Abstract
Animals signal their reproductive status in a range of sensory modalities. Highly social animals, such as primates, have access not only to such signals, but also to prior experience of other group members. Whether this experience affects how animals interpret reproductive signals is unknown. Here, we explore whether familiarity with a specific female affects a male's ability to assess that female's reproductive signals. We used a preferential looking procedure to assess signal discrimination in free-ranging rhesus macaques, a species in which female facial luminance covaries with reproductive status. We collected images of female faces throughout the reproductive cycle, and using faecal hormone analysis to determine ovulation, categorized images as coming from a female's pre-fertile, ovulating, or post-fertile period. We printed colour-calibrated stimuli of these faces, reproducing stimuli perceptually the same in colour and luminance to the original appearance of females. These images were presented to males who were either unfamiliar or familiar with stimuli females. Overall, males distinguished ovulatory from pre-ovulatory faces. However, a significant proportion of males did so only among males familiar with stimuli females. These experiments demonstrate that familiarity may increase a receiver's ability to use a social partner's signals to discern their reproductive status.
Keywords: familiarity, experience, cognition, reproductive signals, ovulation, discrimination
1. Introduction
Females in many animal species use signals to reveal their reproductive status to males. Examples include the chirps of giant pandas [1], the visual signals of some social insect species [2] and the sexual swellings of baboons [3]. Group-living social animals, such as many primates, receive such signals from group members with whom they have a history of previous interactions. The socially derived knowledge that such primates acquire and maintain about other group members can include information about a fellow group member's social rank, kinship relations, and current friendships and associations [4]. However, little is known about whether male familiarity with a specific female can influence his ability to interpret that female's reproductive signals.
Here, we examine whether male rhesus macaques may benefit from having experience of specific females when assessing their reproductive signals. Female catarrhine primates often offer cues or signals to the timing of their fertile period, either through changes in their hindquarter regions [5] or faces (e.g. humans [6]). Female rhesus macaques are known to display variation in facial parameters that could indicate ovulation; females show marked changes in facial luminance (lightness) during the reproductive cycle, and variation in this is related to the timing of the female fertile period [7,8]. Such changes therefore have the potential to indicate this timing to males, though it is unclear whether they do so as this has not been tested experimentally. For female primates living in promiscuous multi-male–multi-female societies it may not be in their interest to signal fertility unambiguously to all males. Instead, females in such social systems may benefit most from issuing graded signals of reproductive status that offer only probabilistic assurances of paternity [9,10]. These may serve to assure some males that they have a high probability of paternity, while still offering others some chance of paternity [9,10]. The benefits of this to females are that probable fathers can favour their offspring by tolerating them at food sources and protecting them from attack [11], while the small probability of paternity offered to other males may prevent them from hindering an infant's access to resources, and from attacking it.
For male rhesus macaques, which immigrate into new groups in search of mating opportunities, both dominance rank and tenure length are important determinants of mating success [12]. New immigrants are typically low-ranking, and increase in rank with increasing tenure length. Like many social primates, rhesus macaques accumulate information about consistent interaction partners, characteristics of whom they are able to recall from viewing pictures of their faces (e.g. social status [13]). If such familiarity helps males to assess a familiar female's reproductive signals, this gives these males a clear advantage, helping them to ensure that they mate with a female only during fertile periods, and that they undertake energetically costly reproductive behaviours [14] only at the most appropriate times. Such effects would provide advantages to resident males, and the learning opportunities obtained by immigrant males would help explain why such males accept the available low-ranking peripheral positions when first moving into new groups. Preliminary evidence that familiarity may affect male mating success comes from wild long-tailed macaques, where group males time their mating activities to female fertile periods better than non-group males [15]. Similarly, in wild baboons, males of longer group residencies consort more frequently with females during conceptive cycles than newer group males do [16]. However, such evidence is correlative, and factors such as female choice, behaviour of other group members, and differences in access to females may cause such effects. There is, to our knowledge, no good evidence to show that familiarity can affect how reproductive signals themselves are interpreted in any species (including humans).
We tested the interaction between male social experience and interpretation of reproductive signals experimentally in free-ranging rhesus macaques on the island of Cayo Santiago. As adult males on this island are familiar with their own group members, but are unfamiliar with members of other social groups, this provides an opportunity to explore the effects of familiarity on signal assessment. We obtained colour-calibrated digital images of female faces and hindquarters throughout ovarian cycles, and showed previously that female facial (but not hindquarter) luminance changes covaried with female fertility (with faces darkening around ovulation) [7,8]. Our analyses revealed a great deal of inter-individual variation shown by females, with some females far darker outside their fertile phase than other females are at any stage of their cycle, even when they are ovulating. Further, some females showed more than twice as much variation in facial luminance in just two days as others showed across the whole cycle. As a consequence, there are seemingly no easy rules for males—a simple cognitive rule such as ‘always prefer the female with the darker face’ would often lead to misidentification of fertile females (see the electronic supplementary material, figure S1).
We reproduced images as experimental stimuli, modelling rhesus macaque vision to create images likely to be indiscriminably different in colour and luminance to rhesus macaques to the colour originally displayed by females. We showed these images to male subjects, in which individual males were presented with two images simultaneously representing one female in either a pre-ovulatory (PRE) versus ovulatory (OV) or an OV versus post-ovulatory (POST) condition. We predicted that males would prefer OV to PRE or POST faces, but that this effect would be stronger for older males (a general experience effect), and strongest in males familiar with the females shown in the images (a specific experience effect). We predicted no results for hindquarter experiments, as previous work demonstrated that variation in hindquarter colour and luminance was unrelated to female ovulation [7,8]. However, we included these as some previous studies of rhesus macaque signalling have either focused on or included stimuli of this area (e.g. [13,17]).
2. Material and methods
(a). Study site and subjects
We studied free-ranging rhesus macaques on Cayo Santiago, Puerto Rico [18], which are habituated to both behavioural and experimental research, and are identifiable through tattoos and earnotches, in addition to other specific individual features. Currently, rhesus macaques on Cayo Santiago undergo a six-month mating period from March to August, followed by a six-month birth period from September to February [19]. Throughout the study period around 1000 animals resided on the island living in six self-formed social groups. Original images of females and endocrine data were collected between 22 April and 12 July 2007 in group V. Experiments in which images were presented to males took place in all groups across the island between 3 June and 10 August 2009. At this time around 105 individuals were in group V, including 15 adult males. Male ages and periods of residence within different social groups were available from the Cayo Santiago database.
(b). Collection of images and determination of female reproductive status
Full details of image collection and endocrine methods have been presented elsewhere [7,8]. Briefly, we collected digital images from 10 ovarian cycles from eight females, capturing pictures of both faces and hindquarters taken from a distance of 1–3 m using a Canon EOS Digital Rebel XTi camera with a 10.1 megapixel CMOS censor and an EF28-135 mm f/3.5-5.6 IS USM lens [7,8]. Photographs were taken in RAW format, and we employed the ‘sequential method’ [20] to standardize images for ambient light and camera settings.
Faecal samples were collected from focal females directly after defecation, homogenized, placed on ice and stored at −20°C. Samples were analysed at the German Primate Centre for progestogen metabolites using a validated pregnanediol glucuronide (PdG) microtiterplate enzymeimmunoassay [7]. Ovulation was considered to have occurred when PdG concentrations rose above a threshold of the mean + 2 s.d. of three to five preceding baseline values, maintained for at least three consecutive samples [21,22]. Given a time lag of 24–56 h in hormone metabolite excretions in faeces [23] and to account for oocyte lifespan, we defined an ‘ovulation window’ as days 2/3 relative to the defined PdG rise (e.g. [22]). We considered each female to have been fertile for a 5 day period including the 2 day ovulation window and the 3 days preceding it to account for sperm lifespan in the female tract [24]. The 5 days preceding and the 5 days following the fertile phase were defined as pre- and post-fertile phases. For experiments, per female, we took one image from OV one from PRE and one from POST conditions.
(c). Production of stimuli
It was essential to ensure that we reproduced the colour and luminance values exhibited by females at different times of the ovarian cycle accurately in our stimuli. As rhesus macaques perceive colour very differently to red, green, blue cameras, we undertook modelling of the rhesus visual system [25]. Briefly, we first modelled the quantal catch of the rhesus macaque retinal receptors in response to the colours seen, using the program ColourWorker (http://www.chrometrics.com) to extract a reflectance spectrum of the signal from the images [8], and then calculating the quantal catch of the rhesus longwave, mediumwave and shortwave cones in response (for further details see the electronic supplementary material and [8]).
Using the above calculated quantal catch data, we then used a log form of the Vorobyev–Osorio receptor noise model [25] to produce stimuli in which colour and luminance were as close as possible to being indiscriminably different to rhesus macaques from the colour and luminance values originally displayed by females. We printed stimuli, measured them using a spectrometer, and then compared the difference in response for each receptor type to each female colour and corresponding stimulus colour according to equations published elsewhere [25] and given in the electronic supplementary material. We undertook an iterative printing process, in which stimuli were printed, measured using a spectrometer, assessed against the original female colour using the model, adjusted, reprinted and reassessed, until the colour and luminance values in the stimuli were all within one to two just noticeable differences ([25], see the electronic supplementary material and [8]) of the original female colour and luminance values as perceived by rhesus macaques. Images were printed at roughly A4 size (approximately the size of real rhesus faces) on waterproof paper (HP LaserJet Tough Paper; Hewlett Packard, Palo Alto, USA) on a Hewlett Packard Colour LaserJet 2605 dn printer at 300 dpi. We selected two sets of the resulting images, representing the ovarian cycles of two different females, for use in experiments. These sets of images were selected on the basis that each female was looking away from the camera, in very similar positions, and with similar expressions (neutral, mouth closed), in all images from the different stages of the cycle.
(d). Experimental test procedure
To assess human infant preferences, researchers typically present two images side-by-side and determine which image attracts longer looking time, with longer time indicating preference in interest or attractiveness [26]. This technique has also been used in a number of studies of rhesus macaques (e.g. [13,17]), and has been validated by several approaches, including by experiments showing that rhesus look longer at images of other rhesus faces and perinea than they do at controls [13]. We presented male macaques with two pictures attached to opposite ends of a white board (140 cm long × 35 cm high) with two frames on each end in which a picture could be placed. The content of each picture frame could be hidden with a black occluder just larger than the size of the images themselves (20 × 30 cm). During each session, a male subject was presented with two images simultaneously, which represented one female in two different phases of her cycle. Each male was either presented with a PRE versus OV, or an OV versus POST, condition. We counterbalanced the location of these images (left or right) across trials.
Each test session required both a presenter and a cameraperson, with the former managing the apparatus and the target stimuli, and the latter recording the subject's looking behaviour with a digital video camera. Before each experiment began, the presenter randomly chose a condition, and then arranged the selected images occluded in the frames. Potential subjects were identified when they were seated and alone, at which point the presenter set up the experimental apparatus within 2–3 m of the subject, and the cameraperson, standing behind the presenter, began recording. When the subject was attending to the display, the presenter simultaneously removed the occluders from the images. The cameraperson then recorded the subject for 30 seconds. The cameraperson was blind to experimental condition, and thus could make decisions about when to abort a session without bias. Sessions were aborted when subjects walked away from the apparatus prematurely, or if they were inattentive or distracted. Those males who saw stimuli but who did not complete a trial were identified and not retested. Each 30 second video clip for each session was later coded frame-by-frame by a coder who was also blind to condition. This coder also discarded trials if the video quality was at any point too poor to discriminate the looking behaviour of the subject. During trials males commonly looked back and forth between the two images.
There are approximately 200 adult males on Cayo Santiago, but only 15 males within group V, of which we were able to test 14. Outside group V, male subjects completed only a single comparison test and so were only ever tested once across all conditions. However, owing to a smaller sample size within group V, males completed two tests in succession. When testing group V males, the presenter loaded the apparatus with two comparison pairs from our experimental set. Male subjects in group V therefore saw, in balanced order, one comparison pair (OV versus PRE or OV versus POST) of one female, and then the opposite comparison pair of the second female. Otherwise, the experimental procedure was as described above. While double exposure may theoretically lead to decreased overall looking time for the second set of pictures, this should not affect relative looking time between sets of pictures. In total (including all males), we completed coded and used 115 trials for face experiments, and 96 trials for hindquarter experiments.
(e). Categorizing males as members of group V
As males on Cayo Santiago transfer between groups, it is inappropriate to categorize males as group V males (experienced with females in images) or Non-V males (inexperienced with females in images) merely based on their group residency on the day of testing. We used census data available for the field site to make these categorizations. We categorized males as members of group V if they had spent at least one of the two previous years' breeding seasons (2007, 2008) within group V and as Non-V males if they had not. As such it was at least 3 years (2006) since any Non-V males may have had access to either of the study females used for experiments. As the experience effect being tested is extensive and specific (variation in facial luminance shown by a specific female when cycling), we considered this to be an appropriate cutoff point for considering which males may have good information of this nature. The vast majority of males tested were found within groups in which they had been resident for the previous several breeding seasons. For OV versus PRE experiments, the above assessment resulted in three males tested in group V being considered Non-V males, and vice versa. For OV versus POST experiments, the above assessment resulted in one male tested in group V being considered a Non-V male, and three males tested outside of group V considered group V males.
(f). Data analysis
We used paired t-tests to assess looking time of individuals at OV versus Non-OV images, and two-way ANOVAs to test for differences in looking time at OV versus Non-OV images according to female stimuli identification (ID) and male group category. To assess the numbers of males preferring different stimuli, we used χ2-tests to determine whether proportions preferring one category (e.g. OV) over another (e.g. PRE) were significantly higher than would be expected by chance, both overall and separated by group. To assess whether male age (as surrogate for general breeding experience) affected preference for OV faces, we used logistic regression to see whether male age determined likelihood of OV preference, and a Pearson's correlation to see whether male age was correlated with looking time difference in favour of OV images. To assess whether males spent total longer times looking at different experiments according to experiment type, or group (V or Non-V) type (a specific experience effect), we undertook independent t-tests. For these tests we assessed Levene's test for equality of variance. Where the two samples did not differ significantly in variance, we assumed equal variances. Where the two samples were significantly different in variance, we did not assume equal variances; in this latter case we state that we have not assumed equal variances when presenting results.
All tests were two-tailed, and were undertaken in SPSS 16.0, and using an online interactive χ2 tool [27], with p < 0.05 considered significant in all tests.
3. Results
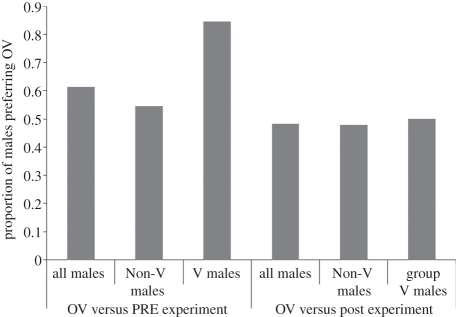
Paired t-tests showed that across all males tested, subjects spent significantly longer looking at OV than PRE faces (mean looking times, OV = 76 ± 9 frames, PRE = 58 ± 6 frames, t56 = 2.209, p = 0.031), and χ2 analyses showed that there was a trend towards a higher total proportion of males preferring OV faces to PRE faces (61%, 35/57; χ2 = 3.0, p = 0.085; figure 1). Together, these results (especially the looking time data) suggest that males distinguish between these two facial categories (table 1; see the electronic supplementary material figure S1). A paired t-test showed no significant difference in the time males spent looking at OV compared with POST faces (mean looking times, OV = 98 ± 12 frames, POST = 98 ± 10 frames, t56 = 0.022, p = 0.983), a result confirmed by looking at the proportion of males preferring OV to POST faces (table 1, figure 1; χ2 = 0.07, p = 0.793). Consistent with our result, males looked for longer at OV versus POST experiments (197 ± 19 frames) than OV versus PRE experiments (134 ± 13 frames) (independent t-test, equal variances not assumed, t99.3 = −2.760, p = 0.007) suggesting that males may have found distinguishing between the two stimuli in the former experiment more challenging. As predicted, there were no significant results related to hindquarter images (all p > 0.1).
Figure 1.
The proportion of males preferring OV faces in the OV versus PRE and OV versus POST experiments. In the PRE versus OV experiment, a trend towards a significant proportion of males preferring OV was found for all males (p = 0.085), but this was driven by a significant relationship among group V males only (p = 0.012). No significant differences were found in the OV versus POST experiment.
Table 1.
Number of males preferring OV versus PRE or POST faces in the two face experiments. (Males are grouped as all males, Non-V males only and group V males, and are also separated to show results for both females separately as well as combined.)
| male grouping | female ID | OV versus PRE face experiment |
OV versus POST face experiment |
||
|---|---|---|---|---|---|
| OV | PRE | OV | POST | ||
| all males | 78I | 16 | 8 | 18 | 19 |
| 31G | 19 | 14 | 10 | 11 | |
| combined | 35 | 22 | 28 | 30 | |
| Non-V males | 78I | 11 | 7 | 14 | 16 |
| 31G | 13 | 13 | 8 | 8 | |
| combined | 24 | 20 | 22 | 24 | |
| V males | 78I | 5 | 1 | 4 | 3 |
| 31G | 6 | 1 | 2 | 3 | |
| combined | 11 | 2 | 6 | 6 | |
Following these results, we explored results of PRE versus OV face experiments in further detail. Male age was unrelated to preference for OV over PRE faces (logistic regression, W = 0.059, p = 0.809) or looking time difference between OV and PRE faces (Pearson's correlation, r = 0.103, p = 0.477). Two-way ANOVA showed no significant differences in overall time individuals spent looking at OV when compared with PRE faces according to female stimuli ID (F1,54 = 1.45, p = 0.233), or male group category (V versus Non-V, F1,54 = 0.05, p = 0.825). However, analysis of the proportion of individuals preferring OV over PRE faces revealed clear differences according to whether males were or were not in the stimulus female's group. The proportion of group V males showing a preference for OV over PRE faces was greater than would be expected by chance (85%, 11 out of 13; χ2 = 6.2, p = 0.012). By contrast, the proportion of Non-V males showing such a preference was not significantly different than chance (55%, 24 out of 44; χ2 = 0.4, p = 0.546). As such, the overall differences in the proportion of males preferring OV to PRE detected above were driven by a strong effect among males who were in the same group as the stimulus female. There were no significant differences between group V males and Non-V males in the total amount of time they spent looking at stimuli in experiments (mean looking times, group V males = 158±30 frames, Non-V males = 168 ± 13 frames; independent t-test, t113 = −0.315, p = 0.722; PRE versus OV experiment only, t55 = −1.143, p = 0.258; PRE versus POST experiment only, t56 = 0.412, p = 0.682), suggesting that novelty of faces was not a factor in the results.
4. Discussion
Our previous assessments of colour measurements suggested that variation in facial (but not hindquarter) appearance is associated with ovulation in rhesus macaque females [7,8]. Our experimental results are consistent with these results, but go beyond previous work to show that variation in facial parameters is sufficient for males to discern ovulating from pre-fertile, but not ovulating from post-fertile, faces. Our results also demonstrate a potential role for a male's prior experience of a particular female. Analysis of the proportion of males preferring OV to PRE shows clear differences between males according to their experience with stimuli females. These results also demonstrate that general experience is not the same as specific experience. Here, age (as proxy for general, non-specific, breeding experience) had no effect on male signal discrimination; instead it is the highly specific social experience that develops from repeated observation and interaction with particular individuals that may affect male response. All females do not show the same variation in facial luminance, and without prior experience of a female's variation though the cycle, it may be difficult for males to discern whether an image represents that female in a particularly marked state of appearance. This would not mean that males unfamiliar with a female are unable to detect the variation between the two images. Rather, we believe it far more likely that most males are able to detect this variation, but that as a rule for simply preferring a darker face when presented with two faces should not be favoured (see the electronic supplementary material, figure S1), they are unlikely just to prefer a darker face per se. Instead, recognizing that a particular level of darkening within a female's face is highly important variation for that female may require direct experience of that female by males.
Males who have spent mating seasons in the same group as the highly promiscuous females are likely to have observed the luminance variation shown by a specific female, and developed associations between levels of facial luminance and female behavioural proceptivity and receptivity [28], as well as other signals of ovulation (e.g. olfactory signals [29]). In humans, prior experience with individuals creates specific episodic memories [30,31] which allow us to react in the most appropriate way when receiving signals from known individuals. Similar mechanisms may take place in other primates to allow familiarity to nuance how individuals respond to signals from familiar social partners. During the 2007 mating season, group V females (n = 22) exhibited an average of 2.96 (range 1–6) periods of behavioural oestrus/reddening, including 2.58 (range 1–5) pre-conception oestrus periods. As such, opportunities for learning intra-cycle variation of specific females do exist. Further, males may obtain information about the probable variation that may be shown by females during non-cycling periods, as whether a female is typically dark or light coloured may provide information on whether a dark image is or is not likely to represent significant colour variation for a particular female.
Regardless of whether we consider looking times or absolute preferences, males did not discriminate between images from OV and post-fertile periods. The finding that males looked longer at the POST versus OV experiment than the PRE versus OV experiment is consistent with the idea that males found the POST versus OV discrimination task more challenging, and more perplexing. It is worth noting here that these two periods are different from an endocrine perspective—the PRE period represents follicular phase images, when oestrogen levels are high and progestogen levels low, whereas the POST period represent luteal phase images, when oestrogen levels are low and progestogen levels high. Both these hormones are known to affect primate sexual signalling directly, with oestrogen causing darkening (e.g. [32]) and (for those species exhibiting sexual swellings) increased swelling size, while progesterone inhibits coloration and swelling (e.g. [33]). The pattern of performance exhibited in the present study (discrimination of OV versus PRE, but not OV versus POST) was not as predicted, but fits with similar findings in some other primate species. For example, male chimpanzees found female sexual swellings from fertile periods more attractive than those from pre-fertile periods, but did not distinguish between fertile and post-fertile phases, although in this case sexual swelling size also did not differ between fertile and post-fertile phases [34]. Although we calibrated images for colour and luminance, it is possible that other features of the face also change across the cycle. In humans, facial attractiveness is determined, in part, by symmetry (e.g. [35]), and is also related to oestrogen levels [36], such that facial symmetry could be another potential trait that males attend to. If any facial trait differs between OV and PRE phases, but not OV and POST phases (as in chimpanzee sexual swelling size [34]), then this might explain the failure of males to discriminate between OV and POST images. Changes in luminance across the cycle are not linear, with females exhibiting perceptually salient darkening periods both during the pre-fertile and post-fertile phases, which lighten again before the main period of OV darkening [8]. We have suggested that this could be a strategy of female rhesus macaques for confusing paternity [8]. As such, colours exhibited during PRE- and POST-periods do not necessarily represent exactly the same level of contrast with the OV period. That said, the OV period is darker than both PRE- and POST-periods, and there were, on average, no difference in the images used in experiments in the contrast between PRE and OV and POST and OV. Our results further demonstrate that males do not follow a simple rule of ‘always prefer the female with the darker face’; if this were the case, then males would have preferred OV faces to POST faces, as OV faces are darker [7,8]. As predicted, males were unable to detect ovulation from hindquarter images, consistent with our previous results showing that hindquarter coloration does not covary with ovulation [7,8]. As a relatively terrestrial primate species, rhesus macaques often have their hindquarters on the ground, perhaps favouring exhibition of such signals in facial rather than hindquarter skin [7].
Another innovation of our study concerns the methods used to present images to field-living primates. In many studies of animal signalling, signal information content may be inferred by showing covariation with a particular aspect of condition (e.g. wing patch size in collared flycatchers [37]). By using visual modelling techniques to produce colour and luminance likely to be indiscriminably different to the target receivers from the original colours observed, we provide a case study for how colours can be reproduced as stimuli appropriate for target receivers. Only by displaying signals accurately to receivers experimentally can we determine whether variation is salient to receivers, and hence should really be considered a ‘signal’. A previous study of rhesus macaques tested experimentally as to whether applying additional red colour to female face images caused an increase in male attention, found no such change [17]. We build on these earlier approaches by showing that variation in facial luminance displayed by females throughout real reproductive cycles is salient to males.
Our study makes a contribution to the debate on whether intra-individual signals of female primate fertility can be used by males as inter-individual indicators of female quality [38,39]. If familiarity is required for a male to be able to place a certain level of signal expression into an appropriate context, it is difficult to see how they might be able to compare aspects of unfamiliar females on the basis of their signal expression. Our results suggest a benefit to males of remaining in new groups and accepting low-ranking status rather than moving on; the learning opportunities provided may prove important to future mating success. Consistent with this, male rhesus mating success improves in the second, not first, year after immigration [12]. The paradigm used in our study could be used in future studies of other primate and non-primate species in which changes in female reproductive status are communicated with visual, auditory or olfactory signals or cues. For example, in humans, research has suggested that a women's voice [40] and her body odour [41] potentially contain information about her fertility. Assessing how familiarity with particular females affects a male's ability to discern key social and reproductive characteristics from such cues would seem to be worthwhile.
Acknowledgements
All procedures were approved by the University of Puerto Rico's Institutional Animal Care and Use Committee (IACUC) (approved protocol number 8310106), and complied with all laws of Puerto Rico and the United States, and the ABS/ASAB guidelines for the ethical treatment of animals.
We thank Adrienne Lighten for assistance with experiments in the field, Petroc Sumner for reflectance spectra for calibrating ColourWorker from http://vision.psychol.cam.ac.uk/spectra/, and Daniel Osorio for advice on modelling and using ColourWorker more generally. We also thank several reviewers for commenting on a previous version of this manuscript. M.S. was supported by a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1) and Churchill College, Cambridge; L.J.N.B. and C.D. were supported by the Natural Sciences and Engineering, and the Social Sciences and Humanities, Research Councils of Canada, respectively. This publication was made possible by grant no. CM-20-P40RR003640 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Charlton B. D., Keating J. L., Li R. G., Yan H., Swaisgood R. R. 2010. Female giant panda (Ailuropoda melanoleuca) chirps advertise the caller's fertile phase. Proc. R. Soc. B 277, 1101–1106 10.1098/rspb.2009.1431 (doi:10.1098/rspb.2009.1431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tannure-Nascimento I. C., Nascimento F. S., Zucchi R. 2008. The look of royalty: visual and odour signals of reproductive status in a paper wasp. Proc. R. Soc. B 275, 2555–2561 10.1098/rspb.2008.0589 (doi:10.1098/rspb.2008.0589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higham J. P., MacLarnon A. M., Ross C., Heistermann M., Semple S. 2008. Baboon sexual swellings: information content of size and color. Horm. Behav. 53, 452–462 10.1016/j.yhbeh.2007.11.019 (doi:10.1016/j.yhbeh.2007.11.019) [DOI] [PubMed] [Google Scholar]
- 4.Cheney D. L., Seyfarth R. M. 2007. Baboon metaphysics: the evolution of a social mind. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Dixson A. F. 1998. Primate sexuality: comparative studies of prosimians, monkeys, apes, and human beings. Oxford, UK: Oxford University Press [Google Scholar]
- 6.Roberts S. C., Havlicek J., Flegr J., Hruskova M., Little A. C., Jones B. C., Perrett D. I., Petrie M. 2004. Female facial attractiveness increases during the fertile phase of the menstrual cycle. Proc. R. Soc. Lond. B 271, S270–S272 10.1098/rsbl.2004.0174 (doi:10.1098/rsbl.2004.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubuc C., Brent L. J. N., Accamando A. K., Gerald M. S., MacLarnon A., Semple S., Heistermann M., Engelhardt A. 2009. Sexual skin color contains information about the timing of the fertile phase in free-ranging rhesus macaques. Int. J. Primatol. 30, 777–789 10.1007/s10764-009-9369-7 (doi:10.1007/s10764-009-9369-7) [DOI] [Google Scholar]
- 8.Higham J. P., Brent L. J. N., Dubuc C., Accamando A. K., Engelhardt A., Gerald M. S., Heistermann M., Stevens M. 2010. Color signal information content and the eye of the beholder: a case study in the rhesus macaque. Behav. Ecol. 21, 739–746 10.1093/beheco/arq047 (doi:10.1093/beheco/arq047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bercovitch B. F. 1995. Female co-operation, consortship maintenance, and male mating success in savanna baboons. Anim. Behav. 50, 137–149 10.1006/anbe.1995.0227 (doi:10.1006/anbe.1995.0227) [DOI] [Google Scholar]
- 10.Nunn C. 1999. The evolution of exaggerated sexual swellings in primates and the graded signal hypothesis. Anim. Behav. 58, 229–246 10.1006/anbe.1999.1159 (doi:10.1006/anbe.1999.1159) [DOI] [PubMed] [Google Scholar]
- 11.Van Schaik C. P., Paul A. 1996. Male care in primates: does it ever reflect paternity? Evol. Anthropol. 5, 152–156 (doi:10.1002/(SICI)1520-6505(1996)5:5<152::AID-EVAN3>3.0.CO;2-H) [DOI] [Google Scholar]
- 12.Berard J. 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta). Primates 40, 159–175 10.1007/BF02557708 (doi:10.1007/BF02557708) [DOI] [PubMed] [Google Scholar]
- 13.Deaner R. O., Khera A. V., Platt M. L. 2005. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr. Biol. 15, 543–548 10.1016/j.cub.2005.01.044 (doi:10.1016/j.cub.2005.01.044) [DOI] [PubMed] [Google Scholar]
- 14.Bercovitch F. B. 1997. Reproductive strategies of rhesus macaques. Primates 38, 247–263 10.1007/BF02381613 (doi:10.1007/BF02381613) [DOI] [Google Scholar]
- 15.Engelhardt A., Hodges J. K., Heistermann M. 2007. Post-conception mating in wild long-tailed macaques (Macaca fasicularis): characterization, endocrine correlates and functional significance. Horm. Behav. 51, 3–10 10.1016/j.yhbeh.2006.06.009 (doi:10.1016/j.yhbeh.2006.06.009) [DOI] [PubMed] [Google Scholar]
- 16.Weingrill T., Lycett J. E., Barrett L., Hill R. A., Henzi S. P. 2003. Male consortship behaviour in chacma baboons: the role of demographic factors and female conceptive probabilities. Behaviour 140, 405–427 10.1163/156853903321826701 (doi:10.1163/156853903321826701) [DOI] [Google Scholar]
- 17.Waitt C., Gerald M. S., Little A. C., Kraiselburd E. 2006. Selective attention toward female secondary sexual color in male rhesus macaques. Am. J. Primatol. 68, 738–744 10.1002/ajp.20264 (doi:10.1002/ajp.20264) [DOI] [PubMed] [Google Scholar]
- 18.Rawlins R. G., Kessler M. J. (eds) 1986. The Cayo Santiago macaques: history, behavior and biology. Albany, NY: SUNY [Google Scholar]
- 19.Hoffman C. L., Ruiz-Lambides A. V., Davila E., Maldonado E., Gerald M. S., Maestripieri D. 2008. Sex differences in survival costs of reproduction in a promiscuous primate. Behav. Ecol. Sociobiol. 62, 1711–1718 10.1007/s00265-008-0599-z (doi:10.1007/s00265-008-0599-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens M., Stoddard M. C., Higham J. P. 2009. Studying primate color: towards visual system-dependent methods. Int. J. Primatol. 30, 893–917 10.1007/s10764-009-9356-z (doi:10.1007/s10764-009-9356-z) [DOI] [Google Scholar]
- 21.Jeffcoate S. L. 1983. Use of rapid hormone assays in the prediction of ovulation. In Ovulation: methods for its prediction and detection (ed. Jeffcoate S. L.), pp. 67–82 Chichester, UK: Wiley [Google Scholar]
- 22.Heistermann M., Uhrigshardt J., Husung A., Kaumanns A., Hodges J. K. 2001. Measurement of faecal steroid metabolites in the lion-tailed macaque (Macaca silenus): a non-invasive tool for assessing female ovarian function. Primate Rep. 59, 27–42 [Google Scholar]
- 23.Wasser S. K., Monfort S. L., Southers J., Wildt D. E. 1994. Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces. J. Reprod. Fertil. 101, 213–220 10.1530/jrf.0.1010213 (doi:10.1530/jrf.0.1010213) [DOI] [PubMed] [Google Scholar]
- 24.Wilcox A. J., Weinberg C. R., Baird D. D. 1995. Timing of sexual intercourse in relation to ovulation: effects on the probability of conception, survival of the pregnancy, and sex of the baby. N. Engl. J. Med. 333, 1517–1522 10.1056/NEJM199512073332301 (doi:10.1056/NEJM199512073332301) [DOI] [PubMed] [Google Scholar]
- 25.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fantz R. 1965. Visual perception from birth as shown by pattern selectivity. Ann. NY Acad. Sci. 118, 793–814 10.1111/j.1749-6632.1965.tb40152.x (doi:10.1111/j.1749-6632.1965.tb40152.x) [DOI] [PubMed] [Google Scholar]
- 27.Preacher K. J. 2001. Calculation for the chi-square test: an interactive calculation tool for chi-square tests of goodness of fit and independence (computer software). See http://www.quantpsy.org
- 28.Keverne E. B. 1976. Sexual receptivity and attractiveness in the rhesus monkey. Adv. Study Behav. 7, 155–200 10.1016/S0065-3454(08)60167-9 (doi:10.1016/S0065-3454(08)60167-9) [DOI] [Google Scholar]
- 29.Michael R. P., Keverne E. B. 1968. Pheromones in the communication of sexual status in primates. Nature 218, 746–749 10.1038/218746a0 (doi:10.1038/218746a0) [DOI] [PubMed] [Google Scholar]
- 30.Gobbini M. I., Haxby J. V. 2007. Neural systems for recognition of familiar faces. Neuropsychologia 45, 32–41 10.1016/j.neuropsychologia.2006.04.015 (doi:10.1016/j.neuropsychologia.2006.04.015) [DOI] [PubMed] [Google Scholar]
- 31.Gobbini M. I., Lebenluft E., Santiago N., Haxby J. V. 2004. Social and emotional attachment in the neural representation of faces. Neuroimage 22, 1628–1635 10.1016/j.neuroimage.2004.03.049 (doi:10.1016/j.neuroimage.2004.03.049) [DOI] [PubMed] [Google Scholar]
- 32.Rhodes L., Argersinger M. E., Gantert L. T., Friscino B. H., Hom G., Pikounis B., Hess D. L., Rhodes W. L. 1997. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, and aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J. Reprod. Fertil. 111, 51–57 10.1530/jrf.0.1110051 (doi:10.1530/jrf.0.1110051) [DOI] [PubMed] [Google Scholar]
- 33.Gillmann J. 1940. The effect of multiple injections of progesterone on the turgescent perineum of the baboon (Papio porcarius). Endocrinology 26, 1072–1077 10.1210/endo-26-6-1072 (doi:10.1210/endo-26-6-1072) [DOI] [Google Scholar]
- 34.Deschner T., Heistermann M., Hodges K., Boesch C. 2004. Female sexual swelling size, timing of ovulation and male behavior in wild West African chimpanzees. Horm. Behav. 46, 204–215 10.1016/j.yhbeh.2004.03.013 (doi:10.1016/j.yhbeh.2004.03.013) [DOI] [PubMed] [Google Scholar]
- 35.Grammer K., Thornhill R. 1994. Human (Homo sapiens) facial attractiveness and sexual selection: the role of symmetry and averageness. J. Comp. Psychol. 3, 233–242 10.1037/0735-7036.108.3.233 (doi:10.1037/0735-7036.108.3.233) [DOI] [PubMed] [Google Scholar]
- 36.Law-Smith M. J., et al. 2006. Facial appearance is a cue to oestrogen levels in women. Proc. R. Soc. B 273, 135–140 10.1098/rspb.2005.3296 (doi:10.1098/rspb.2005.3296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Török J., Hegyi G., Garamszegi L. Z. 2003. Depigmented wing patch size is a condition-dependent indicator of viability in male collared flycatchers. Behav. Ecol. 14, 382–388 10.1093/beheco/14.3.382 (doi:10.1093/beheco/14.3.382) [DOI] [Google Scholar]
- 38.Pagel M. 1994. The evolution of conspicuous oestrus advertisement in Old World monkeys. Anim. Behav. 27, 1–36 [Google Scholar]
- 39.Domb L. G., Pagel M. 2001. Sexual swellings advertise female quality in wild baboons. Nature 410, 204–206 10.1038/35065597 (doi:10.1038/35065597) [DOI] [PubMed] [Google Scholar]
- 40.Pipitone R. N., Gallup G. G. 2008. Women's voice attractiveness varies across the menstrual cycle. Evol. Hum. Behav. 29, 268–274 10.1016/j.evolhumbehav.2008.02.001 (doi:10.1016/j.evolhumbehav.2008.02.001) [DOI] [Google Scholar]
- 41.Havlicek J., Dvorakova R., Bartos L., Flegr J. 2006. Non-advertized does not mean concealed: body odour changes across the human menstrual cycle. Ethology 112, 81–90 10.1111/j.1439-0310.2006.01125.x (doi:10.1111/j.1439-0310.2006.01125.x) [DOI] [Google Scholar]