Abstract
In response to cellular insult, several pathways can be activated, including necrosis, apoptosis, and autophagy. Because glucocorticoids (GCs) have been shown to induce both osteocyte apoptosis and autophagy, we sought to determine whether osteocyte cell fate in the presence of GCs was dose dependent by performing in vivo and in vitro studies. Male Swiss-Webster mice were treated with slow-release prednisolone pellets at 1.4, 2.8, and 5.6 mg/kg/d for 28 d. An osteocyte cell line, MLO-Y4 cells, was treated with various doses of dexamethasone. We found that GC treatments dose dependently decreased activation of antioxidant-, autophagy-, and antiapoptosis-focused RT-PCR gene pathways in mouse cortical bone. The activation of antioxidant genes was correlated with autophagy gene expression after the GC treatments. The presence of osteocyte autophagy, as detected by immunostaining for LC3, increased ∼50% at the distal femur cortical bone region but not at trabecular bone region at the 1.4 and 2.8 mg/kg/d GC dose levels. The number of apoptotic osteocytes was increased at the cortical bone region by ∼40% initially observed at the 2.8 mg/kg/d dose level. In addition, the presence of the osteocyte autophagy was associated with an increased protein level of cathepsin K in vitro after the GC treatments. In summary, we found that GC treatment dose-dependently decreased antioxidant gene expression, with lower GC doses activating autophagy, whereas a higher dose increased apoptosis. These data suggest that autophagy may provide a mechanism for osteocytes to survive the stress after GC exposure and provide further insight into how GCs alter bone cell fate.—Jia, J., Yao, W., Guan, M., Dai, W., Shahnazari, M., Kar, R., Bonewald, L., Jiang, J. X., Lane, N. E. Glucocorticoid dose determines osteocyte cell fate.
Keywords: osteoporosis, apoptosis, autophagy, antioxidant, MLO-Y4 cell, LC3
Glucocorticoids (GCs) are frequently used in clinical medicine to treat noninfectious inflammatory diseases. Epidemiological studies show that 50% of patients with rheumatoid arthritis treated with chronic GCs will have an osteoporotic fracture; baseline data from randomized clinical trials report the prevalence in vertebral fracture is nearly 30% (1–4). Patients treated with GCs may require the treatment for a long period of time, thereby increasing their risk of fractures. Clinical studies of GC-treated subjects observed that the initiation of GC treatment is associated with a change in bone metabolism. In turn, this leads to a rapid reduction in bone mass at sites rich in trabecular bone, e.g., the vertebrae and femur, with incident vertebral fracture risk elevated within 1 yr of initiation of GC treatment (5–7). However, the loss of trabecular mass and architecture does not explain the increase in fracture risk in individuals treated with GCs, because these GC-treated subjects frequently experience fractures with higher bone mineral density values than do women with postmenopausal osteoporosis (6). Therefore, a more comprehensive understanding of the biology of GC-induced bone loss could empower clinicians to effectively prevent and treat this disease.
Osteocytes, the most abundant type of cells in bone, are buried in the bone matrix and are now known to contribute bone mineral homeostasis (8). Osteocytes are connected to one another and to the bone surface. Osteocyte lacunae have been reported to change size in clinical situations when there is a calcium deficiency, including with lactation, GC treatment, hypophosphatemic rickets, and prolonged estrogen deficiency (9–12). The increase in osteocyte lacunae size in these metabolic states may occur because of an insufficient trabecular and endocortical bone surface area for osteoclasts to reabsorb bone and maintain serum calcium balance when calcium is in high demand (13). For example, in the case of lactation, osteocyte lacunae are enlarged during lactation and return to the normal size postlactation, presumably after the calcium demand from lactation is diminished (10). Osteocytes synthesize and secrete a number of “osteocytic” specific proteins, such as dentin matrix acidic phosphoprotein, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23, that contribute to the regulation of both calcium and phosphorus metabolism (14–18), and the synthesis of these proteins by the osteocyte has been associated with changes in the perilacunar mineral around the osteocyte. However, it is unclear whether GC would affect osteocyte cell fates and would alter localized perilacunar mineralization changes around the osteocyte and whole bone strength.
Aging and glucocorticoid treatments are associated with accumulations of destroyed proteins, damaged nucleic acids, and accumulated oxygen radicals (19–21). Cells rely on autophagy, the only known intracellular degradative mechanism, to remove the dysfunctional organelles and/or oxidized proteins (22–24). The autophagic process is also activated when the cell is under stress, as an attempt to survive (25, 26). Once the autophagic process is initiated, parts of the cytoplasm and intracellular organelles are sequestered within autophagic vacuoles, which are eventually delivered to lysosomes for bulk degradation (27). Although the process of autophagy can preserve cell viability as a survival strategy, it can also lead to a self-destructive process, resulting in programmed cell death with excessive activation of this self-degrading system (28, 29). Although autophagy may prolong cell survival under stressful conditions, it is an inefficient process, and over time cells accumulate metabolic debris, which results in a decline in both cell and organ functions (30). Endogenous GCs, secreted by the adrenal glands, are essential in the body's ability to respond to stress. GCs are known to impair the enzymatic antioxidant defenses or directly induce oxidative stress in various tissues (31–36) and are associated with cell fate in a number of disease states. In lymphoid malignancies, Laane et al. (37) reported that dexamethasone (Dex) induced lymphoid cell death through the induction of autophagy before apoptosis. The induction of osteocyte apoptosis was thought to be the primary mechanism for GC-induced osteoporosis and changes in bone quality (38–42). We recently reported that prolonged GC treatment in mice resulted in osteocyte autophagy. Inhibition of autophagy with 3-methyladenine, an inhibitor of endogenous protein degradation, led to osteocyte apoptosis (19, 43). Based on these data, we hypothesized that autophagy is one of the pathways in which osteocytes respond to GC exposure. The purpose of this investigation was to further characterize the dose-dependent effects of GC-induced osteocyte cell fates in vivo and in vitro.
MATERIALS AND METHODS
Animals and experimental procedures
Three-month-old male Swiss-Webster mice were obtained from Charles River, Inc. (San Jose, CA, USA). The mice were maintained on commercial rodent chow (22/5 Rodent Diet; Teklad, Madison, WI, USA) with 0.95% calcium and 0.67% phosphate, available ad libitum. Mice were housed in a room that was maintained at 20°C with a 12-h light-dark cycle. The mice were randomly assigned to 4 experimental groups of 6–16 animals/group. Slow-release pellets (Innovative Research of America, Sarasota, FL, USA) of prednisolone (GC) were implanted as follows: group 1, the control group, was implanted with a placebo pellet (PL); group 2 was implanted with a 2.5 mg/60 d slow-release GC pellet, which is equivalent to 1.4 mg/kg/d for 28 d; group 3 was implanted with a 5 mg/60 d slow-release GC pellet, which is equivalent to 2.8 mg/kg/d for 28 d; and group 4 was implanted with a 10 mg/60 d slow-release GC pellet, which is equivalent to 5.6 mg/kg/d for 28 d. At 48 h before the mice were sacrificed, a fluorescent-conjugated monoclonal antibody for the autophagy marker, LC3 (LC3-5F10; 30 μg/mouse; NanoTools, San Diego, CA, USA) was injected into 3 mice in each of the 4 experimental groups to label the autophagic osteocytes. All animals were treated according to the U.S. Department of Agriculture animal care guidelines with the approval of the University of California at Davis Committee on Animal Research.
Biochemical methods
Serum levels for cortisol (R&D Systems, Minneapolis, MN, USA), osteocalcin (Biomedical Technology, Stoughton, MA, USA), and cathepsin K (Alpco, Salem, NH, USA) were measured in duplicate by ELISA, following the manufacturers' instructions. The within-run variations in our laboratory are between 4 and 6%, and between-run variations are ∼5%, which allow us to determine true changes between treatment groups (43–50).
Osteocyte culture and experiments
MLO-Y4 cells were cultured on collagen-coated (rat tail collagen type I, 0.15 mg/ml) surfaces (BD, Franklin Lakes, NJ, USA) and were grown in phenol red-free α-modified essential medium (α-minimal essential medium) supplemented with 2.5% FBS and 2.5% bovine calf serum (Invitrogen, Carlsbad, CA, USA) and incubated in a 5% CO2 incubator at 37°C as described previously. The cells were treated with Dex (Sigma-Aldrich Corp., St. Louis, MO, USA) at 10−8 to 10−5 M for 24 h (19, 51).
For the cell GFP-LC3 transfection experiments, the cells were transfected with the GFP-LC3 vector for 48 h. The cells were then treated with 10−8 to 10−6 M doses of Dex for 24 h, fixed with 4% paraformaldehyde, and examined under an Olympus BX61 motorized reflected fluorescence microscope (Olympus, Tokyo, Japan) with an AMCA filter (excitation, 350 nm; emission, 460 nm) for DAPI and FITC filter (excitation, 480 nm; emission, 535 nm) using SlideBook4.1 software (Intelligent Imaging Innovations, Denver, CO, USA). Autophagic cells were quantified by counting cells exhibiting GFP-LC3 punctate staining.
To evaluate the colocalization of the LC3 and lysosomes, MLY-O4 cells were plated on coverslips in a 12-well plate at 1 × 104 cells/cm2. After 24 h of plating, the cells were transfected with 1 μg of GFP-LC3 using 5 μl of lipofectamine in optimum I reduced serum medium (Invitrogen). Both GFP-LC3 and lipofectamine were diluted in optimum I reduced serum medium in two separate tubes to a final volume of 100 μl. After 5 min of incubation, GFP-LC3 and lipofectamine were mixed together in one tube and were incubated for 15 min at room temperature. Then the mixture of DNA-lipofectamine was added to cells dropwise in 1 ml of optimum I medium, and the plates were swirled gently and incubated in a 37°C incubator. After 3 h of transfection, medium was replaced with full-growth medium. After 24 h of transfection, cells were treated with 10−8 to 10−6 M Dex in 1% serum (FBS+bovine calf serum) phenol red-free medium for 24 h. Serum starvation was used as a positive control for autophagy (52, 53). The cells were then incubated with 1 μM LysoSensor Blue (Invitrogen) that was added directly to the treatment medium. The medium was removed after 30 min of incubation with LysoSensor Blue. Cells were washed once with fresh medium, and live cell images were taken to visualize the colocalization of GFP-LC3 puncta and the lysosomes.
Real-time RT-PCR
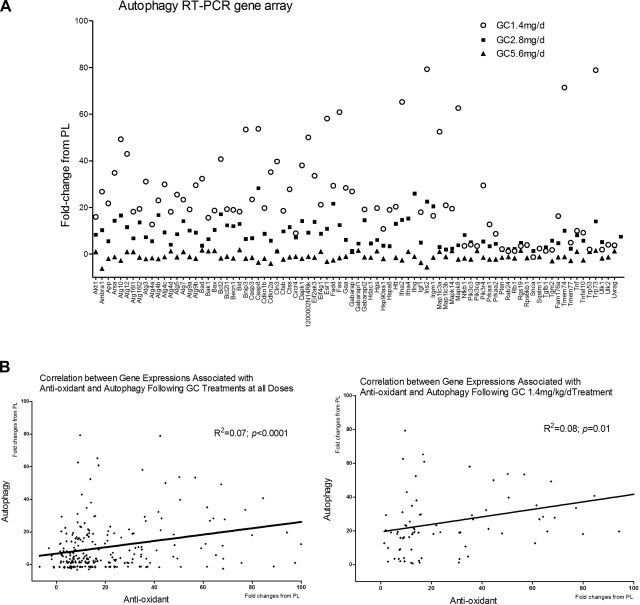
Total RNA was obtained from the tibiae or from MLO-Y4 cell cultures. For the tibiae, joint and bone marrow was removed, and total RNA was isolated using a modified two-step purification protocol with homogenization (PRO250 Homogenizer, 10 mm×105-mm generator; PRO Scientific Inc., Oxford, CT, USA) in TRIzol (Invitrogen) followed by purification over an RNeasy column (Qiagen, Valencia, CA, USA). The antioxidation autophagy and antiapoptosis focus RT-PCR gene pathway arrays were purchased from SABioscience (Frederick, MD, USA). Each pathway gene array has a preselected panel of 96 genes, which are related to antioxidant, autophagy, or apoptosis pathways, housekeeping genes, and no primer or cDNA controls. Detailed gene information can be found online (http://www.sabiosciences.com/RTPCR.php). We excluded genes that had values of Ct ≥ 35 because low expression levels can result in large fold changes, but the differences were not significant. After the exclusions, we reported 80 test genes in the autophagy RT-PCR gene array (see Fig. 2) and genes that were significantly different from PL after GC treatments for antioxidant and antiapoptosis (Tables 1 and 2).
Figure 2.
RNA was extracted from the tibial shafts of PL- or GC-treated mice at d 28. RT-PCR gene arrays for antioxidants and autophagy were performed. A) RT-PCR data are expressed as fold changes from the PL-treated mice. Approximately 70% of the autophagic genes expressed were significantly different from the those for PL. B) Correlation of fold changes from placebo between antioxidant and autophagy gene expression was performed on the same samples for all three GC doses or at the 1.4 mg/kg/d dose level. n = 6/group.
Table 1.
Antioxidant gene expression after GC treatment (fold change from PL)
| Symbol | Description | 1.4 mg | 2.8 mg | 5.6 mg |
|---|---|---|---|---|
| Als2 | Amyotrophic lateral sclerosis 2 (juvenile) homolog (human) | 12.87 | 8 | 7.53 |
| Apc | Adenomatosis polyposis coli | 12.29 | 4.04 | 7.11 |
| Apoe | Apolipoprotein E | 67.28 | 26.44 | 29.19 |
| Aqr | Aquarius | 10.31 | 8.86 | 6.58 |
| Cat | Catalase | 13.32 | 7.65 | 5.29 |
| Ctsb | Cathepsin B | 10.06 | 8.17 | 5.76 |
| Cygb | Cytoglobin | 79.92 | 26.05 | 23.79 |
| Fancc | Fanconi anemia, complementation group C | 12.59 | 7.14 | 4.55 |
| Fmo2 | Flavin containing monooxygenase 2 | 84.42 | 13.15 | 29.47 |
| Gab1 | Growth factor receptor bound protein 2-associated protein 1 | 23.55 | 15.79 | 13.08 |
| Gpx3 | Glutathione peroxidase 3 | 56.67 | 31.72 | 21.45 |
| Gpx5 | Glutathione peroxidase 5 | 50.16 | 42.9 | 14.53 |
| Hbq1 | Hemoglobin, θ 1 | 68.71 | 67.88 | 38.25 |
| Ift172 | Intraflagellar transport 172 homolog (Chlamydomonas) | 14.09 | 10.67 | 5.79 |
| Il19 | Interleukin 19 | 44.51 | 17.21 | 21.33 |
| Il22 | Interleukin 22 | 77.14 | 37.96 | 36.59 |
| Kif9 | Kinesin family member 9 | 42.55 | 40.83 | 23.56 |
| Lpo | Lactoperoxidase | 35.09 | 34.72 | 27.84 |
| Mb | Myoglobin | 36.01 | 29.65 | 15.8 |
| Noxa1 | NADPH oxidase activator 1 | 33.46 | 36.66 | 12.56 |
| Noxo1 | NADPH oxidase organizer 1 | 31.01 | 16.23 | -6.91 |
| Nxn | Nucleoredoxin | 19.21 | 9.78 | 11.74 |
| Park7 | Parkinson disease (autosomal recessive, early onset) 7 | 11.58 | 8.62 | 8.41 |
| Prdx1 | Peroxiredoxin 1 | 9.62 | 3.56 | 5.98 |
| Prnp | Prion protein | 9.21 | 7.12 | 6.73 |
| Recql4 | RecQ protein-like 4 | 7.69 | 5.07 | 4.36 |
| Slc41a3 | Solute carrier family 41, member 3 | 16.12 | 8.71 | 8.48 |
| Sod1 | Superoxide dismutase 1, soluble | 12.59 | 6.93 | 9.52 |
| Sod2 | Superoxide dismutase 2, mitochondrial | 15.07 | 6.45 | 6.8 |
| Sod3 | Superoxide dismutase 3, extracellular | 30.87 | 23.55 | 11.67 |
| Tpo | Thyroid peroxidase | 129.29 | 59.1 | 41.94 |
| Txnip | Thioredoxin interacting protein | 34.52 | 24 | 15.81 |
| Txnrd3 | Thioredoxin reductase 3 | 9.39 | 4.17 | 3.29 |
| Ucp3 | Uncoupling protein 3 (mitochondrial, proton carrier) | 42.34 | 14.71 | 13.25 |
All genes expressed significantly from PL (P<0.05).
Table 2.
Antiapoptotic gene expression after GC treatment (fold change from PL)
| Symbol | Description | 1.4 mg | 2.8 mg | 5.6 mg |
|---|---|---|---|---|
| Api5 | Apoptosis inhibitor 5 | 1.37 | −2.33 | −2.15 |
| Bag1 | Bcl2-associated athanogene 1 | −1.31 | −1.12 | −3.70 |
| Bag3 | Bcl2-associated athanogene 3 | 1.18 | −1.41 | −1.94 |
| Bcl2 | B-cell leukemia/lymphoma 2 | 1.24 | −1.64 | −3.05 |
| Bcl2l1 | Bcl2-like 1 | −1.11 | −1.14 | −3.15 |
| Bcl2l10 | Bcl2-like 10 | 3.79 | −1.60 | −8.97 |
| Bcl2l2 | Bcl2-like 2 | 1.04 | −1.38 | −3.36 |
| Birc2 | Baculoviral IAP repeat-containing 2 | 1.03 | −1.79 | −1.90 |
| Birc3 | Baculoviral IAP repeat-containing 3 | 1.05 | 1.05 | −1.76 |
| Bnip2 | BCL2/adenovirus E1B interacting protein 2 | −1.23 | 1.11 | −2.18 |
| Bnip3 | BCL2/adenovirus E1B interacting protein 3 | 2.26 | −1.43 | −1.36 |
| Casp2 | Caspase 2 | −1.28 | −1.30 | −1.88 |
| Cflar | CASP8 and FADD-like apoptosis regulator | 3.14 | −1.11 | −3.60 |
| Dad1 | Defender against cell death 1 | 4.22 | −2.59 | −10.18 |
| Tsc22d3 | TSC22 domain family, member 3 | −1.16 | −1.50 | −2.27 |
| Fas | Fas (TNF receptor superfamily member 6) | 3.84 | −2.61 | −2.81 |
| Il10 | Interleukin 10 | 3.53 | −6.28 | −35.84 |
| Lhx4 | LIM homeobox protein 4 | 2.40 | −2.86 | −3.86 |
| Mcl1 | Myeloid cell leukemia sequence 1 | 3.03 | −1.23 | 1.06 |
| Nfkb1 | Nuclear factor of κ light polypeptide gene enhancer in B-cells 1, p105 | −1.36 | −1.96 | −5.45 |
| Nme5 | Nonmetastatic cells 5, protein expressed in (nucleoside-diphosphate kinase) | 5.02 | −2.02 | −5.36 |
| Pak7 | P21 (CDKN1A)-activated kinase 7 | 2.72 | −2.47 | −3.50 |
| Pim2 | Proviral integration site 2 | 2.84 | −1.71 | −5.34 |
| Polb | Polymerase (DNA directed), beta | 1.02 | 1.48 | −4.64 |
| Prdx2 | Peroxiredoxin 2 | −1.21 | 1.36 | −3.17 |
| Rnf7 | Ring finger protein 7 | 1.64 | −3.53 | −7.89 |
| Sphk2 | Sphingosine kinase 2 | −1.77 | 1.20 | −19.57 |
| Tnf | Tumor necrosis factor | 2.45 | −1.12 | −3.93 |
| Cd40lg | CD40 ligand | 2.02 | −6.15 | −10.40 |
| Zc3hc1 | Zinc finger, C3HC type 1 | −1.37 | −1.43 | −2.14 |
Immunohistochemistry
The right distal femurs were decalcified in 10% EDTA for 2 wk and embedded in paraffin. Sections (4 μm) were prepared for immunohistochemistry using primary antibodies against the autophagy protein LC3-phosphatidylethanolamine conjugate antibody (LC3B antibody; Cell Signaling Technology, Danvers, MA, USA). LC3 detection was performed using a Vectastain ABC system (Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with methyl green. Apoptosis was determined using an In Situ Fluorescein Cell Death Detection Kit (Roche, Indianapolis, IN, USA) following the manufacturer's instructions. Results are presented as the percentage of the positive stained osteocytes/trabecular or cortical bone volume 0.5 to 3 mm distal to the growth plate at the distal femurs. A Bioquant imaging analyzing system (Bioquant, Nashville, TN, USA) was used for the measurements.
Western blot
The cells were lysed in RIPA buffer with homogenization. The bone lysates or cell lysates were resolved on SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. Membranes were incubated with primary antibodies that included β-actin (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-LC3 (1:1000; Cell Signaling Technology) or anti-cathepsin K (Cell Signaling Technology) followed by species-specific horseradish peroxidase secondary antibody. Anti-LC3 antibody recognizes both LC3-I, which is cytoplasmic and LC3-II that binds to the autophagic membranes. Immunoreactive materials were detected by chemiluminescence (Pierce Laboratories, Thermo Fisher Scientific, Rockford, IL, USA), imaged with a Kodak Gel Logic 100 Digital Imaging System, and quantitated by Kodak 1D 3.6 image analysis software (Eastman Kodak, Rochester, NY, USA).
Statistical analysis
Group means and sds were calculated for all outcome variables. The nonparametric Kruskal-Wallis test was used to determine the differences between the groups. The 2-tailed Spearman correlation test was used to determine the association between the activation (fold changes from PL) for all genes in the antioxidation, autophagy, or antiapoptosis gene arrays (version 12; SPSS Inc., Chicago, IL, USA).
RESULTS
Dose-dependent effects of GC on serum chemistry values, bone turnover, and strength
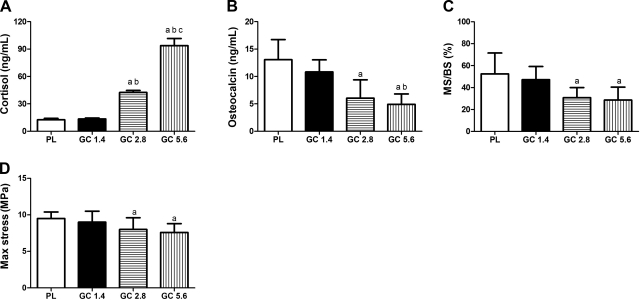
The serum cortisol level was not changed at the 1.4 mg/kg/d GC dose level but increased by >200% at the 2.8 mg/kg/d dose level and 600% at the 5.6 mg/kg/d dose level after 28 d of treatment. Bone formation, measured by the serum osteocalcin level or surface-based mineralizing surface at the distal femurs, decreased nonsignificantly by ∼10–40% at a 1.4 mg/kg/d GC dose level and 40–60% at the two higher GC dose levels (P<0.05). The maximum vertebral compressive stress was significantly lowered at the 5.6 mg/kg/d GC dose level compared with that for PL (Fig. 1).
Figure 1.
Three-month-old male Swiss-Webster mice were treated with a placebo pellet (PL), or a 2.5 mg/60 d (1.4 mg/kg/d), 5 mg/60 d (2.8 mg/kg/d), or 10 mg/60 d (5.6 mg/kg/d) slow-release prednisolone pellet. A, B) Mice were sacrificed at d 28, and serum was collected for cortisol (A) and osteocalcin (B). C, D) Bone histomorphometry was performed on the distal femur; surface-based bone turnover was assessed by mineralizing surface (MS/BS; C), and the sixth lumbar vertebra was tested for compressive strength (max stress; D). n = 6–16/group. aP < 0.05 vs. PL; bP < 0.05 vs. GC 1.4 mg; cP < 0.05 vs. GC 2.8 mg.
Excess GC induced an osteocyte autophagy in vivo
To evaluate the dynamic and integrative nature of GCs on osteocyte stress response and cell fate, we obtained RNA from the long bones (tibiae, n=6/group) of mice treated with PL or 3 doses of GCs for 28 d and performed real time RT-PCR gene arrays for antioxidants, autophagy, and antiapoptosis. We found that GC dose-dependently decreased the activation of oxidative stress responsive gene expressions (Table 1). For example, the expression of superoxide dismutase (Sod) 1, soluble increased ∼12-fold at the 1.4 mg/kg/d dose level and increased 6- and 9-fold, respectively, at the 2.8 and 5.6 mg/kg/d GC dose levels, Sod2 (mitochondrial) increased 15 fold at the 1.4 mg/kg/d dose level and increased 6-fold at the 2.8 and 5.6 mg/kg/d GC dose levels; and Sod3 (extracellular) increased 30-fold at the 1.4 mg/kg/d GC dose level and increased 23- and 11-fold, respectively, at 2.8 and 5.6 mg/kg/d GC dose levels. GC at the 1.4 mg/kg/d dose level activated a number of genes that are associated with autophagy by an average increase of 20- to 30-fold from the PL treatment and by ≤10-fold at the 2.8 mg/kg/d dose level and was similar to PL (1-fold) at the 5.6 mg/kg/d dose level (Fig. 2A). Activation of the antioxidant gene pathway was positively correlated with activation of the autophagic gene array, especially at the 1.4 mg/kg/d GC dose level (Fig. 2B). For example, the expressions of autophagy-related proteins (Atg) 12 and 7, both of which are essential for the formation of double-membrane vesicles and autophagosomes, increased 23- and 42-fold, respectively, at the 1.4 mg/kg/d GC dose level and increased 14- and 11-fold, respectively, at the 2.8 mg/kg/d GC dose but did not differ from PL at the 5.6 mg/kg/d GC dose level. In contrast, the expression of genes associated with antiapoptosis increased by ≤5-fold from PL at the 1.4 mg/kg/d GC group but were significantly decreased by an average of −1 to −6-fold in the 2.8 mg/kg/d GC dose group and −2 to −30-fold in the 5.6 mg/kg/d GC dose group (Table 2). For example, the expression of B-cell leukemia/lymphoma 2 (Bcl2) was not changed at the 1.4 mg/kg/d GC dose level but decreased 1.6-fold at the 2.8 mg/kg/d GC dose and decreased 3-fold at the 5.6 mg/kg/d GC dose level; the expression of IL-10 increased 3-fold at the 1.4 mg/kg/d GC dose level but decreased 6-fold at the 2.8 mg/kg/d GC dose and decreased 35-fold at the 5.6 mg/kg/d GC dose level. There was no significant correlation between the activation of antioxidant and antiapoptosis genes. In addition, there was no significant correlation between activation of autophagy and antiapoptosis genes (data not shown).
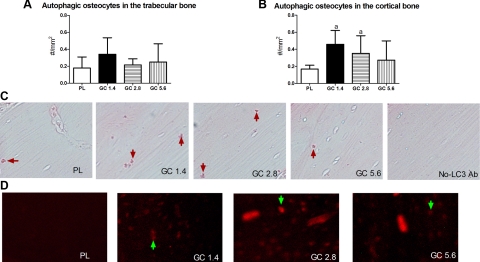
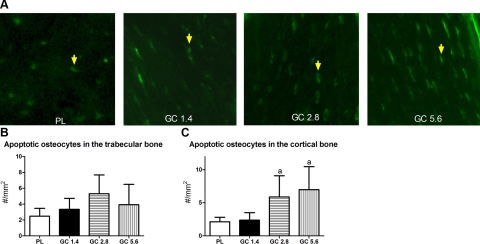
GC treatment at all three doses did not increase the percentage of LC3+ osteocytes in the trabecular bone region (Fig. 3A). However, GC treatment increased autophagic osteocytes in the cortical bone region of the distal femurs at the 1.4 and 2.8 mg/kg/d (Fig. 3B) measured by immunohistochemical staining against LC3 (Fig. 3C) or by injecting the fluorescent-conjugated LC3 antibody (LC3-5F10) into the mice (Fig. 3D). GC exposure did not significantly change osteocyte apoptosis in the trabecular bone region of the distal femur at either dose level but increased apoptotic osteocytes significantly in the cortical bone region at the 2.8 and 5.6 mg/kg/d GC dose levels (Fig. 4).
Figure 3.
Distal femurs from PL- or GC-treated mice were embedded in paraffin at d 28. A, B) Numbers of LC3+ osteocytes present in a defined trabecular bone area (A) or cortical bone area (B) were calculated. C) Immunohistochemical staining was performed using an anti-LC3 antibody (LC3+ cells were stained in red; red arrows). D) Autophagic osteocytes were also quantitated by injecting fluorescent-conjugated monoclonal antibody for LC3 (LC3–5F10; green arrows) into mice at 48 h before sacrifice. n = 8/group, 3 sections/animal were analyzed. aP < 0.05 vs. PL.
Figure 4.
Presence of apoptosis in the trabecular and cortical bone of the right distal femurs in mice treated with PL or GC. Apoptosis was determined by in situ fluorescence TUNEL staining. Apoptotic osteocytes (A, yellow arrows) in both the trabecular bone (B) and cortical bone (C) regions were measured. n = 8/group. aP < 0.05 vs. PL.
GC increased osteocyte autophagy and cathepsin K secretion in vitro
To determine the dose-dependent effect of GCs on autophagy in vitro, osteocytic MLO-Y4 cells were transfected by GFP-LC3 for 48 h and then were treated with various concentrations of Dex (10−8 to 10−6 M) for 24 h. GFP-LC3 was diffusely distributed in the cytoplasm in the absence of Dex (control). In contrast, treatment with Dex increased the number of GFP-LC3 puncta, indicating that LC3 was recruited and aggregated in the cytoplasm (Fig. 5A). Increased colocalization of GFP-LC3 was seen, and lysosomes were observed accumulating in the cytoplasm in Dex-treated cells, especially in 10−7 to 10−6 M Dex (Fig. 5B). Western blot confirmed the increase in GFP-LC3-II levels after Dex treatment at all doses starting from the lowest dose (10−8 M), and the maximal response was seen at 10−7 M Dex (Fig. 5C, D). The enzyme for matrix metabolism, cathepsin K, was increased in the MLO-Y4 cells (Fig. 5C, D) treated with Dex, as well as in the culture medium, which was most significant at the 10−7 M dose level (Fig. 5E). Interestingly, when the MLO-Y4 cells were treated with Dex (10−8 to 10−5 M) and evaluated for the antioxidant gene expressions, we did not find that Dex treatment in vitro activated the antioxidant pathway significantly (data not shown).
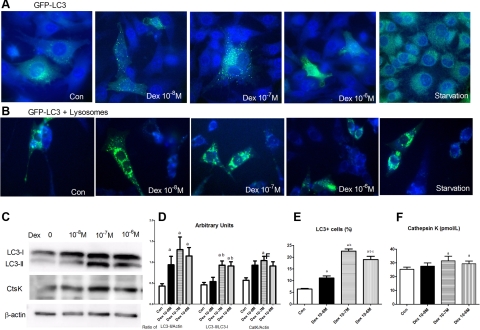
Figure 5.
In vitro assessment of autophagy in ML0-Y4 cells treated with GCs. A) MLO-Y4 cells were cultured on collagen-coated plates and grown in phenol red-free α-modified essential medium supplemented with 2.5% FBS and 2.5% bovine calf serum. Cells were transfected with GFP-LC3 for 48 h before they were treated with Dex at 10−8 to 10−6 M and LysoSensor for 24 h. A few autophagosomes (GFP-LC3+ dots, yellow arrows) were seen in the cytoplasm in the control (Con) cells. Treatment with Dex increased in GFP-LC3+ vacuoles (×100 original view for all images). B) GFP-LC3+ vacuoles (stained in green) and lysosomes (stained in blue) were increased in 10−7 to 10−6 M Dex dose levels. C) Western blots of LC3 and cathepsin K (CtsK) contents in MLO-Y4 cells treated with DEX, as indicated, for 24 h. D) Arbitrary units of Western blots in C for LC3-II/actin, LC3-II/LC3-I, and cathepsin K (CatK)/actin. LC3-I protein level was not changed by Dex treatment, whereas LC3-II protein level was increased after treatment with 10−8–10−6 M Dex. Maximum increase was observed with 10−7 and 10−6 M Dex. Cathepsin K levels were also increased after Dex treatment, measured by Western blot in cell lysates. E) LC3+ cell numbers. Percentage of LC3+ cells was measured by ELISA in the culture medium. F) Cathepsin K levels measured by ELISA in the culture medium. Cathepsin K levels were increased after Dex treatment. Each experiment was repeated 4 times. aP < 0.05 vs. control; bP < 0.05 vs. 10−8 M Dex.
DISCUSSION
After 28 d of low-dose GC treatment in mice, there was significant activation of the autophagy pathway and increased osteocyte autophagy in the cortical bone region of the distal femur that were significantly different from placebo treatment. A decreased antiapoptosis response and osteocyte apoptosis were observed at the cortical bone region after the higher-dose GC treatment.
Weinstein et al. (38, 42) reported that reduced bone formation in GC-treated mice was associated with increased apoptosis of osteoblasts and osteocytes. Plotkin et al. (54) also reported in vitro evidence of apoptosis of osteocytes exposed to GCs using 3 separate assays (trypan blue exclusion, nuclear morphology, and annexin V/propidium iodine ratios by FACS analysis) to accurately confirm that there was osteocyte apoptosis present (54). However, these investigations were performed before reagents were available to assess the presence of autophagy. Our study evaluated the dose response of osteocytes to GCs and found that osteocyte apoptosis increased after a higher dose of GC at the cortical bone region. Our results (43) are similar to those reported by Weinstein et al. (38, 42), who also reported that in male mice treated with higher doses of GCs nearly 20% of the osteocytes at the cortical bone region of the tibiae had undergone apoptosis. These investigators also demonstrated that the increased osteocyte apoptosis was significantly associated with a reduction in whole bone strength.
In additional to apoptosis, we found that autophagic osteocytes were observed in the cortical bone region of the lumbar vertebral bodies in mice that had received 56 d of GC treatment (19). The autophagy pathway is one of the most important biological processes that enables the cells to survive stress and starvation and helps to maintain cellular homeostasis by degrading damaged organelles (22, 25, 28, 55). The hallmark of autophagy is the formation of autophagosomes, also known as autophagic vacuoles that are lined by two membranes with the recruitment of LC3-II to the autophagosomal membranes, a characteristic for autophagosomes, whereas LC3-I remains in the cytoplasm (56). Aging is associated with an increase in the intracellular overabundance of oxidative products, including reactive oxygen species (e.g., oxygen ions and free radicals) and increased defense mechanisms for oxidative stress such as glutathione (GSH), thioredoxin, and GSH peroxidase, which convert the peroxides to harmless materials, and the expressions of superoxide dismutase, the antioxidant enzymes in bone tissues (57–62). Likewise, GCs also activate the oxidative pathway and accelerate the aging process in bone tissue (34, 36, 63–65). GC treatment in vitro did not activate the antioxidant pathway but significantly increased the overall stress level in vivo in mice by increasing the systemic circulating cortisol levels. On the basis of our data, it appears that antioxidative responses were provoked by GC-induced stress, and the osteocytes may have responded with autophagy. An increased LC3-II protein level and autophagic osteocytes were observed primarily in the cortical bone regions. This autophagic attempt to “survive” the insult from the GC exposure may be successful if the dose is low. However, with the increasing doses of GCs, the cells' antioxidant defenses were overwhelmed, and the ability of the osteocyte to survive was reduced, which could then direct the cells to apoptosis. Other investigators have suggested that osteocyte cell fate may be related to the dose of GC (66, 67). Our data clearly demonstrate the dynamics of the osteocyte cell fate with different GC doses within the cortical bone. The lack of osteocyte response in the trabecular bone regions may due to the fact that trabecular and cortical bone have different remodeling rates, and these two bone compartments have different responses to various stimuli, such as immobilization and exercise (68–72). The life spans of trabecular bone osteocytes and cortical bone osteocytes are also different: cortical bone osteocytes live significantly longer, and this may help to explain the differing response to GC treatment (73). The activation of the autophagic gene pathway and osteocyte autophagy was significantly increased when the cells or mice were treated with a low dose of GC. The higher doses of GC activated the gene pathway for apoptosis and osteocyte apoptosis was then significantly increased.
During the initial autophagic process, cells may be able to remain viable during periods of metabolic stress (26, 74, 75). However, in the later stage of autophagy, the digestion of autophagic materials involves the fusion of autophagosomes with lysosomes to form autolysosomes or the degradative autophagic vacuole. This catabolic process releases cathepsins and other hydrolases from the lysosomal lumen into the cytosol or, if the autolysosome fuses with the plasma membrane, into the surrounding tissue (76, 77). This extrusion of the contents of the phagolysosomes is in some degree similar to the virus shedding from a lymphocyte (78, 79). We observed increased colocalization of GFP-LC3 and lysosomes in osteocytes treated with Dex as well as increased cathepsin K levels both in the osteocytes and in the circulation. Our findings suggest that GC increased the release of the cathepsins and other hydrolases secreted into the perilacunar bone matrix. Over time, the localized release of cathepsin K with other hydrolases from the lysosome may change the perilacunar matrix composition, and over time it may also change the localized material properties of the bone. Therefore, osteocyte cell fate through autophagy may have a different effect on the localized perilacunar composition and bone quality than death by apoptosis. Histological sections of osteocyte death by apoptosis generally have only empty lacunae. However, the apoptotic cell may send signals to the bone cell surface to activate osteoclast-mediated bone remodeling. Unlike necrosis and possibly autophagy, the cellular debris from apoptosis does not appear to damage the bone tissue and may not alter the perilacunar matrix. On the other hand, osteocyte autophagy may induce the autolysosomal secretion of degradative enzymes and hydrolases into the perilacunar region that may eventually affect localized bone mineralization after chronic GC exposure (43).
In summary, GC treatment dose dependently decreased antioxidant, autophagy, and antiapoptosis responses. A low GC dose induced osteocyte autophagy, and higher GC doses induced osteocyte apoptosis, principally in the cortical bone. Although GC-induced autophagy has been reported, the dose response of autophagy and apoptosis within the cortical bone is novel, and additional studies are now warranted to elucidate these findings and determine whether interventions directed to alter autophagy or the inhibition of cathepsin K may be a useful approach to reduce GC-induced bone fragility.
Acknowledgments
The authors thank Dr. Frank Chuang (Center for Biophotonics and Science Technology, University of California at Davis Medical Center) for generously providing GFP-tagged LC3 expression vector and photographing the cells.
This work was funded by U.S. National Institutes of Health grants 1K12-HD05195801 (cofunded by the National Institute of Child Health and Human Development, the Office of Research on Women's Health, the Office of Dietary Supplements, and the National Institute of Aging), R01-AR043052-07, K24-AR048841, 5R21-AR57515-2, and P01-AR46798 (to L F.B. and J.X.J), and Welch Foundation grant AQ-1507 (to J.X.J.).
REFERENCES
- 1. Saag K. G. (2002) Glucocorticoid use in rheumatoid arthritis. Curr. Rheumatol. Rep. 4, 218–225 [DOI] [PubMed] [Google Scholar]
- 2. Saag K. G., Shane E., Boonen S., Marin F., Donley D. W., Taylor K. A., Dalsky G. P., Marcus R. (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 357, 2028–2039 [DOI] [PubMed] [Google Scholar]
- 3. Lane N. E., Sanchez S., Modin G. W., Genant H. K., Pierini E., Arnaud C. D. (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J. Clin. Invest. 102, 1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane N. E., Sanchez S., Modin G. W., Genant H. K., Pierini E., Arnaud C. D. (2000) Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: results of a randomized controlled clinical trial. J. Bone Miner. Res. 15, 944–951 [DOI] [PubMed] [Google Scholar]
- 5. Dalle Carbonare L., Arlot M. E., Chavassieux P. M., Roux J. P., Portero N. R., Meunier P. J. (2001) Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J. Bone. Miner. Res. 16, 97–103 [DOI] [PubMed] [Google Scholar]
- 6. Van Staa T. P., Laan R. F., Barton I. P., Cohen S., Reid D. M., Cooper C. (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 48, 3224–3229 [DOI] [PubMed] [Google Scholar]
- 7. Chiodini I., Carnevale V., Torlontano M., Fusilli S., Guglielmi G., Pileri M., Modoni S., Di Giorgio A., Liuzzi A., Minisola S., Cammisa M., Trischitta V., Scillitani A. (1998) Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: study in eumenorrheic patients with Cushing's syndrome. J. Clin. Endocrinol. Metab. 83, 1863–1867 [DOI] [PubMed] [Google Scholar]
- 8. Bonewald L. (2006) Osteocytes as multifunctional cells. J. Musculoskelet. Neuronal Interact. 6, 331–333 [PMC free article] [PubMed] [Google Scholar]
- 9. Boass A., Ramp W. K., Toverud S. U. (1981) Hypocalcemic, hypophosphatemic rickets in rat pups suckling vitamin D-deprived mothers. Endocrinology 109, 505–512 [DOI] [PubMed] [Google Scholar]
- 10. Qing H., Bonewald L. F. (2009) Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int. J. Oral. Sci. 1, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lester D. R., Jr., Seifert M. F. (1996) Maternal high calcium diet fails to reverse rickets in the osteosclerotic mouse. Clin. Orthop. Relat. Res. 330, 271–280 [DOI] [PubMed] [Google Scholar]
- 12. Rasmussen P. (1977) Calcium deficiency, pregnancy, and lactation in rats. Microscopic and microradiographic observations on bones. Calcif. Tissue Res. 23, 95–102 [DOI] [PubMed] [Google Scholar]
- 13. Teti A., Zallone A. (2009) Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone 44, 11–16 [DOI] [PubMed] [Google Scholar]
- 14. Belanger L. F. (1969) Osteocytic osteolysis. Calcif. Tissue Res. 4, 1–12 [DOI] [PubMed] [Google Scholar]
- 15. Bonewald L. F. (2007) Osteocytes as dynamic multifunctional cells. Ann. N. Y. Acad. Sci. 1116, 281–290 [DOI] [PubMed] [Google Scholar]
- 16. Feng J. Q., Ye L., Schiavi S. (2009) Do osteocytes contribute to phosphate homeostasis? Curr. Opin. Nephrol. Hypertens. 18, 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukumoto S. (2009) The role of bone in phosphate metabolism. Mol. Cell. Endocrinol. 310, 63–70 [DOI] [PubMed] [Google Scholar]
- 18. Fukumoto S., Martin T. J. (2009) Bone as an endocrine organ. Trends Endocrinol. Metab. 20, 230–236 [DOI] [PubMed] [Google Scholar]
- 19. Xia X., Kar R., Gluhak-Heinrich J., Yao W., Lane N. E., Bonewald L. F., Biswas S. K., Lo W. K., Jiang J. X. (2010) Glucocorticoid induced autophagy in osteocytes. J. Bone Miner. Res. 25, 2479–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harman D. (1992) Role of free radicals in aging and disease. Ann. N. Y. Acad. Sci. 673, 126–141 [DOI] [PubMed] [Google Scholar]
- 21. Harman D. (1988) Free radicals in aging. Mol. Cell. Biochem. 84, 155–161 [DOI] [PubMed] [Google Scholar]
- 22. Hotchkiss R. S., Strasser A., McDunn J. E., Swanson P. E. (2009) Cell death. N. Engl. J. Med. 361, 1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He C., Klionsky D. J. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen J. L., Lin H. H., Kim K. J., Lin A., Ou J. H., Ann D. K. (2009) PKCδ signaling: a dual role in regulating hypoxic stress-induced autophagy and apoptosis. Autophagy 5, 244–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinet W., Agostinis P., Vanhoecke B., Dewaele M., De Meyer G. R. (2009) Autophagy in disease: a double-edged sword with therapeutic potential. Clin. Sci. (Lond.) 116, 697–712 [DOI] [PubMed] [Google Scholar]
- 26. Todde V., Veenhuis M., van der Klei I. J. (2009) Autophagy: principles and significance in health and disease. Biochim. Biophys. Acta 1792, 3–13 [DOI] [PubMed] [Google Scholar]
- 27. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsujimoto Y., Shimizu S. (2005) Another way to die: autophagic programmed cell death. Cell Death Differ. 12(Suppl. 2), 1528–1534 [DOI] [PubMed] [Google Scholar]
- 29. Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, 741–752 [DOI] [PubMed] [Google Scholar]
- 30. Shibata M., Lu T., Furuya T., Degterev A., Mizushima N., Yoshimori T., MacDonald M., Yankner B., Yuan J. (2006) Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J. Biol. Chem. 281, 14474–14485 [DOI] [PubMed] [Google Scholar]
- 31. Walther F. J., Jobe A. H., Ikegami M. (1998) Repetitive prenatal glucocorticoid therapy reduces oxidative stress in the lungs of preterm lambs. J. Appl. Physiol. 85, 273–278 [DOI] [PubMed] [Google Scholar]
- 32. Ferioli M. E., Pinotti O., Pirona L. (1999) Polyamine oxidase activity in lymphoid tissues of glucocorticoid-treated rats. Biochem. Pharmacol. 58, 1907–1914 [DOI] [PubMed] [Google Scholar]
- 33. Ogasawara M., Nomura K., Shibata N., Ujihara M., Kobayashi M., Demura H. (1999) Surgical stress increases renal glutathione content via increased glucocorticoid, and resistance to subsequent oxidative injury in the rat: significant link between endocrine response and cell defense system under the stress. Endocr. J. 46, 99–106 [DOI] [PubMed] [Google Scholar]
- 34. Adcock I. M., Ito K. (2005) Glucocorticoid pathways in chronic obstructive pulmonary disease therapy. Proc. Am. Thorac. Soc. 2, 313–319; discussion 340–341 [DOI] [PubMed] [Google Scholar]
- 35. Lee C. W., Chuang J. H., Wang P. W., Chang N. K., Wang H. C., Huang C. C., Tiao M. M., Lo S. K. (2006) Effect of glucocorticoid pretreatment on oxidative liver injury and survival in jaundiced rats with endotoxin cholangitis. World J. Surg. 30, 2217–2226 [DOI] [PubMed] [Google Scholar]
- 36. Ong S. L., Zhang Y., Whitworth J. A. (2008) Reactive oxygen species and glucocorticoid-induced hypertension. Clin. Exp. Pharmacol. Physiol. 35, 477–482 [DOI] [PubMed] [Google Scholar]
- 37. Laane E., Tamm K. P., Buentke E., Ito K., Khahariza P., Oscarsson J., Corcoran M., Bjorklund A. C., Hultenby K., Lundin J., Heyman M., Soderhall S., Mazur J., Porwit A., Pandolfi P. P., Zhivotovsky B., Panaretakis T., Grander D. (2009) Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 16, 1018–1029 [DOI] [PubMed] [Google Scholar]
- 38. O'Brien C. A., Jia D., Plotkin L. I., Bellido T., Powers C. C., Stewart S. A., Manolagas S. C., Weinstein R. S. (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145, 1835–1841 [DOI] [PubMed] [Google Scholar]
- 39. Weinstein R. S. (2001) Glucocorticoid-induced osteoporosis. Rev. Endocr. Metab. Disord. 2, 65–73 [DOI] [PubMed] [Google Scholar]
- 40. Weinstein R. S. (2007) Is long-term glucocorticoid therapy associated with a high prevalence of asymptomatic vertebral fractures? Nat. Clin. Pract. 3, 86–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weinstein R. S., Chen J. R., Powers C. C., Stewart S. A., Landes R. D., Bellido T., Jilka R. L., Parfitt A. M., Manolagas S. C. (2002) Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J. Clin. Invest. 109, 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinstein R. S., Jilka R. L., Parfitt A. M., Manolagas S. C. (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 102, 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lane N. E., Yao W., Balooch M., Nalla R. K., Balooch G., Habelitz S., Kinney J. H., Bonewald L. F. (2006) Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J. Bone Miner. Res. 21, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lane N. E., Yao W., Nakamura M. C., Humphrey M. B., Kimmel D., Huang X., Sheppard D., Ross F. P., Teitelbaum S. L. (2005) Mice lacking the integrin β5 subunit have accelerated osteoclast maturation and increased activity in the estrogen-deficient state. J. Bone Miner. Res. 20, 58–66 [DOI] [PubMed] [Google Scholar]
- 45. Yao W., Cheng Z., Shahnazari M., Dai W., Johnson M. L., Lane N. (2010) Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates PTH bone anabolic effects. J. Bone Miner. Res. 25, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao W., Cheng Z., Busse C., Pham A., Nakamura M. C., Lane N. E. (2008) Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 58, 1674–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao W., Cheng Z., Koester K. J., Ager J. W., Balooch M., Pham A., Chefo S., Busse C., Ritchie R. O., Lane N. E. (2007) The degree of bone mineralization is maintained with single intravenous bisphosphonates in aged estrogen-deficient rats and is a strong predictor of bone strength. Bone 41, 804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yao W., Cheng Z., Pham A., Busse C., Zimmermann E. A., Ritchie R. O., Lane N. E. (2008) Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 58, 3485–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalajzic I., Braut A., Guo D., Jiang X., Kronenberg M. S., Mina M., Harris M. A., Harris S. E., Rowe D. W. (2004) Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone 35, 74–82 [DOI] [PubMed] [Google Scholar]
- 50. Rowe P. S., Matsumoto N., Jo O. D., Shih R. N., Oconnor J., Roudier M. P., Bain S., Liu S., Harrison J., Yanagawa N. (2006) Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone 39, 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kato Y., Windle J. J., Koop B. A., Mundy G. R., Bonewald L. F. (1997) Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 12, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 52. Edinger A. L. (2009) Starvation in the midst of plenty: making sense of ceramide-induced autophagy by analysing nutrient transporter expression. Biochem. Soc. Trans. 37, 253–258 [DOI] [PubMed] [Google Scholar]
- 53. Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plotkin L. I., Weinstein R. S., Parfitt A. M., Roberson P. K., Manolagas S. C., Bellido T. (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 104, 1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eisenberg-Lerner A., Bialik S., Simon H. U., Kimchi A. (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 16, 966–975 [DOI] [PubMed] [Google Scholar]
- 56. Tanida I., Ueno T., Kominami E. (2004) LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 36, 2503–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Almeida M., Han L., Martin-Millan M., Plotkin L. I., Stewart S. A., Roberson P. K., Kousteni S., O'Brien C. A., Bellido T., Parfitt A. M., Weinstein R. S., Jilka R. L., Manolagas S. C. (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 282, 27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muthusami S., Ramachandran I., Muthusamy B., Vasudevan G., Prabhu V., Subramaniam V., Jagadeesan A., Narasimhan S. (2005) Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta 360, 81–86 [DOI] [PubMed] [Google Scholar]
- 59. Jilka R. L., Almeida M., Ambrogini E., Han L., Roberson P. K., Weinstein R. S., Manolagas S. C. (2010) Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 9, 851–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Almeida M., Han L., Ambrogini E., Bartell S. M., Manolagas S. C. (2010) Oxidative stress stimulates apoptosis and activates NF-κB in osteoblastic cells via a PKCβ/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol. Endocrinol. 24, 2030–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Manolagas S. C. (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Almeida M., Martin-Millan M., Ambrogini E., Bradsher R., 3rd, Han L., Chen X. D., Roberson P. K., Weinstein R. S., O'Brien C. A., Jilka R. L., Manolagas S. C. (2010) Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERα. J. Bone. Miner. Res. 25, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orzechowski A., Ostaszewski P., Wilczak J., Jank M., Balasinska B., Wareski P., Fuller J., Jr. (2002) Rats with a glucocorticoid-induced catabolic state show symptoms of oxidative stress and spleen atrophy: the effects of age and recovery. J. Vet. Med. A Physiol. Pathol. Clin. Med. 49, 256–263 [DOI] [PubMed] [Google Scholar]
- 64. Jilka R. L., Weinstein R. S., Parfitt A. M., Manolagas S. C. (2007) Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J. Bone Miner. Res. 22, 1492–1501 [DOI] [PubMed] [Google Scholar]
- 65. Komatsu F., Kudoh H., Kagawa Y. (2007) Evaluation of oxidative stress and effectiveness of low-dose glucocorticoid therapy on exacerbation of chronic obstructive pulmonary disease. J. Gerontol. A Biol. Sci. Med. Sci. 62, 459–464 [DOI] [PubMed] [Google Scholar]
- 66. Planey S. L., Abrams M. T., Robertson N. M., Litwack G. (2003) Role of apical caspases and glucocorticoid-regulated genes in glucocorticoid-induced apoptosis of pre-B leukemic cells. Cancer Res. 63, 172–178 [PubMed] [Google Scholar]
- 67. Bonapace L., Bornhauser B. C., Schmitz M., Cario G., Ziegler U., Niggli F. K., Schafer B. W., Schrappe M., Stanulla M., Bourquin J. P. (2010) Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J. Clin. Invest. 120, 1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ke H. Z., Jee W. S., Ito H., Setterberg R. B., Li M., Lin B. Y., Liang X. G., Ma Y. F. (1993) Greater bone formation induction occurred in aged than young cancellous bone sites. Bone 14, 481–485 [DOI] [PubMed] [Google Scholar]
- 69. Jee W. S., Li X. J., Schaffler M. B. (1991) Adaptation of diaphyseal structure with aging and increased mechanical usage in the adult rat: a histomorphometrical and biomechanical study. Anat. Rec. 230, 332–338 [DOI] [PubMed] [Google Scholar]
- 70. Jee W. S., Li X. J. (1990) Adaptation of cancellous bone to overloading in the adult rat: a single photon absorptiometry and histomorphometry study. Anat. Rec. 227, 418–426 [DOI] [PubMed] [Google Scholar]
- 71. Yao W., Jee W. S., Chen J., Liu H., Tam C. S., Cui L., Zhou H., Setterberg R. B., Frost H. M. (2000) Making rats rise to erect bipedal stance for feeding partially prevented orchidectomy-induced bone loss and added bone to intact rats. J. Bone Miner. Res. 15, 1158–1168 [DOI] [PubMed] [Google Scholar]
- 72. Yao W., Jee W. S., Chen J., Tam C. S., Setterberg R. B., Frost H. M. (2000) Erect bipedal stance exercise partially prevents orchidectomy-induced bone loss in the lumbar vertebrae of rats. Bone 27, 667–675 [DOI] [PubMed] [Google Scholar]
- 73. Bonewald L. F. (2011) The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Srinivas V., Bohensky J., Zahm A. M., Shapiro I. M. (2009) Autophagy in mineralizing tissues: microenvironmental perspectives. Cell Cycle 8, 391–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsuchihara K., Fujii S., Esumi H. (2009) Autophagy and cancer: dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 278, 130–138 [DOI] [PubMed] [Google Scholar]
- 76. Kroemer G., Jaattela M. (2005) Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 5, 886–897 [DOI] [PubMed] [Google Scholar]
- 77. Gonzalez-Polo R. A., Boya P., Pauleau A. L., Jalil A., Larochette N., Souquere S., Eskelinen E. L., Pierron G., Saftig P., Kroemer G. (2005) The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J. Cell Sci. 118, 3091–3102 [DOI] [PubMed] [Google Scholar]
- 78. Bardeguez A. D., Skurnick J. H., Perez G., Colon J. M., Kloser P., Denny T. N. (1997) Lymphocyte shedding from genital tract of human immunodeficiency virus-infected women: immunophenotypic and clinical correlates. Am. J. Obstet. Gynecol. 176, 158–165 [DOI] [PubMed] [Google Scholar]
- 79. Evans B. J., McDowall A., Taylor P. C., Hogg N., Haskard D. O., Landis R. C. (2006) Shedding of lymphocyte function-associated antigen-1 (LFA-1) in a human inflammatory response. Blood 107, 3593–3599 [DOI] [PubMed] [Google Scholar]