Abstract
The vascular endothelium responds to damage through activation of multiple signaling events that restore cell-cell adhesion and vascular integrity. However, the molecular mechanisms that integrate these events are not clearly defined. Herein, we identify a previously unexpected role for adenosine monophosphate-activated protein kinase (AMPK) in pulmonary microvascular endothelial cell (PMVEC) repair. PMVECs selectively express the AMPKα1 catalytic subunit, pharmacological and short hairpin RNA-mediated inhibition of which attenuates Ca2+ entry in these cells induced by the inflammatory Ca2+-signaling mimetic thapsigargin. We find that AMPKα1 activity is required for the formation of PMVEC cell-cell networks in a prorepair environment and for monolayer resealing after wounding. Decreasing AMPKα1 expression reduces barrier resistance in PMVEC monolayers, results consistent with a role for AMPKα1 in cell-cell adhesion. AMPKα1 colocalizes and coimmunoprecipitates with the adherens junction protein N-cadherin and cofractionates with proteins selectively expressed in caveolar membranes. Assessment of permeability, by measuring the filtration coefficient (Kf) in isolated perfused lungs, confirmed that AMPK activation contributes to barrier repair in vivo. Our findings thus provide novel evidence for AMPKα1 in Ca2+ influx-mediated signaling and wound repair in the endothelium.—Creighton, J., Jian, M., Sayner, S., Alexeyev, M., Insel, P. A. Adenosine monophosphate-activated kinase α1 promotes endothelial barrier repair.
Keywords: cadherins, calcium, capillary, caveolin, microdomains
Endothelial response to injury is a critical determinant of the balance between normal and pathological vascular repair. Although the development of vascular injury has been actively studied, relatively little is known regarding its resolution. In the pulmonary circulation, the capillary bed consists of a single layer of endothelial cells and a basement membrane directly adjacent to the alveolar epithelium, creating a thin structure that tightly regulates vascular permeability to maintain efficient gas exchange. This capillary environment relies heavily on the capacity of its endothelium to maintain vessel integrity and restore vascular homeostasis after injury. Such dependence implies that barrier-disruptive signaling mechanisms must also activate endothelial-derived repair. Ca2+ entry-mediated pathways target cytoskeletal elements that result in reorganization of protein-protein interactions, structural realignment, and loss of cell-cell contact (1). Paradoxically, Ca2+ influx is also required for the rearrangement of cytoskeletal proteins involved in cell migration and for the promotion of cadherin interactions and cell-cell adhesion (2, 3). An important feature of Ca2+ signaling is the restricted localization of signaling components in specific subcellular compartments, e.g., caveolae/lipid rafts. Redistribution of key molecules (e.g., Ca2+ channels) away from caveolar microdomains alters the physiological outcome of Ca2+ signaling (4, 5).
Adenosine monophosphate-activated protein kinase (AMPK), a highly conserved eukaryotic serine/threonine kinase, was originally identified as a metabolic sensor. However, recent evidence links AMPK activity with cellular and vascular homeostasis (6–8). AMPK targets cytoskeletal proteins and counters the destabilizing effects of increased cytosolic Ca2+ concentrations ([Ca2+]i) by promoting protein-protein interactions (8–10). The kinase is activated by agonist-induced elevations in [Ca2+]i and may integrate [Ca2+]i signaling pathways and limit vascular permeability by blocking injury to the endothelial lining (10, 11).
We hypothesized that AMPK contributes to vascular endothelial repair. Here, we show that AMPKα1 initiates Ca2+ entry after a stimulus that activates Ca2+ influx in pulmonary microvascular endothelial cells (PMVECs) and promotes the restoration of cell-cell adhesions after barrier disruption in vitro and in the intact lung in vivo.
MATERIALS AND METHODS
Materials
AMPKα1 and AMPKα2 rabbit antibodies were purchased from Bethyl Laboratories (Montgomery, TX, USA). Vascular endothelial (VE)-cadherin (CD144) and pan-cadherin rabbit antibodies were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Caveolin-1 and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). N-cadherin mouse antibody was purchased from Zymed Laboratories (San Francisco, CA, USA). Secondary Alexa Fluor antibodies were purchased from Invitrogen/Molecular Probes (Eugene, OR, USA). Compound C (6-[4-(2-piperidin-1-yl ethoxy) phenyl]-3-pyridin-4-yl pyrazolo[1,5-a]pyrimidine) was obtained from EMD Biosciences (San Diego, CA, USA). N1-(β-d-Ribofuranosyl)-5-aminoimidizole-4-carboxamide (AICAR) was purchased from Tocris Biosciences (Ellisville, MO, USA). 4α-Phorbol-12,13-didecanoate (4αPDD) was purchased from Sigma-Aldrich. ATP levels were determined using a ViaLight luciferase reporter assay from Cambrex Bio Science (Rockland, ME, USA). AMPK activity was assayed using an AMPKα [pT172] immunoassay kit from Invitrogen (Camarillo, CA, USA). Unless otherwise noted, all other materials and reagents were obtained from Sigma-Aldrich.
Animals
Male Sprague-Dawley rats (200–250 g, Charles River Breeding Laboratories, Wilmington, MA, USA) were anesthetized with Nembutal following an approved protocol and in accordance with the University of Alabama at Birmingham's institutional animal care and use policies. After sternotomy, the heart and lungs were removed en bloc for isolated lung studies.
Cell culture
Rat pulmonary vascular cells were isolated and cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin as described previously (12–14).
Immunoprecipitation assays
Whole-cell lysate (500 μg of protein) in 1.5 ml of modified Tris buffer (40 mM Tris base, 130 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 50 mM N-octyl-β-d-glucopyranoside, 1 mM MnCl2, 100 mM KI, protease inhibitor cocktail, and 1% Triton X-100, pH 7.5) was incubated with 10 μg of antibody and 20 μg of Ezview Red Protein A Affinity Gel. The samples were rocked at 4°C for 12 h. Beads were washed 3 times with 1% Triton in PBS and resuspended in 100 μl of Tris buffer with SDS loading buffer. Samples were frozen at −80°C until used for immunoblot analysis. Whole-cell lysate (25 μg protein/lane) in Tris buffer with SDS loading buffer was used as a positive control for protein expression in immunoblots.
Isolation of membrane fractions
PMVECs were grown to confluence in T75 75-cm2 flasks, and caveolin-enriched and non-caveolin-enriched membranes were isolated using a previously described method (15). Samples were adjusted to equal protein concentration, prepared for immunoblot analysis by the addition of SDS sample buffer, and stored at −80°C. Samples were heated at 37°C for 30 min before use.
Immunoblot analysis
Protein samples from whole-cell lysates, sucrose gradients, or immunoprecipitated proteins from the affinity gel were loaded onto 7% precast Novex gels (Invitrogen). After transfer to nitrocellulose membranes, protein binding by primary antibodies was detected using horseradish peroxidase-labeled secondary antibodies and enhanced chemiluminescence (Amersham Biosciences Corp., Piscataway, NJ, USA; refs. 12, 13).
Immunocytochemistry
Cells were seeded onto 12-mm coverslips and grown to confluence over 4–5 d. After methanol fixation (1 min), cells were rehydrated in PBS + 5% milk and then were permeabilized in 0.1% Triton X-100. After a PBS rinse, cells were incubated at 4°C overnight with AMPKα1 or AMPKα2 antibody diluted 1:250 in PBS + 5% milk. Cells were rinsed in PBS + 5% milk + 0.1% Triton X-100 and incubated with secondary antibody (Alexa Fluor 555) at 1:500 for 1 h. After a rinse with PBS + 5% milk + 0.1% Triton X-100, antibody to pan-cadherin or N-cadherin was added at 1:100 for 1 h. Secondary antibody (Alexa Fluor 488) for the cadherins was applied in the same manner as for the APMKα1 secondary antibody. After an additional rinse in PBS, cells were mounted onto glass microscope slides using Dako (Carpinteria, CA, USA) fluorescent mounting medium. Cells were imaged using a Leica TCs SP2 confocal microscope fitted with a ×63 oil-immersion objective and Leica Microsystems Confocal Software (version 2.61; Leica Microsystems Heidelberg, GmbH, Heidelberg, Germany).
PCR
Total RNA was isolated from pulmonary vascular cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. RNA was quantitated by absorbance at 260 nm, and RT-PCR reactions were set up using a AccessQuick RT-PCR System (Promega, Madison, WI, USA). Each reaction contained 1 μg of template RNA and 1 μM concentrations of forward and reverse primers: AMPKα1 forward, AMPK1F (RT-PCR) ACCATTCTTGGTTGCCGAAACACC, and reverse, AMPKα1R (RT-PCR) GGTTCTTCCTTCGCACACGCAAAT (expected PCR product size was 224 bp); AMPKα2 forward, AMPKα2F (RT-PCR) TGTCCACTGGATGCACTCAACACA, and reverse, AMPKα2R (RT-PCR) AGACAGTGAATGGTTCTCGG-CTGT (expected PCR product size was 396 bp). For each reaction, a control (without the RT step) was set up to identify potential contamination with genomic DNA.
AMPKα activity
Cells were seeded onto 24-well culture plates (Corning, Corning, NY, USA) and used at confluence 3–4 d later. Medium was aspirated and replaced with fresh medium containing the vehicle control DMSO, the AMPK inhibitor compound C (50 μM), or the AMPK activator AICAR (2 mM). Incubation was stopped at various times posttreatment to determine the optimal effect of AICAR and compound C on AMPKα phosphorylation. Based on these studies, the optimal treatment time was determined to be 1.5 h for AICAR and 2.25 h for compound C. For the studies with thapsigargin and Ca2+, medium was changed 3 h before the start of the experiment (i.e., addition of thapsigargin) and replaced with either medium containing normal (1.8 mM) Ca2+ or medium buffered to 10 μM Ca2+ using EGTA. Then, 1.5 h later and 1.5 h before the start of the experiment, the phosphatase inhibitor okadaic acid (10 nM) was added to all wells to better identify the direct effects of thapsigargin on phosphorylation of AMPKα at T172. Incubation was stopped by cell lysis and AMPKα activity was determined following the manufacturer's protocol. Experiments were conducted in triplicate and repeated at least 3 times.
ATP measurements
Following the same protocol as for AMPKα activity, ATP levels were determined using the manufacturer's method and a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) to detect luciferase bioluminescence.
Calcium measurements
Cells were seeded onto 25-mm glass coverslips and used at confluence 3–4 d postseeding. Changes in [Ca2+]i were determined using Fura-2AM (13, 16). Compound C (EC100, 50 μM), AICAR (EC100, 2mM), or the vehicle control DMSO in DMEM was incubated with cells at optimal treatment times (1.5 h for AICAR and 2.25 h for compound C) before initiation of calcium studies. Data were acquired using a Proton Technology International (Birmingham, NJ, USA) system and an Olympus IX70 microscope fitted with a ×40 oil-immersion objective (Olympus, Tokyo, Japan).
Resistance studies
Trans-endothelial barrier resistance was determined by electric cell impedance sensing (ECIS; Applied Biophysics, Troy, NY, USA). Cells were seeded onto ECIS arrays and used at 60 or 100% confluence. The cells were maintained in a standard cell culture incubator with 5% CO2 at 37°C with normal culture medium during the experiment. Monolayer impedance (Ω) measurements were taken every 2 min. After each experiment, 3 wells were chosen at random and trypsinized for cell counts.
Matrigel studies
Matrigel basement membrane matrix (BD Biosciences; Bedford, MA, USA) was applied to the bottom of 24-well culture dishes following the manufacturer's recommendations for the thin-gel method. Cells were seeded at 1 × 105 cells/cm2 in 200 μl of culture medium that contained vehicle DMSO, 50 μM compound C, or 2 mM AICAR. Images were taken at 4 h using an Olympus IX70 microscope and a ×40 oil-immersion lens. Spot 4.0.9 software (Diagnostics Instruments, Sterling Heights, MI, USA) was used to acquire and edit image sequences.
Scratch-wound assays
Cells were plated on 25-mm coverslips and grown to confluence. Monolayers were scratched with a sterile 200-μl pipette tip, and coverslips were fitted into an Attofluor cell chamber (Invitrogen, Eugene, OR, USA). Experiments were performed in cell culture medium with DMSO as the vehicle control or compound C (EC100, 50 μM) under environmentally controlled conditions (5% CO2, 37°C). Images were taken at 2-min intervals for 18 h using an Olympus IX70 microscope and a ×40 oil-immersion lens. Spot 4.0.9 was used to acquire and edit image sequences.
Retroviral constructs
AMPKα1 expression was reduced using short hairpin RNA (shRNA)-mediated knockdown. shRNA oligos were annealed and cloned in a pSilencer 5.1-H1 Retro vector (Ambion, Austin, TX, USA) according to the manufacturer's recommendations. After verification of inserts by sequencing, retrovirus-containing supernatants were generated by CaPO4-mediated transfection of Phoenix Ampho cells (17). Forward and reverse primer sets for each of the 6 shRNA oligos are listed in Supplemental Data.
Isolated perfused lungs
Isolated lungs were cannulated, the airways were ventilated, and the vascular system was perfused at constant flow with Earle's balanced salt solution containing 4% BSA at pH 7.4 (37°C). Hemodynamic parameters (pulmonary artery, capillary, venous, and airway pressures) and lung weight gain were monitored during the experiment using AD Instruments PowerLab 8/30 and LabChart Pro software. Filtration coefficients (Kf), calculated as the rate of weight gain obtained after a 10-cm H2O increase in pulmonary venous pressure, were normalized to lung dry weight (g). Kf values were measured at 15 min (baseline) and 45 min after addition of drug (final). Lungs were divided into 5 experimental groups: DMSO control; 2 mM AICAR alone; 3 μM 4αPDD alone; 30-min 4αPDD treatment followed by 15-min AICAR treatment (4αPDD+AICAR); and 15-min AICAR treatment followed by 30-min 4αPDD treatment (AICAR+4αPDD).
Graphing and statistical analysis
Analytical data are reported as means ± se. Where appropriate, a paired or unpaired t test was used to compare 2 means. Comparisons between >2 data groups was accomplished using 1-way ANOVA, in conjunction with a Bonferroni post hoc test as needed. In all cases, values of P < 0.05 were considered significant. Data were graphed using GraphPad Prism 5.01 for Windows (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
PMVECs express AMPKα1
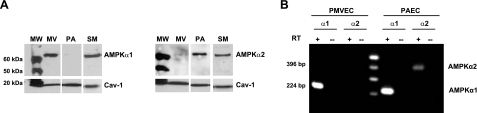
Functional AMPK is an α,β,γ (1:1:1) heterotrimeric complex, with a catalytic α subunit and β/γ regulatory subunits. There are 2 isoforms each of the catalytic (α1 and α2) and β subunits (β1 and β2) and 3 isoforms of the γ subunit (γ1, γ2, and γ3). We initially determined the expression profiles of AMPKα1 and AMPKα2 protein in cells from different pulmonary vascular segments (Fig. 1): conduit-derived pulmonary artery endothelial cells (PAECs) predominantly express AMPKα2, capillary-derived PMVECs express AMPKα1, and pulmonary artery smooth muscle cells (PASMCs) express both α subunits (Fig. 1A). RT-PCR confirmed that only the mRNA for the AMPKα1 isoform is present in PMVECs, but PAECs express the mRNA for both AMPKα isoforms (Fig. 1B). Because PMVECs selectively express the AMPKα1 catalytic subunit, our subsequent studies focused on its activity and function in these cells.
Figure 1.
Pulmonary vascular cells express different AMPKα subunits. A) Immunoblot analysis reveals that the AMPK catalytic α1 (AMPKα1) subunit is selectively expressed by PMVECs (MV). PAECs (PA) express AMPKα2, whereas PASMCs (SM) express AMPKα1 and AMPKα2. Caveolin-1 (Cav-1) was used as a loading control. MW, molecular weight markers. B) AMPKα1, but not AMPKα2, RNA is detected in PMVECs. AMPKα1 and AMPKα2 RNAs are detected in PAECs. RT, with (+) or without (−) reverse transcriptase added to reaction mix.
AMPKα1 activity in PMVECs
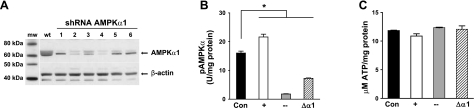
We used shRNA and pharmacological approaches to manipulate AMPKα1 activity. Retroviral vector systems expressing shRNAs were used to decrease AMPKα1 mRNA and protein levels. Constructs targeted to 6 distinct regions of AMPKα1 mRNA were stably transfected into PMVECs. Cells expressing the shRNAs were selected using 20 μg/ml puromycin (12); a decrease in AMPKα1 protein expression was confirmed by immunoblot analysis (Fig. 2A). Cells expressing shRNA construct 4 (designated as Δα1) displayed the greatest reduction in AMPKα1 protein and were used in further studies.
Figure 2.
AMPKα1 protein and activity are decreased in PMVECs by shRNA. A) Immunoblot of control (wt, wild-type) PMVECs and PMVECs engineered to express shRNA constructs (lanes 1–6) targeted to 6 different regions of AMPKα1 mRNA. β-actin, loading control. B) Basal AMPKα activity (Con) is sensitive to stimulation by AICAR (+) and inhibition by compound C (−). Cells expressing shRNA to AMPKα1 (Δα1) have reduced AMPKα1 activity (n=3). *P < 0.05. C) Treatments that alter AMPK activity do not decrease ATP content (n=3). Values are means ± se.
We assessed responses to the AMPK activator AICAR and the AMPK inhibitor compound C to establish conditions for their use with PMVECs. Phosphorylation status of T172 of the AMPKα subunit (pAMPKα) was used as an indicator of AMPK activity. Figure 2B shows results using optimal treatment conditions for AICAR (2 mM, 1.5 h) and compound C (50 μM, 2.25 h). AICAR increased AMPK activity ∼35%; compound C and shRNA to AMPKα1 decreased activity by ∼90 and 55%, respectively, compared with control. Although AMPK regulates the synthesis and utilization of ATP (18), basal [ATP] was not decreased by any of our experimental conditions (Fig. 2C).
AMPKα1 is activated by Ca2+ influx
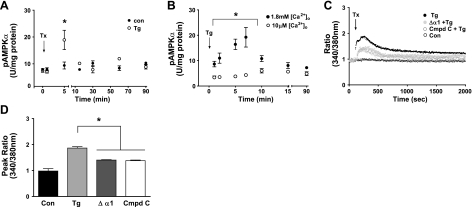
Ca2+ influx is an important second messenger in inflammation-promoted pathways and can activate AMPK (11). We asked whether thapsigargin (1 μM), which mimics inflammatory Ca2+ signaling by inducing store-operated Ca2+ entry, also activates AMPKα1 in PMVECs. Addition of thapsigargin (19) transiently increased pAMPKα 2-fold (Fig. 3A). To determine the role of Ca2+ influx, we tested the AMPK response to thapsigargin in the presence of low (10 μM) extracellular Ca2+ ([Ca2+]o) and found that, unlike results obtained in the presence of physiological (1.8 mM) [Ca2+]o, thapsigargin did not increase pT172 AMPKα levels (Fig. 3B). Thus, increased Ca2+ entry is sufficient to activate AMPKα1 in PMVECs.
Figure 3.
AMPKα1 is involved in multiple Ca2+ signaling mechanisms. A) AMPKα1 is activated by thapsigargin (Tg). B) AMPKα1 response to thapsigargin is dependent on Ca2+ influx (n=3). Con, vehicle control; (+), 1.8 mM [Ca2+]o; (−), 10 μM [Ca2+]o. C) AMPKα1 inhibition using compound C (Cmpd C, 50 μM, 2.25-h pretreatment) or shRNA (Δα1) attenuates thapsigargin-induced Ca2+ entry (ratio), assayed using Fura-2AM. Tx, time thapsigargin or vehicle control added to culture medium (n>5, tracings represent mean for each group, error bars not shown). D) Peak thapsigargin-induced Ca2+ entry is reduced by 50% in the presence of AMPKα1 inhibition (Δα1, AMPKα1 shRNA-expressing PMVECs) (n>5). Values are means ± se. *P < 0.05.
AMPKα1 inhibition attenuates Ca2+ entry in PMVECs
Store-operated Ca2+ channels (SOCs) in endothelial cells are an important mechanism for regulating [Ca2+]i. Inflammatory agonists initiate Ca2+ release from the endoplasmic reticulum, thereby activating SOCs located on the cell surface and increasing influx of extracellular Ca2+ (20). This influx is sufficient to disrupt the endothelial barrier and increase permeability in conduit-derived PAECs but not PMVECs (13, 21), implying that PMVECs possess mechanisms that mitigate barrier disruption by Ca2+ influx. Addition of thapsigargin (1 μM) increased Ca2+ entry (Fig. 3C), as assessed by changes in the ratio of Ca2+-bound (340 nm) to Ca2+-unbound (380 nm) Fura-2AM dye. The thapsigargin-induced peak increase in [Ca2+]i was attenuated 50% (Fig. 3D) in cells pretreated with compound C (50 μM, 2.25 h) or expressing shRNA against AMPKα1, suggesting that AMPK activity contributes to thapsigargin-induced Ca2+ entry response in PMVECs. Neither AMPK inhibition (compound C, 50 μM) nor AMPK activation (AICAR, 2 mM) altered basal Ca2+ levels in the absence of thapsigargin (Supplemental Fig. S1). Moreover, pretreatment with AICAR (2 mM, 1.5 h) to activate AMPK maximally did not enhance the thapsigargin-induced Ca2+ response (Supplemental Fig. S1) compared with thapsigargin alone (Fig. 3C). Thus, AMPKα1 activity contributes to thapsigargin-induced Ca2+ entry; however, added stimulation of AMPK activity does not augment the level of Ca2+ entering the cell. AMPKα1 in PMVECs responds to a Ca2+ influx stimulus by promoting Ca2+ entry, suggesting a dual role for AMPKα1 in Ca2+ signaling.
AMPKα1 contributes to a tight PMVEC barrier
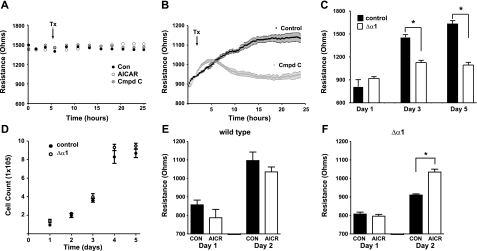
Cis and trans homotypic binding of cadherin molecules are important for the assembly of adherens junction complexes, tight cell-cell contacts, and formation of a strong endothelial barrier. Extracellular Ca2+ is required for cadherin-cadherin ectodomain binding and Ca2+ influx is required for formation of intracellular cadherin junctional complexes (22). To determine whether AMPKα1 activity contributes to barrier strength, we assessed trans-endothelial barrier resistance using ECIS. We found that barrier resistance was constant (∼1450 Ω) in confluent PMVEC monolayers and was not altered by treatment with AICAR (2 mM) or compound C (50 μM) (Fig. 4A). In contrast, resistance (initially ∼900 Ω) increased in subconfluent monolayers as the barrier formed, and the adherens junctions tightened but did not reach control levels if compound C was added (Fig. 4B). These results are consistent with findings in epithelial cells in which AMPK contributes to assembly but not maintenance of tight junctions in cell-cell adhesion (23, 24). Reduction in AMPKα1 protein by the shRNA construct decreased resistance compared with that of control cells (Fig. 4C) without altering cell proliferation (Fig. 4D). Stimulation of AMPK had no effect on the development of barrier resistance in control cells (Fig. 4E) but restored barrier resistance to control levels in cells expressing shRNA directed against AMPKα1 (Fig. 4F). AMPKα1 activity thus influences the development of cell-cell adhesions and barrier tightness in PMVECs.
Figure 4.
AMPKα1 contributes to the development, but not maintenance, of barrier resistance. A) Manipulating AMPK activity does not alter barrier resistance in confluent PMVEC monolayers. B) In subconfluent PMVEC monolayers, AMPKα1 inhibition [compound C (Cmpd C)] impairs development of barrier resistance. Con, vehicle control; AICAR, AMPK stimulation; Tx, time treatment added. C) shRNA (Δα1), which decreases AMPKα1 expression, lowers barrier resistance. D) shRNA-mediated inhibition of AMPKα1 protein synthesis does not alter cell growth. E, F) AMPK stimulation using AICAR (AICR) does not enhance resistance in control cells (E) but restores resistance to control levels in shRNA-expressing cells (F). Bars indicate days postseeding (n=12). Values are means ± se. *P < 0.05.
AMPKα1 is necessary for PMVEC barrier repair
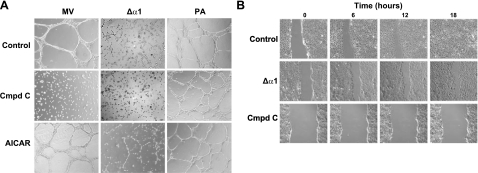
To determine the role of AMPK activity in capillary-derived PMVECs, we cultured cells on dishes coated with the growth-enhancing matrix Matrigel to mimic establishment of an endothelial barrier. We then tested network formation in the presence of AMPK activation (AICAR, 2 mM), in the presence of AMPK inhibition (compound C, 50 μM), or in cells expressing AMPKα1 shRNA (Δα1). Control PMVECs formed cell-cell networks by 4 h (Fig. 5A, top left panel), but compound C attenuated cell spreading and network formation (Fig. 5A, middle left panel). Network formation was attenuated in cells expressing the Δα1 shRNA construct: small groups of cell-cell attachments formed, but long cell-cell networks were absent (Fig. 5A, top center panel). In the presence of compound C, shRNA-expressing cells did not form cell-cell attachments (Fig. 5A, middle center panel), akin to results for compound C-treated control cells. AICAR-treated PMVECs resembled controls (Fig. 5A, bottom left panel) and AICAR partially restored the ability of Δα1 PMVECs to form networks (Fig. 5A, bottom center panel).
Figure 5.
PMVEC monolayer repair requires AMPKα1. A) Decreasing AMPKα1 activity, by inhibition (using compound C) or shRNA (Δα1), blocks formation of cell-cell networks in PMVECs (MV), but not PAECs (PA), on Matrigel (middle panels). AMPK activation using AICAR enhances network formation in shRNA-expressing cells, but not in control MV or PA (bottom panels). Micrographs taken 4 h postseeding. B) AMPK inhibition impairs wound resealing in PMVECs. Control monolayers reseal 18 h after wounding (top panels and Supplemental Video S1). Treatment with shRNA (Δα1) to AMPKα1 impairs wound resealing (middle panels and Supplemental Video S2). More complete inhibition of AMPKα1 using compound C produces greater blockade of cell migration and barrier repair (bottom panels and Supplemental Video S3).
Specificity of AMPKα1 function in PMVECs was assessed using endothelial cells derived from a different vascular segment, conduit-derived PAECs, which express the AMPKα2 isoform and form cell-cell networks similar to PMVEC controls (Fig. 5A, top right panel). In contrast to what occurred with PMVECs, compound C had no effect on network formation in PAECs (Fig. 5A, middle right panel) and AICAR-treated PAECs formed cell-cell networks similar to controls (Fig. 5A, bottom right panel).
Thus, AMPKα isoforms (and AMPK activity) appear to have distinct functions in different types of endothelial cells. In capillary-derived PMVECs, AMPKα1 is involved in the formation of cell-cell networks and endothelial barrier repair. This is not the case for the AMPKα2 isoform (Fig. 1) expressed in large vessel-derived PAECs, in which neither stimulation nor inhibition of AMPK affects network formation.
Cells grown to confluence on coverslips were injured using a scratch-wound technique (25) to confirm that AMPKα1 in PMVECs influences endothelial barrier repair. Images were generated at 2-min intervals over 18 h to create movies of the light micrographic appearance of the cells at each time point. Control monolayers, but not PMVECs expressing shRNA to AMPKα1, resealed within 18 h after injury (Fig. 5B, top vs. middle panels and Supplemental Videos S1 and S2). Time-lapse movies revealed that cells along the leading edge in the shRNA-expressing cell monolayers migrated across the gap in the monolayer, but stopped their forward movement as gaps formed between them and trailing cells. Attenuated wound resealing in the shRNA-expressing cells paralleled the (∼55%) reduction in AMPK activity achieved by shRNA (Fig. 2B). Cells treated with compound C provided further evidence that AMPKα1 is necessary for wound resealing. Consistent with its greater inhibition of AMPK activity (Fig. 2B), compound C (50 μM, added at start of experiment) more profoundly inhibited barrier repair, completely blocking cell migration and wound resealing with cells at the leading edge remaining stationary in response to injury (Fig. 5B, bottom panels and Supplemental Video S3).
AMPK stimulation repairs Ca2+ entry-induced permeability in vivo
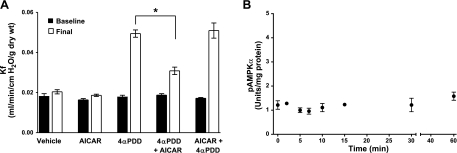
To determine whether AMPK activity is necessary for barrier repair in vivo, we used 4αPDD to induce Ca2+entry-mediated microvascular permeability in the intact lung (26–29). Perfusion of isolated rat lungs with 3 μM 4αPDD induced a 2.5-fold increase in vascular permeability (Fig. 6A) that was attenuated by the addition of AICAR (2 mM). Pretreatment of the lung with AICAR before addition of 4αPDD had no effect on permeability compared with that with 4αPDD alone. These data suggest that AMPK is activated in response to a barrier-disruptive insult but does not protect from subsequent barrier injury. If this idea were correct, then 4αPDD, which induces Ca2+ influx-mediated capillary permeability in vivo, should not activate AMPKα1 in PMVECs. Indeed, 4αPDD does not activate AMPKα1 (Fig. 6B). Taken together, our data indicate that AMPK activation in response to Ca2+ influx initiates barrier repair in the capillary circulation of the lung.
Figure 6.
AMPK activation attenuates Ca2+ entry-induced permeability in the isolated lung. A) 4αPDD increases permeability in the isolated perfused lung (final, open bar vs. baseline, solid bar). Addition of AICAR to stimulate AMPK attenuates 4αPDD-induced lung permeability (final 4αPDD vs. final 4αPDD+AICAR). Pretreatment of the lung with AICAR before addition of 4αPDD does not prevent lung injury (final AICAR+4αPDD vs. final 4αPDD+AICAR). Baseline, initial Kf measurement; final, final Kf measurement (n=5 animals). *P < 0.05. B) 4αPDD does not stimulate AMPK activity (n=3). Values are means ± se.
AMPKα1 colocalizes with N-cadherin
Barrier repair requires coordinated changes in the orientation and composition of cytoskeletal elements located at cell-cell and cell-matrix junctions (9, 30, 31). Molecules that orchestrate these events colocalize with the molecules that mediate the responses. We used confocal microscopy and an AMPKα1-specific antibody to define its subcellular location in PMVECs (Fig. 7) and found that it localized at the plasma membrane near PMVEC cell-cell borders (Fig. 7A, top left panel) and colocalization of membrane-associated AMPKα1 with N-cadherin (Fig. 7A, top right panel). Fluorescence microscopy of PAECs, which express neither N-cadherin nor detectable levels of AMPKα1 protein, demonstrated that AMPKα2 is predominately cytosolic (Fig. 7A, bottom left panel) and does not colocalize with cadherin proteins at cell-cell borders (Fig. 7A, bottom right panel). Coimmunoprecipitation studies using antibody to N-cadherin followed by immunoblotting confirmed an association of AMPKα1 and N-cadherin (Fig. 7B, top and middle panels) in PMVECs. In contrast, another cell adhesion molecule expressed by PMVECs, VE-cadherin does not coimmunoprecipitate with AMPKα1 (Fig. 7B, bottom panels), suggesting that AMPKα1 selectively localizes with N-cadherin in discrete membrane regions.
Figure 7.
AMPKα1 and N-cadherin colocalize and coimmunoprecipitate in PMVECs. A) Confocal micrographs of fluorescently labeled antibodies to AMPKα1 (red, top left panel) and N-cadherin (green, top center panel) show AMPKα1/N-cadherin colocalization (yellow, overlay image, top right panel) at cell-cell borders. AMPKα2 does not localize to cell-cell borders (red, bottom left panel) or colocalize with cadherins in PAECs (green, bottom right panel). B) Coimmunoprecipitation of AMPKα1 and N-cadherin with antibody used for immunoprecipitation (IP) of N-cadherin and immunoblotting (IB) with antibody to AMPKα1 (top panel) or with IP by antibody to AMPKα1 and IB with N-cadherin antibody (center panel). AMPKα1 and VE-cadherin do not coimmunoprecipitate (bottom panel). Mw, molecular weight markers; Wcl, whole-cell lysate. C) Immunoblots of membrane fractions show N-cadherin and AMPKα1 localized to caveolin-1-enriched membranes (fractions 3, 4, and 5). VE-cadherin is primarily found in fraction 7, which is not enriched in caveolin-1, N-cadherin, or AMPKα1. Numbers refer to the buoyancy of the membrane fractions, from 3 (lightest) to 9 (heaviest).
AMPKα1 and N-cadherin colocalize to caveolin-1 membranes
Sucrose gradient fractionation of cell membranes and immunoblot analysis indicated that N-cadherin and AMPKα1 are both enriched in fractions that also contain caveolin-1, a marker for caveolae (Fig. 7C). AMPKα1 localized almost exclusively in buoyant fractions with caveolin-1 (Fig. 7C, bottom panels). In contrast, VE-cadherin, which does not coimmunoprecipitate with AMPKα1, did not localize in caveolin-1-enriched fractions. Caveolar regions are also enriched in signaling molecules important for regulating cell motility and assembly of cell-cell adhesions (30, 32). Localization of AMPKα1 in caveolin-1-enriched membranes together with the adherens junction protein N-cadherin supports the conclusion that AMPKα1 signaling occurs in a membrane microdomain important for orchestrating barrier repair in PMVECs.
DISCUSSION
Considerable prior effort has been made in seeking to understand the molecular events involved in the abnormal resolution of tissue damage (33), but how such events can be manipulated to enhance the restoration of normal function remains poorly understood. In this study, we demonstrate that AMPKα1 within the vascular endothelium has such a role in lung repair.
AMPK-regulated pathways are classically associated with preservation of cellular homeostasis in highly metabolic tissues, particularly in skeletal muscle and the heart, in which AMPK serves as a sensor and mediator of metabolic processes (18, 34, 35). However, AMPK can be regulated by signal transduction mechanisms that are independent of cellular energy state and AMP levels (36, 37). Stimulation of AMPK by such signaling mechanisms suggests that AMPK may influence subcellular signaling compartments. How fidelity of localized AMPK signaling is achieved is not well defined.
In other signaling systems, lipid rafts and caveolae are examples of signaling organizers. Caveolins anchor proteins and together with caveolar lipids create compartmentalized signaling modules at the cell membrane (38–41). We find that AMPKα1 localizes to fractions that contain caveolae-restricted proteins. Caveolae-compartmentalized signaling is consistent with the notion that AMPKα isoforms serve physiological roles that are determined, at least in part, by their subcellular location and has potentially important implications for AMPKα1 activity and vascular repair. Caveolae in lung endothelium contain components that generate and terminate the signaling molecules cAMP and [Ca2+]i, thereby creating signaling domains that regulate endothelial barrier integrity (12, 13, 42). Calcium entry, especially inflammation-induced Ca2+ entry through SOCs, is associated with endothelial barrier dysfunction (21). The current findings demonstrate another role for Ca2+ signaling in endothelial biology: AMPKα1-dependent Ca2+ entry in PMVECs. Unlike other Ca2+ entry mechanisms, this pathway is important in restoring a disrupted endothelial barrier. AMPKα1 signaling compartmentalized in this microdomain may act as a spatial and temporal feedback mechanism to counter the destabilizing effects of inflammatory Ca2+ signals by activating a discrete Ca2+ entry mechanism that restores cell-cell adhesion.
The current studies implicate adherens junction assembly as the molecular target of this Ca2+ pathway. Our results link N-cadherin to the AMPK microdomain involved in endothelial repair, a finding consistent with evidence that N-cadherin localizes to lipid rafts and regulates motility of adhesion components at cell-cell junctions (30, 43, 44). Of note, N-cadherin modulates voltage-dependent Ca2+ channel activity in neurons (45), another setting in which function of this cell adhesion molecule is associated with Ca2+ entry.
Our results thus reveal a previously unappreciated role of AMPKα1 as a raft/caveolae-localized component of the barrier repair machinery. This AMPKα1/[Ca2+]i microdomain promotes development of cell-cell adhesions in lung capillary endothelium and contributes to repair. This signaling system may serve as a mechanism that enhances resolution of tissue damage and restores normal function in the lung.
Supplementary Material
Acknowledgments
The authors thank Troy Stevens (University of South Alabama College of Medicine, Mobile, AL, USA) for providing cells.
This work was supported by U.S. National Institutes of Health grants HL102296 (to J.C.), RR031286 (to M.A.), and HL007261 (to P.A.I.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Meoli D. F., White R. J. (2009) Thrombin induces fibronectin-specific migration of pulmonary microvascular endothelial cells: requirement of calcium/calmodulin-dependent protein kinase II. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L706–L714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martini A., Bruno R., Mazzulla S., Nocita A., Martino G. (2010) Angiotensin II regulates endothelial cell migration through calcium influx via T-type calcium channel in human umbilical vein endothelial cells. Acta Physiol. (Oxf.) 198, 449–455 [DOI] [PubMed] [Google Scholar]
- 3. Louis M., Zanou N., Van Schoor M., Gailly P. (2008) TRPC1 regulates skeletal myoblast migration and differentiation. J. Cell Sci. 121, 3951–3959 [DOI] [PubMed] [Google Scholar]
- 4. Pani B., Singh B. B. (2009) Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 45, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liao Y., Plummer N. W., George M. D., Abramowitz J., Zhu M. X., Birnbaumer L. (2009) A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc. Natl. Acad. Sci. U. S. A. 106, 3202–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Z., Peng I. C., Cui X., Li Y. S., Chien S., Shyy J. Y. (2010) Shear stress, SIRT1, and vascular homeostasis. Proc. Natl. Acad. Sci. U. S. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viollet B., Mounier R., Leclerc J., Yazigi A., Foretz M., Andreelli F. (2007) Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 33, 395–402 [DOI] [PubMed] [Google Scholar]
- 8. Zou M. H., Wu Y. (2008) AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin. Exp. Pharmacol. Physiol. 35, 535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benz P. M., Blume C., Moebius J., Oschatz C., Schuh K., Sickmann A., Walter U., Feller S. M., Renne T. (2008) Cytoskeleton assembly at endothelial cell-cell contacts is regulated by αII-spectrin-VASP complexes. J. Cell Biol. 180, 205–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutchinson D. S., Summers R. J., Bengtsson T. (2008) Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol. Ther. 119, 291–310 [DOI] [PubMed] [Google Scholar]
- 11. Fisslthaler B., Fleming I. (2009) Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ. Res. 105, 114–127 [DOI] [PubMed] [Google Scholar]
- 12. Creighton J., Zhu B., Alexeyev M., Stevens T. (2008) Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J. Cell Sci. 121, 110–119 [DOI] [PubMed] [Google Scholar]
- 13. Creighton J. R., Masada N., Cooper D. M., Stevens T. (2003) Coordinate regulation of membrane cAMP by Ca2+-inhibited adenylyl cyclase and phosphodiesterase activities. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L100–L107 [DOI] [PubMed] [Google Scholar]
- 14. King J., Hamil T., Creighton J., Wu S., Bhat P., McDonald F., Stevens T. (2004) Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc. Res. 67, 139–151 [DOI] [PubMed] [Google Scholar]
- 15. Crossthwaite A. J., Seebacher T., Masada N., Ciruela A., Dufraux K., Schultz J. E., Cooper D. M. (2005) The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J. Biol. Chem. 280, 6380–6391 [DOI] [PubMed] [Google Scholar]
- 16. Cioffi D. L., Moore T. M., Schaack J., Creighton J. R., Cooper D. M., Stevens T. (2002) Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J. Cell Biol. 157, 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pear W. S., Nolan G. P., Scott M. L., Baltimore D. (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U. S. A. 90, 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardie D. G. (2008) Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 582, 81–89 [DOI] [PubMed] [Google Scholar]
- 19. Witczak C. A., Sharoff C. G., Goodyear L. J. (2008) AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell. Mol. Life Sci. 65, 3737–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou C., Chen H., Lu F., Sellak H., Daigle J. A., Alexeyev M. F., Xi Y., Ju J., van Mourik J. A., Wu S. (2007) Cav3.1 (α1G) controls von Willebrand factor secretion in rat pulmonary microvascular endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L833–L844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cioffi D. L., Stevens T. (2006) Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation 13, 709–723 [DOI] [PubMed] [Google Scholar]
- 22. Ko K. S., Arora P. D., Bhide V., Chen A., McCulloch C. A. (2001) Cell-cell adhesion in human fibroblasts requires calcium signaling. J. Cell Sci. 114, 1155–1167 [DOI] [PubMed] [Google Scholar]
- 23. Zheng B., Cantley L. C. (2007) Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 104, 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang L., Jouret F., Rinehart J., Sfakianos J., Mellman I., Lifton R. P., Young L. H., Caplan M. J. (2011) AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3β (GSK-3β) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. J. Biol. Chem. 286, 16879–16890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yarrow J. C., Perlman Z. E., Westwood N. J., Mitchison T. J. (2004) A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 4, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez D. F., King J. A., Weber D., Addison E., Liedtke W., Townsley M. I. (2006) Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ. Res. 99, 988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamanaka K., Jian M. Y., Weber D. S., Alvarez D. F., Townsley M. I., Al-Mehdi A. B., King J. A., Liedtke W., Parker J. C. (2007) TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L923–L932 [DOI] [PubMed] [Google Scholar]
- 28. Townsley M. I., King J. A., Alvarez D. F. (2006) Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation 13, 725–739 [DOI] [PubMed] [Google Scholar]
- 29. Wu S., Jian M. Y., Xu Y. C., Zhou C., Al-Mehdi A. B., Liedtke W., Shin H. S., Townsley M. I. (2009) Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L650–L657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Causeret M., Taulet N., Comunale F., Favard C., Gauthier-Rouviere C. (2005) N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol. Biol. Cell 16, 2168–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ofori-Acquah S. F., King J., Voelkel N., Schaphorst K. L., Stevens T. (2008) Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc. Res. 75, 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Insel P. A., Patel H. H. (2009) Membrane rafts and caveolae in cardiovascular signaling. Curr. Opin. Nephrol. Hypertens. 18, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gurtner G. C., Werner S., Barrandon Y., Longaker M. T. (2008) Wound repair and regeneration. Nature 453, 314–321 [DOI] [PubMed] [Google Scholar]
- 34. Hardie D. G. (2011) AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem. Soc. Trans. 39, 1–13 [DOI] [PubMed] [Google Scholar]
- 35. Steinberg G. R., Kemp B. E. (2009) AMPK in health and disease. Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 36. Colombo S. L., Moncada S. (2009) AMPKα1 regulates the antioxidant status of vascular endothelial cells. Biochem. J. 421, 163–169 [DOI] [PubMed] [Google Scholar]
- 37. Emerling B. M., Weinberg F., Snyder C., Burgess Z., Mutlu G. M., Viollet B., Budinger G. R., Chandel N. S. (2009) Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 46, 1386–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson R. G. (2008) Transendothelial movement and caveolae. Nat. Biotechnol. 26, 380–381; author reply 381–382 [DOI] [PubMed] [Google Scholar]
- 39. Insel P. A., Head B. P., Patel H. H., Roth D. M., Bundey R. A., Swaney J. S. (2005) Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem. Soc. Trans. 33, 1131–1134 [DOI] [PubMed] [Google Scholar]
- 40. Patel H. H., Murray F., Insel P. A. (2008) Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 48, 359–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fuhs S. R., Insel P. A. (2011) Caveolin-3 undergoes SUMOylation by the SUMO E3 ligase PIASy: sumoylation affects G-protein-coupled receptor desensitization. J. Biol. Chem. 286, 14830–14841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stevens T., Creighton J., Thompson W. J. (1999) Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am. J. Physiol. 277, L119–L126 [DOI] [PubMed] [Google Scholar]
- 43. Nakao S., Platek A., Hirano S., Takeichi M. (2008) Contact-dependent promotion of cell migration by the OL-protocadherin-Nap1 interaction. J. Cell Biol. 182, 395–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taulet N., Comunale F., Favard C., Charrasse S., Bodin S., Gauthier-Rouviere C. (2009) N-cadherin/P120 catenin association at cell-cell contacts occurs in cholesterol-rich membrane domains and is required for RhoA activation and myogenesis. J. Biol. Chem. 284, 23137–23145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marrs G. S., Theisen C. S., Bruses J. L. (2009) N-cadherin modulates voltage activated calcium influx via RhoA, p120-catenin, and myosin-actin interaction. Mol. Cell. Neurosci. 40, 390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.