Abstract
Reverse signaling through the ephrinB ligands is important for several morphogenetic events, such as axon guidance, neuronal plasticity, spine maturation, and synaptogenesis. Signaling is initiated by binding of EphB receptors to ephrinB ligands, stimulating their tyrosine phosphorylation via an unclear mechanism. Here we show that this mechanism involves presenilin1 (PS1)/γ-secretase regulation of phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk binding protein (PAG/Cbp), an adaptor protein that controls the activity of Src kinases. Using immunoprecipitation and Western blot of mouse primary neuronal and human embryonic kidney (HEK293) cell extracts overexpressing PAG/Cbp, we show that EphB2 induces tyrosine dephosphorylation of PAG/Cbp in a γ-secretase-dependent manner. In these cells, PAG/Cbp dephosphorylation is promoted by the PS1/γ-secretase-produced fragment of ephrinB2 cleavage (ephrinB2/CTF2), which forms complexes with PAG/Cbp when introduced exogenously. EphB2-induced tyrosine phosphorylation of ephrinB2 depends on PAG/Cbp because EphB2 cannot increase ephrinB2 phosphorylation in cells treated with anti-PAG siRNA or in PAG/Cbp-knockout (KO) cells. Furthermore, in contrast to WT PS1, familial Alzheimer disease (FAD) PS1 mutants expressed in PS1-KO mouse embryonic fibroblasts inhibited both the EphB2-induced dephosphorylation of PAG/Cbp and the phosphorylation of ephrinB2. PS1 FAD mutations may thus inhibit the function of ephrinB in the brain, promoting neurodegeneration in Alzheimer disease.—Georgakopoulos, A., Xu, J., Xu, C., Mauger, G., Barthet, G., Robakis, N. K. Presenilin1/γ-secretase promotes the EphB2-induced phosphorylation of ephrinB2 by regulating phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk binding protein.
Keywords: Alzheimer disease, PAG/Cbp, Src kinases
EphrinB ligands are transmembrane proteins that, by binding to their cognate EphB receptors, trigger bidirectional signaling, modulating the behavior of both the receptor and the ligand-bearing cell. On binding of EphB to ephrinB, the latter's intracellular domain is phosphorylated on tyrosine residues enabling the binding of SH2/SH3 proteins, such as Grb4, modulating spine morphogenesis and synapse formation (1, 2). EphrinB ligands are expressed in both pre- and postsynaptic neurons in the hippocampus (3), and they may regulate synapse formation, plasticity, LTP, and LTD (4, 5, 6). EphB-induced phosphorylation of ephrinB ligands is mediated by Src kinases, by an unclear mechanism (7).
Src family kinases are involved in a number of signal transduction pathways in various cell types, including neurons promoting neurite outgrowth and regulating LTP and myelination, and they have been implicated in memory formation and neurodegeneration (for review, see refs. 8, 9). Src is kept inactive by the C′-terminal Src kinase (Csk) that binds Src and phosphorylates it at residue Tyr529 (10, 11). Csk is recruited to Src by the transmembrane adaptor protein phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk binding protein (PAG/Cbp) when the latter is phosphorylated on tyrosine (12, 13). Various extracellular stimuli induce the dephosphorylation of PAG/Cbp by phosphatases like Shp2 or CD45 (14) and the subsequent release of Csk from the complex (for review, see ref. 15) allowing the activation of Src.
EphrinB ligands are proteolyticaly processed by presenilin1 (PS1)/γ-secretase (16, 17). PS1, a protein involved in the pathogenesis of familial Alzheimer disease (FAD), regulates the proteolytic cleavage of several substrates via γ-secretase activity (for review, see ref. 18), producing cytoplasmic peptides, many of which are active and participate in various cellular functions (for review, see ref. 19).
We have shown that binding of EphB2 receptor to ephrinB2 ligand stimulates the cleavage of ephrinB2 by PS1/γ-secretase, producing the cytoplasmic peptide ephrinB2/CTF2, which, in turn, promotes ephrinB2 phosphorylation in a Src-dependent manner (16). The mechanism of this event is not understood; however, the dissociation of the inhibitory kinase Csk from Src has been implicated (16).
Here, we present evidence that the mechanism by which PS1/γ-secretase regulates the EphB2-induced phosphorylation of ephrinB2 on tyrosine involves PAG/Cbp. EphrinB2/CTF2 forms complexes with PAG/Cbp and induces its dephosphorylation on tyrosine. EphrinB2/CTF2 peptide also promotes the dissociation of PAG/Cbp from the inhibitory Csk and the subsequent release of Src from Csk and PAG/Cbp. This function allows Src to be autophosphorylated and activated. Since ephrinB2/CTF2 also promotes the ephrinB2 tyrosine phosphorylation in a Src-dependent manner (16), the above data show that PS1/γ-secretase controls the EphB2-induced ephrinB2 phosphorylation by regulating components of the Src regulatory machinery.
We further present evidence that PAG/Cbp is necessary for the EphB2-induced tyrosine phosphorylation of ephrinB2 ligand, since absence or down-regulation of this protein by siRNA abolishes the ability of cells to phosphorylate ephrinB2 ligand on tyrosine in response to EphB2 receptor stimulation. Interestingly, several PS1 mutations found in FAD that inhibit the γ-secretase cleavage of ephrinB2 (16) also inhibit the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine and the EphB2-induced tyrosine phosphorylation of ephrinB2. This finding suggests that these mutations may impair ephrinB/EphB signaling in the brain, promoting neurodegneration in AD.
MATERIALS AND METHODS
Materials and antibodies
L-685,458, compound 19, and lactacystin were obtained from Calbiochem (EMD Biosciences, San Diego, CA, USA). EphB2-Fc fusion form and Fc were from R&D Systems (Minneapolis, MN, USA). Rabbit anti-human Fc, goat anti-mouse light chain specific IgG and mouse anti-rabbit light chain specific IgG were from Jackson Immunoresearch (West Grove, PA, USA). NaF, Na3VO4, sodium pyrophosphate, and microcystin-LR were from Sigma-Aldrich Co. (St. Louis, MO, USA). Mouse monoclonal antibody 33B10 specific for the PS1/CTF was prepared as described previously (20). Rabbit polyclonal antibodies against PAG/Cbp (H-100), Csk (C-20), ephrinB (C-18), β-tubulin (H-235), and Src (SRC-2) and mouse monoclonal antibodies against GAPDH (0411), myc (9E10), and phosphotyrosine (pY99) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibody against N-cadherin (C32) was from BD Biosciences (Franklin Lakes, NJ, USA). Polyclonal antibodies against phospho-Src tyr 418 (pY418) and phospho-Src tyr529 (pY529) were from Biosource International (Invitrogen, Carlsbad, CA, USA). Anti-Src monoclonal antibody (clone GD11) was from Upstate Biotechnology (Millipore, Billerica, MA, USA). Protein A and protein G were from Thermo Scientific (Fair Lawn, NJ, USA).
Recombinant plasmids and constructs
WT-PAG/Cbp or Y317F PAG/Cbp mutant was a gift from Dr. Zhengjun Chen (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Shanghai, China). The cytoplasmic domain of ephrinB2 fused to 3 c-myc tags (ephrinB2/CTF2-myc3; GenScript USA Inc., Piscataway, NJ, USA) was inserted into pcDNA3.1 and pMX vectors. The full-length ephrinB2 cDNA was a generous gift from Dr. George D. Yancopoulos (Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) and was also inserted into pcDNA3.1 and pMX vectors. NICD-myc encodes the cytoplasmic domain of Notch1 fused to a myc tag. N-cadherin/CTF2 (N-cad/CTF2) encodes the cytoplasmic domain of N-cadherin (21). pMX-IRES-EGFP and pMX-IRES-DsRed retroviral vectors were a gift from Dr. Toshio Kitamura (Institute of Medical Science, University of Tokyo, Tokyo, Japan; ref. 22).
Cell lines, transfection, and transduction
HEK293 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Mediatech, Inc., Manassas, VA, USA) plus 10% fetal bovine serum (FBS; Life Technologies, Inc.; Invitrogen, Carlsbad, CA, USA), penicillin and streptomycin (Life Technologies) in 5% CO2 at 37°C. Fibroblasts from PS1+/+, PS1−/−, PAG/Cbp+/+, and PAG/Cbp−/− mice were grown in the same medium in the presence of G418 (Life Technologies). For phosphorylation experiments, cells were transferred to medium with 0.5% FBS 16 h prior to stimulation. PAG/Cbp+/+ and PAG/Cbp−/− mouse fibroblasts were a gift from Dr. Chitose Oneyama (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan).
All transfections were performed using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's protocol. WT-PS1, PS1 FAD mutations, WT-PAG/Cbp, Y317F PAG/Cbp, and ephrinB2 were inserted into pMX retroviral vectors, and retroviral particles expressing the above proteins were prepared as described previously (16). Cells were transduced and selected for expression of the respective proteins using FACS analysis (16).
Primary neuronal culture preparation
Cortical neuronal cultures were prepared from embryonic brains of Wistar rats [embryonic day (E)17–E18] or from E15.5 PS1+/+ and PS1−/− mouse embryonic brains, as described previously (23). Briefly, neocortices and hippocampi were dissected out, treated with trypsin, and mechanically dissociated. The neurons were suspended in Neurobasal medium (Life Technologies) supplemented with B27 (Invitrogen, Carlsbad, CA, USA) and plated on poly-d-lysine-coated 6-well dishes at 1 × 106 cells/well.
Receptor stimulation
Cells were stimulated with 2 μg/ml preclustered EphB2-Fc or control Fc for the indicated times. The preclustered multimers of EphB2-Fc or Fc were generated by preincubation of EphB2-Fc or Fc with rabbit anti-human Fc IgG for 1h at 4°C at a ratio of 10:1.
Cell lysates, immunoprecipitation (IP), SDS-PAGE, and immunoblotting
Cell lysates for Western blotting (WB) were prepared in SDS lysis buffer (100 mM Tris/HCl, pH 7.5; 20 mM NaCl; 10 mM EGTA; 10 mM EDTA; and 1% w/v SDS) containing complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA), and phosphatase inhibitors 20 mM NaF, 5 mM Na3VO4, 1 mM sodium pyrophosphate, and 100 nM microcystin-LR. For IP, cells were lysed in IP buffer (10 mM Hepes, pH 7.4; 150 mM NaCl; 2 mM CaCl2; 0.02% sodium azide; and 1% v/v Triton X-100 in the presence of protease inhibitors and phosphatase inhibitors) for 2 h at 4°C, extracts were precleared with protein A or protein G for 1 h and immunoprecipitated for 16 h with the corresponding antibodies, as described previously (16).
siRNA delivery
HEK293 cells (75×104 cells/well in 6-well plates precoated with 1% gelatin) were seeded in antibiotic-free medium 24 h prior to transfection. Cells were transfected using DharmaFECT1 transfection reagent (Dharmacon RNA Technologies, Lafayette, CO, USA) with 50 nM On-Target plus (set of 4) PAG/Cbp siRNA (Dharmacon RNA Technologies) for 72 h according to the manufacturer's protocol. For controls, 50 nM of On-Target plus nontargeting siRNA and 50 nM of On-Target plus GAPDG siRNA were used (Dharmacon RNA Technologies). Cells were extracted and analyzed by WB at 72 h after transfection.
RESULTS
EphB2 induces dephosphorylation of PAG/Cbp on tyrosine in a PS1/γ-secretase-dependent manner
Binding of EphB receptor to ephrinB ligands stimulates the phosphorylation and activation of Src in the ligand-expressing cells (7). Src activity is controlled by the adaptor protein PAG/Cbp (12, 13). This finding raises the possibility that the EphB effect on Src activity may be mediated by PAG/Cbp. PAG/Cbp is a protein heavily phosphorylated on tyrosine residues, and its regulatory activity toward Src is defined by its phosphorylation state (12, 13, 24). To test whether EphB2 affects the tyrosine phosphorylation of PAG/Cbp, rat primary neuronal cultures at 14 days in vitro (DIV) were treated with either EphB2-Fc or control Fc for 10 min. Cells were extracted, immunoprecipitated with specific anti-PAG/Cbp antibody, and probed by WB for phosphotyrosine. Stimulation with EphB2-Fc resulted in clear reduction of phosphorylation of PAG/Cbp on tyrosine (Fig. 1A, lane 2). Since PS1/γ-secretase regulates the EphB2-induced proteolytic cleavage of ephrinB2 ligand (16), we wanted to test whether the observed effect of EphB2-Fc depends on γ-secretase activity. For that purpose, we repeated the experiment described above in the presence of γ-secretase inhibitor L685,458. This inibitor inhibited the EphB2-Fc-induced dephosphorylation of PAG/Cbp on tyrosine (Fig. 1A, lane 3), and the same result was obtained with a different γ-secretase inhibitor, compound 19 (data not shown). The same effect of EphB2-Fc on PAG/Cbp phosphorylation was also observed in HEK293 cells overexpressing WT-PAG/Cbp where, similar with the primary neuronal cultures, EphB2-Fc induced dephosphorylation of PAG/Cbp on tyrosine (Fig. 1B, lane 2) but not in the presence of γ-secretase inhibitor L685,458 (Fig. 1B, lane 3). Our observations indicate that PS1/γ-secretase cleavage of ephrinB may mediate the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine.
Figure 1.
EphB2-Fc dephosphorylates PAG/Cbp in a γ-secretase-dependent manner. A) Rat primary neurons at 14 DIV were stimulated with Fc or EphB2-Fc for 10 min in the presence or absence of γ-secretase inhibitor L685,458. Cell extracts were immunoprecipitated with anti-PAG/Cbp antibody and probed by WB for phosphotyrosine (top panel) or PAG/Cbp (bottom panel). B) HEK293 cells stably transduced with ephrinB2 were transfected with PAG/Cbp and stimulated with Fc or EphB2-Fc for 10 min in the presence or absence of γ-secretase inhibitor L685,458 as in A. Cell extracts were immunoprecipitated with anti-PAG/Cbp antibody and probed for phosphotyrosine (top panel) or PAG/Cbp (bottom panel) by WB. EphB2-Fc promotes the tyrosine dephosphorylation of PAG/Cbp in both neurons and HEK293 cells, and this is inhibited by γ-secretase inhibitor.
EphrinB2/CTF2 forms complexes with PAG/Cbp and promotes its dephosphorylation on tyrosine
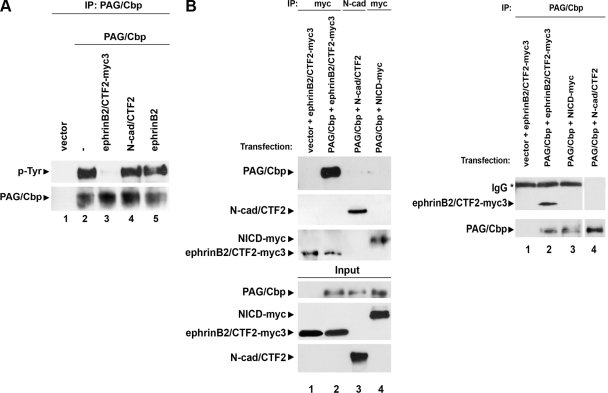
Recent data show that γ-secretase inhibitors block the EphB-induced cleavage of ephrinB and production of ephrinB/CTF2 (16, 17). Here we show that the same γ-secretase inhibitors inhibit the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine (Fig. 1). Our results raise the possibility that the product of the γ-secretase cleavage of ephrinB2 may promote the EphB2-induced tyrosine dephosphorylation of PAG/Cbp. To test this hypothesis, we overexpressed this peptide together with PAG/Cbp in HEK293 cells and detected the tyrosine phosphorylation of PAG/Cbp in cell extracts using IP and WB (Fig. 2A). Peptide ephrinB2/CTF2 decreased tyrosine phosphorylation of PAG/Cbp, while the products of the cleavage of other substrates of γ-secretase, such as N-cadherin (N-cad/CTF2; ref. 21; Fig. 2A, lanes 3, 4) or Notch-1 (NICD; not shown) had no effect. Overexpression of full-length ephrinB2 induced a small decrease in tyrosine phosphorylation of PAG/Cbp, probably due to the low levels of uninduced cleavage of ephrinB2 (Fig. 2A, lane 5). The same results were obtained in HEK293 cells with endogenous PAG/Cbp (data not shown).
Figure 2.
EphrinB2/CTF2 forms complexes with PAG/Cbp and promotes its dephosphorylation on tyrosine. A) HEK293 cells were transiently cotransfected with PAG/Cbp and vector, ephrinB2/CTF2-myc3, N-cadherin/CTF2 (N-cad/CTF2), or full-length ephrinB2. Cell extracts were immunoprecipitated with anti-PAG/Cbp antibody and probed by WB for phosphotyrosine (top panel) or PAG/Cbp (bottom panel). EphrinB2/CTF2-myc3 specifically stimulated PAG/Cbp dephosphorylation on tyrosine. B) HEK293 cells were transiently cotransfected with PAG/Cbp and one of the following: ephrinB2/CTF2-myc3, N-cad/CTF2, or NICD-myc. Cell extracts were immunoprecipitated with anti-myc or anti-N-cadherin antibodies (left) or anti-PAG/Cbp antibody (right), and IPs were probed by WB for PAG/Cbp, N-cadherin, NICD-myc, and ephrinB2/CTF2-myc3, as indicated in the figure. In the reverse experiment, IPs were probed for ephrinB2/CTF2-myc3, N-cadherin, or PAG/Cbp. Input panels (left) show levels of transfected proteins. Asterisk indicates IgG. EphrinB2/CTF2-myc3 specifically forms complexes with PAG/Cbp.
EphrinB2/CTF2 has been found in complexes with Src (16), and Src binds to PAG/Cbp (25). To test whether ephrinB2/CTF2 also forms complexes with PAG/Cbp, we performed co-IP experiments in the above cells. For detection purposes, we used a peptide fused to triple myc tag (ephrinB2/CTF2-myc3; ref. 16). We found that ephrinB2/CTF2-myc3 forms specific complexes with PAG/Cbp since both IPs of cell extracts with anti-myc antibodies precipitated PAG/Cbp (Fig. 2B, left panel, lane 2) and that IPs with anti-PAG/Cbp antibodies precipitated ephrinB2/CTF2 peptide (Fig. 2B, right panel, lane 2). The binding is specific since neither N-cad/CTF2 nor NICD coprecipitated with PAG/Cbp (Fig. 2B, lanes 3, 4). The above data suggest that peptide ephrinB2/CTF2 may affect tyrosine phosphorylation of PAG/Cbp by forming complexes with it.
EphrinB2/CTF2 inhibits binding of Csk to PAG/Cbp
EphB2-induces cleavage of ephrinB2 by PS1/γ-secretase, producing cytoplasmic peptide ephrinB2/CTF2, which binds to PAG/Cbp and promotes its dephosphorylation (ref. 16 and Fig. 2). EphrinB2/CTF2 peptide also promotes Src phosphorylation and activation (16). It is known that Src activation is controlled by a regulatory complex that contains the inhibitory kinase Csk and PAG/Cbp. When the latter is phosphorylated on tyrosine, it recruits Csk to the complex, which in turn binds to and inactivates Src (12, 13). Various extracellular stimuli promote the dissociation of Csk from the complex with PAG/Cbp (14, 26), resulting in Src activation. Since ephrinB2/CTF2 also forms complexes with PAG/Cbp (Fig. 2) and promotes Src phosphorylation and activation (16), we tested whether it affects the binding of Csk to PAG/Cbp. To this end, we coexpressed PAG/Cbp in HEK293 cells together with ephrinB2/CTF2 and analyzed the PAG/Cbp-Csk complexes in cell extracts. Overexpression of ephrinB2/CTF2 specifically inhibited the complex between PAG/Cbp and Csk (Fig. 3A, top panel, lane 3), while N-cad/CTF2 had no effect (Fig. 3A, top panel, lane 4). Interestingly, full-length ephrinB2 had a small effect on the complex (Fig. 3A, top panel, lane 5), consistent with its weak effect on PAG/Cbp dephosphorylation compared to ephrinB2/CTF2 (Fig. 2A). EphrinB2/CTF2 also specifically inhibited the complexes between PAG/Cbp and Src (Fig. 3A, third panel, lane 3), showing that this peptide promotes the dissociation of Src from the complex with PAG/Cbp and Csk. In the reverse experiment, Csk was immunoprecipitated from the above cell extracts, and the precipitates were probed for PAG/Cbp and Src by WB. Overexpression of ephrinB2/CTF2 specifically decreased the amount of PAG/Cbp and Src that was immunoprecipitated with Csk (Fig. 3B, first and third panels, respectively, lane 3). Together the above data indicate that ephrinB2/CTF2 promotes Src activation by inhibiting binding of Csk to PAG/Cbp and by releasing Src form the inhibitory complex with Csk and PAG/Cbp. Consistent with these and our previous observations (16), peptide ephrinB2/CTF2-myc3 promoted Src phosphorylation on tyrosine 418 when overexpressed in HEK293 cells (Fig. 3C, second panel, lanes 1–3). This is an autophosphorylation event known to activate Src (8). In addition, we found that it decreased the inhibitory phosphorylation of Src tyrosine 529 (Fig. 3C, third panel, lanes 1–3), supporting its role as an activator of Src.
Figure 3.

EphrinB2/CTF2 inhibits binding of Csk to PAG/Cbp promoting the dissociation of the PAG/Cbp-Csk-Src complex. A) HEK293 cells stably transduced with PAG/Cbp were transiently transfected with vector, ephrinB2/CTF2-myc3, N-cad/CTF2, or full-length ephrinB2. Cell extracts were immunoprecipitated with anti-PAG/Cbp antibodies and probed by WB for Csk or PAG/Cbp as indicated in the figure. Blots were stripped and reprobed for PAG/Cbp or Src. Input shows the levels of endogenous Csk and Sr. EphrinB2/CTF2-myc3 inhibits Csk and Src binding to PAG/Cbp. B) The above HEK293 cell extracts were immunoprecipitated with anti-Csk antibodies and probed by WB for PAG/Cbp. Blots were stripped and reprobed for Csk or Src as indicated in the figure. Input shows the levels of endogenous Src and overexpressed PAG/Cbp. EphrinB2/CTF2 decreases the PAG/Cbp-Csk complex. C) HEK293 cells stably transduced with pMX or ephrinB2/CTF2-myc3 were incubated in the presence or absence of 10 μM lactacystin as indicated in the figure. Cell extracts were analyzed by WB with antibodies against phospho-Src tyr418 (second panel) or phospho-Src tyr529 (third panel). Top panel shows levels of overexpressed ephrinB2/CTF2-myc3. Fourth and fifth panels show levels of endogenous Src and β-tubulin, respectively. Asterisk indicates nonspecific band. EphrinB2/CTF2-myc3 promotes the phosphorylation of Src on tyrosine 418 and the dephosphorylation of Src on tyrosine 529.
EphB2 promotes the dissociation of Csk from PAG/Cbp in a γ-secretase-dependent manner
EphB2-Fc induces the γ-secretase cleavage of ephrinB2 (16), producing ephrinB2/CTF2 peptide. Here we show that this peptide inhibits binding of Csk to PAG/Cbp (Fig. 3). It is, therefore, expected that stimulation of cells with EphB2-Fc may induce the release of Csk from PAG/Cbp due to production of ephrinB2/CTF2. Indeed, in HEK293 cells overexpressing PAG/Cbp, EphB2-Fc decreased the amount of endogenous Csk coprecipitating with PAG/Cbp (Fig. 4A, lane 2) in a γ-secretase-dependent manner, since the specific γ-secretase inhibitor L685,458 abolished this effect of EphB2-Fc (Fig. 4A, lane 4). This finding implies that the EphB2-induced cleavage of ephrinB2 by γ-secretase may inhibit the complex between Csk and PAG/Cbp via the production of ephrinB2/CTF2 peptide.
Figure 4.
EphB2-Fc induces the dissociation of Csk from PAG/Cbp in a γ-secretase-dependent manner. Left panel: HEK293 cells stably transduced with PAG/Cbp were treated with Fc or EphB2-Fc for 10 min in the presence or absence of γ-secretase inhibitor L685,458. Cell extracts were immunoprecipitated with anti-Csk antibody and probed by WB for PAG/Cbp (top panel) or Csk (middle panel). Bottom panel (input) shows levels of PAG/Cbp. EphB2-Fc promotes the dissociation of Csk from PAG/Cbp, and this is inhibited by the γ-secretase inhibitor. Right panel: densitometric quantification of EphB2-induced decrease in PAG/Cbp-Csk complex. Csk levels in EphB2 treated-cells were normalized to Csk levels in Fc-treated cells. Bars represent means ± se of 3 independent experiments.
EphB2-induced phosphorylation of ephrinB2 is mediated by PAG/Cbp
EphB2 induces tyrosine phosphorylation of ephrinB in a Src-dependent manner (7). Src activity is regulated by PAG/Cbp (12, 13), and EphB2 promotes the tyrosine dephosphorylation of PAG/Cbp (Fig. 1). The above data raise the possibility that PAG/Cbp may regulate the EphB2-induced phosphorylation of ephrinB2. To test this hypothesis, we down-regulated the expression of PAG/Cbp in HEK293 cells using siRNA. Down-regulation of PAG/Cbp specifically inhibited the EphB2-induced phosphorylation of ephrinB2 on tyrosine (Fig. 5A, left panel, lanes 5, 6), although it increased the uninduced (constitutive) levels of ephrinB2 tyrosine phosphorylation (Fig. 5A, left panel, lane 5). This finding is consistent with the constitutive negative regulation of Src by PAG/Cbp (12) and reveals that PAG has opposite effects on the induced and constitutive tyrosine phosphorylation of ephrinB2.
Figure 5.
PAG/Cbp mediates the EphB2-induced phosphorylation of ephrinB2 on tyrosine. A) Left panel: HEK293 cells stably transduced with ephrinB2 and transiently transfected with PAG/Cbp were transfected with PAG/Cbp siRNA, GAPDH siRNA, or a nontargeting sequence as indicated in the figure. Cells were stimulated with Fc or EphB2-Fc for 10 min, and cell extracts were immunoprecipitated with anti-ephrinB antibody. IPs were probed by WB for phosphotyrosine (top panel) or ephrinB2 (second panel). Input shows expression levels of overexpressed PAG/Cbp and ephrinB2 and of endogenous GAPDH. Down-regulation of PAG/Cbp inhibits the EphB2-induced phosphorylation of ephrinB2. Right panel: densitometric quantification of EphB2-induced ephrinB2 tyrosine phosphorylation. Phospho-ephrinB2 levels in EphB2-stimulated cells were normalized to phospho-ephrinB2 levels in Fc-treated cells. Bars represent means ± se of 3 independent experiments. B) Fibroblasts isolated from PAG/Cbp-expressing mice (PAG/Cbp+/+) or PAG/Cbp-knockout (PAG/Cbp KO) mice were cotransduced with ephrinB2 and either vector (pMX), WT-PAG/Cbp, or Y317F PAG/Cbp mutant (Y317F). Cells were stimulated with Fc or EphB2-Fc for 10 min as indicated in the figure, and cell extracts were immunoprecipitated with anti-ephrinB antibody. IPs were probed by WB for phosphotyrosine (top panel) or ephrinB2 (middle panel). Input shows expression levels of WT or mutant PAG/Cbp (bottom panel). Absence of PAG/Cbp or the presence of mutant Y317F PAG/Cbp inhibits the EphB2-induced phosphorylation of ephrinB2.
In a different approach, we transduced fibroblasts obtained from either WT-PAG/Cbp mice or from PAG/Cbp-knockout (KO) mice (25) with either vector pMX or with WT-PAG/Cbp. Figure 5B (lanes 1–4) shows that in the absence of PAG/Cbp, EphB2 cannot increase the tyrosine phosphorylation of ephrinB2 (no induction). Reintroduction of PAG/Cbp into these cells restored their response to EphB2-Fc (Fig. 5B, lanes 5, 6). Consistent with our experiment using PAG/Cbp siRNA (Fig. 5A), absence of PAG/Cbp increased the constitutive (uninduced) levels of ephrinB2 phosphorylation on tyrosine in KO fibroblasts (Fig. 5B, lane 3). The above data show that PAG/Cbp mediates the EphB2-induced phosphorylation of ephrinB2 on tyrosine. The effects of PAG/Cbp on Src activity are mediated by residue Y317, which binds Src inhibitor Csk kinase, thus recruiting it to the PAG/Cbp-Src complex (12). To see whether this tyrosine residue also mediates the EphB-induced phosphorylation of ephrinB2, we transduced PAG/Cbp-KO fibroblasts with mutant PAG/Cbp Y317F and then treated them with Fc or EphB2-Fc. EphB2-Fc failed to promote ephrinB2 tyrosine phosphorylation (Fig. 5B, lanes 7, 8). Furthermore, in contrast to WT PAG/Cbp, mutant PAG/Cbp Y317F did not suppress the constitutive phosphorylation of ephrinB2 in PAG/Cbp-KO cells (Fig. 5B, lanes 5, 7). Together, the above data suggest that Csk binding to PAG/Cbp is necessary for the effect of PAG/Cbp on the EphB2-induced phosphorylation of ephrinB2.
PS1 promotes the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine and PS1 FAD mutations inhibit it
Since PS1/γ-secretase cleaves ephrinB2, producing peptide ephrinB2/CTF2, which promotes Src phosphorylation, and several FAD mutations of PS1 inhibit both the ephrinB2 cleavage and the EphB2-induced Src phosphorylation (16), we aimed to test whether PS1 also affects the EphB2-induced PAG/Cbp dephosphorylation. For that purpose, we treated mouse primary neurons derived from PS1-WT or PS1-KO mouse brains with EphB2-Fc or Fc at 11 DIV and analyzed the tyrosine phosphorylation of PAG/Cbp in neuronal extracts. The neurons from WT PS1 mice responded as expected to EphB2-Fc by dephosphorylating PAG/Cbp on tyrosine (Fig. 6A, lane 2), but the PS1-KO neurons did not respond (Fig. 6A, lane 4). To see whether PS1 FAD mutations inhibit the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine, we transduced fibroblasts from PS1-KO mice with either pMX vector alone, WT-PS1, or one of the following FAD mutations: ΔE9, V82L, A260V, E280A, and G384A in pMX. The cells were transfected transiently with WT-PAG/Cbp and stimulated with either Fc or EphB2-Fc. Absence of PS1 (Fig. 6B, lanes 1, 2) or the presence of PS1 FAD mutations (lanes 7–16) inhibited the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine while reintroduction of WT-PS1 (Fig. 6B, lanes 3, 4) restored the effect. The catalytically inactive PS1 mutant D257A also abolished the response of the cells to EphB2-Fc (Fig. 6B, lanes 5, 6), suggesting that active PS1 is required for the effect. The above data show that PS1 is necessary for the EphB2-induced PAG/Cbp dephosphorylation on tyrosine and that several PS1 FAD mutations that inhibit the γ-secretase cleavage of ephrinB2 also inhibit the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine. In contrast, FAD mutation I213T, which does not inhibit the γ-secretase cleavage of ephrinB2 (16), did not affect the EphB2-induced dephosphorylation of PAG/Cbp in either neurons or fibroblasts (data not shown).
Figure 6.
PS1 promotes the EphB2-induced dephosphorylation of PAG/Cbp, and PS1 FAD mutations inhibit it. A) Top panel: mouse primary neurons (11DIV) from WT PS1-expressing mice (PS1+/+) or from PS1-KO mice (PS1−/−) were stimulated for 10 min with Fc or EphB2-Fc as indicated in the figure. Cell extracts were immunoprecipitated with anti-PAG/Cbp antibody, and IPs were probed by WB for phosphotyrosine (top panel) or PAG/Cbp (bottom panel). EphB2-induced dephosphorylation of PAG/Cbp is inhibited in the absence of PS1. Bottom panel: densitometric quantification of EphB2-induced PAG/Cbp tyrosine dephosphorylation in neurons. Levels of phospho-PAG/Cbp in EphB2-stimulated neurons were normalized to phospho-PAG/Cbp in Fc-treated neurons. B) Left panel: mouse fibroblasts derived from PS1-KO mice were transduced with vector (pMX), WT-PS1, or one of the PS1 mutants, D257A, ΔE9, V82L, A260V, E280A, or G384A. The cells were transiently transfected with PAG/Cbp, stimulated with Fc or EphB2-Fc for 10 min, extracted, and immunoprecipitated with anti-PAG/Cbp antibody. IPs were analyzed with anti-phosphotyrosine antibody (top panel) or anti-PAG/Cbp antibody (second panel) by WB. Input shows the levels of full-length PS1 (third panel) and PS1 CTF (bottom panel). PS1 promotes the EphB2-induced dephosphorylation of PAG/Cbp. PS1 FAD mutations and catalytically inactive PS1 inhibit it. Right panel: densitometric quantification of EphB2-induced PAG/Cbp dephosphorylation on tyrosine in fibroblasts. Levels of phospho-PAG/Cbp in EphB2-stimulated cells were normalized to phospho-PAG/Cbp in Fc-treated cells. Bars represent means ± se of 3 independent experiments.
PS1 FAD mutations inhibit the EphB2-induced phosphorylation of ephrinB2
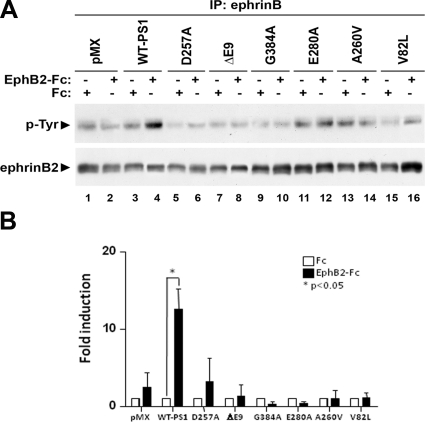
PS1 promotes the EphB2-induced tyrosine phosphorylation of ephrinB2 (16); it regulates the EphB2-induced tyrosine dephosphorylation of PAG/Cbp (Fig. 6), and PAG/Cbp regulates the EphB2-induced tyrosine phosphorylation of ephrinB2 (Fig. 5). Hence, we wanted to test whether PS1 FAD mutations affect the EphB2-induced phosphorylation of ephrinB2 on tyrosine. We treated fibroblasts from PS1 KO mice cotransduced with ephrinB2 and either pMX vector alone, WT-PS1, or one of the PS1 FAD mutations shown in Fig. 7 with Fc or EphB2-Fc, and phosphorylation of ephrinB2 was analyzed in cell extracts using specific antibodies. As shown in Fig. 7, EphB2-Fc stimulated the phosphorylation of ephrinB2 on tyrosine only in the presence of WT-PS1 (lanes 3, 4). In the absence of PS1 (lanes 1, 2) or in the presence of the FAD mutations ΔE9, V82L, A260V, E280A, or G384A (lanes 7–16), EphB2-Fc had no effect. In the presence of the catalytically inactive PS1 D257A, EphB2-Fc also failed to induce phosphorylation of ephrinB2, showing that active PS1 is necessary for this effect (lanes 5, 6).The above results suggest that cleavage of ephrinB2 by PS1/γ-secretase is necessary for the stimulation of ephrinB2 tyrosine phosphorylation by EphB2-Fc.
Figure 7.
PS1 FAD mutations inhibit the EphB2-induced phosphorylation of ephrinB2. A) Mouse fibroblasts derived from PS1-KO mice were cotransduced with ephrinB2 and vector (pMX), WT-PS1, or one of the PS1 mutants, D257A, ΔE9, V82L, A260V, E280A, or G384A. Cells were stimulated with Fc or EphB2-Fc for 10 min, extracted, and immunoprecipitated with anti-ephrinB antibody. IPs were analyzed with anti-phosphotyrosine antibody (top panel) or anti-ephrinB antibody (second panel) by WB. PS1 promotes the EphB2-induced phosphorylation of ephrinB2 on tyrosine. PS1 FAD mutations and catalytically inactive PS1 inhibit it. B) Densitometric quantification of EphB2-induced ephrinB2 phosphorylation on tyrosine. Levels of phospho-ephrinB2 in EphB2-stimulated cells were normalized to phospho-ephrinB2 in Fc-treated cells. Bars represent means ± se of 3 independent experiments.
DISCUSSION
Binding of EphB2 receptor to its cognate ligand ephrinB2 stimulates the phosphorylation and activation of Src and the Src-mediated phosphorylation of ephrinB2. The mechanism of Src activation by EphB receptors has been elusive. In the present study, we show that the regulation of Src activation and ephrinB2 phosphorylation after stimulation of ephrinB2 by EphB2 receptor is mediated by the product of PS1/γ-secretase cleavage of ephrinB2, the ephrinB2/CTF2 peptide. This peptide interacts with the Src regulatory complex, which is a multicomponent determinant of Src activation and function, consisting of the adaptor protein PAG/Cbp and the inhibitory kinase Csk. PAG/Cbp recruits Csk to the plasma membrane, where it phosphorylates and inactivates Src, turning off Src-mediated signaling events. Here we present evidence that ephrinB2/CTF2 peptide promotes Src and ephrinB2 phosphorylation by affecting the stability of the Src regulatory complex. A schematic representation of the hypothesized interactions between ephrinB2/CTF2 and this complex is shown in Fig. 8.
Figure 8.
PS1/γ-secretase promotes the EphB2-induced ephrinB2 phosphorylation by regulating PAG/Cbp. Left: tyrosine phosphorylated PAG/Cbp keeps Src inactive by recruiting the inhibitory Csk to the complex. Right: binding of EphB2 receptor to ephrinB2 ligand induces the γ-secretase cleavage of ephrinB2, producing ephrinB2/CTF2 peptide. EphrinB2/CTF2 forms complexes with PAG/Cbp and Src and promotes dephosphorylation of PAG/Cbp on tyrosine, dissociation of Csk and Src from PAG/Cbp, activation of Src, and phosphorylation of ephrinB2 ligand on tyrosine.
We describe a mechanism through which EphB2 promotes the Src-mediated ephrinB2 phosphorylation on tyrosine. The mechanism involves PAG/Cbp, a protein involved in various interactions in the cell initiating different signaling pathways. This protein regulates early signaling, eventually affecting vital cellular processes, such as cell proliferation, survival, and migration. Accumulating data suggest that PAG/Cbp affects signaling via interaction with the actin cytoskeleton (for review, see ref. 27). This finding raises the possibility that it may participate in the structural integrity of the cytoskeleton at the synapse, which interestingly is the main point of loss in AD. The best-studied interaction of PAG/Cbp is the one with the Src kinases whose function it regulates. It was reported recently that PAG/Cbp regulates the oncogenic potential of Src, acting as a potential tumor suppressor (25).
Here we provide evidence for a new function of PAG/Cbp, which is to promote the tyrosine phosphorylation of ephrinB2 ligand after its binding to EphB2 receptor. Down-regulation or absence of PAG/Cbp in ephrinB2-expressing cells results in loss of the ability of these cells to increase tyrosine phosphorylation of ephrinB2 as a response to EphB2 stimulation (Fig. 5), which shows that PAG/Cbp is necessary for this effect. We show that this function of PAG/Cbp is regulated by PS1/γ-secretase. This condition is achieved by cleavage of ephrinB2 and production of ephrinB2/CTF2. This peptide promotes the dephosphorylation of PAG/Cbp on tyrosine and the dissociation of Csk and Src from the complex with PAG/Cbp (Fig. 3) and is expected to promote Src activity, as Src is not inhibited anymore by the PAG/Cbp-Csk complex. Consistent with these data, ephrinB2/CTF2 decreases the inhibitory phosphorylation of Src on tyrosine 529 and increases the phosphorylation on tyrosine 418 (Fig. 3D), an autophosphorylation event known to activate Src (8). That event promotes Src activity and eventually the Src-mediated ephrinB2 phosphorylation. This fine-tuning of Src activation is specific to ephrinB, since the γ-secretase products of other substrates tested, such as N-cad/CTF2 and NICD, did not affect PAG/Cbp phosphorylation (ref. 16 and Fig. 2). However, since γ-secretase has a multitude of substrates, we cannot exclude the possibility that the cleavage products of other γ-secretase substrates may also regulate the phosphorylation of PAG/Cbp and affect Src activity. It will be interesting to explore this possibility in the future. EprhinB2/CTF2 forms complexes with Src as well (ref. 16; for schematic representation, see Fig. 8), raising the possibility that the regulation of Src phosphorylation and activation by ephrinB2/CTF2 may involve both decrease of the PAG/Cbp-Csk-Src complex and increased complex formation between ephrinB2/CTF2 and Src.
It is not clear how ephrinB2/CTF2 promotes tyrosine dephosphorylation of PAG/Cbp. A number of phosphatases have been proposed to target PAG/Cbp phosphorylated tyrosines, such as Shp2 and CD45 (14, 26). We have obtained preliminary evidence that Shp2 phosphatase may be involved in the ephrinB2/CTF2-stimulated tyrosine dephosphorylation of PAG/Cbp since ephrinB2/CTF2 stimulates the complex between PAG/Cbp and Shp2 (unpublished results). Further analysis could establish whether Shp2 and/or other phosphatases may dephosphorylate PAG/Cbp in response to EphB2 and ephrinB2/CTF2 stimulation.
Active PS1 is necessary for the effect of EphB2 on PAG/Cbp and ephrinB2 phosphorylation, since mutant PS1 D257A, which is proteolyticaly inactive, fails to promote the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine (Fig. 6B) and the EphB2-induced tyrosine phosphorylation of ephrinB2 (Fig. 7). FAD mutations of PS1 that inhibit the cleavage of ephrinB2 (16) also inhibit the EphB2-induced tyrosine dephosphorylation of PAG/Cbp and subsequently the EphB2-induced tyrosine phosphorylation of ephrinB2, which suggests that the functional integrity of the PS1/γ-secretase system is necessary for the regulation of ephrinB2 phosphorylation via Src. Interestingly, the PS1 FAD mutant I213T, which does not affect the γ-secretase cleavage of ephrinB2 (16), also does not affect the EphB2-induced dephosphorylation of PAG/Cbp on tyrosine (unpublished results). This finding supports the hypothesis that the EphB2-induced cleavage of ephrinB2 by γ-secretase is necessary for the effect.
Src kinases have been implicated in neuronal function and degeneration since loss of their function causes neuronal defects, abnormalities in the hippocampus, and LTP impairment (28, 29). They also mediate glutamate-induced neurodegeneration (30). Src kinases exert their neuronal function by interacting with NMDAR. They form complexes and phosphorylate the NMDA receptor, affecting its function (31, 32). This finding is intriguing because NMDAR signaling pathways have been implicated in AD, and targeting them with pharmacological agents has been a main focus in AD treatment (33). It is, therefore, possible that the failure of PS1 FAD mutations to promote the EphB2-induced Src phosphorylation by affecting PAG/Cbp may compromise the functional integrity of Src, promoting some of the neuronal abnormalities observed in AD.
EphrinB ligands are expressed in both pre- and postsynaptic neurons (3). EphB2-induced tyrosine phosphorylation of ephrinB cytoplasmic domain promotes spine maturation via ephrinB reverse signaling (2), and it is also necessary for normal LTP and NMDAR phosphorylation in hippocampus (34). PS1 may therefore affect these functions in the brain by regulating the cleavage and phosphorylation of ephrinB ligands. Recently, it was shown that PS1/γ-secretase promotes NMDAR tyrosine phosphorylation via the cleavage of EphB2 receptor and production of EphB2 intracellular domain (EphB2/CTF2), which directly phosphorylates NMDAR subunits (35). It is, therefore, intriguing that PS1 may regulate NMDAR phosphorylation and activation by affecting both forward and reverse ephrinB/EphB signaling.
Our work provides evidence that the mechanism of induction of ephrinB2 phosphorylation after interacting with its cognate receptor EphB2 involves PS1/γ-secretase and PAG/Cbp. PS1/γ-secretase promotes the EphB2-induced activation of Src kinase and ephrinB2 phosphorylation by directly affecting the physiology of PAG/Cbp through the cleavage of ephrinB2 and the production of ephrinB2/CTF2 peptide. The existence of multiple Src substrates in the cell raises the possibility that the mechanism described here may apply to other pathways as well and that PS1 may regulate the response of various Src substrates to extracellular stimuli. It will be interesting to explore this possibility in the future. Several mutations of PS1, which are found in FAD, inhibit the response of PAG/Cbp and ephrinB2 to EphB2 stimulation and raise the possibility that they may also impair the response of Src regulatory machinery to other extracellular stimuli. Since PAG/Cbp regulates various cellular functions such as proliferation, survival, and migration, it will be worth exploring the role of PS1 in these functions in the future.
Acknowledgments
This work has been supported by U.S. National Institutes of Health grants P50 AG005138 to A.G. and AG008200 and AG017926 to N.K.R. and by Alzheimer's Association grant IIRG-07-59620 to A.G.
REFERENCES
- 1. Cowan C. A., Henkemeyer M. (2001) The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 413, 174–179 [DOI] [PubMed] [Google Scholar]
- 2. Segura I., Essmann C. L., Weinges S., Acker-Palmer A. (2007) Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat. Neurosci. 10, 301–310 [DOI] [PubMed] [Google Scholar]
- 3. Grunwald I. C., Korte M., Wolfer D., Wilkinson G. A., Unsicker K., Lipp H. P., Bonhoeffer T., Klein R. (2001) Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 32, 1027–1040 [DOI] [PubMed] [Google Scholar]
- 4. Grunwald I. C., Korte M., Adelmann G., Plueck A., Kullander K., Adams R. H., Frotscher M., Bonhoeffer T., Klein R. (2004) Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 7, 33–40 [DOI] [PubMed] [Google Scholar]
- 5. Rodenas-Ruano A., Perez-Pinzon M. A., Green E. J., Henkemeyer M., Liebl D. J. (2006) Distinct roles for ephrinB3 in the formation and function of hippocampal synapses. Dev. Biol. 292, 34–45 [DOI] [PubMed] [Google Scholar]
- 6. Aoto J., Ting P., Maghsoodi B., Xu N., Henkemeyer M., Chen L. (2007) Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation. J. Neurosci. 27, 7508–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer A., Zimmer M., Erdmann K. S., Eulenburg V., Porthin A., Heumann R., Deutsch U., Klein R. (2002) EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol. Cell 9, 725–737 [DOI] [PubMed] [Google Scholar]
- 8. Thomas S. M., Brugge J. S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- 9. Ali D. W., Salter M. W. (2001) NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr. Opin. Neurobiol. 11, 336–342 [DOI] [PubMed] [Google Scholar]
- 10. Okada M., Nakagawa H. (1989) A protein tyrosine kinase involved in regulation of pp60c-src function. J. Biol. Chem. 264, 20886–20893 [PubMed] [Google Scholar]
- 11. Cary L. A., Cooper J. A. (2000) Molecular switches in lipid rafts. Nature 404, 945 [DOI] [PubMed] [Google Scholar]
- 12. Kawabuchi M., Satomi Y., Takao T., Shimonishi Y., Nada S., Nagai K., Tarakhovsky A., Okada M. (2000) Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404, 999–1003 [DOI] [PubMed] [Google Scholar]
- 13. Brdicka T., Pavlistova D., Leo A., Bruyns E., Korinek V., Angelisova P., Scherer J., Shevchenko A., Hilgert I., Cerny J., Drbal K., Kuramitsu Y., Kornacker B., Horejsi V., Schraven B. (2000) Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191, 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang S. Q., Yang W., Kontaridis M. I., Bivona T. G., Wen G., Araki T., Luo J., Thompson J. A., Schraven B. L., Philips M. R., Neel B. G. (2004) Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341–355 [DOI] [PubMed] [Google Scholar]
- 15. Horejsi V. (2004) Transmembrane adaptor proteins in membrane microdomains: important regulators of immunoreceptor signaling. Immunol. Lett. 92, 43–49 [DOI] [PubMed] [Google Scholar]
- 16. Georgakopoulos A., Litterst C., Ghersi E., Baki L., Xu C., Serban G., Robakis N. K. (2006) Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 25, 1242–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomita T., Tanaka S., Morohashi Y., Iwatsubo T. (2006) Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol. Neurodegener. 1, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarthy J. V., Twomey C., Wujek P. (2009) Presenilin-dependent regulated intramembrane proteolysis and gamma-secretase activity. Cell Mol. Life Sci. 66, 1534–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marambaud P., Robakis N. K. (2005) Genetic and molecular aspects of Alzheimer's disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav. 4, 134–146 [DOI] [PubMed] [Google Scholar]
- 20. Georgakopoulos A., Marambaud P., Efthimiopoulos S., Shioi J., Cui W., Li H. C., Schutte M., Gordon R., Holstein G. R., Martinelli G., Mehta P., Friedrich V. L., Jr., Robakis N. K. (1999) Presenilin-1 forms complexes with the cadherin/catenin cell-cell adhesion system and is recruited to intercellular and synaptic contacts. Mol. Cell 4, 893–902 [DOI] [PubMed] [Google Scholar]
- 21. Marambaud P., Wen P. H., Dutt A., Shioi J., Takashima A., Siman R., Robakis N. K. (2003) A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114, 635–645 [DOI] [PubMed] [Google Scholar]
- 22. Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. (2003) Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31, 1007–1014 [PubMed] [Google Scholar]
- 23. Baki L., Neve R. L., Shao Z., Shioi J., Georgakopoulos A., Robakis N. K. (2008) Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J. Neurosci. 28, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeuchi S., Takayama Y., Ogawa A., Tamura K., Okada M. (2000) Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 275, 29183–29186 [DOI] [PubMed] [Google Scholar]
- 25. Oneyama C., Hikita T., Enya K., Dobenecker M. W., Saito K., Nada S., Tarakhovsky A., Okada M. (2008) The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol. Cell 30, 426–436 [DOI] [PubMed] [Google Scholar]
- 26. Davidson D., Bakinowski M., Thomas M. L., Horejsi V., Veillette A. (2003) Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell Biol. 23, 2017–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svec A. (2008) Phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk-binding protein: a protein that matters. Pathol. Res. Pract. 204, 785–792 [DOI] [PubMed] [Google Scholar]
- 28. Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. (1992) Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258, 1903–1910 [DOI] [PubMed] [Google Scholar]
- 29. Lu Y. M., Roder J. C., Davidow J., Salter M. W. (1998) Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279, 1363–1367 [DOI] [PubMed] [Google Scholar]
- 30. Khanna S., Roy S., Park H. A., Sen C. K. (2007) Regulation of c-Src activity in glutamate-induced neurodegeneration. J. Biol. Chem. 282, 23482–23490 [DOI] [PubMed] [Google Scholar]
- 31. Suzuki T., Okumura-Noji K. (1995) NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem. Biophys. Res. Commun. 216, 582–588 [DOI] [PubMed] [Google Scholar]
- 32. Yu X. M., Askalan R., Keil G. J., 2nd, Salter M. W. (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275, 674–678 [DOI] [PubMed] [Google Scholar]
- 33. Kemp J. A., McKernan R. M. (2002) NMDA receptor pathways as drug targets. Nat. Neurosc. 5(Suppl.), 1039–1042 [DOI] [PubMed] [Google Scholar]
- 34. Bouzioukh F., Wilkinson G. A., Adelmann G., Frotscher M., Stein V., Klein R. (2007) Tyrosine phosphorylation sites in ephrinB2 are required for hippocampal long-term potentiation but not long-term depression. J. Neurosci. 27, 11279–11288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu J., Litterst C., Georgakopoulos A., Zaganas I., Robakis N. K. (2009) Peptide EphB2/CTF2 generated by the gamma-secretase processing of EphB2 receptor promotes tyrosine phosphorylation and cell surface localization of N-methyl-D-aspartate receptors. J. Biol. Chem. 284, 27220–27228 [DOI] [PMC free article] [PubMed] [Google Scholar]