeric receptor should not influence one another's dissociation kinetics (34). In contrast, allosteric modulation between two simultaneously bound and interacting sites, either within a receptor monomer or across a receptor dimer/oligomer, can alter ligand dissociation (6). In this study, we have employed dissociation kinetic analysis as a powerful method to detect allosteric interactions between the orthosteric binding sites of a GPCR across the dimeric interface of A3-AR homodimers/oligomers in single living cells. We have also used a kinetic model of GPCR homodimerization to provide a mechanistic framework within which to quantify the allosteric interactions across the dimeric interface of GPCRs in terms of affinity and cooperativity. This should provide a powerful basis for future drug discovery efforts.
MATERIALS AND METHODS
Materials
ABA-X-BY630 was from CellAura Technologies Ltd (Nottingham, UK). Fetal calf serum was from PAA Laboratories (Pasching, Austria); l-glutamine and trypsin, from Lonza (Verviers, Belgium). Lipofectamine 2000 transfection reagent and Optimem were from Invitrogen (Paisley, UK). All other chemicals were from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Generation of adenosine-receptor constructs
The A3-ARN-YFP, A3-ARC-YFP, A3-ARYFP, and A3-ARGFP constructs were generated using cDNA encoding the full-length human A3-AR, which was fused in frame with a C-terminal truncated version of YFP (N-YFP; aa 1–155), N-terminal truncated version of YFP (C-YFP; 156–231), full-length version of YFP or full-length version of GFP, respectively (with the methionine start signal removed) and subcloned into pcDNA3.1. The N250A mutation was introduced into the A3-ARYFP gene using the QuikChange site-directed mutagenesis kit (Aglient Technologies, Cheshire, UK) and was confirmed by DNA sequencing. Full-length A3(N250A)-ARYFP DNA was excised and subcloned into a native pcDNA3.1 vector.
Confocal microscopy
Biomolecular fluorescence complementation (BiFC)
Chinese hamster ovary (CHO-K1) cell lines were maintained as described previously (33). For BiFC experiments, CHO-K1 cells were seeded onto Lab-Tek 8-well plates (Nunc Nalgene, Rochester, NY, USA). Cells were transfected for 24 h with 0.15 μg of DNA using Lipofectamine (Invitrogen) according to the manufacturer's instructions, and subsequently incubated for 24 h at 30°C to facilitate correct folding of the YFP fragments. Following washing of the cells in HEPES-buffered saline solution (HBSS; 25 mM HEPES, 10 mM glucose, 146 mM NaCl, 5 mM KCl, 1 mM MgSO4, 2 mM sodium pyruvate, and 1.3 mM CaCl2), images of BiFC were obtained on a Zeiss LSM510 confocal microscope using a c-Apochromat ×40 1.2 NA water-immersion objective (Carl Zeiss, Oberkochen, Germany), using 514-nm excitation and a pinhole diameter of 1 Airy unit.
Influence of competitive ligands on ABA-X-BY630 association and dissociation at A1-ARs and A3-ARs
CHO-K1 cell lines stably expressing either the human A1-AR (CHO–A1; Bmax=3350±315 fmol/mg protein; ref. 35) or the human A3-AR (CHO–A3; Bmax=765±22 fmol/mg protein; ref. 36), respectively, were maintained in Dulbecco's modified Eagle's medium/nutrient mix F12 (DMEM-F12) containing 10% fetal calf serum (FCS) and 2 mM l-glutamine at 37°C in a humidified incubator containing 5% CO2. When confluent, cells were harvested (0.25% trypsin) and centrifuged (1000 g for 5 min), and the resulting pellet was resuspended in DMEM-F12 containing 10% FCS and 2 mM glutamine. Prior to assaying, CHO-A1 and CHO-A3 cells were seeded onto 32-mm circular coverslips in DMEM-F12 containing 10% FCS and 2 mM glutamine for ≥18 h and grown to 80–100% confluence.
Live cell imaging was performed using a Zeiss LSM 510 laser scanning confocal microscope and a Zeiss Plan-Neofluar ×40 1.3 NA oil-immersion objective. A 633-nm HeNe laser was used for the excitation of ABA-X-BY630 (a BODIPY630/650 conjugate) with emission being detected using a 650-nm long-pass filter. Dissociation kinetic assays were performed according to the previously described method of infinite dilution using a temperature-controlled perfusion system (33). This method maintains a constant flow rate while exposing cells to HBSS in the absence and presence of 30 nM ABA-X-BY630 and/or competitive ligands. In each case, the temperature was maintained at 37°C, the flow rate was ≥5 ml/min, and the pinhole diameter (1 Airy unit; 1.1-μm optical slice), laser power, and gain remained constant between experiments.
Live-cell kinetic experiments for CHO-A1 and CHO-A3 cells involved a 30-s perfusion of HBSS to obtain a basal fluorescence reading followed by a 2-min exposure of cells to 30 nM ABA-X-BY630 in the absence or presence of 5′-(N-ethyl carboxamido)adenosine (NECA; 10 nM to 10 μM), xanthine amine congener (XAC; 10 nM to 1 μM), or adenosine (1–10 μM). ABA-X-BY630 (30 nM) dissociation was initiated through exchanging the perfusion from fluorescent ligand to HBSS in the absence or presence of NECA (10 nM to 100 μM), XAC (1 nM to 10 μM), or adenosine (100 nM to 100 μM). Phase and fluorescence images were captured every 2 s for the duration of the experiment. Within each field of view, a region of interest was drawn around the plasma membrane of 10 cells and the changes in fluorescence intensity estimated over time (Zeiss AIM 4.2 software).
Influence of the A3-AR mutation, N250A, on the dissociation of ABA-X-BY630
CHO cells transiently transfected with A3-ARGFP and either A3-ARYFP or A3(N250A)-ARYFP were excited simultaneously using a 488-nm argon laser and imaged as a λ stack with wavelength range of 495–666 nm. Spectral unmixing was used to separate YFP and GFP fluorescence and estimate the GFP/YFP fluorescence ratio at a single-cell level. A corresponding λ stack of A3-ARYFP or A3-ARGFP transiently transfected into CHO cells alone provided a reference spectrum for each fluorescent protein. Live-cell kinetic experiments involved HBSS perfusion to obtain basal fluorescence, initiation of association through exposure of cells to 30 nM ABA-X-BY630 for 2 min, and then initiation of dissociation through perfusion of HBSS alone for 2 min. Cells were subsequently reexposed to 30 nM ABA-X-BY630 for 2 min, followed by perfusion of HBSS in the presence of 1 μM NECA for 2 min. Phase and fluorescence images were captured every 2 s for the duration of the experiment. Within each field of view, a region of interest was drawn around the plasma membrane of transfected cells, and the changes in fluorescence intensity were estimated over time (Zeiss AIM 4.2 software).
Data analysis
Dissociation of 30 nM ABA-X-BY630 was fit to the monoexponential decay in Eq. 1 using Prism 5 (GraphPad, San Diego, CA, USA):
| (1) |
where Y0 represents the level of ABA-X-BY630 binding at time t = 0, plateau is the level of binding at infinite time, and koff is the dissociation rate constant of ABA-X-BY630.
Concentration-response curves of the influence of adenosine, NECA, and XAC on the dissociation of 30 nM ABA-X-BY630 were fit to Eq. 2 using Prism 5:
| (2) |
where Emax is the maximal response and EC50 is the molar concentration of nonfluorescent ligand required to generate 50% of Emax.
Dynamic model of receptor homodimerization
ABA-X-BY630 association and dissociation kinetics were subsequently analyzed using a kinetic model of receptor homodimerization to estimate equilibrium association constants KA and KB and the cooperativity factors α, β, and γ (Fig. 1A). The following dynamic models describe the binding of one or two ligands to a homodimeric GPCR, with forward reactions denoted by a plus sign and backward reactions with a minus sign. Supplemental Fig. S1A describes the binding of ABA-X-BY630 (represented as A) in terms of both the affinity of A for the unoccupied homodimer, KA = ka+/ka−, and the cooperativity, α = α+/α−, between the binding sites when both sites on the homodimeric receptor are simultaneously occupied. System dynamics are given by solutions to the following system of ordinary differential equations, where each R represents a homodimeric receptor:
| (3) |
| (4) |
| (5) |
The association and dissociation of 30 nM ABA-X-BY630 were simultaneously analyzed, and ka−, ka+, α−, α+, parameter estimates were constrained for all subsequent analysis.
Figure 1A shows a schematic for the influence of a second ligand (B) on the dissociation of A, described by the following system of ordinary differential equations where, as previously, each R represents a homodimeric receptor:
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
The affinity of B for the unoccupied homodimer is represented by KB = kb+/kb−, and the cooperativity between binding sites on the homodimer when two B simultaneously binds the receptor is β = β+/β−. The reciprocal influence of the binding of A on the affinity of B and the binding of B on the affinity of A is reflected by γ = γ+/γ−. Within this analysis, ka−, ka+, α−, α+, were fixed to previous estimations in the absence of B. In fitting to this model alone, γ+kb+ could only be estimated as a composite value, and β values cannot be estimated from bound fraction data.
Simultaneous addition of A and B during association (Fig. 1C) is described by the following system of ordinary differential equations where, as previously, each R represents a homodimeric receptor:
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
d[BRB]/dt is described by Eq. 10. All forward and backward reaction rate constants and cooperativity factors are as described previously. Within this analysis, all parameters for drug A were fixed as previously estimated. The parameter estimation method was then applied to the dissociation model, Eqs. 6–11, and the association model, Eqs. 12–16, simultaneously to recover parameters for B, including the cooperativity β. The parameter estimation routine employed uses a bounded Nelder-Mead method with 100 initial guesses in each case.
Statistical analysis
Statistical analysis involved 1-way ANOVA or unpaired t tests, where appropriate, with statistical significance reflecting P < 0.05 (Prism 5).
RESULTS
Detecting A3-AR complexes using BiFC
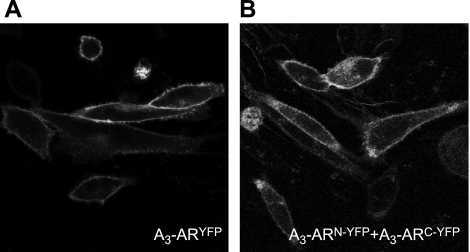
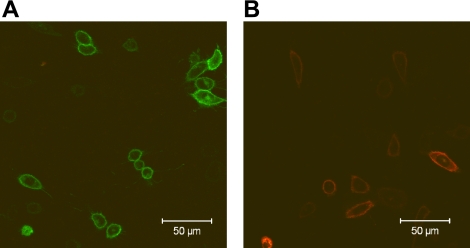
BiFC describes the ability of 2 nonfluorescent fragments of yellow fluorescent protein (YFP), when in proximity, to irreversibly interact with one another to reconstitute fluorescent YFP (37). BiFC has been used previously to demonstrate the ability of A1-ARs to form receptor-receptor complexes (38). Using a similar approach, the current study has used BiFC to detect the ability of A3-ARs to form receptor-receptor complexes. A3-ARs fused at the C terminus with the full-length YFP, the C-YFP fragment, or the N-YFP fragment to form A3-ARYFP, A3-ARC-YFP, and A3-ARN-YFP, respectively, were generated and transiently transfected into native CHO-K1 cells. No fluorescence above basal levels could be detected on single transfection of A3-ARC-YFP or A3-ARN-YFP. In contrast, on cotranfection of both A3-ARC-YFP and A3-ARN-YFP, fluorescence could be visualized at 24–28 h post-transfection, indicating that reconstitution of YFP had taken place. Similar to the full-length A3-ARYFP (Fig. 2A), reconstituted YFP fluorescence obtained following cotransfection of both A3-ARC-YFP and A3-ARN-YFP was predominantly distributed on the plasma membrane (Fig. 2B).
Figure 2.
Cell surface A3-AR homomeric interactions. A) CHO cells transiently expressing A3-ARYFP. B) CHO cells transiently expressing A3-ARN-YFP and A3-ARC-YFP. Images are single confocal equatorial slices, representative of those obtained from 3 wells in each of 4 separate transfections.
Differential effects of competitive ligands on the dissociation kinetics of ABA-X-BY630 from the human A1-AR and A3-AR
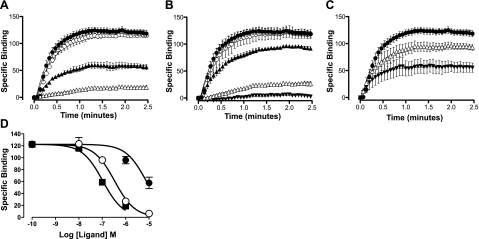
Investigation of the dissociation kinetics of ligand binding represents a sensitive method to detect changes in receptor conformation due to allosterism within a receptor monomer or across a receptor dimer/oligomer interface. This study has used a perfusion system in conjunction with confocal microscopy to quantify the dissociation kinetics of the adenosine BODIPY630/650 conjugate, ABA-X-BY630, from the A1-AR and A3-AR under conditions of infinite dilution (33). The equilibrium and functional properties of ABA-X-BY630 have been characterized previously (33, 39). The advantage of using the method of infinite dilution as compared to isotopic dilution is that the influence of competitive ligands on ABA-X-BY630 dissociation kinetics can be investigated. Under conditions of infinite dilution, the dissociation kinetics of ABA-X-BY630 should not be influenced by the addition of a second ligand, provided both are competing for the same site on a GPCR monomer. In contrast, if ligands bind simultaneously to two interacting sites, such as in the case of receptor dimerization or allosterism, cooperative interactions can mediate reciprocal changes to the rate of ligand dissociation. In the absence of competitive ligands, the dissociation rate constant of ABA-X-BY630 from A1-AR and A3-AR was 1.45 ± 0.05 and 0.57 ± 0.07 min−1 respectively (n=6 and 11 separate experiments, respectively, where each replicate represents the average of 10 cells). At CHO-A3 cells, the nonselective adenosine receptor antagonist, XAC, the nonselective adenosine receptor agonist, NECA, and the endogenous agonist, adenosine, mediated a concentration-dependent increase in the dissociation rate of ABA-X-BY630 (Figs. 3 and 4A–C). In contrast to the large increases observed at CHO-A3 cells, at CHO-A1 cells small but significant increases in the dissociation rate of ABA-X-BY630 were observed in the presence of 10 μM XAC, 100 μM NECA, and 100 μM adenosine (Fig. 4D–F).
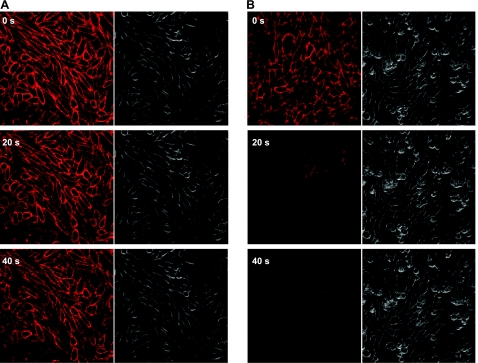
Figure 3.
Enhanced dissociation of a fluorescent adenosine derivative from the A3-AR in the presence of a competitive ligand. Binding of 30 nM ABA-X-BY630 to CHO-A3 cells after 0, 20 and 40 s of infinite dilution in the absence (A) or presence (B) of 1 μM XAC. Rate of perfusion was maintained at >12 complete fluid exchanges/min.
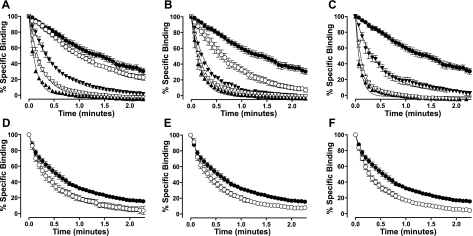
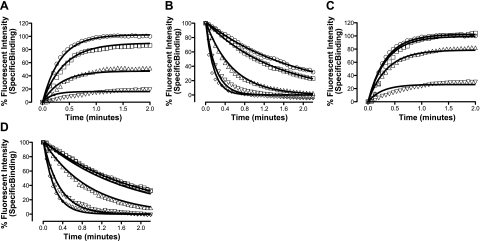
Figure 4.
Competitive ligands significantly enhance the dissociation of 30 nM ABA-X-BY630 from the A3-AR. A–C) ABA-X-BY630 (30 nM) dissociation kinetics from CHO-A3 cells in the absence (●) or presence of XAC (A; ○ 10 nM, ▾ 100 nM, ▿ 1 μM, ▴ 10 μM); NECA (B; ○ 100 nM, ▾ 1 μM, ▿ 10 μM, ▴ 100 μM); or adenosine (C; ▾ 1 μM, ▿ 10 μM, ▴ 100 μM). D–F) ABA-X-BY630 (30 nM) dissociation kinetics from CHO-A1 cells in the absence (●) or presence (○) of XAC (D; 10 μM), NECA (E; 100 μM) or adenosine (F; 100 μM). Rate of perfusion was maintained at >12 complete fluid exchanges/min. Specific binding data was normalized as a percentage of fluorescent intensity at t =0 and globally analyzed according to a monophasic exponential decay. Data points are expressed as means ± se from 3–11 separate experiments; each replicate represents the average of 10 cells.
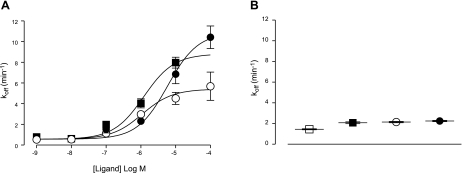
The concentration-dependent relationship between competitive ligand concentration and the dissociation rate constant of ABA-X-BY630 can clearly be seen in Fig. 5A. From these curves, the midpoint location parameter represents the affinity of the competitive ligands for the ABA-X-BY630-occupied receptor unit. Global analysis estimated the pEC50 for XAC, NECA, and adenosine at the A3-AR as 6.0 ± 0.1, 5.9 ± 0.1 and 5.2 ± 0.1, respectively (n=3–11 separate experiments, where each replicate represents the average of 10 cells). The fold-increase in the dissociation rate of ABA-X-BY630 for XAC, NECA, and adenosine at the A3-AR was ∼15, 9, and 19, respectively. At the A1-AR, the fold increase in the dissociation rate of ABA-X-BY630 in the presence of XAC (10 μM), NECA (100 μM), and adenosine (100 μM) was 1.5, 1.4, and 1.5, respectively (Fig. 5B).
Figure 5.
Concentration-koff relationship of ABA-X-BY630 dissociation in the absence and presence of competitive ligands at the A3-AR and A1-AR. Dissociation rate koff of 30 nM ABA-X-BY630 from CHO-A3 (A) and CHO-A1 cells (B) in the absence (□) or presence of XAC (■), NECA (○) and adenosine (●). Values for koff were derived from global analysis of dissociation kinetic data to a monophasic exponential decay.). Rate of perfusion was maintained at >12 complete fluid exchanges/min. Data points are expressed as means ± se from 3–11 separate experiments; each replicate represents the average of 10 cells.
Influence of competitive ligands on the association of ABA-X-BY630 to the human A3-AR stably transfected into CHO cells
The influence of XAC, NECA, and adenosine on the association of ABA-X-BY630 at the A3-AR was then investigated. Within these experiments, the perfusion system was used to expose CHO-A3 cells to 30 nM ABA-X-BY630 in the absence and presence of XAC (10 nM to 1 μM), NECA (10 nM to 10 μM), or adenosine (1–10 μM). XAC, NECA, and adenosine mediated a concentration-dependent inhibition in the binding and, as such, the plasma membrane fluorescence of 30 nM ABA-X-BY630 (Fig. 6A–C). Global analysis of the inhibition of plasma membrane fluorescence after 2 min exposure of 30 nM ABA-X-BY630 in the absence or presence of XAC (10 nM to 1 μM), NECA (10 nM to 10 μM), or adenosine (1–10 μM) estimated a pIC50 of 7.0 ± 0.1, 6.5 ± 0.1, and 5.1 ± 0.1, respectively (Fig. 6D; n=2–9 separate experiments, where each replicate represents the average of 10 cells).
Figure 6.
Competitive ligands inhibit the binding of 30 nM ABA-X-BY630 to the A3-AR. A–C) ABA-X-BY630 (30 nM) association kinetics at CHO-A3 cells in the absence (●) or presence of XAC (A; ○ 10 nM, ▴ 100 nM, ▵ 1 μM); NECA (B; ○ 10 nM, ▴ 100 nM, ▵ 1 μM, ▾ 10 μM); or adenosine (C; ▵ 1 μM, ▴ 10 μM). D) ABA-X-BY630 (30 nM) specific binding at 2 min association in the presence of XAC (■), NECA (○) and adenosine (●). Rate of perfusion was maintained at >12 complete fluid exchanges/min. Data points are expressed as means ± se from 2 to 11 separate experiments; each replicate represents the average of 10 cells.
Decreasing cooperative A3-AR homomeric interactions with a nonligand binding A3-AR, A3(N250A)-ARYFP
An enhancement in tracer dissociation kinetics can be indicative of allosteric interactions both within a monomeric receptor and/or across a receptor dimer. Distinguishing between the two different types of allosterism can be difficult. However, in contrast to interactions occurring within a monomeric receptor, cooperative interactions resulting from receptor–receptor interactions can potentially be inhibited through coexpression of a nonbinding receptor variant. The A3(N250A)-ARYFP is a C-terminal fusion of the A3-AR that contains an asparagine 250 to alanine point mutation, corresponding to position 6.55 using the numbering system of Ballesteros and Weinstein (40), which has been shown previously to decrease agonist and antagonist binding to nonspecific binding levels (41). As such, the A3(N250A)-ARYFP should act as a dominant negative if the cooperative interactions between ABA-X-BY630 and XAC, NECA, and adenosine are mediated across a receptor dimer .
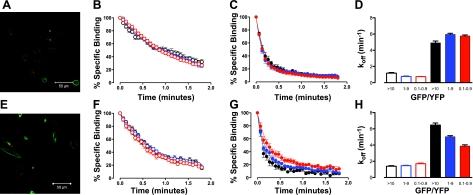
Spectral unmixing of GFP and YFP fluorescence when the A3-ARGFP was expressed alone lacked any detectable fluorescence in the YFP channel (Fig. 7A). Similarly, on expression of the A3(N250A)-ARYFP alone, no fluorescence was detectable in the GFP channel on spectral unmixing (Fig. 7B). The integrity of ABA-X-BY630 dissociation on coexpression of GFP- and YFP-tagged A3-ARs was assessed by cotransfection of the C-terminal A3-AR fusions, A3-ARGFP and A3-ARYFP. A3-ARGFP and A3-ARYFP were expressed predominantly on the cell surface (Fig. 8A). Within the one field view, GFP and YFP fluorescence varied considerably from cell to cell, and as such, individual cells were grouped into GFP/YFP fluorescence ratios of 0.1–0.9, 1–9, and >10. Within treatment groups, the dissociation curves of 30 nM ABA-X-BY630 in the absence (Fig. 8B) or presence (Fig. 8C) of 1 μM NECA were virtually superimposed for each GFP/YFP fluorescence ratio. Similar to previous experiments, the dissociation rate of 30 nM ABA-X-BY630 from CHO cells expressing A3-YFP and A3-GFP was significantly enhanced in the presence of 1 μM NECA (Fig. 8D) at all GFP/YFP fluorescence ratios.
Figure 7.
Cell surface expression of A3-AR fusions. Expression of A3-ARGFP (A; green channel) and A3(N250A)-ARYFP (B; red channel) in CHO cells. Images are single confocal equatorial slices.
Figure 8.
Nonbinding A3(N250A)-AR can disrupt cooperative interactions between ABA-X-BY630 and NECA at the A3-AR. A) Expression of A3-ARGFP (green channel) and A3-ARYFP (red channel) in CHO cells. B, C) Influence of A3-ARGFP/A3-ARYFP fluorescence ratio (red, >10; blue, 1–9; black, 0.1–0.9) on dissociation of 30 nM ABA-X-BY630 in the absence (B) and presence (C) of 1 μM NECA. D) Dissociation rate of 30 nM ABA-X-BY630 in the absence (open bars) and presence (solid bars) of 1 μM NECA; cells are grouped according to their A3-ARGFP/A3-ARYFP fluorescence ratio. E) Expression of A3-ARGFP (green channel) and A3(N250A)-ARYFP (red channel) in CHO cells. F, G) Influence of A3-ARGFP/A3(N250A)-ARYFP fluorescence ratio (red, >10; blue, 1–9; black, 0.1–0.9) on dissociation of 30 nM ABA-X-BY630 in the absence (F) and presence (G) of 1 μM NECA. H) Dissociation rate of 30 nM ABA-X-BY630 in the absence (open bars) and presence (solid bars) of 1 μM NECA; cells are grouped according to their A3-ARGFP/A3(N250A)-ARYFP fluorescence ratio. Rate of perfusion was maintained at >12 complete fluid exchanges/min. Data points are expressed as means ± se from 5 to 38 cells from ≥3 separate experiments.
On transient transfection of A3(N250A)-ARYFP alone, YFP fluorescence was predominantly observed at the cell surface (Fig. 7B). Despite being expressed at the cell surface, binding of ABA-X-BY630 (30 nM) above nonspecific binding levels was not observed at the A3(N250A)-ARYFP (data not shown). After coexpression of the C-terminal A3-AR fusions, A3-ARGFP and A3(N250A)-ARYFP, both GPCRs were predominantly expressed at the surface of healthy cells (Fig. 8E). Similar to previous experiments, individual cells within the same field of view were grouped into GFP/YFP fluorescence ratios of 0.1–0.9, 1–9, and >10. Under conditions of infinite dilution, 30 nM ABA-X-BY630 dissociation curves were virtually superimposed for all GFP/YFP fluorescence ratios (Fig. 8F). In contrast, in the presence of 1 μM NECA, the dissociation of 30 nM ABA-X-BY630 was slowed progressively when the proportion of A3(N250A)-ARYFP at the cell surface was increased (Fig. 8G). No significant difference in the dissociation rate of 30 nM ABA-X-BY630 was observed in the absence of NECA. In contrast, the dissociation rate of 30 nM ABA-X-BY630 in the presence of 1 μM NECA was significantly different at each GFP/YFP fluorescence ratio (Fig. 8H).
Application of a dynamic 2-drug model of receptor homodimerization to quantify allosterism across homodimeric A3-ARs
The study has used mathematical models to analyze ligand binding to receptors that exist as homodimers. Analytical solutions can be found for both the steady-state and dynamic model of receptor dimerization. Within this study, a parameter estimation method derived from the dynamic model of receptor dimerization was applied to time-course data for 30 nM ABA-X-BY630 in the absence and presence of XAC or NECA at the A3-AR (Fig. 9). This analysis provided estimates for the affinity constants for ABA-X-BY630, XAC, and NECA and cooperativity factors between A3-AR binding sites when the homodimer was simultaneously occupied by 2 ligands.
Figure 9.
A kinetic 2-drug model of GPCR homodimerization can fit experimental data. Parameter estimation methods were applied to the kinetic 2-drug model of GPCR homodimerization fitting the association (A) and dissociation (B) of 30 nM ABA-X-BY630 in the presence of increasing concentrations of XAC and the association (C) and dissociation (D) of 30 nM ABA-X-BY630 in the presence of increasing concentrations of NECA. Data points are expressed as the mean from 3 to 11 separate experiments; each replicate represents the average of 10 cells.
Kinetic data were analyzed using a sequential method. A computational parameter estimation method applied to 30 nM ABA-X-BY630 specific binding data alone estimated the equilibrium association constant for ABA-X-BY630, KA, and the cooperativity between the binding of 2 molecules of ABA-X-BY630, α (Table 1). These ABA-X-BY630 parameters were then constrained for subsequent global analysis of association and dissociation kinetic data in the presence of XAC and NECA (Table 1). Together, this sequential method of parameter estimation allowed estimation of ABA-X-BY630, XAC, and NECA equilibrium association constants as well as the cooperativity between binding sites on dual occupancy of the A3-AR homodimer (Table 1).
Table 1.
Affinity and cooperativity parameters for ABA-X-BY630, NECA, and XAC at A3-AR homodimers derived from a kinetic 2-drug model of GPCR homodimerization
| Agonist/antagonist | Log KA | α | β | γ |
|---|---|---|---|---|
| ABA-X-BY630 | 7.9 | < 0.01 | ||
| NECA | 7.0 | 0.18 | 0.13 | |
| XAC | 7.6 | 0.02 | 0.07 |
KA, logarithm of equilibrium association constants; α, cooperativity when 2 molecules of ABA-X-BY630 simultaneously bind the homodimer (value of α tended to the bounds set during the optimization, with α insensitive to relatively large increases in negative cooperativity above 0.010); β, cooperativity when homodimer is simultaneously bound by 2 molecules of the corresponding ligand; γ, cooperativity when homodimer is simultaneously bound by 1 molecule of ABA-X-BY630 and 1 molecule of the corresponding ligand.
DISCUSSION
This study has used confocal microscopy in conjunction with a rapid-exchange perfusion system to investigate cooperative interactions resulting from homodimerization of A1-ARs or A3-ARs at the surface of live cells. In contrast to the A1-AR, highly cooperative interactions between competitive ligands were observed at the A3-AR. These effects could be decreased significantly on coexpression of A3-ARGFP with the non-ligand-binding A3(N250A)-ARYFP, suggesting that the cooperativity between competitive ligands arises as a result of interactions between A3-ARs at the cell surface. Kinetic data were subsequently fit to a dynamic mathematical model of constitutive dimerization to allow, for the first time, quantification of the interactions between competitive ligands at a homodimeric GPCR in live cells.
Within classical GPCR pharmacology, ligands are defined by two system-independent properties, affinity and intrinsic efficacy. However, recent studies have identified GPCR ligand binding and signaling profiles that do not conform to this theoretical framework. The discovery of allosteric ligands and functionally selective orthosteric ligands introduced the phenomenon of probe dependence and, as such, the requirement for careful consideration of the tracer ligand and functional assays employed when screening for drug candidates (6). Additional layers of complexity are introduced when describing ligand binding and intracellular signaling events at GPCR dimers or higher order oligomers. Homomeric and heteromeric interactions between GPCRs have the potential to be dependent on probe, receptor density, and tissue. However, these system-dependent properties of GPCR-GPCR interactions may also represent potential novel therapeutic avenues for developing drug candidates that modulate GPCR intracellular signaling with greater functional and/or spatiotemporal selectivity (12).
To date, the majority of studies investigating GPCR dimerization in live cells have employed techniques such as resonance energy transfer (RET) or BiFC. Within the adenosine receptor family, BiFC between YFP fragments suggest that both the A1-AR and the A3-AR can form homomeric complexes (Fig. 2 and ref. 38). Although BiFC and RET between GPCRs can be very powerful in detecting receptor proximity in live cells, when used alone, these methods cannot establish the pharmacological or physiological consequences of GPCR-GPCR interactions. Cooperative interactions must be present for GPCR dimerization to have an influence on ligand binding and/or function; however, few studies have investigated this phenomenon in live cells (11, 12). Quantification of probe-dependent cooperativity factors can provide a mechanistic framework within which to understand the influence of dimerization on ligand-GPCR interactions and, as such, develop structure-activity relationships for ligands interacting across a dimer interface.
Investigation of the dissociation kinetics of a tracer ligand in the absence and presence of a second ligand represents a sensitive method to detect cooperative interactions between two topographically distinct binding sites. The fluorescent adenosine derivative ABA-X-BY630 used within this study has been well characterized previously (33, 39). At both CHO-A1 and CHO-A3 cells, ABA-X-BY630 retained the ability to stimulate calcium mobilization and ERK1/2 phosphorylation in a concentration-dependent manner. The association and dissociation specific binding kinetics of ABA-X-BY630 concentrations within the nanomolar range follow a monoexponential association and decay respectively. Together, these results suggest that, similar to endogenous adenosine, ABA-X-BY630 acts as a classical orthosteric agonist at both the A1-AR and A3-AR. The nonselective adenosine receptor antagonist XAC and the nonselective adenosine receptor agonists NECA and adenosine had varying influences on the dissociation of ABA-X-BY630, at the A1-AR and A3-AR. At the A3-AR, each of the nonselective ligands mediated a >9-fold enhancement in ABA-X-BY630 dissociation (Fig. 5A). In contrast, at the A1-AR, each of the ligands mediated a small, ∼1.5-fold, increase in ABA-X-BY630 dissociation (Fig. 5B). The influence of competitive ligands on ABA-X-BY630 dissociation at the A3-AR cannot reflect ABA-X-BY630 rebinding under conditions of infinite dilution or A3-AR sequestration from the cell surface. This is because the combination of ABA-X-BY630 association alone and ABA-X-BY630 dissociation in the presence of a saturating concentration of antagonist do not conform to the rules of simple mass action; that is, the observed association rate is slower than the dissociation rate. Furthermore, the significant difference between the A1-AR and A3-AR must be receptor specific, as the fluorescent ligand, competitive ligands, and cell background remain constant. Interestingly, cooperative interactions are unlikely to be a consequence of high receptor density, as the receptor expression is ∼4-fold greater in A1-CHO cells as compared to A3-CHO cells.
Cooperative interactions at the A3-AR receptor between ABA-X-BY630 and NECA could be inhibited in a concentration-dependent manner by the presence of the non-ligand-binding mutant A3-AR at the cell surface. These experiments involved cotransfection of A3-ARGFP and A3(N250A)-ARYFP into CHO cells. Spectral unmixing of confocal images represented a robust method to separate the fluorescence resulting from simultaneously excited GFP and YFP. Within a field of view, GFP/YFP fluorescence intensity ratios varied greatly from cell to cell. As such, single-cell analysis of cooperative interactions were determined for cells where the only variable was the ratio of A3-ARGFP to A3(N250A)-ARYFP. Increasing the proportion of A3(N250A)-ARYFP at the cell surface, which likely correlates with an increase of A3-ARGFP/A3(N250A)-ARYFP dimers, mediated a progressive decrease in the cooperativity between ABA-X-BY630 and NECA. Allosteric interactions within a monomeric receptor should not be influenced by the presence of a nonbinding mutant receptor. The observed decrease in cooperativity in the presence of A3(N250A)-ARYFP suggests the existence of A3-ARGFP/A3(N250A)-ARYFP dimers and no influence of the non-ligand-binding protomer on the ligand-binding protomer. These results support the suggestion that in CHO-A3 cells, the observed allosteric interactions are being mediated across an A3-AR homomeric complex.
The similar effects of agonists and antagonists with respect to the increase in ABA-X-BY630 dissociation from the A3-AR are consistent with a number of previous studies describing homomeric and heteromeric interactions and suggest that cooperative interactions across a dimeric GPCR interface do not necessarily require receptor activation (11, 12). A recent study at the dopamine D2 receptor, investigating the downstream signaling consequences of GPCR-GPCR interactions, observed opposing cooperative interactions between agonist-agonist and agonist-inverse agonist binding to the D2 receptor homodimer (42). In contrast to dopamine D2 receptor homdimers, cooperativity at adenosine A3 receptor homodimers does not correlate with ligand efficacy. However, given the complex allosteric nature of GPCR homomers and heteromers, it is perhaps not surprising that allosteric interactions can vary depending on the receptor, probe, and tissue investigated.
To quantify allosteric interactions, a mechanistic framework is required. The general aim of mathematical modeling within GPCR pharmacology is to develop a model that can describe allosteric interactions adequately using a minimum number of parameters. The simplest model describing allosteric modulation of ligand affinity at constitutive heterodimers is the ternary complex model. In the case of constitutive homodimerization, the ternary complex model must be extended to allow simultaneous occupancy of the homodimer by 2 molecules of the same ligand. Within this model, binding is dependent on the affinity of the ligand for both the free and singularly occupied homodimer. When describing the binding of 2 structurally different ligands, the simple model of constitutive GPCR homodimerization is extended, now described by 2 affinity constants and 3 cooperativity factors. A kinetic version of constitutive GPCR homodimerization was derived within these studies to quantify cooperative interactions between competitive ligands at A3-AR homodimers. Within this model, the assumption that A3-ARs exist as constitutive homodimers is consistent with previous studies suggesting that GPCRs are born, function, and die as dimers (43, 44). The receptor homodimerization model yields analytical solutions for bound fractions as functions of the binding parameters and ligand concentrations, and also of time for the dynamic systems. A relatively inexpensive computational parameter estimation routine could therefore be employed to obtain ligand rate constant and cooperativity estimates. Interactions between simultaneously bound homodimeric A3-AR sites were generally found to be negatively cooperative. Analysis of kinetic data represents a high content approach to investigating GPCR-GPCR interactions. Furthermore, kinetic analysis of GPCR pharmacology may represent a more physiologically relevant approach to understanding endogenous ligand-binding events that are rarely at equilibrium within the body.
An unexpected observation within these studies is the contrasting influence of nonselective ligands on the dissociation kinetics of ABA-X-BY630 from the A1-AR as compared to the A3-AR. The A3-AR is more rapidly desensitized when compared to the other members of the AR (45). Enhanced dissociation on 2 molecules of adenosine binding to an A3-AR homodimer may reflect an additional physiological mechanism to reduce the residence time of the endogenous adenosine at pathophysiologically high concentrations. With respect to drug discovery, increased A3-AR expression within a number of different types of tumor cells has been suggested to have a role in cell proliferation and migration. As such, if A3-AR homomerization is receptor density and/or tissue dependent, ligands that distinguish between A3-AR monomers and homomers may represent a novel therapeutic strategy for selective targeting of tumor cells in the treatment of cancer (46).
The A3-AR is a potential novel therapeutic target for a number of conditions, including inflammatory disorders and cancer (46, 47). This study has identified and quantified novel homomeric interactions between native A3-ARs. The kinetic and mathematical approach used within this study represents a powerful method to detect and interpret cooperative interactions between allosteric and/or orthosteric ligands. An additional advantage of this approach is that it is readily amenable to study interactions between endogenously expressed GPCRs within a physiological context.
Supplementary Material
Acknowledgments
The authors thank N.D. Holliday for useful discussions.
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BB/D008522) and the UK Medical Research Council (G0800006). L.T.M. is an Australian National Health and Medical Research Council Postdoctoral Research Fellow. L.T.M. designed and performed kinetic fluorescent ligand-binding assays and analysis. L.J.B. developed and performed parameter estimation methods. L.A.S., S.J.B., and S.J.H. generated the AR constructs. S.J.B. performed the BiFC experiments. L.T.M., L.J.B., S.J.B., and S.J.H. designed experiments and interpreted results. All authors participated in the writing and editing of the manuscript. S.J.H. managed the overall project.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Bortolato A., Mobarec J. C., Provasi D., Filizola M. (2009) Progress in elucidating the structural and dynamic character of G protein-coupled receptor oligomers for use in drug discovery. Curr. Pharm. Des. 15, 4017–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Overington J. P., Al-Lazikani B., Hopkins A. L. (2006) How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 [DOI] [PubMed] [Google Scholar]
- 3. Kenakin T., Onaran O. (2002) The ligand paradox between affinity and efficacy: can you be there and not make a difference? Trends Pharmacol. Sci. 23, 275–280 [DOI] [PubMed] [Google Scholar]
- 4. Birdsall N. J., Lazareno S. (2005) Allosterism at muscarinic receptors: ligands and mechanisms. Mini Rev. Med. Chem. 5, 523–543 [DOI] [PubMed] [Google Scholar]
- 5. Christopoulos A. (2002) Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 1, 198–210 [DOI] [PubMed] [Google Scholar]
- 6. May L. T., Leach K., Sexton P. M., Christopoulos A. (2007) Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 47, 1–51 [DOI] [PubMed] [Google Scholar]
- 7. Kenakin T. (2007) Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol. Sci. 28, 407–415 [DOI] [PubMed] [Google Scholar]
- 8. Whalen E. J., Rajagopal S., Lefkowitz R. J. (2011) Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol. Med. 17, 126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casado V., Cortes A., Mallol J., Perez-Capote K., Ferre S., Lluis C., Franco R., Canela E. I. (2009) GPCR homomers and heteromers: a better choice as targets for drug development than GPCR monomers? Pharmacol. Ther. 124, 248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Springael J. Y., Urizar E., Costagliola S., Vassart G., Parmentier M. (2007) Allosteric properties of G protein-coupled receptor oligomers. Pharmacol. Ther. 115, 410–418 [DOI] [PubMed] [Google Scholar]
- 12. Smith N. J., Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol. Rev. 62, 701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Attwood T. K., Findlay J. B. (1994) Fingerprinting G-protein-coupled receptors. Protein Eng. 7, 195–203 [DOI] [PubMed] [Google Scholar]
- 14. Kolakowski L. F., Jr. (1994) GCRDb: a G-protein-coupled receptor database. Receptors Channels 2, 1–7 [PubMed] [Google Scholar]
- 15. Pin J. P., Galvez T., Prezeau L. (2003) Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 98, 325–354 [DOI] [PubMed] [Google Scholar]
- 16. Birdsall N. J. (2010) Class A GPCR heterodimers: evidence from binding studies. Trends Pharmacol. Sci. 31, 499–508 [DOI] [PubMed] [Google Scholar]
- 17. Milligan G. (2009) G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 158, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pin J. P., Neubig R., Bouvier M., Devi L., Filizola M., Javitch J. A., Lohse M. J., Milligan G., Palczewski K., Parmentier M., Spedding M. (2007) International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 59, 5–13 [DOI] [PubMed] [Google Scholar]
- 19. Kuszak A. J., Pitchiaya S., Anand J. P., Mosberg H. I., Walter N. G., Sunahara R. K. (2009) Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J. Biol. Chem. 284, 26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U. S. A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whorton M. R., Jastrzebska B., Park P. S., Fotiadis D., Engel A., Palczewski K., Sunahara R. K. (2008) Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J. Biol. Chem. 283, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Armstrong D., Strange P. G. (2001) Dopamine D2 receptor dimer formation: evidence from ligand binding. J. Biol. Chem. 276, 22621–22629 [DOI] [PubMed] [Google Scholar]
- 23. Brea J., Castro M., Giraldo J., Lopez-Gimenez J. F., Padin J. F., Quintian F., Cadavid M. I., Vilaro M. T., Mengod G., Berg K. A., Clarke W. P., Vilardaga J. P., Milligan G., Loza M. I. (2009) Evidence for distinct antagonist-revealed functional states of 5-hydroxytryptamine(2A) receptor homodimers. Mol. Pharmacol. 75, 1380–1391 [DOI] [PubMed] [Google Scholar]
- 24. Chidiac P., Green M. A., Pawagi A. B., Wells J. W. (1997) Cardiac muscarinic receptors. Cooperativity as the basis for multiple states of affinity. Biochemistry 36, 7361–7379 [DOI] [PubMed] [Google Scholar]
- 25. Christopoulos A., Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 54, 323–374 [DOI] [PubMed] [Google Scholar]
- 26. Durroux T. (2005) Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol. Sci. 26, 376–384 [DOI] [PubMed] [Google Scholar]
- 27. Franco R., Casado V., Mallol J., Ferrada C., Ferre S., Fuxe K., Cortes A., Ciruela F., Lluis C., Canela E. I. (2006) The two-state dimer receptor model: a general model for receptor dimers. Mol. Pharmacol. 69, 1905–1912 [DOI] [PubMed] [Google Scholar]
- 28. Franco R., Casado V., Mallol J., Ferre S., Fuxe K., Cortes A., Ciruela F., Lluis C., Canela E. I. (2005) Dimer-based model for heptaspanning membrane receptors. Trends Biochem. Sci. 30, 360–366 [DOI] [PubMed] [Google Scholar]
- 29. Rovira X., Pin J. P., Giraldo J. (2010) The asymmetric/symmetric activation of GPCR dimers as a possible mechanistic rationale for multiple signalling pathways. Trends Pharmacol. Sci. 31, 15–21 [DOI] [PubMed] [Google Scholar]
- 30. Rovira X., Vivo M., Serra J., Roche D., Strange P. G., Giraldo J. (2009) Modelling the interdependence between the stoichiometry of receptor oligomerization and ligand binding for a coexisting dimer/tetramer receptor system. Br. J. Pharmacol. 156, 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wreggett K. A., Wells J. W. (1995) Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J. Biol. Chem. 270, 22488–22499 [DOI] [PubMed] [Google Scholar]
- 32. May L. T., Briddon S. J., Hill S. J. (2010) Antagonist selective modulation of adenosine A1 and A3 receptor pharmacology by the food dye Brilliant Black BN: evidence for allosteric interactions. Mol. Pharmacol. 77, 678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. May L. T., Self T. J., Briddon S. J., Hill S. J. (2010) The effect of allosteric modulators on the kinetics of agonist-G protein-coupled receptor interactions in single living cells. Mol. Pharmacol. 78, 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christopoulos A., Lanzafame A., Ziegler A., Mitchelson F. (1997) Kinetic studies of co-operativity at atrial muscarinic M2 receptors with an “infinite dilution” procedure. Biochem. Pharmacol. 53, 795–800 [DOI] [PubMed] [Google Scholar]
- 35. Cordeaux Y., Briddon S. J., Megson A. E., McDonnell J., Dickenson J. M., Hill S. J. (2000) Influence of receptor number on functional responses elicited by agonists acting at the human adenosine A(1) receptor: evidence for signaling pathway-dependent changes in agonist potency and relative intrinsic activity. Mol. Pharmacol. 58, 1075–1084 [DOI] [PubMed] [Google Scholar]
- 36. Cordeaux Y., Briddon S. J., Alexander S. P., Kellam B., Hill S. J. (2008) Agonist-occupied A3 adenosine receptors exist within heterogeneous complexes in membrane microdomains of individual living cells. FASEB J. 22, 850–860 [DOI] [PubMed] [Google Scholar]
- 37. Rose R. H., Briddon S. J., Holliday N. D. (2010) Bimolecular fluorescence complementation: lighting up seven transmembrane domain receptor signalling networks. Br. J. Pharmacol. 159, 738–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Briddon S. J., Gandia J., Amaral O. B., Ferre S., Lluis C., Franco R., Hill S. J., Ciruela F. (2008) Plasma membrane diffusion of G protein-coupled receptor oligomers. Biochim. Biophys. Acta 1783, 2262–2268 [DOI] [PubMed] [Google Scholar]
- 39. Middleton R. J., Briddon S. J., Cordeaux Y., Yates A. S., Dale C. L., George M. W., Baker J. G., Hill S. J., Kellam B. (2007) New fluorescent adenosine A1-receptor agonists that allow quantification of ligand-receptor interactions in microdomains of single living cells. J. Med. Chem. 50, 782–793 [DOI] [PubMed] [Google Scholar]
- 40. Ballesteros J. A., Weinstein H. (1995) Integrated methods for the construction of three dimensional models and computational probing of structure function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 [Google Scholar]
- 41. Gao Z. G., Chen A., Barak D., Kim S. K., Muller C. E., Jacobson K. A. (2002) Identification by site-directed mutagenesis of residues involved in ligand recognition and activation of the human A3 adenosine receptor. J. Biol. Chem. 277, 19056–19063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han Y., Moreira I. S., Urizar E., Weinstein H., Javitch J. A. (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 5, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Agnati L. F., Ferre S., Lluis C., Franco R., Fuxe K. (2003) Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol. Rev. 55, 509–550 [DOI] [PubMed] [Google Scholar]
- 44. Bouvier M. (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2, 274–286 [DOI] [PubMed] [Google Scholar]
- 45. Gao Z. G., Jacobson K. A. (2008) Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol. Res. 57, 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobson K. A., Gao Z. G. (2006) Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5, 247–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasko G., Linden J., Cronstein B., Pacher P. (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.