Abstract
Recent declines in biodiversity have increased interest in the link between biodiversity and the provision and sustainability of ecosystem services across space and time. We mapped the complex network of interactions between herbivores and parasitoids to examine the relationship between parasitoid species richness, functional group diversity and the provision of natural pest control services. Quantitative food webs were constructed for 10 organic and 10 conventional farms. Parasitoid species richness varied from 26 to 58 species and we found a significant positive relationship between parasitoid species richness and temporal stability in parasitism rates. Higher species richness was associated with lower variation in parasitism rate. A functional group analysis showed significantly greater parasitoid species complementarity on organic farms, with on average more species in each functional group. We simulated parasitoid removal to predict whether organic farms experienced greater robustness of parasitism in the face of local extinctions. This analysis showed no consistent differences between the organic and conventional farm pairs in terms of loss of pest control service. Finally, it was found that the different habitats that make up each farm do not contribute equally to parasitoid species diversity, and that hedgerows produced more parasitoid species, significantly more so on organic farms.
Keywords: functional redundancy, biological insurance, parasitoid, pest control, ecosystem services, complementarity
1. Introduction
A precautionary approach to biodiversity management is often justified on the basis that the maintenance of a diversity of species is useful for both the provision of a particular ecosystem service now, and as a form of biological ‘insurance’ against disturbances in the future [1–3]. The recent concern over global declines in biodiversity has led to renewed interest in the link between biodiversity and the provision and sustainability of ecosystem services across space and time. One mechanism by which greater diversity may lead to increased ecosystem services is through complementarity among particular species [4]. The newly added species use different resources than those that are already present [5]. Parasitoid wasp species use host resources in a complementary fashion, for example, when they attack hosts at different stages or generations. Temporal complementarity could also occur, for example, when the abundance of different generalist parasitoids changes over the field season, leading to a positive relationship between parasitoid diversity and host mortality. Alternatively, parasitoid species may overlap in the host resources they use across space and time, and so lead to interspecific competition. In this situation, the addition of extra species leads to no gain in function despite an increase in diversity [6]. However, it is important to understand that these ‘redundant’ species may become more important as environmental conditions change. This change could be in the form of novel species invading a community or disturbances that cause local extinctions of species. So both biodiversity (species richness per se) and functional diversity may be critical for maintaining important ecosystem services both now and in the future.
Natural pest control is an ecosystem service that is thought to be threatened by the very process that relies on this service: agricultural intensification [7]. The process of agricultural intensification involves the removal or isolation of remnant perennial vegetation patches and hedgerows, larger field sizes and the greater use of agrochemicals to control pests. These practices in combination and across large spatial scales have led to decreases in natural enemy diversity, and may be linked to increased pest outbreaks [7].
We have shown in a previous study that organic farms with greater parasitoid species richness do not show greater provision of pest control services [8]. Here, we ask whether increased parasitoid species richness provides greater temporal stability in parasitism rates and whether organic farms are more resilient to species loss. While some previous studies have examined the impact of species loss on food web structure (e.g. [9]), here we are more interested in how species loss impacts the provision of an ecosystem service: natural pest control. We examine the complex network of interactions between insect herbivores and their parasitoids on 20 farms that have a gradient of parasitoid species richness partly as a result of farming system (organic or conventional) and local variation in species richness. Organic farming is thought to lead to increased species diversity of up to 30 per cent for some taxa [10,11], and to increased natural enemy species evenness [12], probably owing to the greater areas of semi-natural vegetation [13] and reduced agrochemical usage. The first prediction we make from our data is that there will be a strong relationship between parasitoid species richness and variability in parasitism rate across time. We predict that the organic farms, which show greater parasitoid diversity [8], will also show greater temporal stability in parasitism rates. Our second prediction is that organic farms have greater levels of functional redundancy (numbers of parasitoid species within functional groups) and this will lead to more reliable pest control services under environmental change. We test this by examining the parasitoid communities' robustness to simulated species loss as a surrogate for a disturbance event. Finally, we ask whether the different habitats that make up each farm contribute equally to parasitoid species diversity and the provision of pest control services. We predict that habitats with greater plant diversity (non-crop habitats, e.g. hedgerows) contribute proportionally greater numbers of parasitoid species.
2. Material and methods
(a). Field sites and insect sampling
Ten pairs of organic and conventional farms that contained a mixture of both arable crops and livestock were selected in southwest England. Organic farms had been certified for an average of 7.3 years (range 3–12 years) at the start of the study. Organic farms were selected first, then paired with a non-adjoining conventional farm of similar size within 5 km [13]. The farms were mapped and stratified by habitat type and each habitat was randomly sampled using transects (i.e. a stratified random method) [13]. The habitats sampled included all arable crop types, grass leys and other pasture fields, field margins, set-aside fields, game cover patches, orchards, hedgerows, woodlands, and areas not used for production purposes (the latter referred to as rough ground). In 2005, a total transect area of 150 × 1 m was sampled on each farm for plants and herbivores on six occasions (April–September) and all habitats were sampled on each visit. In 2006, a subset of habitats was sampled (arable fields, woodlands and hedgerows) and a total transect area of 100 × 1 m was sampled per farm on five occasions (May–September). The transects were divided according to the area occupied by each landscape element per farm; however, total sampling effort was equal between farm pairs. The position of each transect on the farm was determined by randomly choosing a different starting point each visit, then selecting the closest representative patches of each habitat type (starting with the pasture field), as close as possible to this point [13]. In each transect, we recorded plant diversity and abundance in the field, and collected herbivores for rearing in the laboratory. All vegetation (below approx. 2.5 m) was visually inspected, sweep netted and, if tall enough, beaten over a tray, and the herbivores were collected (leaf-mining Lepidoptera, externally feeding Lepidoptera and leaf-mining Diptera). Herbivores were reared individually under ambient conditions in a laboratory until either an adult herbivore or parasitoid emerged. All parasitoids were identified by specialist taxonomists (see acknowledgements at the end of this paper).
(b). Testing prediction 1: organic farms show greater temporal stability in parasitism rates over time
The coefficient of variation (CV) was used as a measure of the variability in parasitism rates across time [14]. The dataset was separated into nine time periods across the 2 years of the study in order to provide adequate host numbers per time period (R1, April 2005; R2, May 2005; R3, June 2005; R4, July 2005; R5, August 2005; R6, September 2005; R7, May 2006; R8, June and July 2006; R9, August and September 2006). Parasitism rate (as a percentage) was calculated for each time point, and included successful rearing events only (i.e. those herbivores that died prior to an adult host or parasitoid emerging were removed from the analysis). CV per farm was calculated as the standard deviation in parasitism rate divided by the mean parasitism rate across time periods. The total number of parasitoid species per farm, the mean number of parasitoid species per time point and CV for the number of parasitoid species at each time point were calculated. Using general linear models, we tested the relationship between CV parasitism rate (as the response variable) and the explanatory variables: mean number of parasitoid species per time period and farming system (organic or conventional), total number of parasitoid species per farm period and farming system, and CV number of parasitoid species and farming system. We also tested the relationship between overall percentage parasitism at the whole-farm level and the total number of parasitoid species per farm period, including farming system in the model. Data were transformed to satisfy the assumptions of the test, with parasitism rate (expressed as a proportion) being arcsine square root transformed. A non-parametric paired t-test (Wilcoxon matched-pairs test in GenStat) was used to test whether there was any relationship between CV and farming system.
(c). Testing prediction 2: organic farms have greater levels of functional redundancy and this leads to more reliable parasitism under environmental change
(i). Functional group analysis
There are a variety of approaches for defining functional groups, which reflect the ecological question being addressed and the state of knowledge of the focus organisms [15,16]. Here, we assigned each parasitoid species to one of 14 functional groups according to host range, host stage killed and the feeding niche of the host (adapted from published methods [17,18]). Using our rearing data, we assigned functional groups to each species based on the feeding niche of the hosts collected in the study (electronic supplementary material, table S1). Host stage killed was determined using our own data and by searching the literature using Web of Science (http://thomsonreuters.com/products_services/science/science_products/a-z/web_of_science), the Universal Chalcidoidea Database (http://www.nhm.ac.uk/jdsml/research-curation/research/projects/chalcidoids), and papers by Shaw & Aeschlimann [19] and Shaw & Huddleston [20]. If a reference at species level was found this was used, otherwise references were found that provided information on families of parasitoids with shared developmental strategies. Where no host records could be found in the literature, our rearing records alone were used. Individuals identified to genus only were excluded from the analysis. Data from both years of the study were combined and used to calculate the total number of functional groups present per farm (referred to as total functional group) and the average number of parasitoid species per functional group per farm (referred to as functional group species richness). A third predictor (referred to as functional group diversity) based on the Shannon diversity index was calculated using the number of functional groups per farm and the number of species in each functional group. A paired MANOVA (repeated measures MANOVA test in GenStat) was used to test whether there was any relationship between total functional group, functional group species richness or functional group diversity and farming system.
(ii). Robustness to parasitoid loss
We used an in silico simulation approach to assess whether future changes in parasitoid diversity (for example, as a result of changes in climate, pesticide regimes or agricultural intensification) could potentially change the provision of natural pest control services. To do this, we simulated species loss from each farm by removing parasitoid species one by one from each network and then calculating the proportion of herbivore species thereby released from biological control. The stages of this process are as follows: each interaction between a herbivore and a parasitoid was scored for its frequency of observation in the field. For a given herbivore, its level of control by parasitoids was then taken to be the sum of its interactions. For example, a single herbivore species may be attacked on 10 occasions by parasitoid species 1, and on five occasions by parasitoid species 2; the total level of control by parasitoids is therefore 15. As parasitoid species were removed in the simulation, the total number of interactions between a given herbivore and its parasitoids was reduced (for the example above, if parasitoid species 2 is removed, the control level is reduced from 15 to 10, a 33% reduction in control). We arbitrarily scored a herbivore as no longer controlled if the number of interactions between it and its parasitoids dropped to less than 50 per cent of those in the original web. While an arbitrary cut-off point, this allowed us to make quantitative comparisons between networks (similar to the ‘robustness’ measure of Dunne et al. [21]). The proportion of all herbivore species with less than 50 per cent control was recorded after the removal of a parasitoid species.
Parasitoid species were made extinct sequentially following one of three rules: least connected first (least-to-most), most connected first (most-to-least) and random. These three scenarios are the ones commonly used in the literature (e.g. [22]). In this context, the least-to-most scenario represents a realistic extinction pattern in that the least connected species in the networks are often rare, and therefore more vulnerable to extinction. The most-to-least scenario represents a catastrophic event in which species that are highly connected are lost (a real-world example is the decline in honeybees around the world [23]), and the random scenario represents a null model [21,23,24]. In the random case and to accommodate ties or equal ranking in the other two cases, we carried out 50 extinction iterations for each of the 20 webs.
Despite presenting the results using proportions (i.e. the proportion of herbivore species released from biological control), it remains a possibility that any observed differences between webs derived from organic and conventional farms are a consequence of differences in the total web size (total number of species per web), rather than structural properties of the webs. We used a novel, whole-web rarefaction procedure that used a stochastic process to reduce the larger web within a pair until it matched the size of the smaller web in a pair (see the electronic supplementary material). For each pair, we generated 100 webs in this way, then conducted our extinction scenarios on both the randomly reduced webs and the actual webs.
To determine whether there was a consistent difference in behaviour between the organic and conventional members of each pair of farms, the curves derived from the robustness analysis were compared. For each pair of graphs in turn, at 50 equally spaced evaluation points along the x-axis the difference between the loss of biocontrol for the organic web and the conventional web was calculated. These 10 sets of differences (one for each pair) were pooled into a single dataset and plotted against proportion of parasitoids removed using a Gaussian smoothing kernel with parameter = 0.02. In all cases, the variable representing the proportion of herbivores with less than 50 per cent control was arcsine square root transformed in order to equalize variance across the full range of parasitoid removal.
(d). Testing prediction 3: non-crop habitats will provide greater levels of parasitoid species richness
Rarefaction was used to determine the relative productivity of each habitat in terms of parasitoid species richness. Four habitats (arable fields, grass fields, hedgerows and woodlands) had sufficient numbers of individuals (greater than two individuals and a small degree of difference between the numbers of individuals in each pair) collected in eight or more pairs of farms for analysis. For each habitat, the total number of individuals and number of parasitoid species collected were calculated (across all 20 farms), and rarefaction used to estimate the number of species that would have been recorded if the same number of individuals were collected in all habitats (200). A rarefaction calculator was used (accessed online at http://www2.biology.ualberta.ca/jbrzusto/rarefact.php; web page by John Brzustowski; the calculator is loosely based on the program RAREFACT.FOR, written by Charles J. Krebs [25]) that employed the non-parametric Chao estimator [26]. To assess whether the productivity of each habitat was different between the two farming systems, rarefaction was again used, but this time at the farm pair level. Within each habitat, the minimum number of individuals collected within an organic and conventional farm pair was used in the rarefaction. If either member of the pair had very few individuals collected then that pair was excluded from the analysis. A Wilcoxon signed-rank test was used to test whether there was any significant difference between the estimated species richness in each pair of organic and conventional farms.
3. Results
(a). Testing prediction 1: organic farms show greater temporal stability in parasitism rates
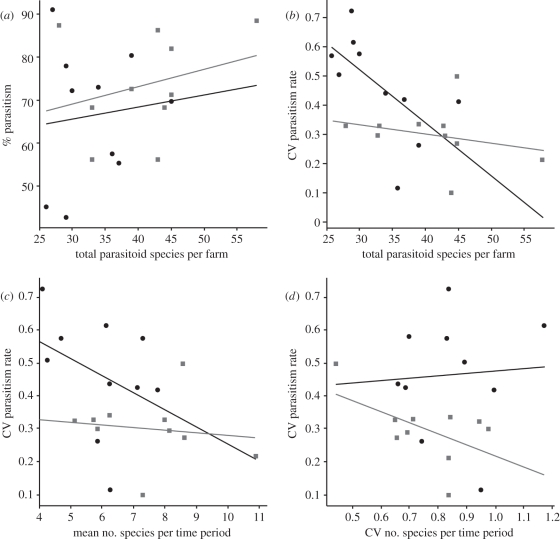
Total parasitism rate ranged from 91 per cent to 43 per cent host mortality; there was, however, no significant relationship between total parasitism rate and parasitoid species richness (F2,19 = 0.78, p = 0.473; figure 1a). Parasitism rates varied across time for all 20 farms, with CV ranging from 0.10 to 0.73. There was a significant relationship between CV in parasitism rates and parasitoid species richness at the whole farm level when farming system was included in the model (adjusted r2 = 0.328, F2,19 = 5.65, p = 0.013; figure 1b). On farms with a greater number of parasitoid species we saw significantly lower variability in parasitism rates. The same pattern was seen for CV in parasitism rates and mean parasitoid species richness at each time point (adjusted r2 = 0.236, F2,19 = 3.93, p = 0.040; figure 1c). There was no significant relationship between CV in parasitism rates and CV in parasitoid species richness at each time point (adjusted r2 = 0.189, F2,19 = 3.21, p = 0.066; figure 1d). There was a significant relationship between CV in parasitism rates and farming system (Wilcoxon signed-rank test t = 6, n = 10, p = 0.027). Organic farms had lower CV in parasitism rate (median = 0.312, maximum = 0.497, minimum = 0.098) compared with their conventional pairs (median = 0.472, maximum = 0.727, minimum = 0.113).
Figure 1.
(a) The relationship between the number of parasitoid species on each farm and the total parasitism rate per farm. (b) The relationship between total parasitoid richness per farm and coefficient of variation (CV) in parasitism rates of insect herbivores across time. (c) The relationship between CV in parasitism rates and mean parasitoid richness per time point. (d) The relationship between CV in parasitism rates and CV in the number of species per time period. The black circles and black regression line represent conventional farms and the grey squares and grey regression line represent organic farms.
(b). Testing prediction 2: organic farms have greater levels of functional redundancy and this leads to more reliable parasitism under environmental change
(i). Functional group analysis
We reared 195 parasitoid species from the 20 farms from eight families of Hymenoptera. Three parasitoid species could not be assigned to a single functional group and were excluded from the analysis. The total number of functional groups was not significantly higher on organic farms (table 1); however, within functional groups, the organic farms had significantly greater functional group species richness and diversity, with, on average, one extra species per functional group (F1,9 = 6.17, p = 0.035). While the latter result was significant at α = 0.05, it was not significant when adjusting for multiple comparisons with α = 0.02. However, we believe the adjusted α is too conservative for ecological studies of this kind, running a real risk of a type II error (i.e. a false negative [27]). Given that this appears to be an ecologically interesting pattern we discuss this result further below.
Table 1.
Results of parasitoid functional group analysis showing the difference between organic and conventional farms in terms of total number of functional groups, and richness and diversity within functional groups. Data were analysed using a paired MANOVA. 10 pairs of organic and conventional farms (20 farms in total) were used in the analysis, with farming system as the treatment factor. All variables were log10-transformed for analysis, but the range values shown are derived from untransformed data. Bold values indicate results that were significant at α = 0.05. Multivariate test, Wilks's λ = 0.358, p = 0.054. Adjusted univariate test α = 0.02.
| range organic | range conventional | F1,9 | p | |
|---|---|---|---|---|
| total functional group (total number of functional groups per farm) | 5–12 | 6–11 | 4.09 | 0.074 |
| functional group species richness (mean number of parasitoid species per functional group per farm) | 1.85–4.15 | 1.77–3.15 | 6.17 | 0.035 |
| functional group diversity (Shannon diversity index incorporating the number of functional groups and the number of species within each functional group) | 1.31–2.13 | 1.37–1.96 | 9.35 | 0.014 |
(ii). Robustness to parasitoid loss
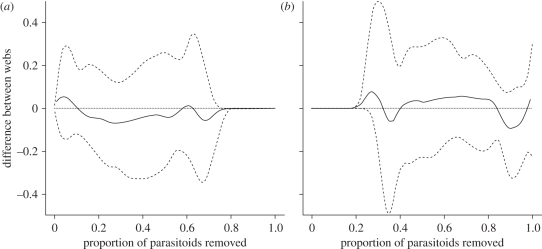
When comparing the response of the organic and conventional members of each pair to species loss, there is generally little difference between them that cannot be attributed to the effect of variation in species richness (figure 2). In fact, for five of the pairs subjected to most-to-least extinction and two of the pairs subjected to least-to-most extinction, the response of the less speciose web falls entirely within the 95 per cent confidence interval of random webs derived from the more speciose web, or where it does differ, it does so to a very small extent (see the electronic supplementary material). Thus, using a species loss simulation approach, there is no evidence to suggest that organic farms will have more resilient pest control services under environmental change.
Figure 2.
Differences between the organic and conventional farm pairs in terms of the proportion of herbivores that lose control as parasitoid species and are removed from the networks. Solid line shows the mean and the dotted lines the 95% confidence intervals. (a) Most-to-least scenario; (b) least-to-most scenario. When the line is above the zero point the organic networks have lost proportionally more control and when it is below, the conventional farms have lost proportionally more control.
(c). Testing prediction 3: non-crop habitats will provide greater levels of parasitoid species richness
The hedgerows and woodlands on farms produced the greatest number of parasitoid species. Hedgerows produced 56 species of parasitoid per 200 individuals collected compared with grass fields that produced just 11 (table 2). In comparison, the arable fields and field margins produced fewer parasitoid species for a given number of individuals collected. For the four landscape elements analysed, only hedgerows showed significantly greater species productivity on the organic farms when compared with hedgerows on the paired conventional farms (organic, median = 9; conventional, median = 5; p = 0.004). For grass fields, arable fields and woodlands there was no significant difference between farming systems.
Table 2.
Productivity of each landscape element in terms of parasitoid species richness. The field-collected total number of species includes all 20 farms (10 organic and conventional). Rarefaction (using the Chao estimator) was used to estimate the number of species (rounded up to nearest whole number) that would have been recorded if a standard number of individuals (200) had been collected per landscape element.
| field collection |
post-rarefaction |
||||
|---|---|---|---|---|---|
| abundance | species richness | mean no. species | s.d. | no. individuals | |
| hedgerows | 600 | 96 | 57 | 3.67 | 200 |
| woodland and trees | 979 | 88 | 44 | 3.49 | 200 |
| arable fields | 1846 | 78 | 35 | 3.18 | 200 |
| rough ground | 200 | 30 | — | — | — |
| field margins | 212 | 28 | 28 | 0.75 | — |
| grass field | 11 479 | 43 | 12 | 1.44 | 200 |
| orchard | 76 | 13 | — | — | — |
| game cover | 14 | 9 | — | — | — |
| vegetables | 122 | 5 | — | — | — |
| set-aside | 7 | 4 | — | — | — |
4. Discussion
Despite a large gradient in terms of parasitoid species richness per farm (range of 26–58 species), we found no significant relationship between species richness and overall percentage parasitism per farm, or between farming system and overall percentage parasitism. However, we did find that farms with a greater number of parasitoid species (at the whole farm level and averaged for each time period) experienced significantly less variability in parasitism rate across time. So while organic farming does not lead to increased overall pest control services, it may lead to more reliable pest control services across time. That said, we did not find any evidence to suggest that organic farms are more resilient to environmental changes that lead to species loss. We first discuss the limitations of our approach and then consider our results in the light of our three predictions.
(a). Limitations
Here, we have used a rich dataset that details herbivore–parasitoid interactions from a range of habitat types across 20 farms to answer questions relating to stability and robustness of ecosystem services. However, any assessment of the stability of pest control services across time is limited by the quality of the temporal data, and our study was restricted to two field seasons. That said, the average generation time of a pest species is often very short, usually far shorter than a year, and so while ‘year’ is a widely used variable, it may not be the most appropriate when considering pest control. Ideally, though, a longer-term dataset that maintains this same level of spatial resolution would be the most suitable for addressing these types of questions. With respect to our functional group analysis, other methods of classifying traits (e.g. foraging mode, starvation capability, phenology [15]) may lead to different conclusions. We used a standardized simulation approach to assess whether potential future changes in parasitoid diversity (illustrated by species removal according to three scenarios) would lead to changes to the provision of natural pest control services in each of our farming systems. Of course this simulation procedure represents an over-simplification of a real-world scenario. For example, in our scenarios there was no possibility of compensation (i.e. following a parasitoid extinction the remaining species from that functional group could potentially use the newly available host resource at the same rate), whereas there would be that possibility in real life. Being able to incorporate how the remaining species in a community respond to extinction is critical to providing more realistic scenarios [22,28]. Furthermore, the simulation scenarios (most-to-least, least-to-most and random), while widely used and with a biological basis, do not incorporate many of the factors that predispose a species to a higher probability of extinction [29]. Despite these limitations, this is the first attempt to comprehensively examine the impact of species loss on the provision of an ecosystem service at the whole-farm scale.
(b). Prediction 1: organic farms show greater temporal stability in parasitism rates
Looking at stability overall, our results suggest that parasitoid species diversity stabilized the provision of natural pest control services across the time period studied. A similar trend was seen by Veddeler et al. [30], who found that increased natural enemy diversity was related to reduced temporal variability in parasitism rates by wasps in a coffee agroforestry context, but was not related to temporal variation in bee parasitism. By contrast, Rodriguez & Hawkins [14] reported that species-rich parasitoid communities did not result in higher parasitism rates than species-poor communities, although their study was restricted to one host group (grass-feeding chalcid wasps). Given that our study includes data from a large number of Lepidopteran and Dipteran host species, and from a range of parasitoid functional groups, evidence of such a stabilizing effect of species diversity is significant. Our prediction that organic farms would show lower variability in parasitism rates across time was proved correct, with significantly lower CV values when paired with conventional farms.
(c). Prediction 2: organic farms have greater levels of functional redundancy and this leads to more reliable parasitism under environmental change
The functional group analysis revealed greater parasitoid species complementarity on organic farms, with on average more species within each functional group. We did not find a significantly greater number of functional groups on organic farms, but rather the extra parasitoid species on the organic farms resulted in more species within each functional group (but did not increase the number of functional groups). It is difficult to predict what effect this result will have on pest control services in each farming system. In the short term, increased diversity within functional groups might increase the magnitude of some agroecosystem processes [5]; however, our analysis above does not suggest that there is a greater level of overall parasitism rate on these farms. It may be that the functional complimentarity within functional groups is already maximized, so the extra species within each functional group seen on the organic farms offer little in terms of added resilience. Alternatively, in the long term, this greater level of within-functional-group species complementarity could provide a form of biological ‘insurance’ against disturbance events [4]. The fact that farms with greater numbers of parasitoid species experienced less variability in pest control services across time suggests that the extra species within functional groups on organic farms are important for stabilizing pest control services across time. In theory, species within functional groups share similar host resources. Thus, if one species was to go locally extinct, then another species within the functional group could still provide control of the same pest control. To test this theory empirically is, however, a challenge, as replicated networks of high resolution collected across a long time frame are needed. Here, we employed a simulated species removal approach in silico to start to address this question.
Our simulated removal of parasitoid species demonstrated that the organic farms, with greater levels of parasitoid diversity, did not experience increased pest control protection when faced with the loss of parasitoid species. Thus, when species were removed one by one according to our extinction scenarios, there was no significant difference between the different farming systems in the pest control service that remained. Despite the artificial nature of this simulation scenario, the results clearly demonstrate that there is no difference between the relative responses of the replicated networks from different farming systems to a standardized extinction scenario. Moreover, to our knowledge our approach is the first time a community-level species loss simulation has been used to predict the impact of species loss on ecosystem service provision. There may be a threshold of parasitoid species richness below which no change in the robustness of these communities to species removal can be observed. Our organic farms, despite having extra parasitoid diversity, may not have reached this threshold.
(d). Prediction 3: non-crop habitats will provide greater levels of parasitoid species richness
The hedgerows and woodlands are highly productive in terms of parasitoid species diversity. Clearly, maintenance of hedgerows and woodlands is important for the conservation of overall parasitoid biodiversity on farms. Moreover, organic hedgerows are producing significantly more parasitoid species than their conventional counterparts. Gibson et al. [13] reported that hedgerows on organic farms did not have greater plant species diversity or two-dimensional area in comparison to conventional hedgerows. However, there is evidence that organic hedgerows are cut less frequently (and so are taller) and have a greater three-dimensional area than conventional hedgerows [31]. This may have some impact on the number and diversity of parasitoid species produced. For other crop habitats (e.g. grass pasture fields) there appears to be no difference in the productivity of these areas under the two farming systems.
(e). Conclusions
Different components of organic farm management clearly have an impact on parasitoid species richness at the whole-farm scale. Determining what benefit, if any, this provides the farmer in terms of ecosystem services such as pest control, both in the short term and long term, is a difficult task. Increased levels of parasitoid species richness did not lead to an improvement in pest control services in terms of mean parasitism rate, but did provide greater temporal stability in parasitism rates. From a farmer's perspective, the notion that increased diversity leads to a greater temporal stability in ecosystem service provision is an appealing proposition, and should enable the explicit incorporation of these ‘free’ services into management plans. Identifying the stabilizing mechanism(s) underlying this pattern should be a priority for future research (but see [2]). On the organic farms, we observed a significant increase in the number of species within existing functional groups. However, using a simulated species removal approach, we found no evidence to support the theory that organic farm networks are better able to cope with disturbance events that cause the local extinction of parasitoid species. From this we cannot conclude that these extra species within functional groups are truly ‘redundant’, as we did not examine response diversity within or between functional groups. This response diversity may allow apparently redundant species to react differently to catastrophic events or long-term environmental shifts that cause species loss. Furthermore, we make no distinction about the relative importance of individual parasitoid species for controlling particular herbivore species that may be of greater concern in terms of pest management. We see these results as a first step towards a more prescriptive understanding of ecosystem service provision, and crucial for determining the resilience of these services to environmental change.
Acknowledgements
We would like to thank the many field assistants involved, especially Ray Barnett and Steve Palmer. Taxonomists who examined specimens included Dick Askew, Mike Bailey, Hannes Baur, Gavin Broad, John Deeming, Christer Hansson and Phil Quinn. Mark Shaw provided advice regarding the parasitoid functional group classification scheme used. This work was funded by a Biotechnology and Biological Sciences Research Council grant (BBS/B/01782). The anonymous referees offered many helpful suggestions that greatly improved the manuscript. Finally, many thanks go to the land owners who allowed us access to their farms in order to complete this study.
References
- 1.Tscharntke T., Bommarco R., Clough Y., Crist T. O., Kleijn D., Rand T. A., Tylianakis J., Nouhuys S., Vidal S. 2007. Conservation biological control and enemy diversity on a landscape scale. Biol. Control 43, 294–309 10.1016/j.biocontrol.2007.08.006 (doi:10.1016/j.biocontrol.2007.08.006) [DOI] [Google Scholar]
- 2.Winfree R., Kremen C. 2009. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc. R. Soc. B 276, 229–237 10.1098/rspb.2008.0709 (doi:10.1098/rspb.2008.0709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yachi S., Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 10.1073/pnas.96.4.1463 (doi:10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder G. B., Finke D. L., Snyder W. E. 2008. Predator biodiversity strengthens aphid suppression across single- and multiple-species prey communities. Biol. Control 44, 52–60 10.1016/j.biocontrol.2007.09.006 (doi:10.1016/j.biocontrol.2007.09.006) [DOI] [Google Scholar]
- 5.Moonen A. C., Barberi P. 2008. Functional biodiversity: an agroecosystem approach. Agri. Ecosyst. Environ. 127, 7–21 10.1016/j.agee.2008.02.013 (doi:10.1016/j.agee.2008.02.013) [DOI] [Google Scholar]
- 6.Straub C. S., Finke D. L., Snyder W. E. 2008. Are the conservation of natural enemy biodiversity and biological control compatible goals? Biol. Control 45, 225–237 10.1016/j.biocontrol.2007.05.013 (doi:10.1016/j.biocontrol.2007.05.013) [DOI] [Google Scholar]
- 7.Wilby A., Thomas M. B. 2002. Natural enemy diversity and pest control: patterns of pest emergence with agricultural intensification. Ecol. Lett. 5, 353–360 10.1046/j.1461-0248.2002.00331.x (doi:10.1046/j.1461-0248.2002.00331.x) [DOI] [Google Scholar]
- 8.Macfadyen S., Gibson R. H., Polaszek A., Morris R. J., Craze P. G., Planque R., Symondson W. O. C., Memmott J. 2009. Do differences in food web structure between organic and conventional farms affect the ecosystem service of pest control? Ecol. Lett. 12, 229–238 10.1111/j.1461-0248.2008.01279.x (doi:10.1111/j.1461-0248.2008.01279.x) [DOI] [PubMed] [Google Scholar]
- 9.Tylianakis J. M., Tscharntke T., Lewis O. T. 2007. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445, 202–205 10.1038/nature05429 (doi:10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson J., Ahnstrom J., Weibull A. C. 2005. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J. Appl. Ecol. 42, 261–269 10.1111/j.1365-2664.2005.01005.x (doi:10.1111/j.1365-2664.2005.01005.x) [DOI] [Google Scholar]
- 11.Hole D. G., Perkins A. J., Wilson J. D., Alexander I. H., Grice F., Evans A. D. 2005. Does organic farming benefit biodiversity? Biol. Conserv. 122, 113–130 10.1016/j.biocon.2004.07.018 (doi:10.1016/j.biocon.2004.07.018) [DOI] [Google Scholar]
- 12.Crowder D. W., Northfield T. D., Strand M. R., Snyder W. E. 2010. Organic agriculture promotes evenness and natural pest control. Nature 466, 109–123 10.1038/nature09183 (doi:10.1038/nature09183) [DOI] [PubMed] [Google Scholar]
- 13.Gibson R. H., Pearce S., Morris R. J., Symondson W. O. C., Memmott J. 2007. Plant diversity and land use under organic and conventional agriculture: a whole-farm approach. J. Appl. Ecol. 44, 792–803 10.1111/j.1365-2664.2007.01292.x (doi:10.1111/j.1365-2664.2007.01292.x) [DOI] [Google Scholar]
- 14.Rodriguez M. A., Hawkins B. A. 2000. Diversity, function and stability in parasitoid communities. Ecol. Lett. 3, 35–40 10.1046/j.1461-0248.2000.00115.x (doi:10.1046/j.1461-0248.2000.00115.x) [DOI] [Google Scholar]
- 15.Bell J. R., Mead A., Skirvin D. J., Sunderland K. D., Fenlon J. S., Symondson W. O. C. 2008. Do functional traits improve prediction of predation rates for a disparate group of aphid predators? Bull. Entomol. Res. 98, 587–597 10.1017/s0007485308005919 (doi:10.1017/s0007485308005919) [DOI] [PubMed] [Google Scholar]
- 16.Hooper D. U., et al. 2002. Species diversity, functional diversity, and ecosystem functioning. In Biodiversity and ecosystem functioning: synthesis and perspectives (eds Loreau M., Naeem S., Inchausti P.) pp. 195–208 New York, NY: Oxford University Press. [Google Scholar]
- 17.Mills N. J. 1992. Parasitoid guilds, life-styles, and host ranges in the parasitoid complexes of tortricoid hosts (Lepidoptera, Tortricoidea). Environ. Entomol. 21, 230–239 [Google Scholar]
- 18.Stephens C. J., Schellhorn N. A., Wood G. M., Austin A. D. 2006. Parasitic wasp assemblages associated with native and weedy plant species in an agricultural landscape. Aust. J. Entomol. 45, 176–184 10.1111/j.1440-6055.2006.00519.x (doi:10.1111/j.1440-6055.2006.00519.x) [DOI] [Google Scholar]
- 19.Shaw M. R., Aeschlimann J. P. 1994. Host ranges of parasitoids (Hymenoptera, Braconidae and Ichneumonidae) reared from Epermenia chaerophyllella (Goeze) (Lepidoptera, Epermeniidae) in Britain, with description of a new species of Triclistus (Ichneumonidae). J. Nat. Hist. 28, 619–629 10.1080/00222939400770281 (doi:10.1080/00222939400770281) [DOI] [Google Scholar]
- 20.Shaw M. R., Huddleston T. 1991. Classification and biology of braconid wasps. London, UK: Royal Entomological Society of London [Google Scholar]
- 21.Dunne J. A., Williams R. J., Martinez N. D. 2002. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567 10.1046/j.1461-0248.2002.00354.x (doi:10.1046/j.1461-0248.2002.00354.x) [DOI] [Google Scholar]
- 22.Kaiser-Bunbury C. N., Muff S., Memmott J., Müller C. B., isch A. 2010. The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecol. Lett. 13, 442–452 10.1111/j.1461-0248.2009.01437.x (doi:10.1111/j.1461-0248.2009.01437.x) [DOI] [PubMed] [Google Scholar]
- 23.Biesmeijer J. C., et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and The Netherlands. Science 313, 351–354 10.1126/science.1127863 (doi:10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 24.Memmott J., Waser N. M., Price M. V. 2004. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 10.1098/rspb.2004.2909 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs C. J. 1989. Ecological methodology. New York, NY: Harper & Row [Google Scholar]
- 26.Chao A. 1984. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270 [Google Scholar]
- 27.Moran M. D. 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403–405 10.1034/j.1600-0706.2003.12010.x (doi:10.1034/j.1600-0706.2003.12010.x) [DOI] [Google Scholar]
- 28.Hughes J. B., Ives A. R., Norberg J. 2002. Do species interactions buffer environmental variation (in theory)? In Biodiversity and ecosystem functioning: synthesis and perspectives (eds Loreau M., Naeem S., Inchausti P.) pp. 92–101 New York, NY: Oxford University Press. [Google Scholar]
- 29.Srinivasan U. T., Dunne J. A., Harte J., Martinez N. D. 2007. Response of complex food webs to realistic extinction sequences. Ecology 88, 671–682 10.1890/06-0971 (doi:10.1890/06-0971) [DOI] [PubMed] [Google Scholar]
- 30.Veddeler D., Tylianakis J., Tscharntke T., Klein A. M. 2010. Natural enemy diversity reduces temporal variability in wasp but not bee parasitism. Oecologia 162, 755–762 10.1007/s00442-009-1491-x (doi:10.1007/s00442-009-1491-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller R. J., et al. 2005. Benefits of organic farming to biodiversity vary among taxa. Biol. Lett. 1, 431–434 10.1098/rsbl.2005.0357 (doi:10.1098/rsbl.2005.0357) [DOI] [PMC free article] [PubMed] [Google Scholar]