Abstract
CC chemokine ligand 2 (CCL2), a ligand of CC chemokine receptor 2 (CCR2), is essential to mount an adequate inflammatory response to repair acute skeletal muscle injury. We studied the mechanisms by which CCL2 regulates muscle inflammation and regeneration. Mobilization of monocytes/macrophages (MOs/MPs) but not lymphocytes or neutrophils was impaired from bone marrow to blood and from blood to injured muscles in Ccl2−/− mice. This was accompanied by poor phagocytosis, reduced up-regulation of insulin-like growth factor-1 (IGF-1), and impaired muscle regeneration. Bone marrow transfer from wild-type mice to irradiated Ccr2−/− but not Ccl2−/− mice restored muscle inflammation. Intravenously injected CCL2-deficient bone marrow monocytes could not enter wild-type injured muscles as well as wild-type bone marrow monocytes. Intravenously injected wild-type bone marrow monocytes could not enter CCL2-deficient injured muscles as well as wild-type injured muscles. CCL2 stimulated IGF-1 expression by wild-type but not CCR2-deficient intramuscular macrophages. A single intramuscular injection of IGF-1, but not PBS, markedly improved muscle regeneration in Ccl2−/− mice. We conclude that CCL2 is a major ligand of CCR2 to recruit MOs/MPs into injured muscles to conduct phagocytosis and produce IGF-1 for injury repair. CCL2 needs to be expressed by bone marrow cells, circulating monocytes, and injured muscle tissue cells to recruit MOs/MPs into injured muscles. CCL2/CCR2 signaling also up-regulates IGF-1 expression by intramuscular macrophages to promote acute skeletal muscle injury repair.—Lu, H., Huang, D., Ransohoff, R. M., Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair.
Keywords: chemokine, inflammation, IGF-1
Acute skeletal muscle injury is a relatively common clinical condition, which can be caused by trauma, contraction, chemicals, myotoxins, and ischemia. Acute skeletal muscle injury repair is a tightly regulated process, which mainly consists of muscle inflammation, regeneration, and angiogenesis (1).
CC chemokine ligand 2 (CCL2), also called macrophage chemoattractant protein 1 (MCP-1), is a ligand of CC chemokine receptor 2 (CCR2). CCL2/CCR2 signaling is critical for tissue recruitment of monocytes/macrophages (MOs/MPs) upon inflammation and infection (2, 3). It plays a significant pathogenic role in chronic inflammatory diseases, including multiple sclerosis (4–6), atherosclerosis (2, 7), rheumatoid arthritis (8, 9), and bronchitis obliterans syndrome (10). Conversely, CCL2/CCR2-mediated inflammatory response is essential to repair acute skeletal muscle injury. Ccr2−/− or Ccl2−/− mice showed markedly reduced macrophage infiltration in response to acute muscle injuries induced by ischemia (11, 12) or myotoxic agents (13–15), and the diminished inflammatory response was accompanied by poor muscle regeneration.
We recently characterized the mechanism by which CCR2 regulates acute skeletal muscle injury repair, and demonstrated that CCR2 exerts proregenerative function primarily by recruiting Ly-6C+ MOs/MPs from bone marrow to blood and further from blood to injured muscles. Within injured muscles, these cells conduct phagocytosis, contribute to accumulation of Ly-6C− MPs, and produce a high level of insulin-like growth factor 1 (IGF-1) to promote muscle regeneration. We further showed that CCR2 is mainly expressed by infiltrating macrophages but not by activated regenerating myogenic cells or capillary endothelial cells in injured muscles to directly affect muscle regeneration or angiogenesis associated with injury repair (16).
CCL2/CCR2 signaling has become a salient target for treating chronic inflammatory disorders (17, 18). CCR2 antagonists have been under active preclinical and clinical development (17–20), and targeting CCL2 to induce CCL2 deficiency has also been advocated for treating human diabetic kidney disease (21) and vascular complications of obesity (22). Poor muscle injury repair may be a significant side effect of this line of therapies. Murine CCR2 has 3 main ligands, including CCL2, CCL7, and CCL12. CCR2 is the only receptor identified for CCL2 and CCL12, while CCL7 can bind and function through other chemokine receptors. These CCR2 ligands may play redundant and/or complementary roles in mediating diverse functions of CCR2. It is thus important to precisely determine how CCL2 regulates muscle inflammation and regeneration, so that we can develop a targeted approach to prevent or control the potential muscle side effects in the setting of future therapeutic CCL2 inhibition. Additionally, insights into CCL2/CCR2 pathways to muscle regeneration may undercover fundamental restorative function that can be exploited therapeutically. In the present study, we addressed whether CCL2 is a major ligand that mediates the function of CCR2 in acute skeletal muscle injury repair, which cells need to express CCL2 to mount an adequate inflammatory and regenerative response for acute skeletal muscle injury repair, and how to improve poor muscle regeneration resulting from CCL2 deficiency.
MATERIALS AND METHODS
Animals
Wild-type (Wt) C57BL/6J mice and enhanced green fluorescent protein transgenic mice (β-actin-GFP) were derived from the Jackson Laboratory (Bar Harbor, ME, USA). Ccr2−/− and Ccl2−/− mice were derived and crossed into C57BL/6J background as described previously (23, 24). We followed the guide for the care and use of laboratory animals of Cleveland Clinic, and the study was approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Acute muscle injury and IGF-1 treatment
To induce acute skeletal muscle injury, 100 μl barium chloride (BaCl2; 1%) was injected into the right quadriceps muscle of each mouse (age 8–10 wk). For IGF-1 treatment, each mouse received an injection of IGF-1 (0.5 μg/100 μl/muscle) or PBS into the right quadriceps muscle at 3 d after the BaCl2 injection.
Histopathological analysis
Mice were sacrificed at d 1, 3, 5, 7, 14, 21, and 42 after BaCl2 injections. Quadriceps muscles were collected and fresh frozen in liquid nitrogen-cooled isopentane, sectioned at 8 μm, stained with hematoxylin and eosin, and viewed under a bright-field microscope at ×20.
Quantification of muscle regeneration
Muscle regeneration was quantified by measuring muscle volume, percentage of regenerative area, and muscle fiber cross-sectional area (CSA) in muscles 21 and 42 d after BaCl2 injections, using the method previously described (16).
Quantification of muscle necrosis
Muscle necrosis was quantified by measuring the necrotic fiber density (number of necrotic fibers per square millimeter) and the percentage of necrotic area in muscles at 7 d after BaCl2 injections. Briefly, 5 sections from each specimen separated by at least 50 μm were stained with H&E, and 5 nonoverlapping areas of each section were digitally captured. These images were analyzed using ImageJ software (http://rsbweb.nih.gov/ij/) to measure the total area, number of necrotic fibers, and area of necrosis. The necrotic fiber density and the percentage of necrotic area were then calculated. The necrotic fibers were identified on H&E stain as myofibers with reduced eosinophilic staining, which may be surrounded by activated satellite cells/regenerating fibers.
Flow cytometry
Flow cytomety was performed using muscle single-cell suspension, blood, and bone marrow, as previously described (16). We used 2 definitions for MOs/MPs: 7/4+ Ly-6G− cells (7); and F4/80+ cells (25). As bone marrow MO/MP precursor cells do not express high levels of F4/80, we used 7/4+ Ly-6G− to indicate MOs/MPs in bone marrow or blood. As MOs/MPs do not universally express 7/4, we also used F4/80 as a marker for MOs/MPs when studying blood and muscle tissue. We used Ly-6G+ as a marker for neutrophils (25), CD3+ for T cells, and CD19+ for B cells. We also analyzed F4/80+ Ly-6C+ and F4/80+ Ly-6C− cells to study 2 subsets of MOs/MPs (26, 27).
Generation of radiation bone marrow chimeras
Generation of radiation bone marrow chimeras was performed using the protocol previously described (28). Briefly, bone marrow cells were harvested from femurs of donor mice. Red blood cells were lysed, and the remaining cells were washed with PBS and resuspended in RPMI 1640 with antibiotics. Each recipient mouse received 900 rad of irradiation. After 2–3 h of recovery, each mouse received 3.0 × 107 donor bone marrow cells via intravenous injection. Five types of chimeric mice were generated. Wt → Wt, Wt → Ccr2−/−, Wt → Ccl2−/−, Ccl2−/− → Wt, and Ccl2−/− → Ccl2−/−.
Adoptive bone marrow mononuclear cell transfer
Adoptive bone marrow mononuclear cell transfer was performed as described previously (16). Bone marrow cells were harvested from femurs of donor mice. Red blood cells were lysed, and the remaining cells were washed with PBS, resuspended in RPMI 1640, and injected intravenously into wild-type recipient mice. Each mouse received 3.0 × 107 cells at the time of receiving BaCl2 injection.
ELISA
Murine CCL2, CCL7, CCL12, and IGF-1 ELISA were performed using the kits purchased from R&D Systems (Minneapolis, MN, USA; CCL2: MJE00; CCL12: MCC120; and IGF-1: MG100) and Bender Medsystems (San Diego, CA, USA; CCL7: BMS6006INST). We used the protocols provided by the manufacturers.
Intramuscular MP isolation, culture, and CCL2 treatment
Muscles were removed from Wt or Ccr2−/− mice at d 3 after BaCl2 injections and lysed using the collagenase solution (0.5 mg/ml type IA collagenase and 0.5 mg/ml type IV collagenase; Invitrogen, Carlsbad, CA, USA). The top liquid layers were filtered through a 70-μm basket, brought up to 40 ml with PBS, and centrifuged at 670 g for 10 min. The pallets were resuspended in 15 ml of PBS, filtered through a 40-μm cell strainer twice, layered on 15 ml of Lympholyte-M (Cedarlane, Burlington, NC, USA), and centrifuged at 2095 g for 45 min. Cells at the interface were collected and sorted using mouse CD45 MicroBeads (cat. no. 130-052-301; Miltenyi Biotec, Bergisch Gladbach, Germany) to obtain CD45+ cells. The beads were conjugated to the monoclonal rat anti-mouse CD45 antibody (rat IgG2b, clone 30-F11.1), which reacts with all isoforms of CD45. The sorted CD45+ cells were cultured using DMEM with 10% fetal bovine serum (FBS), penicillin–streptomycin (100 U/ml), and 2.5 mM l-glutamine (Invitrogen) with or without CCL2 (10 ng/ml/106 cells; R&D Systems) for 24 h.
Immunostaining
Cells were blocked in 5% goat serum for 2 h, followed by overnight incubation in rat-anti-mouse F4/80 antibody (1:10; Serotec, Raleigh, NC, USA). The cells were then incubated with goat anti-rat IgG labeled with Alexa Fluor 594 (1:1000; Invitrogen) for 1 h at room temperature, followed by staining with DAPI (Invitrogen). Percentage of F4/80+ MPs was calculated as F4/80+DAPI+ cells divided by DAPI+ cells.
Quantitative RT-PCR
Quantitative RT-PCR was performed using the protocol previously described (29) and the following primers: IGF-1, forward 5′-CTACAAAAGCAGCCCGCTCT-3′ and reverse 5′-CTTCTGAGTCTTGGGCATGTCA-3′; and GAPDH, forward 5′-CATGGCCTTCCGTGTTCCTA-3′ and reverse 5′-ATGCCTGCTTCACCACCTTCT-3′. Mouse GAPDH was used as an internal control. Reaction specificity was determined by product melting curves. The PCR products were verified by running 3% agarose gels.
Statistical analyses
SPSS11.5 software (SPSS, Inc. Chicago, IL, USA) was used for statistical analyses. Results are expressed a means ± sd. Differences in various parameters between different experimental groups were evaluated by ANOVA analysis with least significant difference and Student-Newman-Keul's as post hoc tests if there were 3 or more groups in each experiment, or by Student's t test after testing for normality if there were 2 groups in each experiment. Values of P < 0.05 were considered significant.
RESULTS
Ccl2−/− mice showed reduced muscle inflammation, poor phagocytosis, and impaired muscle regeneration after acute injury induced by BaCl2
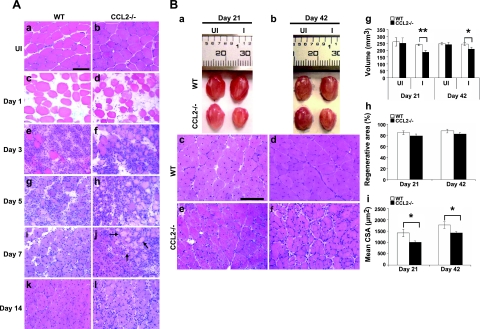
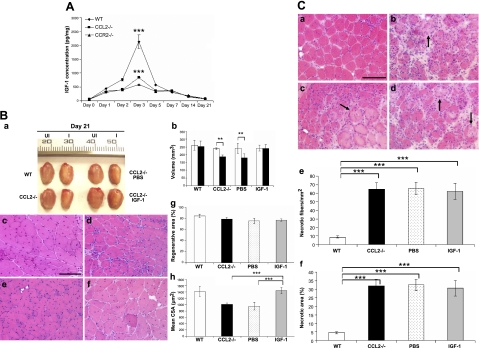
In wild-type mice (Fig. 1A), after BaCl2 injections, robust inflammatory cell infiltrates along with necrotic fibers were noted at d 3. Necrotic fibers were largely cleared and replaced by central-nucleated regenerating fibers by d 7, and inflammation was resolved and regenerated fibers were predominant in the injured areas by d 14. At d 21 and 42 (Fig. 1B), regenerated fibers became more mature, and the injured areas recovered their normal architecture. Ccl2−/− mice, however, exhibited markedly reduced inflammation at d 3, which lingered through d 14 (Fig. 1A). Abundant necrotic fibers were still present at d 7. At d 21 and 42 (Fig. 1B), although the injured areas were filled with central-nucleated regenerated fibers, the size of these fibers was significantly smaller than that in wild-type mice. Muscle regeneration, as quantified by the volume of injured muscles (mm3) after regeneration, and mean muscle fiber CAS (μm2) were poor in Ccl2−/− mice as compared with wild-type controls, with no difference in the percentage of regenerative area (Fig. 1B). These findings demonstrated that CCL2 deficiency resulted in significant reduction of muscle inflammation, poor phagocytosis, and impaired muscle regeneration after acute injury induced by BaCl2. The effects appeared less severe than seen in Ccr2−/− mice (16).
Figure 1.
Ccl2−/− mice showed markedly reduced muscle inflammation, delayed phagocytosis, and impaired muscle regeneration after acute injury induced by BaCl2. A) Compared to uninjured wild-type (a) and ccl2−/− mice (b), H&E staining showed myofiber necrosis and tissue edema in injured muscles at d 1 of both wild-type (c) and ccl2−/− mice (d). While robust inflammatory infiltrates were seen at d 3 in injured muscles of wild-type mice (e), fewer inflammatory cells were seen in Ccl2−/− mice (f). Necrotic fibers were gradually replaced by mononucleated and multinucleated myoblasts and myotubes at d 5 (g) and 7 (i) in wild-type mice; many of them (arrows) remained in Ccl2−/− mice with scattered endomysial inflammation (h, j). While the injured areas were filled with central-nucleated regenerated myofibers with no necrotic fibers or inflammatory cells seen in wild-type mice at d 14 (k), scattered inflammatory cells were still present in Ccl2−/− mice (l). B) At d 21 and 42, the injured muscles (a, b) and regenerated muscle fibers (c–f) were smaller in Ccl2−/− mice (a, b, e, f) than in wild-type mice (a–d). Quantitative analyses showed that the volume of injured muscles (g) and the mean CSA of regenerated fibers (i) were significantly reduced in Ccl2−/− mice as compared with wild-type controls. There was no difference in the percentage of regenerative area (h). WT, wild-type; UI, uninjured; I, injured; CSA, cross-sectional area. Scale bars = 50 μm. n = 5–7 mice/group/time point. *P < 0.05; **P < 0.01.
CCL2 deficiency impaired recruitment of MOs/MPs into injured muscles
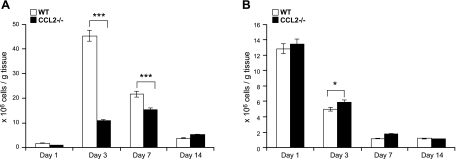
CCL2 deficiency reduced inflammatory response to acute skeletal muscle injury. We first addressed which subpopulations of inflammatory cells were affected by CCL2 deficiency. We measured the numbers of intramuscular macrophages, neutrophils, T cells, and B cells in injured muscles by flow cytometry (Fig. 2). In wild-type mice, the numbers of macrophages (Fig. 2A) were remarkably elevated at d 3, to a lesser degree at d 7, and almost returned to baseline at d 14. The accumulation of intramuscular macrophages was markedly reduced in Ccl2−/− mice, with a small peak at d 7 rather than a large peak at d 3. The numbers of neutrophils (Fig. 2B) in injured muscles of Ccl2−/− mice were increased modestly but significantly only at d 3, as compared with wild-type controls. Increased neutrophil accumulation was probably due both to increased neutrophil recruitment in Ccr2−/− mice and reduced neutrophil phagocytosis by intramuscular macrophages. There was no significant difference in the numbers of intramuscular T cells or B cells between Ccl2−/− mice and wild-type mice (data not shown). Therefore, as seen with CCR2 deficiency (16), CCL2 deficiency primarily limited muscle recruitment of MOs/MPs in response to acute injury.
Figure 2.
CCL2 deficiency blocked MO/MP recruitment into injured muscles. A) Flow cytometry showed markedly increased number of intramuscular macrophages in wild-type mice at d 3 and 7, with the peak at d 3. Number of intramuscular macrophages in Ccl2−/− mice was also increased after injury, but was significantly reduced at d 3 and 7 as compared with wild-type mice. B) Number of neutrophils in Ccl2−/− mice was increased at d 3 as compared with wild-type mice. *P < 0.05; ***P < 0.001.
Recruitment of MOs/MPs to injured tissues consists of mobilization of these cells from bone marrow to blood and further from blood to injured tissues. There are two main subsets of MOs/MPs in blood, based on the expression of Ly-6C: Ly-6C+ cells, which infiltrate injured tissues; and Ly-6C− cells, which mainly contribute to tissue-resident macrophages (26, 27). CCR2 deficiency impaired Ly-6C+ precursors to enter blood and then enter injured muscles (16). To address whether CCL2 deficiency caused the same impairment, we performed flow cytometry using bone marrow, blood, and single-cell suspensions of injured muscles at 3 d after BaCl2 injections, which corresponded to the peak of inflammation in wild-type mice.
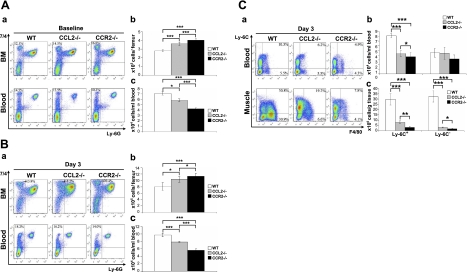
To address the level at which the mobilization of MOs/MPs was blocked, we used 7/4+Ly-6G− to identify MOs/MPs (7). At baseline, 7/4+Ly-6G− cells were significantly increased in bone marrow and reduced in blood in Ccl2−/− mice, and more so in Ccr2−/− mice, as compared with wild-type controls (Fig. 3A). At d 3 postinjury, 7/4+Ly-6G− cells were also significantly increased in bone marrow but reduced in blood in Ccl2−/− mice, and more so in Ccr2−/− mice (Fig. 3B). These findings indicate that CCL2 deficiency causes baseline monocytopenia in blood and also impairs the mobilization of 7/4+Ly-6G− MOs/MPs from bone marrow to blood in response to acute muscle injury. The effects were less severe in Ccl2−/− mice than in Ccr2−/− mice.
Figure 3.
Ccl2−/− mice showed impaired MO/MP recruitment from bone marrow to blood and from blood to injured muscles, less severe than seen in Ccr2−/− mice. A) Flow cytometry at baseline showed that numbers of 7/4+Ly-6G− MOs/MPs (a, b) were increased in bone marrow but reduced in blood (a, c) in Ccl2−/− mice (n=15), and more so in Ccr2−/− mice (n=15), as compared with wild-type controls (n=15). B) At d 3 postinjury, numbers of 7/4+Ly-6G− MOs/MPs were also increased in bone marrow (a, b) and reduced in blood (a, c) in Ccl2−/− mice (n=12), and more so in Ccr2−/− mice (n=13), as compared with wild-type controls (n=15). C) Flow cytometry at d 3 showed that the numbers of F4/80+ MOs/MPs were reduced in blood and in injured muscles of Ccl2−/− mice (n=15) and more severely reduced in Ccr2−/− mice (n=15) as compared with wild-type controls (n=15), among which the Ly-6C+, but not the Ly-6C−, subset was significantly reduced in blood (a, b). However, both Ly-6C+ and Ly-6C− subsets were significantly reduced in injured muscles of Ccl2−/− mice and more severely reduced in Ccr2−/− mice (a, c). *P < 0.05; **P < 0.01; ***P < 0.001.
We further addressed which subset of MOs/MPs was primarily affected by CCL2 deficiency (Fig. 3C). At d 3, F4/80+ MOs/MPs were significantly reduced in blood and in injured muscles of Ccl2−/− mice, among which the Ly-6C+ but not the Ly-6C− subset was significantly reduced in blood. However, both Ly-6C+ and Ly-6C− macrophages were significantly reduced in injured muscles of Ccl2−/− mice. These effects were also less severe than seen in Ccr2−/− mice.
Expression of CCL2 by bone marrow cells, circulating monocytes, and injured muscle tissue cells was required for recruiting MOs/MPs into injured muscles
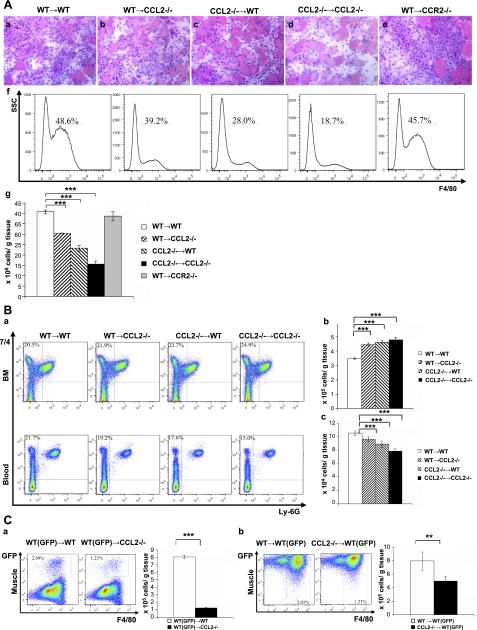
To address whether CCL2 expression by bone marrow cells and/or injured muscle tissue cells is required for muscle recruitment of MOs/MPs, we generated bone marrow chimeras and evaluated muscle inflammation at d 3 by histopathological analysis and flow cytometry (Fig. 4A). Wt → Wt chimeras showed robust inflammatory infiltrates in inured muscles, whereas Ccl2−/− → Ccl2−/− showed markedly reduced inflammation. While wild-type bone marrow-derived cells completely restored muscle inflammation in Ccr2−/− mice, they only partially restored muscle inflammation in Ccl2−/− mice. Muscle inflammation was also reduced in Ccl2−/− → Wt chimeras. The number of F4/80+ MPs in injured muscles (Fig. 4Ag) was significantly reduced in Wt → Ccl2−/−, Ccl2−/− → Wt, and Ccl2−/− → Ccl2−/− but not in Wt → Ccr2−/− chimeras as compared to that in Wt → Wt chimeras. These findings indicate that CCL2 expression by both bone marrow and injured muscle tissue cells is required for recruiting MOs/MPs into acutely injured muscles.
Figure 4.
CCL2 expression by bone marrow cells, circulating monocytes, and injured muscle resident tissue cells was required for recruiting MOs/MPs from bone marrow to blood and from blood to injured muscles. A) H&E staining showed that the inflammatory infiltrates at d 3 were markedly reduced in Ccl2−/− → Ccl2−/− bone marrow chimeras (d) and partially reduced in Wt → Ccl2−/− (b) and Ccl2−/− → Wt (c) chimeras as compared with Wt → Wt chimeras (a). Flow cytometry showed reduced F4/80+ MPs in injured muscles at d 3 in Ccl2−/− →Ccl2−/−, Wt → Ccl2−/−, and Ccl2−/− → Wt chimeras as compared with Wt → Wt chimeras (f, g). Wt → Ccr2−/− chimeras showed an inflammatory response (e) and intramuscular F4/80+ MPs (f, g) similar to those in Wt → Wt chimeras. B) At d 3 postinjury, the percentage (a) and numbers (b, c) of 7/4+Ly-6G− MOs/MPs were increased in bone marrow and reduced in blood in Ccl2−/− → Ccl2−/−, Wt → Ccl2−/−, and Ccl2−/− → Wt chimeras as compared with Wt → Wt chimeras. C) a) Bone marrow monocyte transfer showed that the number of GFP+ monocytes recruited into wild-type injured muscles at d 3 was significantly higher than that recruited into Ccl2−/− injured muscles. b). Number of wild-type GFP− monocytes recruited into wild-type injured muscles at d 3 was significantly higher than that of Ccl2−/− GFP− monocytes. n = 5–7 mice/group/experiment. **P < 0.01; ***P < 0.001.
To determine whether CCL2 expression by bone marrow cells and injured muscle tissue cells is required for MO/MP precursor cells to exit bone marrow and enter blood, we performed flow cytometry using bone marrow and blood cells from bone marrow chimeras 3 d after BaCl2 injections (Fig. 4B). 7/4+Ly-6G− MO/MP population was significantly increased in bone marrow but reduced in blood in Wt → Ccl2−/−, Ccl2−/− → Wt, and Ccl2−/− → Ccl2−/− chimeras as compared with Wt → Wt chimeras. Therefore, expression of CCL2 by both bone marrow cells and injured muscle tissue cells is required for mobilizing MO/MP precursor cells from bone marrow to blood.
We then determined whether CCL2 expression by circulating monocytes and/or injured muscle tissue cells is required for monocyte recruitment from blood to injured muscles (Fig. 4C). We first intravenously injected wild-type (GFP+) bone marrow cells into wild-type (GFP−) or Ccl2−/− mice upon BaCl2 injury and monitored their accumulation (GFP+ MOs/MPs) in injured muscles at d 3. The recruitment of GFP+ wild-type MOs by CCL2-deficient injured muscles was markedly impaired as compared with that by wild-type injured muscles (Fig. 4Ca). We then intravenously injected bone marrow cells from wild-type (GFP−) or Ccl2−/− mice into wild-type (GFP+) mice on BaCl2 injury and monitored their accumulation (GFP− MPs) in injured muscles at d 3. Muscle recruitment of GFP− monocytes from Ccl2−/− mice was also impaired as compared with those from wild-type mice (Fig. 4Cb). The findings indicate that expression of CCL2 by both circulating monocytes and injured muscle tissue cells is required for normal recruitment of monocytes from blood to injured muscles.
Up-regulation of IGF-1 expression was impaired and local IGF-1 replacement improved muscle injury repair in Ccl2−/− mice
To address whether reduced muscle inflammation resulted in diminished up-regulation of IGF-1 in Ccl2−/− mice as seen in Ccr2−/− mice, we performed ELISA. The marked up-regulation of IGF-1 protein in injured muscles of wild-type mice at d 3 was significantly reduced in Ccl2−/− mice and more drastically diminished in Ccr2−/− mice (Fig. 5A). A single injection of IGF-1 (0.5 μg/muscle) into injured muscles at d 3 remarkably improved muscle regeneration in Ccl2−/− mice, as evidenced by increased volume of regenerated quadriceps and mean CSA of regenerated muscle fibers at d 21 (Fig. 5B). However, IGF-1 treatment did not improve necrotic fiber clearance at d 7 (Fig. 5C). The dose of IGF-1 was determined based on the IGF-1 protein level measured by ELISA in injured muscles of wild-type mice at d 3.
Figure 5.
Up-regulation of IGF-1 in injured muscles was impaired and local IGF-1 replacement improved muscle regeneration but not necrotic fiber clearance in Ccl2−/− mice. A) ELISA showed that the level of IGF-1 was increased in injured muscles of wild-type, Ccl2−/−, and Ccr2−/− mice, with the peak at d 3, at which point the IGF-1 level was remarkably higher in wild-type mice than in Ccl2−/− mice and Ccr2−/− mice. Ccr2−/− mice showed a more drastic reduction of IGF-1 up-regulation than Ccl2−/− mice. B) At d 21, while the injured quadriceps muscles of wild-type mice regenerated to reach the size of contralateral uninjured controls, the size (volume) of injured quadriceps in Ccl2−/− mice was significantly smaller than the contralateral uninjured controls (a, b). Regenerated muscle fibers were also smaller in Ccl2−/− mice (e, h) than in wild-type mice (c, h). Intramuscular injection of IGF-1(a, b, f, h), but not PBS (a, b, d, h), improved muscle regeneration with increased muscle volume (a, b) and muscle fiber size (f, h). There was no difference in the percentage of regenerative area in these muscles (g). C) At d 7, necrotic fibers (arrows) were largely cleared in wild-type mice (a, e, f) but not in Ccl2−/− mice (c), which showed significantly increased necrotic fiber density (e) and percentage of necrotic area (f). Neither IGF-1 (b, e, f) nor PBS (d–f) improved necrotic fiber clearance. UI, uninjured; I, injured. n = 5 mice/group/experiment. Scale bars = 50 μm. **P < 0.01; ***P < 0.001.
CCL2 up-regulated IGF-1 mRNA expression by MPs in injured muscles
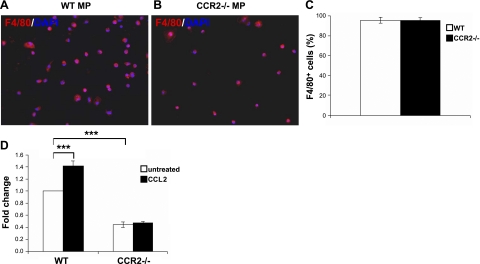
During acute skeletal muscle injury repair, intramuscular infiltrating MPs are the major cellular source of IGF-1 (16). To address whether CCL2/CCR2 signaling also contributes to this biological function of tissue MPs, we treated intramuscular CD45+ cells derived from injured muscles of Wt or Ccr2−/− mice at d 3 after BaCl2 injections with CCL2. We then quantified IGF-1 mRNA expression by qRT-PCR (Fig. 6). After 24 h of in vitro culture, the majority of cells attached to the culture plates were F4/80+ macrophages (wild-type: 95.6%; Ccr2−/−: 95.1%; Fig. 6A–C). IGF-1 mRNA expression was higher by intramuscular MPs from wild-type mice than those from Ccr2−/− mice (Fig. 6D). CCL2 treatment significantly increased IGF-1 expression by wild-type but not CCR2-deficient intramuscular MPs (Fig. 6D). The finding indicates that CCL2/CCR2 signaling does up-regulate IGF-1 mRNA expression in MPs within injured muscles.
Figure 6.
CCL2 up-regulated IGF-1 mRNA expression by macrophages in injured muscles. A–C) Immunostaining of wild-type (A) and Ccr2−/− MPs (B) showed that the intramuscular CD45+ cells attached to the culture plates were mostly F4/80+ MPs (C). D) IGF-1 mRNA expression was higher in wild-type MPs than in Ccr2−/− MPs, and CCL2 treatment increased IGF-1 mRNA expression in wild-type but not Ccr2−/− MPs. Fold change refers to the comparison to wild-type untreated MPs. n = 3 experiments. ***P < 0.001.
Expression of CCL7 and CCL12 in injured muscles suggested potential compensation
Since impaired muscle inflammation and regeneration in Ccl2−/− mice is not as severe as seen in Ccr2−/− mice, and murine CCR2 has 3 main ligands, CCL2, CCL7, and CCL12, we wondered whether CCL7 and CCL12 also play a role in the muscle recruitment of MOs/MPs during acute muscle injury repair. We measured protein expression of these chemokines by ELISA (Fig. 7). The CCL2 protein level started to increase at d 1, peaked at d 2, and gradually returned to baseline at d 14 in wild-type mice, which correlated with the time course of MO/MP infiltration. The CCL7 protein level was increased in both wild-type and Ccl2−/− mice, with a peak at d 3 in wild-type mice and at d 2 in Ccl2−/− mice. The CCL7 level was higher in Ccl2−/− mice than in wild-type mice at d 1 and 5, and lower in Ccl2−/− mice than in wild-type mice at d 3. The CCL12 protein level was also increased in wild-type and Ccl2−/− mice, with a peak at d 3 in both strains. At d 7, the CCL12 level was higher in Ccl2−/− mice than in wild-type mice. Based on the up-regulation of CCL7 and CCL12 protein expression, it is possible that CCL7 and CCL12 may also serve as chemoattractants to recruit MOs/MPs into injured muscles.
Figure 7.
CCL7 and CCL12 protein levels were increased in injured muscles of wild-type and Ccl2−/− mice. A) CCL2 ELISA showed increased CCL2 protein level in injured muscles of wild-type mice, with a peak at d 2. CCL2 was not detected in injured muscles of Ccl2−/− mice. B) CCL7 ELISA showed elevated CCL7 level in injured muscles of both wild-type and Ccl2−/− mice, with a peak at d 3 in wild-type and at d 2 in Ccl2−/− mice. CCL7 level was higher in Ccl2−/− mice at d 2 and d 5 but lower at d 3 than in wild-type mice. C) CCL12 ELISA showed elevated CCL12 level in injured muscles, with a peak at d 3 in both wild-type and Ccl2−/− mice. CCL12 level was higher in Ccl2−/− mice than in wild-type mice at d 7. n = 5–7 mice/group/experiment. *P < 0.05; **P < 0.01.
DISCUSSION
CCL2/CCR2 signaling is essential to acute skeletal muscle injury repair, as Ccl2−/− or Ccr2−/− mice displayed markedly reduced muscle inflammation and poor muscle regeneration. In the present study, we demonstrate that CCL2 needs to be expressed by bone marrow cells, circulating monocytes, and injured muscle tissue cells to adequately recruit MOs/MPs into acutely injured skeletal muscles to conduct phagocytosis and produce IGF-1 to support injury repair.
Our previous study using Ccr2−/− mice showed that CCR2 regulated acute skeletal muscle injury repair primarily by recruiting Ly-6C+ MOs/MPs from bone marrow to blood and further from blood to injured muscles (16). Within injured muscles, these cells conduct phagocytosis, contribute to accumulation of Ly-6C− MPs, and produce a high level of IGF-1 to promote muscle regeneration. Our present study showed that CCL2 deficiency also significantly impaired Ly-6C+ MOs/MPs recruitment from bone marrow to blood and from blood to injured muscles, which was accompanied by delayed phagocytosis, decreased accumulation of Ly-6C− MPs, reduced up-regulation of IGF-1 expression, and poor muscle regeneration. The findings indicate that CCL2 is a major chemokine ligand mediating CCR2 regulation of MO/MP recruitment during acute muscle injury repair. This is consistent with the role of CCL2 in other tissue inflammation models, including atherosclerosis (7), sterile peritonitis (3, 7), and experimental autoimmune encephalomyelitis (5). Our findings further support the notion that inflammation is essential to muscle regeneration (16, 30, 31). However, our studies cannot determine whether muscle regeneration is significantly delayed or blocked by CCL2 or CCR2 deficiency. A recent study showed that the inflammatory response to skeletal muscle injury by whole muscle grafting was diminished at d 5 in geriatric mice. Muscle regeneration was poor at d 5 but was very good at d 10 (31). The finding suggests that the impairment of muscle regeneration from inadequate inflammatory response can be transient as long as myogenic cells have excellent regenerative capacity. However, our model is different in that muscle regeneration in Ccl2−/− or Ccr2−/− mice was still poor at d 42 as compared with wild-type mice. Scattered fat accumulation and mild endomysial fibrosis were also noted by us and others (11, 15, 16). Therefore, it might be difficult for injured muscles in Ccl2−/− or Ccr2−/− mice to repair completely.
It has been shown that CCR2 expression by bone marrow cells, but not by injured muscle tissue cells, is required to mount an inflammatory response following acute skeletal muscle injury (15). However, in addition to bone marrow cells, injured muscle tissue cells also need to express CCL2 to generate adequate muscle inflammation, as muscle inflammation was only partially restored in Wt → Ccl2−/− and Ccl2−/− → Wt chimeras. We also showed that the CCL2 protein expression was remarkably upregulated in injured muscles, with a peak at d 2, right before the peak of MO/MP infiltration at d 3. Up-regulation of CCL2 also corresponded well to inflammatory cell infiltration in other models of muscle injuries induced by ischemia (11, 32), freeze (33), or cardiotoxin (34). These findings strongly support a chemoattracttant role for CCL2 in recruiting CCR2-expressing MOs/MPs from circulation into injured muscles. Injured muscle tissue cells mainly consist of myofibers, myogenic cells, infiltrating inflammatory cells, muscle-resident macrophages, capillary endothelial cells, and fibroblasts. CCL2 has been reported to be localized to inflammatory cells and endothelial cells in muscles injured by ischemia (32). In vitro, activated satellite cells also produced CCL2 (35).
Muscle recruitment of MOs/MPs consists of mobilization of MOs/MPs from bone marrow to blood and from blood to injured muscles. MO/MP mobilization from bone marrow to blood was impaired at baseline and in response to experimental atherosclerosis and sterile peritonitis in Ccl2−/− mice (7). Likewise, MO/MP mobilization from bone marrow to blood was also impaired in response to acute skeletal muscle injury in Ccl2−/− mice, as shown by this study. We further showed that mobilization of MOs/MPs from bone marrow to blood required CCL2 expression by both bone marrow cells and injured muscle tissue cells. The findings suggest that CCL2 expressed by injured muscle tissue cells may be a part of the signals sent by injured muscles for bone marrow monocyte precursor cells to proliferate and enter blood. CCL2 produced by injured muscle tissues may diffuse into blood to increase the plasma CCL2 level to drive the recruitment of 7/4+Ly-6G− monocyte precursor cells from bone marrow to blood. However, the precise mechanism by which CCL2 drives proliferation and/or mobilization of monocyte precursor cells from bone marrow to blood remains elusive.
Our adoptive bone marrow mononuclear cell transfer studies showed that the recruitment of monocytes from blood into injured muscles required expression of CCL2 not only by injured muscle tissue cells but also by circulating monocytes themselves. Intravenously injected CCL2-deficient bone marrow cells could not enter wild-type injured muscles as well as wild-type bone marrow cells, despite normal up-regulation of CCL2 in injured muscles. The finding suggests that CCL2 expressed by circulating monocytes may exert an autocrine function important for these cells to transmigrate from blood to injured muscles. Transmigration of monocytes from blood to injured tissues is a multistep process, which consists of tethering and rolling of circulating monocytes on vascular walls, firm adhesion and arrest of monocytes on microvascular endothelial cells, locomotion of monocytes to the nearest endothelial junctions, and diapedesis. Chemokine and chemokine receptor binding is critical for firm adhesion of monocytes to endothelium, during which the endothelial cell-derived chemokines bind corresponding chemokine receptors on monocytes to trigger instant conformational changes of integrins on monocytes so that the intergrins can bind to their ligands expressed on endothelial cells with high affinities (36). It has been shown that CCL2 is required for firm adhesion of monocytes to endothelial cells to arrest monocytes under flow conditions (37, 38). Ccr2−/− mice showed a severe defect in CCL2-induced leukocyte firm adhesion and extravasation (24). However, it has also been shown that chemokine KC, but not CCL2, triggers monocyte arrest on early atherosclerotic endothelium (39). Nevertheless, our present and previous studies (16) suggest that CCL2/CCR2 binding appears to play a role in monocyte transmigration in response to acute skeletal muscle injury, as CCL2 or CCR2-deficient monocytes were unable to transmigrate from blood to injured muscles as well as wild-type monocytes. Although CCL2 involved in monocyte adhesion has been shown to be derived from endothelial cells rather than plasma, it remains possible as suggested by our present study that CCL2 expressed by transmigrating monocytes themselves might also contribute, especially as a high occupancy of CCR2 by CCL2 is required for inducing conformational changes of integrins (36). However, this hypothesis needs to be tested.
The function of CCL2/CCR2 signaling is thought mainly to recruit MOs/MPs into injured or infected tissues. Our present study demonstrates that within injured tissue, CCL2/CCR2 signaling also regulates the effector function of recruited MOs/MPs. CCL2 up-regulated IGF-1 expression by infiltrating macrophages in injured muscles. The notion that CCL2/CCR2 signaling not only regulates tissue recruitment of circulating monocytes or monocyte derivatives, but also regulates biological function of these cells is supported by a previous study showing that CCL2/CCR2 up-regulated collagen expression by lung fibrocytes recruited via CCR2 to contribute to pulmonary fibrogenesis (40).
The impaired muscle inflammation and regeneration seen in Ccl2−/− mice is less severe than seen in Ccr2−/− mice, suggesting that other CCR2 ligands may also participate in MO/MP recruitment from bone marrow to blood and/or from blood to injured muscles. It has been shown that CCL2 and CCL7 but not CCL12 play an important role in mobilizing MO/MP precursor cells from bone marrow to blood in healthy mice and in mice with experimental atherosclerosis or sterile peritonitis (7). It is conceivable that CCL7 may also play a role in mobilizing MO/MP precursor cells from bone marrow to blood in response to acute skeletal muscle injury, especially in the absence of CCL2. Like CCL2, the protein levels of both CCL7 and CCL12 were up-regulated in injured muscles, suggesting that these ligands might also serve as chemoattractants for CCR2+ MOs/MPs. However, a recent study showed that a CCL12 neutralizing antibody failed to worsen muscle regeneration in Ccl2−/− mice after muscle injury induced by cardiotoxin, suggesting that CCL12 does not significantly participate in muscle inflammation or regeneration during acute muscle injury repair (14). But it remains possible that CCL7 may serve as a chemoattractant to recruit MOs/MPs, especially given the fact that both CCL2 and CCL7 had a peak protein level at d 2, before the peak of MO/MP infiltration at d 3.
Ccl2−/− mice showed a small peak of intramuscular macrophage accumulation at d 7 rather than a large peak at d 3 as seen in wild-type mice. A similar small peak was also seen in Ccr2−/− mice (16), indicating that the macrophage accumulation at d 7 in Ccl2−/− mice was unlikely due to compensation by CCL7 or CCL12 signaling through CCR2. However, CCL7 can bind other chemokine receptors, including CCR1, CCR3, and CCR5. It might function through these receptors rather than CCR2 to contribute to the small peak of macrophage accumulation at d 7. Alternatively, other chemokine ligands, such as RANTES, might also compensate as the expression of this ligand was also up-regulated during acute muscle injury repair (33).
Our finding that up-regulation of IGF-1 was reduced in injured muscles of Ccl2−/− mice and a single intramuscular injection of IGF-1 to locally replace IGF-1 significantly improved muscle regeneration is important. It further supports the notion that the essential role of CCL2/CCR2 signaling in acute skeletal muscle injury repair is to recruit MOs/MPs to produce a high level of IGF-1 to support muscle regeneration. Although IGF-1 is mainly produced by intramuscular macrophages, it may also be expressed by other intramuscular cells, as the IGF-1 protein is noticeably increased at d 1 in Wt, Ccl2−/−, and Ccr2−/− mice, but macrophage infiltration is not evident at d 1. This may also explain why the increase of intracellular macrophage content is greater than the increase of muscle IGF-1 levels at d 3 between Wt and Ccr2−/− or Ccl2−/− mice. IGF-1, an anabolic growth factor, is a key regulator of skeletal muscle growth and size during development and regeneration (41–44). IGF-1 mRNA was markedly increased in regenerating muscles following various injuries (45–47). Transgenic expression of IGF-1 in skeletal muscle accelerated muscle injury repair and also significantly reduced inflammatory cytokine and chemokine expression, suggesting that IGF-1 may also contribute to the resolution of inflammation, which is necessary for normal muscle regeneration (48). Antagonists and inhibitors targeting CCL2/CCR2 have been under active preclinical and clinical development (17–20). Poor muscle injury repair may be a considerable side effect of this line of therapies. IGF-1 may thus represent a useful therapy to control this muscle side effect in future CCL2/CCR2 blockade.
Acknowledgments
This study is supported by U.S. National Institutes of Health grant K08 NS049346 (L.Z.) and Muscular Dystrophy Association grants MDA#91682 (L.Z.) and K24 51400 (R.M.R.). The authors thank Drs. Liping Liu, Georgian Cheng, and Ping Huang and the Flow Cytometry Core of the Cleveland Clinic for superb technical support.
REFERENCES
- 1. Tidball J. G. (2005) Inflammatory processes in muscle injury and repair. Am. J. Physiol. 288, R345–353 [DOI] [PubMed] [Google Scholar]
- 2. Boring L., Gosling J., Cleary M., Charo I. F. (1998) Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 [DOI] [PubMed] [Google Scholar]
- 3. Lu B., Rutledge B. J., Gu L., Fiorillo J., Lukacs N. W., Kunkel S. L., North R., Gerard C., Rollins B. J. (1998) Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187, 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fife B. T., Huffnagle G. B., Kuziel W. A., Karpus W. J. (2000) CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192, 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang D. R., Wang J., Kivisakk P., Rollins B. J., Ransohoff R. M. (2001) Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 193, 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Izikson L., Klein R. S., Charo I. F., Weiner H. L., Luster A. D. (2000) Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsou C. L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007) Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinones M. P., Ahuja S. K., Jimenez F., Schaefer J., Garavito E., Rao A., Chenaux G., Reddick R. L., Kuziel W. A., Ahuja S. S. (2004) Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J. Clin. Invest. 113, 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shahrara S., Proudfoot A. E., Park C. C., Volin M. V., Haines G. K., Woods J. M., Aikens C. H., Handel T. M., Pope R. M. (2008) Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J. Immunol. 180, 3447–3456 [DOI] [PubMed] [Google Scholar]
- 10. Belperio J. A., Keane M. P., Burdick M. D., Lynch J. P., 3rd, Xue Y. Y., Berlin A., Ross D. J., Kunkel S. L., Charo I. F., Strieter R. M. (2001) Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J. Clin. Invest. 108, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Contreras-Shannon V., Ochoa O., Reyes-Reyna S. M., Sun D., Michalek J. E., Kuziel W. A., McManus L. M., Shireman P. K. (2007) Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2-/- mice following ischemic injury. Am. J. Physiol. Cell. Physiol. 292, C953–C967 [DOI] [PubMed] [Google Scholar]
- 12. Shireman P. K., Contreras-Shannon V., Ochoa O., Karia B. P., Michalek J. E., McManus L. M. (2007) MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J. Leukoc. Biol. 81, 775–785 [DOI] [PubMed] [Google Scholar]
- 13. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez C. O., McHale M. J., Wells J. T., Ochoa O., Michalek J. E., McManus L. M., Shireman P. K. (2010) Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. 299, 832–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun D., Martinez C. O., Ochoa O., Ruiz-Willhite L., Bonilla J. R., Centonze V. E., Waite L. L., Michalek J. E., McManus L. M., Shireman P. K. (2009) Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 23, 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu H., Huang D., Saederup N., Charo I. F., Ransohoff R. M., Zhou L. (2011) Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charo I. F., Ransohoff R. M. (2006) The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 [DOI] [PubMed] [Google Scholar]
- 18. Proudfoot A. E. (2002) Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2, 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang T. L., Gordon C. J., Roscic-Mrkic B., Power C., Proudfoot A. E., Moore J. P., Trkola A. (2002) Interaction of the CC-chemokine RANTES with glycosaminoglycans activates a p44/p42 mitogen-activated protein kinase-dependent signaling pathway and enhances human immunodeficiency virus type 1 infectivity. J. Virol. 76, 2245–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xia M., Sui Z. (2009) Recent developments in CCR2 antagonists. Expert. Opin. Ther. Pat. 19, 295–303 [DOI] [PubMed] [Google Scholar]
- 21. Giunti S., Barutta F., Perin P. C., Gruden G. (2010) Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr. Vasc. Pharmacol. 8, 849–860 [DOI] [PubMed] [Google Scholar]
- 22. Charo I. F., Taubman M. B. (2004) Chemokines in the pathogenesis of vascular disease. Circ. Res. 95, 858–866 [DOI] [PubMed] [Google Scholar]
- 23. Cardona A. E., Sasse M. E., Liu L., Cardona S. M., Mizutani M., Savarin C., Hu T., Ransohoff R. M. (2008) Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 112, 256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuziel W. A., Morgan S. J., Dawson T. C., Griffin S., Smithies O., Ley K., Maeda N. (1997) Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. U. S. A. 94, 12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lagasse E., Weissman I. L. (1996) Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197, 139–150 [DOI] [PubMed] [Google Scholar]
- 26. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 [DOI] [PubMed] [Google Scholar]
- 27. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 28. Liu L., Darnall L., Hu T., Choi K., Lane T. E., Ransohoff R. M. Myelin repair is accelerated by inactivating CXCR2 on nonhematopoietic cells. J. Neurosci. 30, 9074–9083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou L., Porter J. D., Cheng G., Gong B., Hatala D. A., Merriam A. P., Zhou X., Rafael J. A., Kaminski H. J. (2006) Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul. Disord. 16, 32–38 [DOI] [PubMed] [Google Scholar]
- 30. Robertson T. A., Maley M. A., Grounds M. D., Papadimitriou J. M. (1993) The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp. Cell Res. 207, 321–331 [DOI] [PubMed] [Google Scholar]
- 31. Shavlakadze T., McGeachie J., Grounds M. D. (2010) Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 11, 363–376 [DOI] [PubMed] [Google Scholar]
- 32. Shireman P. K., Contreras-Shannon V., Reyes-Reyna S. M., Robinson S. C., McManus L. M. (2006) MCP-1 parallels inflammatory and regenerative responses in ischemic muscle. J. Surg. Res. 134, 145–157 [DOI] [PubMed] [Google Scholar]
- 33. Summan M., McKinstry M., Warren G. L., Hulderman T., Mishra D., Brumbaugh K., Luster M. I., Simeonova P. P. (2003) Inflammatory mediators and skeletal muscle injury: a DNA microarray analysis. J. Interferon Cytokine Res. 23, 237–245 [DOI] [PubMed] [Google Scholar]
- 34. Ochoa O., Sun D., Reyes-Reyna S. M., Waite L. L., Michalek J. E., McManus L. M., Shireman P. K. (2007) Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am. J. Physiol. 293, R651–R661 [DOI] [PubMed] [Google Scholar]
- 35. Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A. C., Poron F., Authier F. J., Dreyfus P. A., Gherardi R. K. (2003) Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 163, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laudanna C., Alon R. (2006) Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb. Haemost. 95, 5–11 [PubMed] [Google Scholar]
- 37. Gerszten R. E., Garcia-Zepeda E. A., Lim Y. C., Yoshida M., Ding H. A., Gimbrone M. A., Jr., Luster A. D., Luscinskas F. W., Rosenzweig A. (1999) MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398, 718–723 [DOI] [PubMed] [Google Scholar]
- 38. Maus U., Henning S., Wenschuh H., Mayer K., Seeger W., Lohmeyer J. (2002) Role of endothelial MCP-1 in monocyte adhesion to inflamed human endothelium under physiological flow. Am. J. Physiol. Heart Circ. Physiol. 283, H2584–H2591 [DOI] [PubMed] [Google Scholar]
- 39. Huo Y., Weber C., Forlow S. B., Sperandio M., Thatte J., Mack M., Jung S., Littman D. R., Ley K. (2001) The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J. Clin. Invest. 108, 1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moore B. B., Kolodsick J. E., Thannickal V. J., Cooke K., Moore T. A., Hogaboam C., Wilke C. A., Toews G. B. (2005) CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am. J. Pathol. 166, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 [PubMed] [Google Scholar]
- 42. Musaro A. (2005) Growth factor enhancement of muscle regeneration: a central role of IGF-1. Arch. Ital. Biol. 143, 243–248 [PubMed] [Google Scholar]
- 43. Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L., Rosenthal N. (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 [DOI] [PubMed] [Google Scholar]
- 44. Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D., Gillett N., Stewart T. A. (1993) IGF-I is required for normal embryonic growth in mice. Genes Dev. 7, 2609–2617 [DOI] [PubMed] [Google Scholar]
- 45. Hayashi S., Aso H., Watanabe K., Nara H., Rose M. T., Ohwada S., Yamaguchi T. (2004) Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochem. Cell Biol. 122, 427–434 [DOI] [PubMed] [Google Scholar]
- 46. Heinemeier K. M., Olesen J. L., Haddad F., Schjerling P., Baldwin K. M., Kjaer M. (2009) Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J. Appl. Physiol. 106, 178–186 [DOI] [PubMed] [Google Scholar]
- 47. Sacco A., Doyonnas R., LaBarge M. A., Hammer M. M., Kraft P., Blau H. M. (2005) IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J. Cell Biol. 171, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pelosi L., Giacinti C., Nardis C., Borsellino G., Rizzuto E., Nicoletti C., Wannenes F., Battistini L., Rosenthal N., Molinaro M., Musaro A. (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 21, 1393–1402 [DOI] [PubMed] [Google Scholar]