Abstract
Staphylococcus aureus infections result in abscesses as well as septicemia. Even with therapy, abscesses can persist or even reoccur, as staphylococcal infections fail to induce protective immune responses. Here, we show that prior infection with certain attenuated strains may elicit protective immunity. A closer examination reveals that protection correlates with antibody responses elicited on exposure to particular attenuated variants. Linear regression analysis was used to compare reduction in staphylococcal disease and antibody responses to infection with wild-type and attenuated variants. This analysis identified protective antigens that, when tested as vaccines in mice, elicited disease protection. Protection afforded by attenuated strains correlates in part with the ability of Staphylococcus aureus to modulate B cell responses via protein A (spa encoded). We designate this approach “genetic vaccinology,” since it exploits genetic variants to draw a correlation between disease protection and humoral immune responses for the deduction of vaccine antigens. Genetic vaccinology is particularly useful for microbes that do not elicit natural protective immunity during infection.—Kim, H. K., Kim, H. -Y., Schneewind, O., Missiakas, D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses.
Keywords: genetic vaccinology, protein A, sortase A, clumping factor, fibrinogen-binding protein, SdrD
The survivors of many infectious diseases acquire immunity to the causative microbial agents. Analysis of microbe-specific immune responses has informed the design of effective vaccines and immune therapies (1). These technologies also laid the foundation for a rational appreciation of protective immunity, whereby antibodies neutralizing key virulence traits (2) or fixing complement to kill infectious agents (3) represent correlates for vaccine efficacy. Nevertheless, some microbes, including Staphylococcus aureus, suppress the development of immunity in infected hosts (4). The strategy of identifying protective antigens with immune sera or the rational design of effective vaccines has thus far failed for this pathogen (5).
S. aureus, a gram-positive bacterium that colonizes the skin and nares, gains access to host tissues through hair follicles or cuts, causing purulent abscesses that require surgical intervention and antibiotic therapy (6). Even with therapy, staphylococcal abscesses reoccur in up to 20% of all cases, and prior infection does not trigger protective immune responses in humans (7). Invasive disease, the progressive infiltration of host tissues, is frequently associated with bacteremia; i.e., the isolation of staphylococci from the bloodstream (8). S. aureus sepsis represents the most frequent cause of infectious disease mortality in the United States (8). Staphylococcal isolates resistant to many antibiotic therapies are designated methicillin-resistant S. aureus (MRSA; ref. 9). Vancomycin is considered the antibiotic of last resort for MRSA; however, strains with intermediate or full resistance to vancomycin have been isolated (7, 10). While preventive measures to reduce the burden of S. aureus disease have been needed for many years, an FDA-licensed vaccine with proven clinical efficacy is still not available (11). The investigation of several individual envelope components and secreted products as vaccine antigens [surface proteins, including clumping factor A (ClfA) and iron-regulated surface determinant B (IsdB), capsular polysaccharide, exopolysaccharide poly-N-acetyl-β-1,6-glucosamine (PNAG), α-hemolysin, and leukocidins; 12–16] have not yet proven fruitful. These studies have, however, served to highlight the need for a clear understanding of the features of the host immune response that are requisite for the generation of protective immunity. This knowledge may also be useful for the development of effective antistaphylococcal vaccines. Here, we show that prior infection with particular live-attenuated staphylococcal variants can lead to the generation of protective immunity in animal models of disease. We dissect this finding to derive combinations of defined staphylococcal subunit vaccines.
MATERIALS AND METHODS
Bacterial strains and culturing conditions
Staphylococci were cultured with tryptic soy broth (TSB) or agar at 37°C. Escherichia coli strains DH5α and BL21(DE3) were cultured with Luria broth (LB) or agar at 37°C. Ampicillin (100 μg/ml for pET15b), erythromycin (200 μg/ml for Bursa aurealis variants), and spectinomycin (200 μg/ml for the spa deletion variant) were used for the selection of antibiotic resistance traits.
Mutagenesis
B. aurealis minitransposon insertions from the Phoenix library were transduced into S. aureus Newman (17). The spa gene on the chromosome of S. aureus Newman was deleted by allelic replacement, as described previously (18).
Cloning and purification
Cloning of ClfA, serine-aspartate repeat D (SdrD), fibrinogen binding protein B (FnBPB), and nontoxigenic protein A was described previously (13, 19). Plasmids were transformed into BL21(DE3). Overnight cultures of recombinant E. coli strains were diluted 1:100 into fresh medium and grown at 37°C to OD600 0.5, at which point cultures were induced with 1 mM isopropyl β-d-1-thiogalatopyranoside (IPTG) and grown for an additional 3 h. Bacterial cells were sedimented by centrifugation, suspended in column buffer (50 mM Tris-HCl, pH 7.5, and 150 mM NaCl), and disrupted with a French pressure cell at 14,000 psi. Lysates were cleared of membrane and insoluble components by ultracentrifugation at 40,000 g. Proteins in the soluble lysate were subjected to nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography. Proteins were eluted in column buffer containing successively higher concentrations of imidazole (100–500 mM). Protein concentrations were determined by bicinchonic acid (BCA) assay (Thermo Scientific, Waltham, MA, USA).
Renal abscess model and live-attenuated vaccine
Overnight cultures of S. aureus Newman and its isogenic mutants were diluted 1:100 into fresh TSB and grown for 2 h at 37°C. Staphylococci were sedimented, washed, and suspended in PBS at OD600 0.4 (∼1×108 CFU/ml). Inocula were quantified by spreading sample aliquots on TSA and enumerating colony formation. BALB/c mice (4 wk old, female; Charles River Laboratories, Wilmington, MA, USA) were anesthetized via intraperitoneal injection with 100 mg/ml ketamine and 20 mg/ml xylazine per kilogram of body weight. Mice were infected with 100 μl of bacterial suspension (1×107 CFU) by retroorbital injection. To examine virulence defects, animals were killed by CO2 inhalation on d 18 postinfection. To examine immunization with live attenuated strains, on d 19 following infection, cohorts of mice were treated with antibiotic (chloramphenicol; 1 mg/ml) in water for 3 d. On d 26, mice were challenged with 100 μl of S. aureus Newman (1×107 CFU) by retroorbital injection or bled to analyze adaptive immune response toward components of the antigen matrix, which consists of 26 affinity-purified recombinant His6-tagged staphylococcal antigens, as described earlier (19) and as listed in Supplemental Table S1. Animals were killed by CO2 inhalation on d 30 after initial infection. Both kidneys were removed, and the staphylococcal load in the right kidney was analyzed by homogenizing renal tissue with PBS and 0.1% Triton X-100. Serial dilutions of homogenate were spread on TSA or TSA containing antibiotics and incubated for colony formation. The left kidney was examined by histopathology. Briefly, kidneys were fixed in 10% formalin for 24 h at room temperature. Tissues were embedded in paraffin, thin-sectioned 4 times in 200-μm increments, stained with hematoxylin-eosin, and inspected by light microscopy to enumerate abscess lesions. Abscess lesions were identified as foci with infiltrated immune cells (mainly neutrophils) and/or bacterial community (staphylococcal abscess community). Hyperimmune sera were collected via cardiac puncture and analyzed against components of the antigen matrix, a nitrocellulose membrane blotted with 2 μg of a collection of 26 Ni-NTA affinity purified recombinant His6-taged staphylococcal antigens (19). All animal experiments were performed in accordance with the institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at the University of Chicago.
Active immunization using recombinant antigens
BALB/c mice (3 wk old, female; Charles River Laboratories) were immunized with 25 μg protein emulsified in complete Freund's adjuvant (CFA; Difco, Corpus Christie, TX, USA) by intramuscular injection. For booster immunizations, proteins were emulsified in incomplete Freund's adjuvant (IFA) and injected 11 d following the initial immunization. On d 20 following immunization, 5 mice were bled to obtain sera for specific antibody titers by enzyme-linked immunosorbent assay (ELISA). On d 21, mice were challenged with either 1 × 107 CFU (abscess model) or 1 × 108 CFU (sepsis model) S. aureus Newman. At 4 and 18 d following sublethal challenge, kidneys were removed during necropsy, and renal tissue was analyzed for staphylococcal load or histopathology.
Antibody quantification
For the antigen matrix, nitrocellulose membrane was blotted with 2 μg of a collection of Ni-NTA affinity purified recombinant His6 tagged staphylococcal proteins (19). Signal intensities in mouse sera were quantified and normalized using anti-His6 antibody with the Odyssey imaging system (Li-Cor, Lincoln, NE, USA).
Statistical analysis
Unpaired 2-tailed Student's t tests were performed to analyze the statistical significance. Linear regression analysis was performed using GraphPad Prism (GraphPad, San Diego, CA, USA).
RESULTS
Attenuated strains of S. aureus that elicit protective immune responses in mice
Following injection into the bloodstream of mice, S. aureus seeds abscesses in all organ systems (20). Within 4 d, staphylococci grow at the center of these lesions, surrounded by a pseudocapsule of fibrin deposits as well as layers of live and dead immune cells (ref. 20 and Fig. 1). Over time, abscesses migrate to the surface of organs, where they rupture and release pathogens into body fluids, with ensuing establishment of new lesions (20). Animals with a history of staphylococcal infection do not acquire protective immunity. This can be demonstrated experimentally by infecting mice with S. aureus via intravenous challenge and eventually administering chloramphenicol therapy for animals with abscess lesions. Subsequent intravenous challenge of such animals with S. aureus produces histopathology, bacterial burden, and persistence in a manner that is indistinguishable from the staphylococcal disease of naive mice (ref. 19 and Table 1; compare mock-treated and S. aureus Newman-infected animals). This inability to acquire protection toward subsequent infections correlated with the inability to mount a robust humoral immune response. Indeed, even after 18–30 d, infected hosts mount humoral immune responses against only few staphylococcal antigens (ref. 19 and Supplemental Table S1; data shown following 18 d postinfection). Together, these observations are in agreement with a model whereby S. aureus infection actively suppresses the development of adaptive immune responses (19, 21).
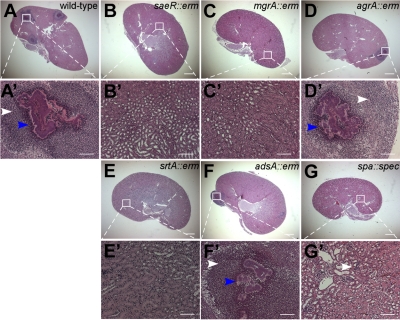
Figure 1.
Virulence defect of S. aureus Newman variants to form abscess lesions in the kidneys of infected mice. BALB/c mice were treated by injection into the retroorbital plexus with 1 × 107 CFU of S. aureus Newman (wild type; A, A′), saeR (B, B′), mgrA (C, C′), agrA (D, D′), srtA (E, E′), adsA (F, F′), or spa variants (G, G′). At 18 d after infection, animals were necropsied. Kidneys were analyzed for staphylococcal load (Supplemental Table S2) as well as histopathology using thin-sectioned, hematoxylin-eosin stained tissue slides. White arrowheads identify polymorphonuclear leukocyte infiltrates. Blue arrowheads identify staphylococcal abscess communities. Animal data are representative of 3 independent experiments. Histopathology images were acquired with light microscopy at ×12.5 (A–G) and ×200 (A′–G′). Scale bars = 1 mm (A–G); 100 μm (A′–G′).
Table 1.
Immunization with live-attenuated S. aureus strains and protective immunity
| Vaccination | Staphylococcal load in renal tissuea |
Abscess formation in renal tissueb |
|||
|---|---|---|---|---|---|
| Load (log10 CFU/g) | P value | Reduction (log10 CFU/g) | Number of abscesses | P value | |
| Mock | 6.09 ± 0.70, n = 12 | — | — | 4.9 ± 1.6, n = 12 | — |
| Wild type | 5.51 ± 0.42, n = 15 | 0.4665 | 0.58 | 4.9 ± 1.3, n = 15 | 0.9808 |
| saeR::erm | 3.91 ± 0.61, n = 15 | 0.0267 | 2.18 | 2.5 ± 1.1, n = 10 | 0.2477 |
| mgrA::erm | 3.49 ± 0.63, n = 15 | 0.0107 | 2.60 | 2.0 ± 0.7, n = 10 | 0.1352 |
| srtA::erm | 1.58 ± 0.55, n = 14 | <0.0001 | 3.48 | 0.3 ± 0.2, n = 10 | 0.0153 |
| spa::spec | 3.93 ± 0.56, n = 14 | 0.0226 | 2.16 | 1.3 ± 0.7, n = 14 | 0.0371 |
Representative data of 3 independent animal experiments are shown. Values are presented as means ± se. Statistical significance was calculated with the unpaired 2-tailed Students t-test; values of P < 0.05 were deemed significant.
Means of staphylococcal load calculated as log10 CFU/g in homogenized renal tissues 4 d after retroorbital injection of 1×107 CFU S. aureus Newman into BALB/c mice that had been immunized. Immunization occurred by prior injection with 1×107 CFU S. aureus Newman, its isogenic variants, or mock (PBS) treatment into the retroorbital space of naïve BALB/c mice. On d 19 following live immunization, animals were treated with chloramphenicol for 3 d. Mice were challenged with S. aureus Newman injection on d 26.
Histopathology of hematoxylene-eosin stained, thin-sectioned kidneys; average number of abscesses per kidney was recorded and averaged again for the final mean.
We wondered whether exposure to strains with altered virulence could be exploited to deduce the identity of protective antigens. We chose to work with 4 mutants, accessory gene regulator A (agrA), S. aureus exoprotein regulator (saeR), multiple gene regulator A (mgrA), and sortase A (srtA), because of their effect on expression of exoproteins and surface protein genes as well as the processing of surface proteins. Specifically, AgrA is the response regulator of the 2-component sensory transduction module (AgrCA) responding to AgrBD-derived quorum signals. AgrA coordinates the expression of exoprotein and surface protein genes (22). Coordination of gene expression is lost in an agrA mutant, resulting in aberrant gene expression. MgrA is the cytoplasmic sensor of oxidative stress, as occurs when S. aureus is phagocytosed by immune cells; expression of exoprotein and surface protein genes is increased in mgrA mutants (23). Another 2-component regulatory system, SaeRS, controls key virulence factors secreted by S. aureus (24). Sortase A is the transpeptidase that anchors surface proteins with LPXTG sorting signals to the cell wall envelope (25, 26). Surface proteins are not displayed in the envelope of srtA mutants and instead are secreted.
As expected from earlier work, mutants lacking saeR or mgrA displayed severe virulence defects (23, 27), allowing infected mice to clear these infections (Fig. 1 and Supplemental Table S2). Nevertheless, animals inoculated with saeR or mgrA mutants did not acquire immunity, as subsequent challenge with wild-type S. aureus resulted in infectious lesions and bacterial burden that was not significantly different from naive mice (Fig. 2 and Table 1). agrA mutants did not display defects in abscess formation and could not be cleared by the host (Fig. 1 and Table 1). agrA mutants were not pursued further as live-attenuated vaccine strains (Fig. 2). Following intravenous challenge of mice, srtA mutants cannot establish abscess lesions and are cleared by the host immune system (ref. 20 and Fig. 1). Inoculation of mice with srtA variants elicited protective immunity, as subsequent challenge with S. aureus Newman resulted in a reduction of abscess lesions and in reduced staphylococcal burden (Fig. 2 and Table 1).
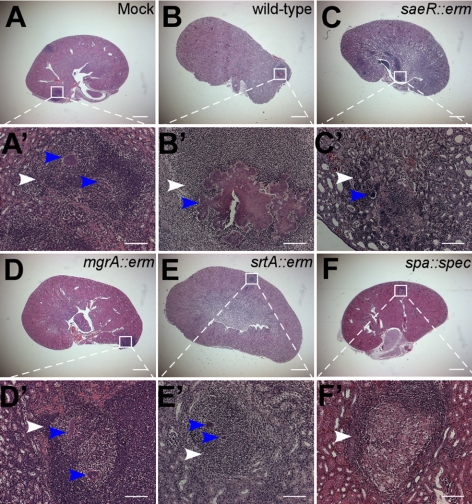
Figure 2.
Attenuated S. aureus strains that elicit protective immunity in mice. BALB/c mice were treated by injection into the retroorbital plexus with either mock treatment (PBS; A, A′) or 1 × 107 CFU of S. aureus Newman (wild type; B, B′), saeR (C, C′), mgrA (D, D′), srtA (E, E′), or spa variants (F, F′). At 18 d after infection, all animals were treated with antibiotic and then challenged by retroorbital injection with 1 × 107 CFU S. aureus Newman. At 4 d after challenge, animals were necropsied. Kidneys were analyzed for staphylococcal load as well as histopathology using thin-sectioned, hematoxylin-eosin stained tissue slides (Table 1). White arrowheads identify polymorphonuclear leukocyte infiltrates. Blue arrowheads identify staphylococcal abscess communities. Animal data are representative of 3 independent experiments. Histopathology images were acquired with light microscopy at ×12.5 (A–G) and ×200 (A′–F′). Scale bars = 1 mm (A–F); 100 μm (A′–F′).
Antibody responses of animals with protective immunity against S. aureus
Serum samples of mice were withdrawn 18 d following inoculation with either wild-type or mutant S. aureus strains and analyzed by immunoblotting with 26 staphylococcal antigens immobilized on a membrane filter (ref. 19 and Supplemental Table S1). Naive mice, which had not been infected with staphylococci, did not reveal any positive signal on the matrix. Mice that had been subjected to S. aureus Newman infection developed antibodies against LukD (Supplemental Table S1). When animals were infected with srtA mutants, they displayed increased antibody responses toward several surface proteins, in agreement with the notion that surface proteins are secreted in the extracellular milieu in this mutant (Supplemental Table S1 and Supplemental Fig. S1).
Protein A is required for staphylococcal persistence in host tissues
Since vaccination with a srtA mutant afforded protective immunity, we examined two sortase A-anchored substrates for their immune suppressive attributes, staphylococcal protein A (SpA) and adenosine synthase A (AdsA). Previous work implicated SpA (28), a B-cell superantigen that binds to the Fc-γ and F(ab)2 portions of VH3+ immunoglobulins, as a factor in the pathogen's strategy to modulate host adaptive immune responses (29–32). mgrA and srtA variants harbor the wild-type spa gene; however srtA, not mgrA, is required for the cell wall anchoring and surface display of protein A (33). AdsA, an enzyme that cleaves nucleotides to trigger the buildup of adenosine in infected tissues, is also anchored by SrtA (21). AdsA activity is thought to signal via adenosine receptors on the surface of lymphocytes as well as cells of the myeloid lineage, interfering with host innate and adaptive immune responses (21). We asked whether the inability of srtA mutants to display wild-type levels of SpA or AdsA is associated with the attribute of eliciting protective immunity. At 18 d after inoculation of mice, the spa mutant displayed a decrease in bacterial load and abscess lesions (Fig. 1). The adsA mutant also displayed a reduction in bacterial load; however, this defect was not significantly different from the wild-type parent (Fig. 1 and Supplemental Table S2). Even though adsA mutants are defective for the establishment of abscess lesion on d 4 following injection, the mutant can persist in infected host tissues (Fig. 1 and Supplemental Table S2) much like the agrA mutant. Thus, adsA mutants were not characterized further as potential live-attenuated vaccine strains.
Protein A suppresses host antibody responses during staphylococcal infection
Mice inoculated with spa mutants were treated with antibiotics and then challenged with S. aureus Newman. Prior immunization with spa mutants significantly reduced the burden of S. aureus Newman (Table 1). Further, histopathology analysis revealed fewer abscessed lesions and a reduction in staphylococcal abscess communities associated with the remaining lesions of mice that had been immunized with the spa mutant (Fig. 2). Sera from animals immunized with spa mutants harbored elevated antibody titers toward several staphylococcal antigens, in particular sortase A-anchored surface proteins (Supplemental Table S1 and Supplemental Fig. S1).
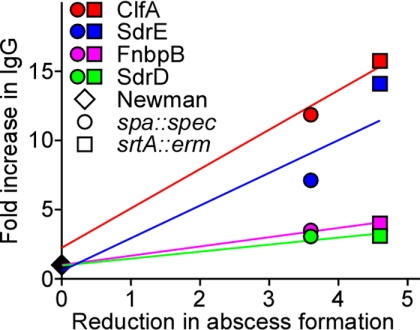
A linear regression analysis of serum antibody titers in animals preimmunized with wild-type, srtA, or spa mutant staphylococci and associated disease protection (reduction in abscess formation in kidney tissue) was used to identify putative protective antigens (Fig. 3). Plots for all candidate antigens analyzed by ELISA were examined for best values of slope and coefficient of determination (r2>0.85). The best correlation was obtained for ClfA, FnBPB and SdrD antigens (r2 values of 0.997, 0.998, 0.954) while SdrE scored the lowest coefficient of determination (r2=0.88). Thus, disease protection in animals appears to correlate with the production of antibodies against ClfA, FnbpB, SdrD, and SdrE on prior exposure to selective attenuated strains.
Figure 3.
Genetic vaccinology identifies protective staphylococcal antigens. Linear regression analysis was performed to correlate protective immunity; i.e., the reduction in abscesses at 4 d after challenge in animals preimmunized with srtA (□) or spa (○) mutants as compared to the wild-type Newman (◊) (as shown in Fig. 2), and the humoral (IgG) immune response in animals at the time of challenge against a matrix consisting of 26 staphylococcal antigens (see Supplemental Table S1 and Supplemental Fig. S1). Four antigens displayed a positive correlation and are shown on the graph: ClfA (red; r2=0.9976), SdrE (blue; r2=0.8819), FnBPB (pink; r2=0.9982), and SdrD (green; r2=0.9546).
Genetic vaccinology identifies protective antigens of S. aureus
The putative protective antigens identified by genetic vaccinology are sortase A-anchored surface proteins with C-terminal LPXTG sorting signals (34). Previous work assessed the contribution of surface proteins to disease pathogenesis and vaccine protection in the murine abscess model. Mutations in sdrD or clfA, but not sdrE or fnbpB, reduced the staphylococcal load in infected renal tissues (20). SdrD and ClfA were both selected for inclusion in combination vaccines. Polyclonal antibodies raised against SdrD cross-react with SdrE (data not shown); SdrD and SdrE share >76% identity in 561 residues. Due to the high degree of sequence identity, SdrE was not pursued for combination vaccines (see below). FnBPB is a homologue of FnBPA (60% sequence identity), and both polypeptides are known to bind fibronectin as well as fibrinogen (35). The contribution of both surface proteins to disease pathogenesis and protective immunity has not yet been assessed, and this prompted the inclusion of FnBPB into a combination vaccine with ClfA and SdrD (combo 1). Previous work identified nontoxigenic protein A (SpAKKAA) as a protective antigen, which elicits neutralizing IgG responses for the Fcγ and VH3+ F(ab)2 binding attributes of SpA (19). We, therefore, added SpAKKAA to the antigen mixture with ClfA, FnBPB and SdrD (combo 2).
Immunization of animals with combo 1 or 2 emulsified in CFA and boosted with the same antigen mixture emulsified in IFA, raised specific IgG responses (Supplemental Table S4). As expected, sera of animals immunized with SdrD reacted with both SdrD and SdrE purified proteins (Supplemental Table S4). Following intravenous challenge with S. aureus Newman, we observed a significant reduction in bacterial load for both vaccines on d 4 after challenge with the wild-type strain S. aureus Newman (Fig. 4A). To monitor the ability of vaccine formulations to prevent staphylococcal persistence, immunized animals were also analyzed 18 d after challenge (Fig. 4B). Again, immunization with either combo 1 or 2 conferred protection against persistent S. aureus Newman infection (Supplemental Table S3).
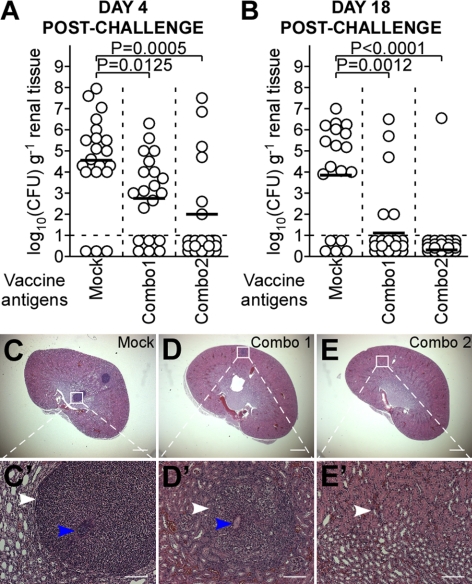
Figure 4.
Active immunization with antigens revealed by genetic vaccinology elicits protection in mice against staphylococcal abscess formation. Cohorts of BALB/c mice (n=18–20) were actively immunized with mock treatment (PBS; C, C′), combo 1 (ClfA, FnBPB, and SdrD; D, D′), or combo 2 (ClfA, FnBPB, SdrD, and SpAKKAA; E, E′) at d 0 and 11. On d 21, animals were challenged by retroorbital injection with 1 × 107 CFU S. aureus Newman. A, B) On d 4 (A) and 18 (B) postchallenge, animals were killed to enumerate staphylococcal burden in renal tissues. C–E′) Representative thin-sectioned, hematoxylin-eosin stained histopathology slides from each cohort (n=10, 4 d postchallenge; Supplemental Table S4). White arrowheads identify polymorphonuclear leukocyte (PMN) infiltrates. Blue arrowheads identify staphylococcal abscess communities. Histopathology images were acquired with light microscopy at ×12.5 (C–E) and ×200 (C′–E′). Scale bars = 1 mm (A–F); 100 μm (A′–F′). See Supplemental Table S5 for antibody titers in response to immunization. Animal data are representative of 2 independent experiments.
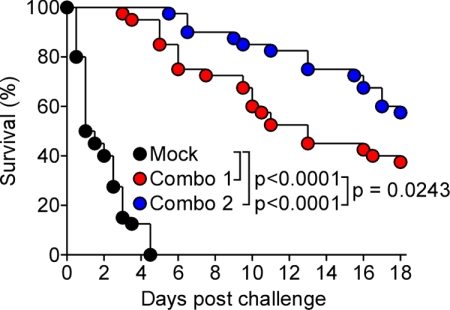
Vaccine protection against staphylococcal sepsis
The mortality of S. aureus infections increases dramatically when the pathogen replicates in blood or on endocardial tissue (8). We therefore asked whether combo 1 and 2 protect animals against lethal S. aureus Newman challenge (36). All mock-immunized animals succumbed to challenge within 4 d (Fig. 5). In contrast, combo 1-immunized mice displayed either a delayed time to death or survived the lethal challenge (Fig. 5). Mice immunized with combo 2 displayed a further increase in protective immunity and delayed time to death in a manner that was statistically significant over combo 1 (Fig. 5). Thus, the combination of antibodies against ClfA, FnBPB, SdrD, and SpA generates significant protection from staphylococcal abscess formation and lethal challenge.
Figure 5.
Active immunization with antigens revealed by genetic vaccinology elicits protection in mice against staphylococcal sepsis. Cohorts of BALB/c mice (n=20) were actively immunized with mock treatment (PBS), combo 1 (ClfA, FnBPB, and SdrD) or combo 2 (ClfA, FnBPB, SdrD, and SpAKKAA) at d 0 and 11. On d 21, animals were challenged by retroorbital injection with 1 × 108 CFU S. aureus Newman and monitored for survival. See Supplemental Table S5 for antibody titers in response to immunization. Animal data are combined from 2 independent experiments.
DISCUSSION
Much progress was derived from the discoveries that antibodies neutralize the virulence strategies of infectious microbes or opsonize invading pathogens. By examining immune responses in the survivors of infectious diseases, researchers could merge studies of microbes with immunology to derive vaccines that eventually had a tremendous effect on human morbidity and mortality (1). However, several infectious agents continue to evolve variations in their key virulence traits, thereby escaping the protective effect of neutralizing antibody responses and establishing disease in individuals already immune to segments of the pathogen's population (37). Reverse vaccinology addresses these issues, as this technology utilizes genome sequence information from all members of a pathogen species to identify and assess its antigenic determinants for protective immunity (38).
Some microbes suppress the development of host immunity, for example by interfering with T- and/or B-cell responses (39). For S. aureus, this has presented a formidable obstacle to the development of vaccines, as live-attenuated vaccine strains failed to induce protective immunity and subunit vaccines could not neutralize the immune suppressive attributes of the pathogen (4, 5). A vaccine that can prevent pulmonary tuberculosis or provide therapeutic clearance of the causative agent, Mycobacterium tuberculosis, is also not available (40). Recent work suggests that M. tuberculosis has evolved the ESAT-6/CFP-10 secretion pathway to suppress host immune responses (41). Neither the protective antigens for tuberculosis nor a strategy that neutralizes pathogen-derived immune suppression have become available (42).
Here, we have utilized S. aureus strain Newman, a clinical isolate from a human infection, to describe genetic vaccinology, a technology for vaccine development against pathogens that suppress host immune responses. Although Newman is known to overproduce several secreted factors, this strain has been extensively used for the discovery of virulence factors in the mouse models of infection. It also shares the same pattern of pathogenicity islands as USA300, the current epidemic MRSA stain in the United States. The general strategy of genetic vaccinology involves the systematic isolation and characterization of pathogen variants for which attenuation correlates with the generation of protective immunity in animal models. Sera of immune and nonimmune animals are studied to identify antibody responses associated with protective immunity. The presumed protective antigens can then be tested as subunit vaccines. Using this approach, we identified S. aureus variants that elicit protective immune responses in mice and circumvent the immune suppressive attributes of this pathogen. Comparative analysis of antibody responses in immune vs. nonimmune animals identifies antigens that, when used in purified form, raised protection against both staphylococcal abscess formation and sepsis. Previous attempts toward the identification of protective antigens by our group targeted sortase A-anchored substrates because of the significant defect of srtA mutants in establishing infection in the murine abscess model of disease (20). The relative protective immunity of individual sortase A-anchored surface protein was assessed in the murine abscess model. This systematic approach identified IsdA, IsdB, SdrD, and SdrE as the best four antigens, and, when used in combination, they conferred additive immune protection in a lethal challenge model in mice (13). Both methods, systematic and genetic, identified SdrD and SdrE as protective antigens of both persistent and lethal staphylococcal diseases. However, protective antigens with toxigenic attributes, such as protein A, cannot be identified through a systematic search but can be revealed by genetic vaccinology. We propose that genetic vaccinology may be useful for the development of vaccines against several different human diseases, including staphylococcal infections, syphilis, tuberculosis, or even leprosy, where classical approaches toward vaccine development have failed.
Supplementary Material
Acknowledgments
The authors thank members of their laboratory for experimental assistance and critical discussion.
This work was supported by grants from the U.S. National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI52747 and AI92711 to O.S. and AI75258 to D.M.). D.M. and O.S. acknowledge membership within and support from the Region V Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (National Institutes of Health award 1-U54-AI-057153).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Behring E. A., Kitasato S. (1890) Über das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch. Med. Wochenschr. 16, 1113–1114 [PubMed] [Google Scholar]
- 2. Smith T. (1907) The degree and duration of passive immunity to diphtheria toxin transmitted by immunized female guinea-pigs to their immediate offspring. J. Med. Res. 16, 359–379 [PMC free article] [PubMed] [Google Scholar]
- 3. Bordet J. (1923) Microbic transmissible autolysis. Br. Med. J. 1, 175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogers D. E., Melly M. A. (1965) Speculation on the immunology of Mudd, S. Capsulation, pseudocapsulation, and the somatic antigens of the surface of Staphylococcus aureus. Ann. N. Y. Acad. Sci. 128, 45–56 [DOI] [PubMed] [Google Scholar]
- 5. Projan S. J., Nesin M., Dunman P. M. (2006) Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr. Opin. Pharmacol. 6, 473–479 [DOI] [PubMed] [Google Scholar]
- 6. Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 [DOI] [PubMed] [Google Scholar]
- 7. Chambers H. F. (2005) Community-associated MRSA–resistance and virulence converge. N. Engl. J. Med. 352, 1485–1487 [DOI] [PubMed] [Google Scholar]
- 8. Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., Fridkin S. K. for the Active Bacterial Core Surveillance (ABCs) MRSA investigators (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 [DOI] [PubMed] [Google Scholar]
- 9. DeLeo F. R., Chambers H. F. (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang S., Sievert D. M., Hageman J. C., Boulton M. L., Tenover F. C., Downes F. P., Shah S., Rudrik J. T., Pupp G. R., Brown W. J., Cardo D., Fridkin S. K. (2003) Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348, 1342–1347 [DOI] [PubMed] [Google Scholar]
- 11. Bubeck Wardenburg J., Schneewind O. (2008) Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fattom A., Fuller S., Propst M., Winston S., Muenz L., He D., Naso R., Horwith G. (2004) Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23, 656–663 [DOI] [PubMed] [Google Scholar]
- 13. Stranger-Jones Y. K., Bae T., Schneewind O. (2006) Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 103, 16942–16947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKenney D., Pouliot K. L., Wang Y., Murthy V., Ulrich M., Doring G., Lee J. C., Goldmann D. A., Pier G. B. (1999) Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284, 1523–1527 [DOI] [PubMed] [Google Scholar]
- 15. Menzies B. E., Kernodle D. S. (1996) Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect. Immun. 64, 1839–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuklin N. A., Clark D. J., Secore S., Cook J., Cope L. D., McNeely T., Noble L., Brown M. J., Zorman J. K., Wang X. M., Pancari G., Fan H., Isett K., Burgess B., Bryan J., Brownlow M., George H., Meinz M., Liddell M. E., Kelly R., Schultz L., Montgomery D., Onishi J., Losada M., Martin M., Ebert T., Tan C. Y., Schofield T. L., Nagy E., Meineke A., Joyce J. G., Kurtz M. B., Caulfield M. J., Jansen K. U., McClements W., Anderson A. S. (2006) A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74, 2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bae T., Banger A. K., Wallace A., Glass E. M., Aslund F., Schneewind O., Missiakas D. M. (2004) Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. U. S. A. 101, 12312–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bae T., Schneewind O. (2005) Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63 [DOI] [PubMed] [Google Scholar]
- 19. Kim H. K., Cheng A. G., Kim H.-Y., Missiakas D. M., Schneewind O. (2010) Non-toxigenic protein A vaccine for methicillin-resistant Staphylococcusaureus infections. J. Exp. Med. 207, 1863–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng A. G., Kim H. K., Burts M. L., Krausz T., Schneewind O., Missiakas D. M. (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thammavongsa V., Kern J. W., Missiakas D. M., Schneewind O. (2009) Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 206, 2417–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Novick R. P., Geisinger E. (2008) Quorum sensing in staphylococci. Annu. Rev. Genet. 42, 541–564 [DOI] [PubMed] [Google Scholar]
- 23. Chen P. R., Bae T., Williams W. A., Duguid E. M., Rice P. A., Schneewind O., He C. (2006) An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat. Chem. Biol. 2, 591–595 [DOI] [PubMed] [Google Scholar]
- 24. Adhikari R. P., Novick R. P. (2008) Regulatory organization of the staphylococcal sae locus. Microbiology 154, 949–959 [DOI] [PubMed] [Google Scholar]
- 25. Mazmanian S. K., Liu G., Ton-That H., Schneewind O. (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285, 760–763 [DOI] [PubMed] [Google Scholar]
- 26. Ton-That H., Liu G., Mazmanian S. K., Faull K. F., Schneewind O. (1999) Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. U. S. A. 96, 12424–12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rampone H., Martinez G. L., Giraudo A. T., Calzolari A., Nagel R. (1996) In vivo expression of exoprotein synthesis with a sae mutant of Staphylococcus aureus. Can. J. Vet. Res. 60, 237–240 [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen K. (1958) A normally occurring staphylococcus antibody in human serum. Acta Path. Microbiol. Scandin. 44, 421–428 [DOI] [PubMed] [Google Scholar]
- 29. Forsgren A. (1968) Protein A from Staphylococcus aureus. VI. Reaction with subunits from guinea pig gamma-1- and gamma-2-globulin. J. Immunol. 100, 927–930 [PubMed] [Google Scholar]
- 30. Forsgren A., Svedjelund A., Wigzell H. (1976) Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur. J. Immunol. 6, 207–213 [DOI] [PubMed] [Google Scholar]
- 31. Goodyear C. S., Silverman G. J. (2003) Death by a B cell superantigen: in vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J. Exp. Med. 197, 1125–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodyear C. S., Silverman G. J. (2004) Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like B lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 101, 11392–11397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazmanian S. K., Liu G., Jensen E. R., Lenoy E., Schneewind O. (2000) Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. U. S. A. 97, 5510–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazmanian S. K., Ton-That H., Su K., Schneewind O. (2002) An iron-regulated sortase enzyme anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99, 2293–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fitzgerald J. R., Loughman A., Keane F., Brennan M., Knobel M., Higgins J., Visai L., Speziale P., Cox D., Foster T. J. (2006) Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol. Microbiol. 59, 212–230 [DOI] [PubMed] [Google Scholar]
- 36. Kim H. K., DeDent A., Cheng A. G., McAdow M., Bagnoli F., Missiakas D. M., Schneewind O. (2010) IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 28, 6382–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lancefield R. (1962) Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 89, 307–313 [PubMed] [Google Scholar]
- 38. Maione D., Margarit I., Rinaudo C. D., Masignani V., Mora M., Scarselli M., Tettelin H., Brettoni C., Iacobini E., Rosini R., D'Agostino N., Miorin L., Buccato S., Mariani M., Galli G., Nogarotto R., Nardi Dei V., Vegni F., Fraser C., Mancuso G., Teti G., Madoff L. C., Paoletti L. C., Rappuoli R., Kasper D. L., Telford J. L., Grandi G. (2005) Identification of a universal group B streptococcus vaccine by multiple genome screen. Science 309, 148–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silverman G. J., Goodyear C. S. (2006) Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat. Rev. Immunol. 6, 465–475 [DOI] [PubMed] [Google Scholar]
- 40. Kernodle D. S. (2010) Decrease in the effectiveness of Bacille Calmette-Guérin vaccine against pulmonary tuberculosis: a consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin. Infect. Dis. 51, 177–184 [DOI] [PubMed] [Google Scholar]
- 41. Simeone R., Bottai D., Brosch R. (2009) ESX/type VII secretion systems and their role in host-pathogen interaction. Curr. Opin. Microbiol. 12, 4–10 [DOI] [PubMed] [Google Scholar]
- 42. Sambandamurthy V. K., Derrick S. C., Hsu T., Chen B., Larsen M. H., Jalapathy K. V., Chen M., Kim J., Porcelli S. A., Chan J., Morris S. L., Jacobs W. R. J. (2006) Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine 24, 6309–6320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.