Abstract
The thyroid-stimulating hormone (TSH) receptor signals via Gs to produce cAMP and via Gq/11 to produce inositol-1,4,5-trisphosphate, which is degraded to inositol monophosphate (IP1; phosphoinositide signaling). The potency of TSH for cAMP signaling is higher than for phosphoinositide signaling, and it was suggested that there are “spare receptors” for cAMP signaling. In a human embryonic kidney macrophage scavenger receptor-expressing (HEK-EM) 293 model system, there are no spare receptors, but the cells still exhibited 100-fold differences in potencies. Dose responses for TSH-stimulated dissociation of prebound 125I-TSH (negative cooperativity; EC50=70 mU/ml), which requires TSH binding to both sites of the TSH receptor (TSHR) homodimer, and TSH-stimulated IP1 production (EC50=50 mU/ml) were indistinguishable. Fluorescence resonance energy transfer (FRET) using tagged receptors showed that TSHR formed homodimers and heterodimers with two binding-deficient mutant TSHRs, L252P and C41S. When L252P or C41S was expressed with TSHR, that is, when TSHR/L252P or TSHR/C41S heterodimers could only bind one TSH, TSH-stimulated IP1 production was decreased relative to cAMP production. The slopes of linear regression analyses comparing fold stimulation by TSH of IP1 vs. cAMP production were 0.044 ± 0.0047, 0.0043 ± 0.0041, and 0.0059 ± 0.0014 for cells expressing TSHR alone, TSHR and L252P, or TSHR and C41S, respectively. We suggest that TSHR coupling to phosphoinositide signaling is dependent on binding 2 molecules of TSH to TSHR homodimer, causing a conformational change allowing coupling to Gq/11.—Allen, M. D., Neumann, S., Gershengorn, M. C. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling.
Keywords: GPCR, inositolphosphates, IP1, Gq/11 coupling
The thyrotropin [thyroid-stimulating hormone (TSH)] receptor (TSHR) in human thyroid cells couples to several G proteins (1), including the stimulatory G protein (Gs), which activates adenylyl cyclase to produce cAMP (cAMP signaling), and Gq/11, which activates phospholipase C to produce inositol-1,4,5-trisphosphate (I-1,4,5-P3), which is rapidly degraded to inositol monophosphate (IP1; phosphoinositide signaling). It has been appreciated for more than 25 yr that the potency for TSH to stimulate cAMP signaling is much higher than for phosphoinositide signaling (2). Similarly, large differences in potencies for activation by their cognate agonists for cAMP and phosphoinositide signaling have been found for some G-protein-coupled receptors (GPCRs), including the calcitonin (3, 4) and secretin receptors (5), but not for others, such as the parathyroid hormone (4) and histamine H2 receptors (6). The reason for the differences in potencies for those receptors that exhibit them is unclear; however, it has been suggested that there may be “spare receptors” for cAMP signaling but not for phosphoinositide signaling. Spare receptors are present when cells are sensitized to agonists because there is an excess number of receptors, and not all receptors need be occupied for maximal signaling.
Another feature that the TSHR shares with the receptor for secretin is that both exhibit negatively cooperative binding of their cognate ligands (7, 8). Negatively cooperative binding is the phenomenon detected when the rate of dissociation of prebound ligand is increased in the presence of added ligand and is, in general, dependent on receptor dimerization (9). (We use the term “dimerization,” although it is possible that the receptor complex contains more than two receptors.) For both TSHR (10) and the secretin receptor (8), negative cooperativity has been shown to be dependent on homodimerization. Moreover, as has been proposed for other GPCRs (11, 12), the secretin receptor appears to couple to G proteins as a homodimer (8). Although it has been shown that heterodimerization with binding to sites on both protomers of a receptor heterodimer leads to activated GPCRs with distinct signaling characteristics (13, 14), including activation of both protomers of a D1/D2 heterodimeric dopamine receptor coupling to Gq, even though monomers or homodimers do not activate Gq (15), to our knowledge, a role for homodimerization and binding of a single natural agonist to both protomers of a naturally occurring receptor homodimer in activating different G proteins has not been demonstrated for any class A GPCR.
In this report, we present evidence that although coupling to Gs protein to activate cAMP signaling may be mediated by TSH binding to one protomer of a TSHR homodimer, coupling to Gq/11 to activate the phosphoinositide pathway appears to be dependent on TSH binding to both protomers of the TSHR homodimer.
MATERIALS AND METHODS
Cell culture and transfection
Human embryonic kidney macrophage scavenger receptor-expressing (HEK-EM) 293 cells were grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 10 μg/ml streptomycin (Life Technologies Corp., Carlsbad, CA, USA) at 37 C in a humidified 5% CO2 incubator. Cells were transiently transfected with plasmids encoding TSHR, L252P, C41S, or “empty” plasmids in 24-well plates (7.5×104 cells/well) with 0.005 to 0.5 μg DNA/well or on poly-d-lysine-coated coverglass culture dishes (MatTek Corp., Ashland, MA, USA) using FuGene6 reagent (Roche, Indianapolis, IN, USA). In an individual experiment, the total plasmid amount was kept constant by adding the appropriate amount of empty plasmid. Experiments were performed 20 to 72 h after transfection.
125I-TSH and antibody binding
To measure 125I-TSH binding, cells were incubated in buffer (HBSS/HEPES containing 2.5% milk powder and 0.2% BSA) with 60,000 cpm bovine 125I-TSH (Brahms Aktiengesellschaft, Hennigsdorf, Germany) for 90 min at room temperature, washed 3 times with 0.5 ml ice-cold HBSS, and lysed with 0.5 ml 0.4N NaOH. The lysates were then counted. Total binding was measured in the absence of unlabeled TSH, and nonspecific binding in the presence of 100 mU/ml unlabeled bovine TSH. Specific binding was calculated as total minus nonspecific binding.
To measure 125I-TSH binding dissociation, cells were incubated in HBSS/HEPES containing 2.5% milk powder and 0.2% BSA with 60,000 cpm bovine 125I-TSH (Brahms Aktiengesellschaft) for 90 min at room temperature, washed 3 times with 0.5-ml ice-cold HBSS, and then incubated in buffer without (control) or with various concentrations of unlabeled TSH (0 to 300 mU/ml) for the designated times. Thereafter, the cells were washed 3 times with 0.5 ml ice-cold HBSS and then lysed with 0.5 ml 0.4N NaOH.
To measure cell surface receptor expression by antibody binding, we used 2 antibodies directed at 2 different epitopes on the TSHR amino-terminal ectodomain: 2C11, targeted to aa 354–359 (Serotec, Oxford, UK), and 4C1, targeted to aa 378–384 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cells were harvested at 48 h after transfection by treatment with 1 mM EDTA/1 mM EGTA in PBS, washed once with PBS containing 0.1% BSA and 0.1% NaN3 (binding buffer), and then incubated in 1:200 dilutions of 2C11 or 4C1 for 30 min. Cells were then washed and incubated for 30 min with a 1:200 dilution of an Alexa-Fluor 488 conjugated F(ab′)2 fragment of goat-anti-mouse IgG (Molecular Probes, Eugene, OR, USA) in buffer. After a final wash, cells were fixed with 1% paraformaldehyde and analyzed 24 h later by FACS (FACSCalibur; BD Biosciences, San Jose, CA). Receptor expression was determined by the percentage of analyzed singlet cells found in a gate that contained few or no positive cells during analysis of empty plasmid transfectants.
cAMP and IP1 production
Cells seeded into 24-well plates at a density of 2.2 × 105 cells/well were cultured for 24 h. Then they were washed with 0.5 ml of 37 C HBSS and incubated in HBSS/10 mM HEPES (pH 7.4) for 30 min. To determine the effects of TSH stimulation on cAMP production, we measured cAMP in cells incubated in a humidified incubator at 37 C for 60 min in 0.2 ml HBSS/HEPES containing various concentrations (0–300 mU/ml) of TSH (bovine TSH; Sigma, St. Louis, MO, USA) and 1 mM 3-isobutyl-1-methylxanthine (IBMX). Total cAMP production was measured by adding 0.20 ml lysis buffer (cAMP-Screen Direct System; Applied Biosystems, Foster City, CA, USA) and measuring cAMP in the samples, according to the manufacturer's protocol. Chemiluminescence was measured in a Victor3 V 1420 multilabel counter (Perkin Elmer, Waltham, MA, USA).
To determine the effects of TSH stimulation on IP1 production, total IP1 was measured under the same conditions as described above for cAMP, with the exception of using 50 mM LiCl instead of IBMX. The incubations were terminated by adding 0.05 ml lysis buffer (IP-One ELISA kit; CIS Bio International, Bagnols-sur-Cèze, France), and IP1 content was determined according to the manufacturer's protocol. Optical density was measured in SpectraMax Plus384 (Molecular Devices, Sunnyvale, CA, USA).
Fluorescent microscopy
Fluorescently tagged TSHRs were generated by PCR amplification of the gene with primers that introduce a 5′-XhoI restriction digest site and a 3′-EcoRI restriction digest site followed by digestion and ligation into likewise cut pAcGFP1-N1 (Clontech, Mountain View, CA, USA) or mCherry-N1 (16) mammalian expression vectors. Expression in both cases is driven by a CMV promoter.
GFP-tagged thyrotropin-releasing hormone receptor subtype 1 (TRHR1) was generated by PCR amplification of the gene with primers that introduce a 5′-HindIII restriction digest site and 3′-BamHI restriction digest site, after which product was digested and ligated into likewise cut pAcGFP1-N1 (Clontech) for mammalian expression.
HEK-EM 293 cells were seeded onto 35-mm MatTek dishes coated with poly-d-lysine at a density of 2 × 105 cells/dish in growth medium. After 24 h, the cells were transiently transfected using FuGene 6 with TSHR tagged with RFP and TSHR variants or thyrotropin-releasing hormone receptor subtype 1 tagged with GFP from Aequorea coerulescens (AcGFP). Notably, GFP and RFP constructs were transfected in a 1:1 ratio (0.5 μg of each plasmid per dish). After 20 h, HBSS supplemented with 20 mM HEPES replaced growth medium, and cells were imaged on a Zeiss 510 NLO/Meta system, using a Plan-Neofluor ×40/N.A. 1.3 objective (Carl Zeiss, Oberkochen, Germany). The pinhole was completely open to generate epifluorescent images. GFP was excited with a 477-nm laser at 75% transmission and detected using a 510/20 emission filter. RFP was excited with a 546-nm laser at 50% transmission and detected with a 600- to 650-nm emission filter. GFP to RFP fluorescence resonance energy transfer (GR FRET) was detected by exciting GFP with the 477-nm laser at 75% transmission, passing RFP emission through not only a 488 dichroic but also a 545-nm dichroic to a 685-nm short pass emission filter. Detector gains were set at 400 for GFP and GR FRET and 600 for RFP detection. Detector gains and microscope parameters remained unchanged throughout all experiments. Cell images were captured every 30 s for up to 5 min to establish a FRET ratio. Cells expressing GFP or RFP alone were used to determine correction coefficients for the bleedthrough of these fluorophores into the FRET channel. With these settings, AcGFP emission made up only 12% of the FRET channel intensity, while mCherry contributed 5%. These contaminants were subtracted from the FRET channel intensity and then the FRET ratio of individual cells was determined. FRET ratios were normalized by finding the percentage of a given ratio to the FRET ratio of TSHR-GFP- and TSHR-RFP-expressing cells.
Statistical analyses
Significance was determined by t test or ANOVA; P < 0.05 was considered significant.
RESULTS
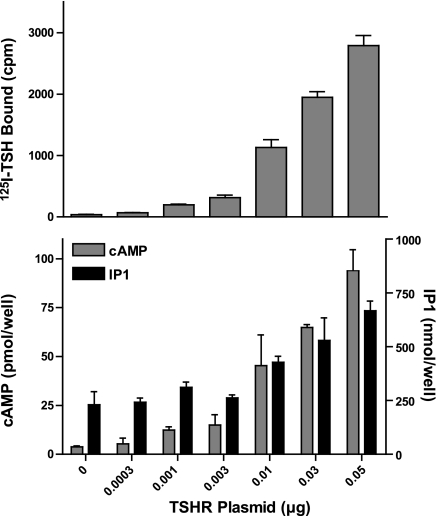
As it has been suggested that the differences in potencies of stimulation of cAMP and phosphoinositide signaling by TSH may be because there are spare receptors for cAMP but not for IP1 production, we established a model cell system in which there were no or few spare receptors for cAMP or phosphoinositide signaling. Figure 1 illustrates the important characteristic of this system, in which transfection of HEK-EM 293 cells with increasing amounts of TSHR-expressing plasmids up to 0.05 μg caused a progressive increase in the level of TSHR expression, as measured by 125I-TSH binding, and in which the levels of cAMP and IP1 production stimulated by TSH increased with increasing TSHR levels. We measured phosphoinositide signaling as IP1 production because I-1,4,5-P3 is rapidly metabolized to IP1, which accumulates in the presence of LiCl, inhibitor of IP1 monophosphatase. This strategy is similar to quantifying cAMP signaling by measuring cAMP accumulation in the presence of IBMX, which inhibits cAMP phosphodiesterases. As there was a progressive increase in the maximal level of cAMP (and IP1) production with increasing TSHR expression, this system does not exhibit spare receptors for cAMP (or phosphoinositide) signaling.
Figure 1.
Absence of spare receptors for cAMP or IP1 production in HEK-EM 293 cells transiently expressing TSHRs under controlled conditions. HEK-EM 293 cells were transiently transfected with the designated amounts of TSHR-expressing plasmids. After 48 h, 125I-TSH binding (top panel) and stimulation of cAMP and IP1 production by 100 mU/ml TSH (bottom panel) were measured as described in Materials and Methods. Results shown are from 1 of 3 experiments performed in triplicate. Bars represent means ± sd.
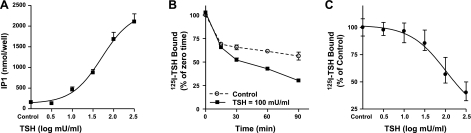
To begin to test the hypothesis that TSH stimulation of phosphoinositide signaling required binding to both protomers of the TSHR homodimer, we compared the dose dependency of TSH stimulation of IP1 production with that of negatively cooperative 125I-TSH binding, which is known to require binding of unlabeled TSH to the low-affinity site on the second protomer (10). Figure 2A illustrates the dose-dependent stimulation of IP1 production. There was a 13-fold stimulation by TSH with an EC50 of ∼50 mU/ml; the EC50 for cAMP production was 0.7 mU/ml (not shown). Figure 2B illustrates the time course and Fig. 2C the dose-dependency of TSH enhancement of dissociation of prebound 125I-TSH. At 90 min, 70% of the originally bound 125I-TSH had dissociated, and the EC50 for negative cooperativity was ∼70 mU/ml. Thus, the potencies of TSH for phosphoinositide signaling and negative cooperativity were similar.
Figure 2.
Comparison between the dose dependencies of TSH stimulation of IP1 production and enhanced dissociation of prebound 125I-TSH (negative cooperativity). Bars represent means ± sd. A) TSH stimulation of IP1 production. IP1 production was measured as described in Materials and Methods. Results shown are from two experiments performed in duplicate. B) Time course of 125I-TSH dissociation. 125I-TSH dissociation was performed as described in Materials and Methods in the absence (control) or presence of 100 mU/ml TSH. Results shown are from 2 experiments performed in duplicate. C) TSH stimulation of 125I-TSH dissociation. 125I-TSH dissociation was performed as described in Materials and Methods in the absence (control) or presence of various concentrations of TSH. Results shown are from 2 experiments performed in triplicate.
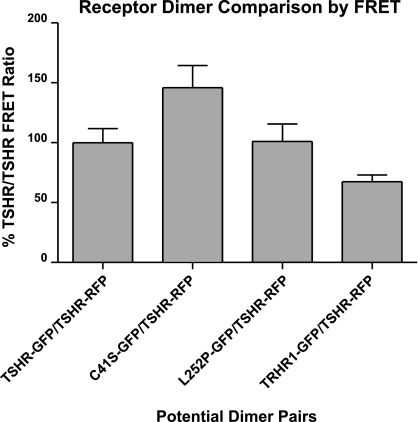
To test the hypothesis more directly, we proposed that heterodimers comprising TSHR and a TSHR mutant that could not bind TSH should be able to stimulate cAMP, but not phosphoinositide signaling. Therefore, we cotransfected plasmids encoding TSHR and a naturally occurring mutant TSHR in which Leu at position 252 was replaced by Pro (L252P; ref. 17), or another naturally occurring mutant TSHR in which Cys at position-41 was replaced by Ser (C41S; ref. 18). L252P and C41S do not bind 125I-TSH, but they signal via the phosphoinositide pathway when activated by a small-molecule receptor agonist (ref. 19 and Supplemental Fig. S1). To show that these two mutant receptors dimerize with TSHR, we measured the propensity of TSHR and L252P or C41S to form dimers using FRET between GFP and RFP as readout. Because it is known that TSHRs can form homodimers, the FRET ratio in cells that express TSHR/TSHR homodimers was used as a standard to normalize heterodimerization of TSHR and a TSHR mutant. As shown in Fig. 3, when cells express either L252P-GFP or C41S-GFP and TSHR-RFP, the resulting FRET ratios are at least that of cells expressing TSHR-GFP and TSHR-RFP. The FRET ratio between C41S-GFP and TSHR-RFP is higher than in the case of the wild-type homodimer, indicating that C41S might have a higher propensity to dimerize with TSHR than another wild-type receptor. Conversely, when cells express tagged TSHR and thyrotropin-releasing hormone receptor subtype 1 (TRHR1), another class A GPCR (20), the FRET ratio was lower than in cells expressing TSHRs, indicating that TSHR and TRHR1 are less likely to dimerize. Thus, TSHR/TSHR, TSHR/L252P, and TSHR/C41S dimers form in HEK-EM 293 cells.
Figure 3.
TSHR/TSHR, TSHR/L252P, and TSHR/C41S dimers form in HEK-EM 293 cells, as detected by FRET microscopy. HEK-EM 293 cells were transiently transfected with TSHR-RFP and TSHR-GFP or L252P-GFP or C41S-GFP or thyrotropin-releasing hormone receptor subtype 1 (TRHR1)-GFP. After 24 h, cells were imaged and the FRET ratio of intensities of GFP to RFP FRET divided by GFP was calculated for each cell. All ratios were normalized to those of cells expressing TSHR-RFP and TSHR-GFP (100%). Bars represent means ± se.
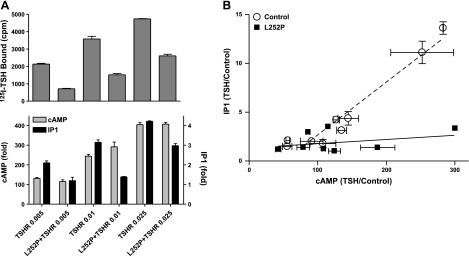
We then measured signaling in cells transfected with 5- to 20-fold more L252P than TSHR to favor formation of TSHR/L252P heterodimers and limit TSHR/TSHR homodimers. L252P monomers and L252P/L252P homodimers do not bind TSH, nor are they activated by TSH and are not well expressed, as very low levels were found on the cell surface in cells transfected with L252P alone (17). To compare signaling, we attempted to express similar levels of TSHR/TSHR and TSHR/L252P dimers that could be activated by TSH. We estimated receptor expression in two ways. First, we assumed that low concentrations of 125I-TSH would bind only to the high-affinity site on homodimers or heterodimers and that 125I-TSH binding would accurately reflect expression levels of TSHR/TSHR and TSHR/L252P dimers; TSHR monomers would bind 125I-TSH also. Second, we estimated receptor expression using two antibodies directed at two different epitopes on the TSHR amino-terminal ectodomain: 2C11, targeted to amino acids from 354 to 359; and 4C1, targeted to amino acids from 378 to 384. Cells cotransfected with more L252P-expressing plasmid than TSHR-expressing plasmid exhibited cell-surface receptor levels 37, 21, or 32% of that in cells transfected with the same amount of TSHR-expressing plasmid alone, as estimated by 125I-TSH, 2C11 antibody, or 4C1 antibody binding, respectively. By using different amounts of TSHR-expressing plasmid when transfected alone than when it was transfected with plasmids expressing L252P, we were able to obtain similar levels of 125I-TSH binding (see, for example, Fig. 5A). Thus, we were able to establish conditions under which there were levels of TSHR/TSHR dimers in cells expressing TSHRs alone that were similar to the levels of TSHR homodimers and TSHR/L252P heterodimers in cells expressing TSHRs and L252Ps.
Figure 5.
Effects of TSH to stimulate cAMP and IP1 production in cells expressing TSHRs alone compared to cells expressing TSHRs and L252Ps. A) Cells were transfected with TSHR alone (0.005, 0.01, or 0.025 μg TSHR plasmid+0.5 μg empty plasmid) or with L252P (0.5 μg plasmid) + TSHR (0.005, 0.01, or 0.025 μg TSHR plasmid). After 48 or 72 h, 125I-TSH binding (top panel) and stimulation of cAMP and IP1 production by 100 mU/ml TSH (bottom panel) were measured as described in Materials and Methods. Results shown are from 1 of 4 experiments performed in duplicate. Bars represent means ± range. B) Comparison of the effects of TSH to stimulate IP1 and cAMP production. Stimulation of cAMP and IP1 production by 100 mU/ml TSH were measured as described in Materials and Methods. Results are presented as fold stimulation over control (TSH/control) from 4 experiments performed in duplicate. Points represent means ± se. Lines represent linear regression analyses of data from cells expressing TSHRs alone (TSHR/TSHR, dotted line) and cells expressing L252Ps and TSHRs (TSHR/L252P, solid line). Slope of the line for TSHR/TSHR is 0.054 ± 0.0036 and for TSHR/L252P is 0.0043 ± 0.0041 (means±sd).
To estimate the fraction of TSHR homodimers in cells coexpressing TSHR and L252P, we determined the effect of TSH on the dissociation of prebound 125I-TSH, because TSHR/TSHR homodimers would exhibit negative cooperativity but TSHR/L252P heterodimers (or TSHR monomers) would not. Figure 4 shows the effect of TSH to increase 125I-TSH dissociation in cells expressing TSHR and L252P compared to cells expressing TSHRs only. In cells expressing TSHR and L252P, the decrease in the amount of 125I-TSH bound to cells caused by unlabeled TSH after 90 min, that is, the increase in 125I-TSH dissociation, was 58% of that in cells expressing only TSHRs. This is consistent with there being 58% TSHR/TSHR homodimers, from which 125I-TSH dissociation was increased by TSH, and 42% TSHR/L252P heterodimers, from which 125I-TSH dissociation was not affected by TSH, in cells expressing both receptors. These values are estimates, because we cannot determine what fraction of the 125I-TSH initially bound was to TSHR monomers; we assumed that there were similar fractions of TSHR monomers in cells expressing TSHR only and in cells expressing TSHR and L252P. Thus, signaling in cells coexpressing TSHR and L252P was mediated by TSHR/L252P and TSHR/TSHR dimers.
Figure 4.
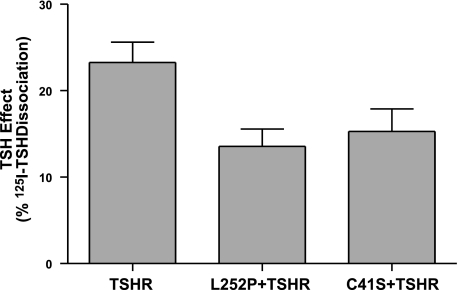
Effect of TSH to enhance dissociation of prebound 125I-TSH in cells expressing TSHRs alone, TSHRs and L252Ps, or TSHRs and C41Ss. Cells were transfected with TSHR alone (0.0025 μg TSHR plasmid+0.5 μg empty plasmid), 0.5 μg L252P plasmid + 0.025 μg TSHR plasmid, or 0.5 μg C41S plasmid + 0.025 μg TSHR plasmid. After 48 or 72 h, 125I-TSH dissociation was performed as described in Materials and Methods in the absence or presence of 100 mU/ml TSH. TSH effect was calculated as the difference between percentages of total 125I-TSH dissociated in the presence and absence of 100 mU/ml TSH. Results shown are from 2 experiments performed in triplicate. Bars represent means ± se.
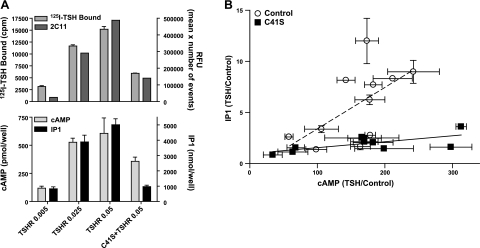
Figure 5 illustrates the results of a series of experiments in which cells expressing various levels of TSHR and L252P were stimulated with a dose of TSH (100 mU/ml) that would activate both the cAMP and phosphoinositide pathways. We were able to express receptors in cells expressing TSHRs alone and in cells expressing TSHRs and L252Ps over a 10-fold range that overlapped in the two cell models (Fig. 5A). There were increases in TSH-stimulated cAMP and IP1 production that was in general proportional to the level of receptor expression. However, there was an important difference in the magnitude of the increases in cAMP and IP1 production in cells expressing TSHRs alone vs. cells expressing TSHRs and L252Ps. Cells expressing TSHRs and L252Ps exhibited lower levels of IP1 relative to cAMP production than cells expressing TSHRs alone. Figure 5A (bottom panel) shows that cells exhibiting similar levels of TSH-stimulated cAMP production showed lower levels of TSH-stimulated IP1 production. A compilation of similar data from 4 independent experiments is illustrated in Fig. 5B. The data were plotted as the fold stimulation by TSH of IP1 production vs. cAMP production and were found to fit linear regression analyses with slopes for cells expressing TSHR alone or TSHR and L252P of 0.054 and 0.0043, respectively. Thus, cells expressing TSHR and L252P responded to TSH stimulation with relatively smaller increases in IP1 production compared to cAMP production than cells expressing TSHRs alone.
To determine whether the observations with cells expressing TSHRs and L252Ps were peculiar to L252Ps, we performed a series of similar experiments with the other naturally occurring mutant, C41S, which, like L252P, does not bind TSH (18). Figure 3 shows that C41S forms heterodimers with TSHR. Figure 4 shows that negative cooperativity is decreased in cells expressing C41S and TSHR. A series of experiments with C41S that were similar to the experiments illustrated in Fig. 5 for L252P are shown Fig. 6. Cells expressing TSHR and C41S responded to TSH stimulation with smaller relative increases in IP1 production compared to cAMP production than cells expressing TSHRs alone. The slopes of the lines of fold stimulation stimulated by TSH of IP1 vs. cAMP production for cells expressing TSHR alone or TSHR and C41S were of 0.041 and 0.0059, respectively. Thus, TSH-stimulated phosphoinositide signaling was reduced relative to cAMP signaling in both cells expressing TSHR and L252P or TSHR and C41S compared to cells expressing TSHR alone.
Figure 6.
Effects of TSH to stimulate cAMP and IP1 production in cells expressing TSHRs alone compared to cells expressing TSHRs and C41Ss. A) Cells were transfected with TSHR alone (0.005, 0.025, or 0.05 μg TSHR plasmid+0.5 μg empty plasmid) or with C41S (0.5 μg plasmid) + TSHR (0.05 μg TSHR plasmid). After 48 or 72 h, 125I-TSH binding and 2C11 antibody binding (top panel) and stimulation of cAMP and IP1 production by 100 mU/ml TSH (bottom panel) were measured as described in Materials and Methods. Results shown are from 1 of 4 experiments performed in triplicate, except 2C11 binding, which was performed singly. Bars represent means ± sd. B) Comparison of the effects of TSH to stimulate IP1 and cAMP production. Stimulation of cAMP and IP1 production by 100 mU/ml TSH were measured as described in Materials and Methods. Results are presented as fold stimulation over control (TSH/control) from 4 experiments performed in duplicate or triplicate. Points represent means ± sd. Lines represent linear regression analyses of data from cells expressing TSHRs alone (TSHR/TSHR, dotted line) and cells expressing C41Ss and TSHRs (TSHR/C41S, solid line). Slope of the line for TSHR/TSHR is 0.041 ± 0.010; slope of the line for TSHR/L252P is 0.0059 ± 0.0014 (means±sd).
DISCUSSION
Our results demonstrate that the difference between potencies for TSH stimulation of the cAMP signaling pathway and of the phosphoinositide signaling pathway is not because there are spare receptors for cAMP production but not for IP1 production. Rather, they are consistent with the idea that stimulation of cAMP production requires only one binding site, the high affinity site, on the TSHR homodimer to be occupied, whereas stimulation of IP1 production occurs only when both binding sites, the high and low affinity sites (10), are occupied by TSH. The observations that support this conclusion are as follow. There are no or few spare receptors for cAMP production because there was a progressive increase in the level of cAMP production with increasing receptor expression in the model system we used. FRET microscopy showed that TSHR/L252P and TSHR/C41S heterodimers form in cells transfected with plasmids expressing TSHR and L252P or TSHR and C41S. The effect of unlabeled TSH to enhance dissociation of prebound 125I-TSH (negative cooperativity) was decreased in cells that expressed TSHR and L252P or TSHR and C41S. Negative cooperativity was previously shown to be dependent on TSHR homodimerization (10), that is, on occupancy of the binding site on the second protomer of the homodimer. As TSHR/L252P and TSHR/C41S heterodimers do not bind TSH at a second site, they would not exhibit negative cooperativity. On the basis of our findings that there were effects of unlabeled TSH to increase the dissociation of prebound 125I-TSH in all cells studied, we conclude that cells expressing TSHRs and L252Ps or TSHRs and C41Ss express heterodimers and TSHR homodimers. In cells expressing both TSHRs and L252Ps or TSHRs and C41Ss, the relative stimulation by TSH of IP1 production was decreased relative to cAMP production. As noted above, all cells expressing TSHR/L252P or TSHR/C41S heterodimers expressed TSHR/TSHR homodimers also. Therefore, we suggest that IP1 production stimulated by TSH in cells expressing TSHRs and L252Ps or TSHRs and C41Ss was caused by activation of TSHR homodimers. However, we cannot exclude the possibility that a low level of IP1 production was caused by activation of heterodimers.
TSHR is a member of a subfamily of class A (or rhodopsin-like) GPCRs that includes the receptors for follitropin [follicle-stimulating hormone (FSH)] and lutropin [luteinizing hormone (LH)]. Although class A GPCRs have consistently been found within cells in vitro as homodimers (14, 21) and, recently, the lutropin receptor was shown to be capable of forming dimers in vivo (22), a role for the homodimer in G protein coupling was initially unclear (11). Specifically, the question of whether the homodimer was involved directly in G protein signaling was negatively affected by the finding that class A GPCRs are capable of coupling to and activating G proteins as monomers (23, 24). However, it is now appreciated that the second protomer of the homodimer may affect the conformation and coupling affinity of the other protomer (25). For example, it was shown that the neurotensin receptor NTS1 monomer couples with higher affinity to Gq than the receptor dimer (26). A study of dopamine receptor chimeras has extended these findings to show that binding to both protomers of the dimer and the nature of the ligand bound to the second protomer affects G protein coupling (12). A complementary finding was made with a mutant of the leukotriene B4 receptor in which binding of the same agonist to both sites on the receptor homodimer caused different conformational changes in each protomer, and this occurred only when the receptor was coupled to a G protein (27). The results of our study provide insights into the role of receptor homodimerization in signaling by showing, to our knowledge for the first time, that binding of the same natural agonist, in our case TSH, to both protomers of the homodimeric form of the class A GPCR TSHR allowed coupling to and activation of a G protein Gq/11 that was not activated by binding to only one protomer of the homodimer.
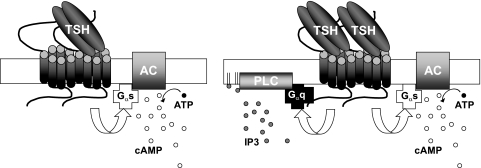
In summary, our findings are most consistent with the following explanation for the marked difference in potencies of TSH to stimulate cAMP and phosphoinositide signaling (Fig. 7). TSHR signals via activation of Gs to stimulate cAMP production but not IP1 production, when TSH binds to the high-affinity binding site on one protomer of the TSHR homodimer. TSHR signals via activation of Gs and Gq/11 to increase both cAMP and IP1 production when TSH binds to both protomers occupying both the high- and low-affinity sites of the TSHR homodimer. This mechanism is consistent with the following conceptualization. The TSHR homodimer binds to and activates a single G protein via one protomer, that is, the stoichiometry of the active complex is 2 TSHR:1 G protein (for review, see ref. 11). Binding of TSH to one protomer induces a receptor conformation that markedly favors binding to and activation of Gs, whereas binding of TSH to both protomers induces a TSHR conformation that can bind to and activate Gs or Gq/11.
Figure 7.
Model of TSHR activation of cAMP and phosphoinositide signaling. TSHR signals via activation of Gs to stimulate cAMP production but not IP1 production when TSH binds to the high-affinity binding site on one protomer of the TSHR homodimer. TSHR signals via activation of Gs and Gq/11 to increase both cAMP and IP1 production when TSH binds to both protomers occupying both the high- and low-affinity sites of the TSHR homodimer.
Supplementary Material
Acknowledgments
The authors thank Stefano Costanzi and Jurgen Wess for helpful comments.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Z01-DK011006), U.S. National Institutes of Health.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Laugwitz K. L., Allgeier A., Offermanns S., Spicher K., Van Sande J., Dumont J. E., Schultz G. (1996) The human thyrotropin receptor: A heptahelical receptor capable of stimulating members of all four G protein families. Proc. Natl. Acad. Sci. U. S. A. 93, 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corda D., Marcocci C., Kohn L. D., Axelrod J., Luini A. (1985) Association of the changes in cytosolic Ca2+ and iodide efflux induced by thyrotropin and by the stimulation of alpha 1-adrenergic receptors in cultured rat thyroid cells. J. Biol. Chem. 260, 9230–9236 [PubMed] [Google Scholar]
- 3. Nussenzveig D. R., Thaw C. N., Gershengorn M. C. (1994) Inhibition of inositol phosphate second messenger formation by intracellular loop one of a human calcitonin receptor. Expression and mutational analysis of synthetic receptor genes. J. Biol. Chem. 269, 28123–28129 [PubMed] [Google Scholar]
- 4. Offermanns S., Iida-Klein A., Segre G. V., Simon M. I. (1996) Gαq family members couple parathyroid hormone (PTH)/PTH-related peptide and calcitonin receptors to phospholipase C in COS-7 cells. Mol. Endocrinol. 10, 566–574 [DOI] [PubMed] [Google Scholar]
- 5. Trimble E. R., Bruzzone R., Biden T. J., Farese R. V. (1986) Secretin induces rapid increases in inositol trisphosphate, cytosolic Ca2+ and diacylglycerol as well as cyclic AMP in rat pancreatic acini. Biochem. J. 239, 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuhn B., Schmid A., Harteneck C., Gudermann T., Schultz G. (1996) G proteins of the Gq family couple the H2 histamine receptor to phospholipase C. Mol. Endocrinol. 10, 1697–1707 [DOI] [PubMed] [Google Scholar]
- 7. Latif R., Graves P., Davies T. F. (2001) Oligomerization of the human thyrotropin receptor. Fluorescent protein-tagged hTSHR reveals post-translational complexes. J. Biol. Chem. 276, 45217–45224 [DOI] [PubMed] [Google Scholar]
- 8. Gao F., Harikumar K. G., Dong M., Lam P. C., Sexton P. M., Christopoulos A., Bordner A., Abagyan R., Miller L. J. (2009) Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol. Pharmacol. 76, 264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Meyts P., Gauguin L., Svendsen A. M., Sarhan M., Knudsen L., Nohr J., Kiselyov V. V. (2009) Structural basis of allosteric ligand-receptor interactions in the insulin/relaxin peptide family: implications for other receptor tyrosine kinases and G-protein-coupled receptors. Ann. N. Y. Acad. Sci. 1160, 45–53 [DOI] [PubMed] [Google Scholar]
- 10. Urizar E., Montanelli L., Loy T., Bonomi M., Swillens S., Gales C., Bouvier M., Smits G., Vassart G., Costagliola S. (2005) Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 24, 1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gurevich V. V., Gurevich E. V. (2008) How and why do GPCRs dimerize? Trends Pharmacol. Sci. 29, 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han Y., Moreira I. S., Urizar E., Weinstein H., Javitch J. A. (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 5, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milligan G. (2009) G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 158, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pin J. P., Neubig R., Bouvier M., Devi L., Filizola M., Javitch J. A., Lohse M. J., Milligan G., Palczewski K., Parmentier M., Spedding M. (2007) International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 59, 5–13 [DOI] [PubMed] [Google Scholar]
- 15. Lee S. P., So C. H., Rashid A. J., Varghese G., Cheng R., Lanca A. J., O'Dowd B. F., George S. R. (2004) Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 279, 35671–35678 [DOI] [PubMed] [Google Scholar]
- 16. Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 17. Tonacchera M., Perri A., De Marco G., Agretti P., Banco M. E., Di Cosmo C., Grasso L., Vitti P., Chiovato L., Pinchera A. (2004) Low prevalence of thyrotropin receptor mutations in a large series of subjects with sporadic and familial nonautoimmune subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 89, 5787–5793 [DOI] [PubMed] [Google Scholar]
- 18. De Roux N., Misrahi M., Brauner R., Houang M., Carel J. C., Granier M., Le Bouc Y., Ghinea N., Boumedienne A., Toublanc J. E., Milgrom E. (1996) Four families with loss of function mutations of the thyrotropin receptor. J. Clin. Endocrinol. Metab. 81, 4229–4235 [DOI] [PubMed] [Google Scholar]
- 19. Neumann S., Huang W., Titus S., Krause G., Kleinau G., Alberobello A. T., Zheng. W., Southall N., Inglese J., Austin C. P., Celi F. S., Gavrilova O., Thomas C. J., Raaka B. M., Gershengorn M. C. (2009) Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc. Natl. Acad. Sci. U. S. A. 106, 12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deflorian F., Engel S., Colson A. O., Raaka B. M., Gershengorn M. C., Costanzi S. (2008) Understanding the structural and functional differences between mouse thyrotropin-releasing hormone receptors 1 and 2. Proteins 71, 783–794 [DOI] [PubMed] [Google Scholar]
- 21. Dalrymple M. B., Pfleger K. D., Eidne K. A. (2008) G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol. Ther. 118, 359–371 [DOI] [PubMed] [Google Scholar]
- 22. Rivero-Muller A., Chou Y. Y., Ji I., Lajic S., Hanyaloglu A. C., Jonas K., Rahman N., Ji T. H., Huhtaniemi I. (2010) Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc. Natl. Acad. Sci. U. S. A. 107, 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ernst O. P., Gramse V., Kolbe M., Hofmann K. P., Heck M. (2007) Monomeric G protein-coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc. Natl. Acad. Sci. U. S. A. 104, 10859–10864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whorton M. R., Jastrzebska B., Park P. S., Fotiadis D., Engel A., Palczewski K., Sunahara R. K. (2008) Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J. Biol. Chem. 283, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Springael J. Y., Urizar E., Costagliola S., Vassart G., Parmentier M. (2007) Allosteric properties of G protein-coupled receptor oligomers. Pharmacol. Ther. 115, 410–418 [DOI] [PubMed] [Google Scholar]
- 26. White J. F., Grodnitzky J., Louis J. M., Trinh L. B., Shiloach J., Gutierrez J., Northup J. K., Grisshammer R. (2007) Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc. Natl. Acad. Sci. U. S. A. 104, 12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Damian M., Martin A., Mesnier D., Pin J. P., Baneres J. L. (2006) Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 25, 5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.