Abstract
Although global declines in frugivores may disrupt seed dispersal mutualisms and inhibit plant recruitment, quantifying the likely reduction in plant regeneration has been difficult and rarely attempted. We use a manipulative factorial experiment to quantify dependence of recruitment on dispersal (i.e. fruit pulp removal and movement of seed away from parental area) in two large-seeded New Zealand tree species. Complete dispersal failure would cause a 66 to 81 per cent reduction in recruitment to the 2-year-old seedling stage, and synergistic interactions with introduced mammalian seed and seedling predators increase the reduction to 92 to 94 per cent. Dispersal failure reduced regeneration through effects on seed predation, germination and (especially) seedling survival, including distance- and density-dependent (Janzen–Connell) effects. Dispersal of both species is currently largely dependent on a single frugivore, and many fruits today remain uneaten. Present-day levels of frugivore loss and mammal seed and seedling predators result in 57 to 84 per cent fewer seedlings after 2 years. Our study demonstrates the importance of seed dispersal for local plant population persistence, and validates concerns about the community consequences of frugivore declines.
Keywords: conservation, frugivore loss, Janzen–Connell effects, mutualism disruption, plant recruitment, seed dispersal
1. Introduction
Worldwide declines of frugivorous birds and mammals [1,2] have prompted concern about the potential consequences for plant persistence and mobility in a changing environment [3,4]. However, it has been difficult to estimate the size of likely impacts on plant reproduction if seed dispersal fails. The effectiveness of animal seed dispersers depends on both the quantity (number of seeds dispersed) and quality (seed treatment after ingestion and quality of deposition sites) of dispersal [5]. There are a number of possible mechanisms by which plant recruitment could be affected if dispersers decline, but their strength and frequency is often unclear [6,7]. Recent observational studies report lower seedling densities in forests with fewer frugivores [8], but these may be affected by confounding factors.
There is abundant evidence of widespread human-caused reductions in frugivores (principally birds and mammals) on most continents [9]. Even where animal mutualists persist, animal numbers may be insufficient for them to function effectively as seed disperser (i.e. they may be functionally extinct) [4,10]. Because gape size tends to limit the size of fruits an animal can swallow, particularly in birds, fewer animal species are capable of dispersing the seeds of large-seeded species [11,12], which consequently are more vulnerable to dispersal failure. Human activities also tend to have stronger effects on populations of larger-bodied vertebrates [2,4].
Although declines of large-bodied frugivores are well documented, the effects on plant recruitment have rarely been demonstrated and remain poorly quantified. At one extreme, dispersal failure might prevent regeneration completely, leaving forests full of ‘living dead’ adult trees [13] and eventually, depending on tree longevity, leading to the collapse and successional replacement of mature forest stands. However, plant regeneration is sometimes surprisingly robust in the face of disperser loss [14], requiring us to determine actual mechanisms and effects for potential declines in regenerative potential. Seed dispersal may be essential for plant recruitment at different spatial scales by facilitating germination [15], enabling seeds and seedlings to escape disproportionate mortality near parent plants (i.e. Janzen–Connell effects) [16,17], enabling colonization of new sites [18] or contributing to gene flow between populations [19]. For germination, concern is sometimes raised about possible germination failure in seeds with an apparent obligate need for gut passage through a frugivore such as the extinct dodo, but experimental evidence is lacking [15,20]. More important is higher mortality of undispersed seeds and seedlings caused by natural enemies, which respond to distance from the parent and/or density, thus limiting recruitment in the vicinity of conspecific adults [16,17]. As a result, in the absence of dispersers, all seeds or seedlings may remain near parents and die, although the consequences of regeneration failure may not become evident for a long time, especially in tree species that can live for 1000 years. However, a recent review found little evidence for consistent Janzen–Connell effects on short-term seed predation even in the tropics [21].
Here, we quantify both dispersal dependence (i.e. the dependence of seedling recruitment on dispersal [22]) and the impacts of dispersal failure (i.e. current reduction in regeneration) in two temperate-zone large-seeded New Zealand trees that are now largely dependent on a single frugivore. Although current frugivore losses are occurring predominantly in the tropics, islands like New Zealand can arguably give a much clearer impression of the impacts, as most of the extinctions have already occurred and alternative dispersers among the immigrant biota are rare, especially for large-seeded plants [23,24]. Human settlement of New Zealand had a huge impact on the avian fauna, driving 41 per cent of endemic forest bird species to extinction, restricting others to pest-free sanctuaries, and reducing the abundance and distribution of many of the survivors [25,26]. Dispersal of five large-seeded tree species (fruit width greater than 14 mm) is now largely dependent on the New Zealand pigeon (Hemiphaga novaeseelandiae Gmelin, Columbidae; figure 1 [24]), whose numbers have declined dramatically since human arrival [25,26]. Consequently, large-seeded trees in New Zealand are vulnerable to dispersal failure. However, as for most bird-dispersed plants, there is no evidence on the likely consequences of dispersal failure for these large-seeded trees or the actual current levels of dispersal service.
Figure 1.
New Zealand pigeon H. novaeseelandiae swallowing B. tarairi fruit (photo: Nga Manu Images).
We studied the two largest-seeded species in the New Zealand flora—Beilschmiedia tarairi (taraire, Lauraceae) and Corynocarpus laevigatus (karaka, Corynocarpaceae) [27]—as these may be the most susceptible to dispersal failure. Introduced animals are not acting as effective replacement dispersers: mammals (e.g. ship rats, Rattus rattus L.; brushtailed possums, Trichosurus vulpecula Kerr) have never been reported dispersing these large seeds [28], and the only contribution by introduced birds is from rare visits by the European blackbird (Turdus merula L.) to C. laevigatus [24]. Gut passage to remove fruit pulp was initially thought to be essential for C. laevigatus germination [29]. However, subsequent tests showed that this effect was an artefact of using Petri dishes [15], suggesting that dispersal failure may not pose as great a risk to C. laevigatus regeneration as previously thought.
We investigated the effects of dispersal failure, and its interaction with introduced mammalian seed and seedling predators, on recruitment of these two large-seeded tree species. We used a manipulative factorial experiment to test the effects of (i) movement of seeds away from adult conspecifics, (ii) seed density, (iii) fruit pulp removal, and (iv) introduced seed and seedling-predator mammals on seed predation, germination and seedling survival and growth of B. tarairi and C. laevigatus for 2 years in the field at Whangarei and Auckland in the North Island, New Zealand. Both sites were managed for conservation and had some control of mammalian pests (possums and/or rats).
2. Material and methods
(a). Study sites and species
We conducted experiments using two large-seeded species, B. tarairi and C. laevigatus. The canopy tree B. tarairi grows up to 20 m or more tall and is a successional climax species in lowland and coastal forest from the north of New Zealand's North Island to latitude 38° S [30]. Ripe B. tarairi drupes are dark purple (mean 19 × 32 mm, width × length) with a single seed averaging 16 × 29 mm [27]. Corynocarpus laevigatus is a mid-successional canopy tree that grows up to 20 m tall, occurs naturally in the northern North Island [31] and extends to latitude 44° S. The bright orange drupes of C. laevigatus average 20 × 28 mm and contain a single seed averaging 16 × 25 mm [27]. Dispersal of C. laevigatus is principally, and of B. tarairi solely, reliant on New Zealand pigeons H. novaeseelandiae, which are large (approx. 650 g) fruit pigeons endemic to New Zealand [24,25]. Although New Zealand pigeons are still widespread throughout New Zealand, their numbers have declined drastically since humans arrived in New Zealand, owing to habitat loss, introduced mammalian predators and illegal hunting [32–34], and the IUCN has classified them as ‘near threatened’ [35].
We conducted research in native lowland forest at Mt Tiger Bush, Whangarei (35°43′ S, 174°23′ E) and Wenderholm Regional Park, near Auckland (36°32′ S, 174°42′ E) from January 2005 to September 2007. The Mt Tiger site was located in a 7 ha privately owned block that forms part of the 267 ha Mt Tiger Bush and ranged in altitude from 140–270 m. Mt Tiger is mainly Streblus banksii (Moraceae)-dominant secondary lowland forest, with other common tree species including C. laevigatus and Rhopalostylis sapida (Arecaceae). During this study, the property owner undertook intensive possum and rat control in one area of the site (where two of five C. laevigatus focal trees (‘parents’) and three of five B. tarairi focal trees were located—see below for details) from late winter to late summer (R. J. Pierce 2007, personal communication). There was no pest control in the rest of the study site, although it would have gained some benefit from the intensive control nearby (R. J. Pierce 2007, personal communication). Wenderholm is a coastal B. tarairi-dominated forest remnant (approx. 60 ha) ranging in altitude from sea level to 140 m [36]. The forest contains a wide range of fleshy-fruited species, including the common tree species C. laevigatus, Vitex lucens (Verbenaceae), Dysoxylum spectabile (Meliaceae) and R. sapida. Forests in northern New Zealand, where both study sites were located, are typically dominated by fleshy-fruited species (woody basal area exceeding 60%) [24]. Introduced brush-tailed possums are controlled to low levels at Wenderholm, while rodents (principally ship rats) are controlled annually [36].
(b). Experimental design
We compared experimentally the fate of seeds using a split-plot full-factorial design with four treatments, each with two levels: (i) under a conspecific adult (referred to as a parent) versus 20 m away, (ii) whole fruits versus seeds with the pulp removed (by hand for B. tarairi, as few pigeon-ingested seeds were found, and by passage through pigeons for C. laevigatus), (iii) high versus low seed density (20 or four seeds, respectively), and (iv) mammal exclusion versus open access. Each parent tree was paired spatially with a location 20 m away. Fruit, density and exclusion treatments were nested within parent tree, with plots under and away from parents. We used a distance of 20 m for ‘away’ plots as we were unable to obtain sufficient replicates using greater distances without coming near another conspecific tree. Previous studies indicate that most parental effects on recruitment are negligible at 20 m and beyond [37–39]. Prior work showed no difference in germination of hand-cleaned versus bird-cleaned seeds in these two species [24].
To prevent seeds from rolling away, we placed them within 7 cm wide strips of lexan polycarbonate (1 mm thick) with the ends stapled together to construct 20 cm diameter tubes (figure 2). Tubes were inserted into the soil approximately 1 m apart, with around 5 cm of the tube remaining above ground. We constructed 30 cm high mammal-proof cages using 5.8 mm aperture galvanized steel weldmesh, which is small enough to exclude all mammals present, including house mice (Mus musculus). Cages were removed from C. laevigatus seedlings after 1 year to allow unrestricted seedling growth. Cages and tubes were secured to the ground with wire pegs. We randomly assigned one of the eight treatment combinations to each tube in the plot. This design was replicated at five parent trees at both Mt Tiger and Wenderholm for C. laevigatus, and at Mt Tiger for B. tarairi. We monitored tubes for 2 years, recording seed disappearance (see below), insect and mammal predation, germination, seedling height and survival for each seed or seedling.
Figure 2.
Intact C. laevigatus fruits at high density in a 20 cm diameter experimental tube (photo: Javi Rodríguez).
We marked B. tarairi fruits and seeds to increase the recovery rate and help determine their fate. We tagged all uncaged seeds by tying one end of a 15 cm length of nylon fishing line to 5 cm strips of pink and black striped flagging tape, and gluing the other end to the seed. We cut the flagging tape to a point at the end attached to the nylon line to decrease snagging and numbered each tag to aid identification. We conducted a pilot study in June 2005 at Wenderholm to determine whether tagging affected B. tarairi seed removal rates. We placed either four hand-cleaned seeds or four whole fruits in each tube. Half of the tubes had tagged seeds and the other half were untagged. We repeated this at two locations at Wenderholm (giving two replicates for each treatment combination) and recorded removal rates after one month. Removal of tagged and untagged seeds did not differ, and we therefore assumed that marking of seeds had no effect on seed removal.
Owing to difficulties in finding isolated C. laevigatus trees, not all parent trees at Wenderholm were fruiting when the experiment was set up. In addition, the B. tarairi fruit crop virtually failed at the Mt Tiger study site in 2005, so B. tarairi parents had few ripe fruits, and fruits were collected from taraire-dominated forests nearby to use in the experiment. For each species, we collected fruits from beneath trees that were generally within 50 m of an adult conspecific and for each site we combined all fruits prior to randomly allocating them to tubes. Beilschmiedia tarairi seeds germinated within one to three months of sowing, while most C. laevigatus seeds took four to six months. We monitored C. laevigatus seeds one week after setting up the experiment, monthly for the first six months, and then at 1 and 2 years. Beilschmiedia tarairi seeds were monitored monthly for the first three months and then at 1 and 2 years. At each visit, we placed any litter found on top of a cage inside the tube to reduce the effect of interception of litter-fall by cages.
Insect-eaten seeds were characterized by small holes in the seed or seed coat and the presence of insects and/or frass. Mammal-eaten seeds generally had 2–3 mm wide tooth marks consistent with rodent predation [40]. Some seeds that were partially eaten by either insects or mammals still germinated. Therefore, we classified only those seeds that suffered fatal predation as being eaten, assigning the fate of each seed prior to germination into four mutually exclusive categories: fatal predation (disappeared, insect-predated or mammal-predated) or uneaten. For some seeds, predation occurred after germination, in which case we classified it (when fatal) as mortality during year 1. For the purpose of the analysis, we assumed seeds that disappeared were killed as there are no reports of seed caching by any animals in New Zealand and we found no evidence of it (cf. [41]). Seventy per cent of B. tarairi seeds that disappeared had their tags recovered, mostly within 1 m of the tube, indicating that the seed had been consumed. A small number of seeds were found to have been moved to outside the tube, where we continued to follow their fate. We classified seeds as germinated upon radicle emergence. A seed was considered alive if it remained firm, and viable C. laevigatus seeds were often green beneath the seed coat.
In contrast to seed predators, seed dispersers consume only the fruit pulp and deposit clean, undamaged seeds. To estimate current percentages of our study species' fruit crops being consumed by frugivores in the field, we used data on the proportion of whole and clean seeds collected over several years around Auckland. Dijkgraaf [42] sampled the seed rain at six sites spread over a 60 km range around Auckland for 2 or 3 years per site between October 1994 and January 1998. Each site had 30 seed traps, with six of the traps beneath mature C laevigatus trees, six beneath B. tarairi and the remaining 18 traps beneath three other tree species. S. H. Anderson (2010, unpublished data) collected 10 fruits or seeds at random from beneath each of 11 B. tarairi trees in 2004 and 15 C. laevigatus trees in 2005 at Wenderholm [24]. Anderson's data may underestimate the proportion of the fruit crop consumed, as whole fruits are more likely to end up beneath the parent than ingested seeds, although this may be somewhat compensated for by seeds dispersed from other trees.
(c). Statistical analysis
We analysed data at each stage of recruitment, i.e. total seed predation (including insect predation, mammal predation and removed seeds), germination of those seeds that were not eaten, survival and growth to 1 year for germinated seeds, and survival and growth from 1 to 2 years for those seedlings alive after 1 year. We used generalized linear mixed models (GLMMs) to analyse seed predation, germination and seedling survival (the binomial response variables) for each species. GLMMs provide a framework for analysing data with a non-normal error distribution and hierarchical random effects. We used linear mixed models (LMMs) to analyse seedling growth during the first and second year. For seed predation, germination and seedling survival GLMMs, we specified a binomial error distribution (and associated logit link), with number of successes and number of failures as the response variable. For seedling growth LMMs, we specified a Gaussian error distribution.
For all response variables, we constructed a (maximal) model that initially included all explanatory variables (location, density, fruit and mammal exclusion) and all two-way interactions as fixed effects. In this paper, we largely focus on the treatment main effects for reasons of clarity and brevity, but all significant interactions are presented in the electronic supplementary material, tables S1–S5 and were used when calculating fitted values (e.g. figure 3). We included plots nested within parent trees as random effects in all models. For C. laevigatus, we also included site as a fixed effect in the maximal model, analysing data for the two sites separately when site had a significant effect.
Figure 3.
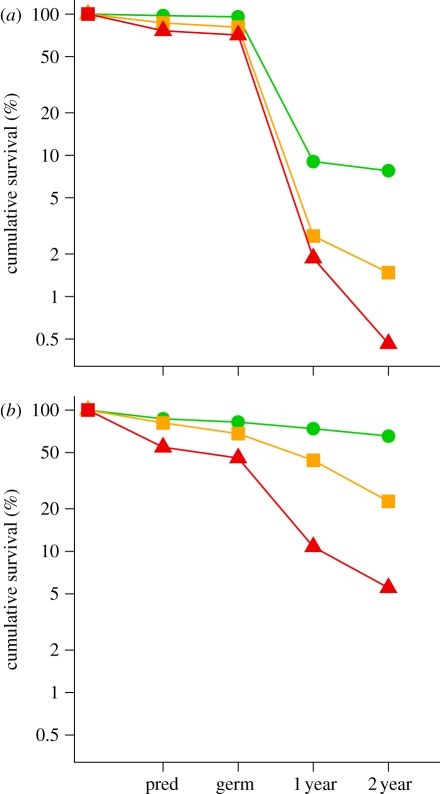
Dispersal failure and introduced mammals both caused a dramatic decrease in survival during the first 2 years for (a) B. tarairi and (b) C. laevigatus (mean of two sites). Mean cumulative survival rates (GLMM fitted values) are plotted through four recruitment stages (post-dispersal seed predation, germination, 1 year and 2 years). Green circles, best-case scenario (seeds dispersed and introduced predatory mammals excluded); amber squares, dispersal failure (introduced mammals excluded); red triangles, worst-case scenario (dispersal failure and introduced mammals). Treatment levels for dispersal were clean seeds, 20 m away from conspecific and low density. Treatment levels for dispersal failure were whole fruits, under conspecific and high density. Note the y-axis log scale. Effects on seedling height (table 1) are additional to those shown here.
We fitted the GLMMs using Laplacian approximation to maximum likelihood (which is more accurate than penalized quasi-likelihood, the only other method that was available in the statistical package [43]), and the LMMs using restricted maximum likelihood. We used model simplification by backward selection to construct final models. We compared the effect of removing each variable from the maximal model on Akaike information criterion (AIC) values. AIC provides a measure of model fit accounting for the sample size and the number of parameters estimated in the model, with smaller values of AIC indicating a better-fitting model [44]. We proceeded with simplification of the model with the lowest AIC value until removing any variable increased the AIC value. We calculated ΔAIC as the difference in AIC between a model and the best-fitting (final) model, which has ΔAIC of 0. As a rule of thumb, models with ΔAIC ≤ 2 have substantial support, those with 4 ≤ ΔAIC ≤ 7 weaker support and those with ΔAIC > 10 virtually no support [44]. Where multiple models had ΔAIC ≤ 2, we selected the model with the lowest AIC value as the final model, except where interaction terms in that model appeared biologically insignificant from graphical inspection of data. We ran all models using the lme4 package [43] in R v. 2.4.1 [45].
To quantify the impact of dispersal failure (sensitivity of reproduction), we modelled mean survival over 2 years as a function of the proportion of fruits consumed by frugivores. This was done twice, in the absence of mammal predation (caged) and in its presence (open). A fraction of seeds consumed by frugivores will be dropped under the parent tree, which affects seed fates. For modelling mean seed fates, we estimated this fraction at 0.13 for New Zealand pigeons based on seed-shadow modelling using gut passage times and fine-scale radiotracking data [46]. Of consumed fruits, 87 per cent were assumed to be dispersed and were given the fitted value for clean–away–low density, while the other 13 per cent were assumed to be defecated under the tree and were given the fitted value for clean–under–high density. Undispersed seeds were assigned whole–near–high density. The weighted mean survival was then expressed relative to the best-case scenario (100% of fruits consumed, no mammals). Although it is unlikely that fruit consumption by frugivores would reach 100 per cent in the wild, two multi-year studies in New Zealand mistletoes [6] came close (means approx. 95% consumption) and the model results would be very little changed (approx. 4%) if 95 per cent removal was used for the best case.
3. Results
Undispersed seed treatments (i.e. whole fruit, under conspecific, high density; 20 seeds) significantly reduced survival of both B. tarairi and C. laevigatus during the first two years by 81 and 66 per cent, respectively, relative to dispersed-treatment seeds (pulp removed, away from conspecific, low density; four seeds; tables 1 and 2 and figure 3). Fruit pulp removal and movement away from the parent were the dominant factors affecting B. tarairi survival (table 1). In C. laevigatus, movement away from parents had the greatest effect, increasing survival at all recruitment stages (table 1). Including interactions, significant effects were found at all stages for both species (table 1 and the electronic supplementary material, table S5), but the largest effects were on seedling survival through year 1 (figure 3). Unexpectedly, some effects of dispersal treatments persisted through the 2 years (e.g. significant effects of fruit pulp removal on seedling height growth during year 2; table 1).
Table 1.
Effect of seed dispersal and mammal exclusion treatments on percentage change in survival and seedling growth in B. tarairi and C. laevigatus (the first number is for Mt Tiger and the second is for Wenderholm). Recruitment stages measured were seed predation, germination of unpredated seeds, survival of germinated seeds to 1 year and seedling survival during the second year. Treatments that were retained in final models are shown in bold.
| recruitment stage | pulp removal | away from conspecific | low density | caged |
|---|---|---|---|---|
| B. tarairi | ||||
| seed predation | +18 | −1 | −5 | +27 |
| germination | +14 | −3 | −2 | −1 |
| survival year 1 | +117 | +134 | −50 | +23 |
| survival year 2 | +3 | +23 | +3 | +25 |
| height year 1 | +24 | +3 | +15 | +5 |
| height year 2 | −123 | −77 | − 75 | +142 |
| C. laevigatus | ||||
| seed predation | +23, +3 | +22, +4 | +4, −3 | +28, +21 |
| germination | −5, −1 | +24, +5 | +3, +1 | 0, −1 |
| survival year 1 | −4, +6 | +122, +31 | +28, −1 | +82, +18 |
| survival year 2 | −13, +1 | +35, +48 | +26, +31 | −1, +7 |
| height year 1 | −8, −3 | +25, +10 | +1, +19 | +12, +1 |
| height year 2 | +11, +32 | +43, +14 | −4, −76 | +52, +46 |
Table 2.
Effect of seed dispersal and mammal exclusion treatments on mean (± s.d.) percentage survival in B. tarairi and C. laevigatus (mean of two sites). Recruitment stages shown are germination of unpredated seeds, survival of germinated seeds to 1 year and seedling survival during the second year. The number of replicates for each treatment are presented in brackets. Treatments that were retained in final models are shown in bold. For seed predation, see figure 3.
| variable | level | recruitment stage |
||
|---|---|---|---|---|
| germination | survival year 1 | survival year 2 | ||
| B. tarairi | ||||
| fruit | whole | 83.5 ± 22.8 (39) | 7.1 ± 14.3 (38) | 64.1 ± 41.3 (13) |
| clean | 95.0 ± 8.2 (39) | 15.4 ± 16.9 (39) | 66.1 ± 40.8 (22) | |
| location | under | 90.7 ± 11.9 (39) | 6.8 ± 10.6 (39) | 55.6 ± 40.2 (15) |
| away | 87.8 ± 22.5 (39) | 15.9 ± 19.4 (38) | 72.8 ± 40.0 (20) | |
| density | high | 90.1 ± 8.4 (40) | 14.9 ± 17.1 (40) | 64.9 ± 37.7 (26) |
| low | 88.4 ± 24.4 (38) | 7.4 ± 14.3 (37) | 66.7 ± 50.0 (9) | |
| mammal access | open | 89.6 ± 19.5 (38) | 10.1 ± 16.1 (37) | 53.6 ± 41.8 (13) |
| cage | 88.9 ± 16.6 (40) | 12.4 ± 16.3 (40) | 72.3 ± 38.8 (22) | |
| C. laevigatus | ||||
| fruit | whole | 90.2 ± 18.2 (77) | 66.0 ± 38.2 (77) | 73.2 ± 31.7 (61) |
| clean | 87.2 ± 18.2 (80) | 67.1 ± 35.8 (79) | 69.0 ± 34.2 (69) | |
| location | under | 82.9 ± 22.5 (77) | 50.5 ± 40.2 (76) | 57.1 ± 35.2 (52) |
| away | 94.3 ± 10.1 (80) | 81.7 ± 25.7 (80) | 81.0 ± 27.4 (78) | |
| density | high | 87.7 ± 14.5 (80) | 63.2 ± 34.7 (80) | 62.9 ± 31.5 (73) |
| low | 89.7 ± 21.4 (77) | 70.2 ± 39.0 (76) | 80.7 ± 32.2 (57) | |
| mammal access | open | 88.9 ± 17.3 (78) | 55.0 ± 37.7 (77) | 69.7 ± 35.3 (59) |
| cage | 88.5 ± 19.2 (79) | 78.0 ± 32.4 (79) | 72.2 ± 31.0 (71) | |
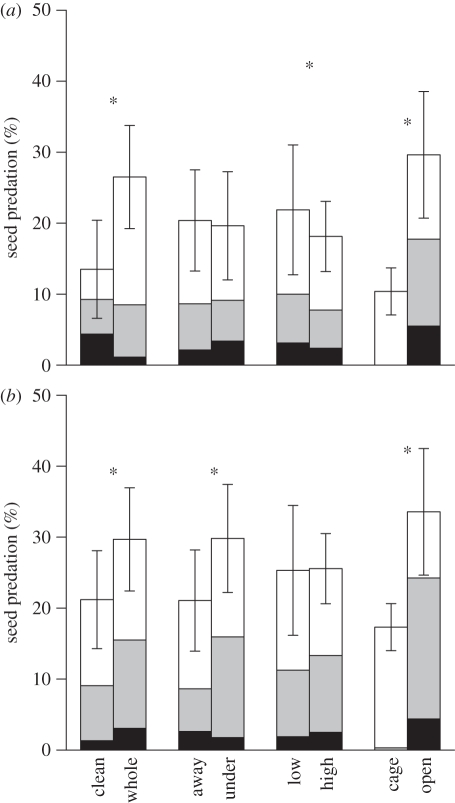
Losses to seed predators totalling 10 to 30 per cent of seeds were caused about equally by insects (native species) and mammals (all introduced species; figure 4). Dispersal reduced a seed's chances of suffering predation in B. tarairi (lower predation of clean seeds and at low density) and in C. laevigatus (lower predation of clean seeds and away from conspecifics; figure 4).
Figure 4.
Treatment main effects on mean seed predation percentage. (a) B. tarairi and (b) C. laevigatus (mean of two sites). Treatments were cleaned seeds versus whole fruits, 20 m away from conspecific versus under conspecific, low seed density (four seeds) versus high seed density (20 seeds) and mammal exclusion versus open access. White, insect predation; grey, removed; black, mammal predation. Error bars are 95% confidence intervals. Asterisks indicate treatments retained in final models.
Mammalian predation (cage effect) was not limited to seeds, but also affected seedling survival and seedling growth in both species (table 1). Mammalian predation interacted synergistically with dispersal failure, with significant interaction terms (cage×distance, cage×pulp removal, cage×density) retained for both species at both the seed predation and first-year seedling stages (see the electronic supplementary material, tables S1, S3 and S5). As a result, overall decreases in survival to 2 years caused by mammalian predators were much larger for undispersed-treatment seeds (69 and 76% reductions in B. tarairi and C. laevigatus, respectively) than for dispersed-treatment seeds (5 and 25% reductions, respectively). Decreases owing to mammals for undispersed-treatment seeds were similar in size to the decreases caused by dispersal failure, even though both study sites had some pest control. The overall decreases in survival with dispersal failure plus mammalian predation were 94 per cent for B. tarairi and 92 per cent for C. laevigatus.
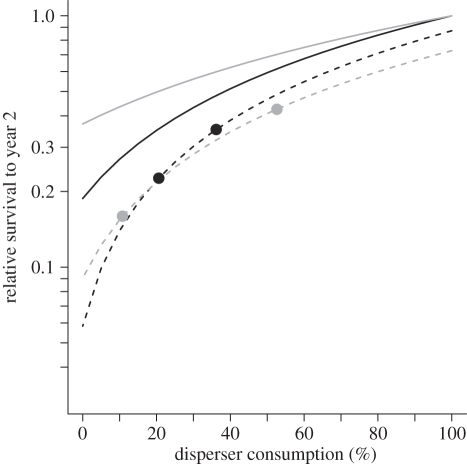
Knowing the survival of seeds under dispersed and undispersed conditions allows us to quantify how changes in dispersal service affect recruitment (figure 5). Relative to the best-case (pre-human) scenario, reductions in survival to age 2 years are small at moderate levels of disperser service, but accelerate greatly as the fraction of fruit crop consumed drops below 30 per cent. With the additional losses owing to interactions with mammalian predators, complete dispersal failure would reduce regeneration to less than 10 per cent of the pre-human level. There are few field estimates of current fruit consumption quantities but two studies reported ranges of 21 to 36 per cent for B. tarairi and 11 to 53 per cent for C. laevigatus [42] (S. H. Anderson 2010, unpublished data). Including the effects of managed densities of pest mammals at these sites, survival to age 2 years is currently being reduced by 65 to 77 per cent and 57 to 84 per cent, respectively (figure 5).
Figure 5.
Sensitivity to and impacts of frugivore loss (alone and in interaction with introduced mammalian seed predators) on survival to year 2 in B. tarairi and C. laevigatus. The solid lines represent the sensitivity (fitted mean seed fate as a function of fruit crop consumed by frugivores) in the absence of mammalian predators (‘cage’), while dotted lines are sensitivity including interactions with mammalian predators (‘open’). The x-axis is per cent of fruit crop consumed by frugivores, but 13% of consumed seeds are assumed dropped under parents (§2). The circles represent the impact based on current field estimates of consumption rates and with mammals present. Survival is relative to best-case condition as in figure 3 (all seeds dispersed, no mammals). Effects on seedling height (table 1) are additional. Black solid line, B. tarairi cage; grey solid line, C. laevigatus cage; black dashed line, B. tarairi open; grey dashed line, C. laevigatus open.
4. Discussion
By using manipulative field-based experiments, we found that recruitment in two large-seeded tree species is very sensitive to both dispersal failure and introduced mammalian seed and seedling predators, and that dispersal failure interacts synergistically with mammalian predation. Our data enable the relative contributions of introduced mammals and dispersal failure to be assessed at each stage in the post-fruit production recruitment cycle. The effects of factors associated with seed dispersal on survival were unexpectedly large and persistent, beyond the dispersal and germination stages. Effects were ubiquitous—there were significant effects of dispersal at every stage (post-dispersal seed predation, germination, seedling survival and height growth) in both species. It is also noteworthy that both species occur in the temperate zone, where escape from parents is thought to be less important than in the tropics [17].
Both forest dominants are highly dependent on seed dispersal (especially in the presence of mammalian predators), and the current impact of disperser loss on recruitment is ecologically considerable. Surviving bird populations appear to be dispersing relatively few seeds, and the magnitudes of present-day impacts on regeneration are 62 to 91 per cent of the worst-case scenario. Data on actual levels of fruit crop removal are few, and are likely to vary among years and across sites, as shown for Beilschmiedia tawa in New Zealand [24]. Although better data on this measure of frugivore service will be valuable, the sensitivity of recruitment to level of crop removal (the curves in figure 5) makes it straightforward to estimate the actual impact for any given level of fruit crop removal. There is little cause for complacency in New Zealand, because the most important frugivore for these large-seeded plants, the New Zealand pigeon, suffers from high levels of nest predation, and also from illegal human hunting [32]. Our analysis reinforces the need to conserve bird populations because of their ecosystem services to plants [4,47,48].
The Janzen–Connell model predicts lower seed and seedling survival beneath parent plants owing to density- and distance-dependent natural enemies [16,17]. Although often reported in the tropics, a meta-analysis of one part of the model (short-term seed predation near and away from conspecifics) found no consistent tendency for higher survival further away [21]. There was some evidence that effects on seedling survival were more consistent and stronger, as reported here. Janzen–Connell distance effects have been demonstrated less often in temperate areas, but examples are known [49,50]. Negative density dependence appears to be widespread in both tropical and temperate regions (e.g. [37,50,51]). In this study, distance- and density-dependent effects both contributed to lower survival of undispersed seeds and seedlings. Several factors are probably responsible for increased mortality under parents in these species. Higher B. tarairi seedling mortality under parents may be due to the deep, persistent litter layer that forms beneath B. tarairi trees, rather than host-specific enemies [52]. In C. laevigatus, higher levels of mammalian seed predation beneath adult conspecifics suggests that rodents may use parent trees to guide foraging [17].
Density-dependent effects on survival differed between C. laevigatus and B. tarairi. The increased germination success of whole B. tarairi fruits at high density compared with low density (see the electronic supplementary material, table S2) is unusual, but may be related to possible anti-feeding or anti-fungal properties in the copious jelly that fresh B. tarairi seeds exude [52]. Reduced B. tarairi seed predation and seedling mortality at high density (table 1) may be due to satiation of mammalian seed predators outside cages and of insect seedling predators inside cages [17]. By contrast, C. laevigatus seedlings exhibited negative density-dependent mortality (table 1), which was greater beneath conspecifics than 20 m away (see the electronic supplementary material, table S3).
Fruit pulp removal was previously thought to be critical for promoting germination (cf. [15]), but in this study it was more important for decreasing seed predation, particularly for C. laevigatus (table 1). Beilschmiedia tarairi fruit pulp removal at the start of the experiment increased not only germination success, but also later seedling survival and growth (table 1), which may have been due to higher levels of predation (which continues post-germination) in the storage tissue of whole fruits. Higher levels of pathogens and fungi can also occur in whole fruits [53], which may increase seedling mortality.
Introduced mammals disrupt regeneration of large-seeded trees both directly through predation of seeds and seedlings (this study), and indirectly by causing a decline in densities of frugivorous birds (like New Zealand pigeons) through predation and competition for food [32]. Even at our Wenderholm site, where pest control is intensive [36], introduced mammals decreased C. laevigatus survival by 28 per cent after 1 year and 34 per cent after 2 years. Ship rats and possums were probably responsible for the mammalian seed predation that we observed [40].
While our results show that local regeneration is highly dependent on seed dispersal, dispersal also plays a key role in plant succession by enabling C. laevigatus (a mid-successional tree) to stay in the landscape. For both B. tarairi and C. laevigatus, movement of seeds between forest patches may prevent local extinction of small, isolated populations and contribute to gene flow among populations. Inbreeding appears to be particularly detrimental in large-statured plants (trees and shrubs), with inbred offspring almost never surviving to maturity [54], including in two New Zealand trees [55]. Consequently, fragmented tree populations may be particularly vulnerable to extinction [54], and even more dependent on their dispersers.
The extent to which dispersers are ecologically redundant will influence how great an effect their disappearance will have [56–58]. If multiple dispersers are performing the same ecological role, then the loss of one disperser may not have a noticeable impact on plant populations [57]. Conserving the full range of dispersers within an ecosystem ensures that ecological redundancy is retained and provides a buffer to plant extinction, which is absent in the dispersal assemblage of these two New Zealand trees. Observational studies are providing mounting evidence that loss of dispersal agents negatively affects plant recruitment in the tropics. Hunted or fragmented forests in India, Peru and Tanzania had fewer frugivores, and fewer seedlings and saplings of animal-dispersed plants, than protected sites or continuous forest [59–61]. Reduced seedling density was particularly pronounced for large-seeded plants [60]. Hunted forests also showed evidence of changing plant community composition, with a higher proportion of seedlings and saplings dispersed by abiotic means than in protected forests [60,62]. Our experimental results are consistent with and support those observational studies.
Seed availability appears to be the main factor limiting seedling recruitment in many plant species [63,64], particularly at greater distances from the parent tree. Nevertheless, because the strength of processes limiting recruitment can change dramatically over a plant's life, seed limitation may be less important for later recruitment stages [63]. Data from the entire life cycle are required to address this question [63], but are obviously difficult to obtain for long-lived trees.
Few studies have managed to demonstrate experimentally that dispersal failure results in significantly lower recruitment [56,65]. Most studies have inferred a detrimental effect of dispersal failure on recruitment using comparative methods [8,59–62,66], which are unable to separate the effects of dispersal from other possible confounding causes. Although elements of our methodology have been often applied in the past (such as recording seed predation and removal in caches placed near and far from parents [21]), we believe the combination of our full-factorial manipulative experiment that followed the survival of seedlings for several years with integrating the results into a risk (or sensitivity) curve, provides a useful solution to the problem of estimating the impacts of frugivore loss.
Our findings highlight the importance of mutualistic interactions in the local regeneration of plant populations. Previously voiced concerns about the possible flow-on effects of functional extinction of bird populations [4] appear to be well founded, so conservation efforts must focus on conserving both individual species and mutualistic interactions [67].
Acknowledgements
We thank Kim McConkey, Bill Lee, Don Drake, Jason Tylianakis and two anonymous referees for helpful comments on earlier drafts, Chris Berry, Javi Rodríguez, Dean Benvenuti, Bev Woods and Reuben Ferguson for field assistance, Richard Duncan and Ashley Sparrow for statistical advice, Sandra Anderson for access to unpublished data, and Maggie Tisch and Jenny Ladley for logistical support. This research was funded by grants from Auckland Regional Council, the Foundation for Research Science and Technology (contract C09X0503), Brian Mason Scientific and Technical Trust, Robert C. Bruce Trust and Canterbury Botanical Society. D.M.W. was supported by a University of Canterbury Doctoral Scholarship, a New Zealand Federation of Graduate Women Fellowship and a Royal Forest and Bird Protection Society (North Canterbury Branch) Stocker Scholarship. Thanks to Ray Pierce, Auckland Regional Council, and Kathy and Don Hutchinson for permission to conduct research.
References
- 1.Peres C., Palacios E. 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39, 304–315 10.1111/j.1744-7429.2007.00272.x (doi:10.1111/j.1744-7429.2007.00272.x) [DOI] [Google Scholar]
- 2.Schipper J., et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230 10.1126/science.1165115 (doi:10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 3.Corlett R. T. 2007. Pollination or seed dispersal: which should we worry about most? In Seed dispersal: theory and its application in a changing world (eds Dennis A. J., Schupp E. W., Green R., Westcott D. W.), pp. 523–544 Wallingford, UK: CABI Publishing [Google Scholar]
- 4.Şekercioğlu C. H., Daily G. C., Ehrlich P. R. 2004. Ecosystem consequences of bird declines. Proc. Natl Acad. Sci. USA 101, 18 042–18 047 10.1073/pnas.0408049101 (doi:10.1073/pnas.0408049101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schupp E. W. 1993. Quantity, quality and the effectiveness of seed dispersal by animals. Vegetation 108, 15–29 [Google Scholar]
- 6.Kelly D., Ladley J. J., Robertson A. W. 2004. Is dispersal easier than pollination? Two tests in New Zealand Loranthaceae. N. Z. J. Bot. 42, 89–103 10.1080/0028825X.2004.9512892 (doi:10.1080/0028825X.2004.9512892) [DOI] [Google Scholar]
- 7.Muller-Landau H. 2007. Predicting the long term effects of hunting on plant species composition and diversity in tropical forests. Biotropica 39, 372–384 10.1111/j.1744-7429.2007.00290.x (doi:10.1111/j.1744-7429.2007.00290.x) [DOI] [Google Scholar]
- 8.Sharam G. J., Sinclair A. R. E., Turkington R. 2009. Serengeti birds maintain forests by inhibiting seed predators. Science 325, 51. 10.1126/science.1173805 (doi:10.1126/science.1173805) [DOI] [PubMed] [Google Scholar]
- 9.Wright S. J., Stoner K. E., Beckman N., Corlett R. T., Dirzo R., Muller-Landau H. C., Nuñez-Iturri G., Peres C. A., Wang B. C. 2007. The plight of large animals in tropical forests and the consequences for plant regeneration. Biotropica 39, 289–291 10.1111/j.1744-7429.2007.00293.x (doi:10.1111/j.1744-7429.2007.00293.x) [DOI] [Google Scholar]
- 10.McConkey K. R., Drake D. R. 2006. Flying foxes cease to function as seed dispersers long before they become rare. Ecology 87, 271–276 10.1890/05-0386 (doi:10.1890/05-0386) [DOI] [PubMed] [Google Scholar]
- 11.Kitamura S., Yumoto T., Poonswad P., Chuailua P., Plongmai K., Maruhashi T., Noma N. 2002. Interactions between fleshy fruits and frugivores in a tropical seasonal forest in Thailand. Oecologia 133, 559–572 10.1007/s00442-002-1073-7 (doi:10.1007/s00442-002-1073-7) [DOI] [PubMed] [Google Scholar]
- 12.Wheelwright N. T. 1985. Fruit size, gape width, and the diets of fruit-eating birds. Ecology 66, 808–818 10.2307/1940542 (doi:10.2307/1940542) [DOI] [Google Scholar]
- 13.Janzen D. H. 1986. The future of tropical ecology. Annu. Rev. Ecol. Syst. 17, 305–324 10.1146/annurev.es.17.110186.001513 (doi:10.1146/annurev.es.17.110186.001513) [DOI] [Google Scholar]
- 14.Janzen D. H., Martin S. 1982. Neotropical anachronisms: the fruits the gomphotheres ate. Science 215, 19–27 10.1126/science.215.4528.19 (doi:10.1126/science.215.4528.19) [DOI] [PubMed] [Google Scholar]
- 15.Robertson A. W., Trass A., Ladley J. J., Kelly D. 2006. Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect. Funct. Ecol. 20, 58–66 10.1111/j.1365-2435.2005.01057.x (doi:10.1111/j.1365-2435.2005.01057.x) [DOI] [Google Scholar]
- 16.Connell J. H. 1971. On the role of natural enemies in preventing competitive exclusion in some marine mammals and forest trees. In Dynamics of populations (eds den Boer P. J., Gradwell G. R.), pp. 298–312 Wageningen, The Netherlands: Centre for Agricultural Publishing and Documentation [Google Scholar]
- 17.Janzen D. H. 1970. Herbivores and number of tree species in tropical forests. Am. Nat. 104, 501–508 10.1086/282687 (doi:10.1086/282687) [DOI] [Google Scholar]
- 18.Howe H. F., Smallwood J. 1982. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228 10.1146/annurev.es.13.110182.001221 (doi:10.1146/annurev.es.13.110182.001221) [DOI] [Google Scholar]
- 19.Shapcott A. 2000. Conservation and genetics in the fragmented monsoon rainforest in the Northern Territory, Australia: a case study of three frugivore-dispersed species. Aust. J. Bot. 48, 397–407 10.1071/BT98081 (doi:10.1071/BT98081) [DOI] [Google Scholar]
- 20.Witmer M. C., Cheke A. S. 1991. The dodo and the tambalacoque tree—an obligate mutualism reconsidered. Oikos 61, 133–137 10.2307/3545415 (doi:10.2307/3545415) [DOI] [Google Scholar]
- 21.Hyatt L. A., et al. 2003. The distance dependence prediction of the Janzen–Connell hypothesis: a meta-analysis. Oikos 103, 590–602 10.1034/j.1600-0706.2003.12235.x (doi:10.1034/j.1600-0706.2003.12235.x) [DOI] [Google Scholar]
- 22.Bond W. J. 1994. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Phil. Trans. R. Soc. Lond. B 344, 83–90 10.1098/rstb.1994.0055 (doi:10.1098/rstb.1994.0055) [DOI] [Google Scholar]
- 23.Kelly D., Robertson A. W., Ladley J. J., Anderson S. H., McKenzie R. J. 2006. The relative (un)importance of introduced animals as pollinators and dispersers of native plants. In Biological invasions in New Zealand (eds Allen R. B., Lee W. G.), pp. 227–245 Berlin, Germany: Springer-Verlag [Google Scholar]
- 24.Kelly D., Ladley J. J., Wotton D. M., Robertson A. W., Anderson S. H., Wiser S. K. 2010. Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit dispersal in New Zealand. N. Z. J. Ecol. 34, 66–85 [Google Scholar]
- 25.Clout M. N., Hay J. R. 1989. The importance of birds as browsers, pollinators and seed dispersers in New Zealand forests. N. Z. J. Ecol. 12(Suppl.), 27–33 [Google Scholar]
- 26.Innes J., Kelly D., Overton J., Gillies C. 2010. Predation and other factors currently limiting New Zealand forest birds. N. Z. J. Ecol. 34, 86–114 [Google Scholar]
- 27.Wotton D. M., Ladley J. J. 2008. Fruit size preference in the New Zealand pigeon (Hemiphaga novaeseelandiae). Austral Ecol. 33, 341–347 10.1111/j.1442-9993.2007.01822.x (doi:10.1111/j.1442-9993.2007.01822.x) [DOI] [Google Scholar]
- 28.Williams P. A., Karl B. J., Bannister P., Lee W. G. 2000. Small mammals as potential seed dispersers in New Zealand. Austral Ecol. 25, 523–532 10.1046/j.1442-9993.2000.01078.x (doi:10.1046/j.1442-9993.2000.01078.x) [DOI] [Google Scholar]
- 29.Burrows C. J. 1996. Germination behaviour of seeds of the New Zealand woody species Alectryon excelsus, Corynocarpus laevigatus and Kunzea ericoides. N. Z. J. Bot. 34, 489–498 [Google Scholar]
- 30.Allan H. H. 1961. Flora of New Zealand, volume I. Wellington, New Zealand: P. D. Hasselberg [Google Scholar]
- 31.Costall J. A., Carter R. J., Shimada Y., Anthony D., Rapson G. L. 2006. The endemic tree Corynocarpus laevigatus (karaka) as a weedy invader in forest remnants of southern North Island, New Zealand. N. Z. J. Bot. 44, 5–22 10.1080/0028825X.2006.9513002 (doi:10.1080/0028825X.2006.9513002) [DOI] [Google Scholar]
- 32.Clout M. N., Karl B. J., Pierce R. J., Robertson H. A. 1995. Breeding and survival of New Zealand pigeons Hemiphaga novaeseelandiae. Ibis 137, 264–271 10.1111/j.1474-919X.1995.tb03248.x (doi:10.1111/j.1474-919X.1995.tb03248.x) [DOI] [Google Scholar]
- 33.Pierce R. J., Atkinson R., Smith E. 1993. Changes in bird numbers in six Northland forests 1979–1993. Notornis 40, 285–293 [Google Scholar]
- 34.McEwen W. M. 1978. The food of the New Zealand pigeon (Hemiphaga novaeseelandiae novaeseelandiae). N. Z. J. Ecol. 1, 99–108 [Google Scholar]
- 35.IUCN 2008. 2008 IUCN red list of threatened species. See www.iucnredlist.org (downloaded on 28 April 2009)
- 36.Lovegrove T. G., Zeiler C. H., Greene B. S., Green B. W., Gaastra R., MacArthur A. D. 2002. Alien plant and animal control and aspects of ecological restoration in a small mainland island: Wenderholm Regional Park, New Zealand. In Turning the tide: the eradication of invasive species (eds Veitch C., Clout M.), pp. 155–163 Auckland, New Zealand: IUCN SSC ISSG [Google Scholar]
- 37.Augspurger C. K. 1983. Seed dispersal of the tropical tree, Platypodium elegans, and the escape of its seedlings from fungal pathogens. J. Ecol. 71, 759–771 10.2307/2259591 (doi:10.2307/2259591) [DOI] [Google Scholar]
- 38.Schupp E. W. 1992. The Janzen–Connell model for tropical tree diversity—population implications and the importance of spatial scale. Am. Nat. 140, 526–530 10.1086/285426 (doi:10.1086/285426) [DOI] [PubMed] [Google Scholar]
- 39.Howe H. F., Schupp E. W., Westley L. C. 1985. Early consequences of seed dispersal for a neotropical tree (Virola surinamensis). Ecology 66, 781–791 10.2307/1940539 (doi:10.2307/1940539) [DOI] [Google Scholar]
- 40.Beveridge A. E. 1964. Dispersal and destruction of seed in central North Island podocarp forest. Proc. N. Z. Ecol. Soc. 11, 48–55 [Google Scholar]
- 41.Vander Wall S. B., Kuhn K. M., Beck M. J. 2005. Seed removal, seed predation, and secondary dispersal. Ecology 86, 801–806 10.1890/04-0847 (doi:10.1890/04-0847) [DOI] [Google Scholar]
- 42.Dijkgraaf A. C. 2002. Phenology and frugivory of large-fruited species in northern New Zealand and the impacts of introduced mammals. PhD thesis, University of Auckland, New Zealand [Google Scholar]
- 43.Bates D., Sarkar D. 2007. lme4: Linear mixed-effects models using S4 classes. R package, v. 0.9975–13. See www.r-project.org.
- 44.Burnham K. P., Anderson D. R. 2001. Kullback–Leibler information as a basis for strong inference in ecological studies. Wildl. Res. 28, 111–119 10.1071/WR99107 (doi:10.1071/WR99107) [DOI] [Google Scholar]
- 45.R Development Core Team 2006. R: a language and environment for statistical computing, v. 2.4.1 Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 46.Wotton D. 2007. Consequences of dispersal failure: kereru and large seeds in New Zealand. PhD thesis, University of Canterbury, New Zealand [Google Scholar]
- 47.Anderson S. H., Kelly D., Ladley J. J., Molloy S., Terry J. 2011. Cascading effects of bird functional extinction reduce pollination and plant density. Science 331, 1068–1071 10.1126/science.1199092 (doi:10.1126/science.1199092) [DOI] [PubMed] [Google Scholar]
- 48.Wenny D. G., Devault T. L., Johnson M. D., Kelly D., Şekercioğlu C. H., Tomback D. F., Whelan C. J. 2011. The need to quantify ecosystem services provided by birds. The Auk 128, 1–14 10.1525/auk.2011.10248 (doi:10.1525/auk.2011.10248) [DOI] [Google Scholar]
- 49.Packer A., Clay K. 2000. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404, 278–281 10.1038/35005072 (doi:10.1038/35005072) [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki M., Iwamoto S., Seiwa K. 2009. Distance-and density-dependent seedling mortality caused by several diseases in eight tree species co-occurring in a temperate forest. Plant Ecol. 201, 181–196 10.1007/s11258-008-9531-x (doi:10.1007/s11258-008-9531-x) [DOI] [Google Scholar]
- 51.Harms K. E., Wright S. J., Calderon O., Hernandez A., Herre E. A. 2000. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404, 493–495 10.1038/35006630 (doi:10.1038/35006630) [DOI] [PubMed] [Google Scholar]
- 52.Myers S. C. 1984. Studies in the ecology of Beilschmiedia tarairi (A. Cunn.) Benth. et Hook. F. ex Kirk. MSc, University of Auckland, New Zealand [Google Scholar]
- 53.Traveset A. 1998. Effect of seed passage through vertebrate frugivores' guts on germination: a review. Perspect. Plant Ecol. Evol. Syst. 1, 151–190 10.1078/1433-8319-00057 (doi:10.1078/1433-8319-00057) [DOI] [Google Scholar]
- 54.Scofield D. G., Schultz S. T. 2006. Mitosis, stature and evolution of plant mating systems: low-ϕ and high-ϕ plants. Proc. R. Soc. B 273, 275–282 10.1098/rspb.2005.3304 (doi:10.1098/rspb.2005.3304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robertson A. W., Kelly D., Ladley J. J. 2011. Futile selfing in the trees Fuchsia excorticata (Onagraceae) and Sophora microphylla (Fabaceae): inbreeding depression over 11 years. Int. J. Plant Sci. 172, 191–198 10.1086/657678 (doi:10.1086/657678) [DOI] [Google Scholar]
- 56.Christian C. E. 2001. Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 413, 635–639 10.1038/35098093 (doi:10.1038/35098093) [DOI] [PubMed] [Google Scholar]
- 57.Loiselle B. A., Blake J. G. 2002. Potential consequences of extinction of frugivorous birds for shrubs of a tropical wet forest. In Seed dispersal and frugivory: ecology, evolution and conservation (eds Levey D. J., Silva W. R., Galleti M.), pp. 397–406 Wallingford, UK: CABI [Google Scholar]
- 58.Brodie J. F., Helmy O. E., Brockelman W. Y., Maron J. L. 2009. Functional differences within a guild of tropical mammalian frugivores. Ecology 90, 688–698 10.1890/08-0111.1 (doi:10.1890/08-0111.1) [DOI] [PubMed] [Google Scholar]
- 59.Sethi P. I. A., Howe H. F. 2009. Recruitment of hornbill-dispersed trees in hunted and logged forests of the Indian eastern Himalaya. Conserv. Biol. 23, 710–718 10.1111/j.1523-1739.2008.01155.x (doi:10.1111/j.1523-1739.2008.01155.x) [DOI] [PubMed] [Google Scholar]
- 60.Terborgh J., Nunez-Iturri G., Pitman N. C. A., Valverde F. H. C., Alvarez P., Swamy V., Pringle E. G., Paine C. E. T. 2008. Tree recruitment in an empty forest. Ecology 89, 1757–1768 10.1890/07-0479.1 (doi:10.1890/07-0479.1) [DOI] [PubMed] [Google Scholar]
- 61.Cordeiro N. J., Howe H. F. 2003. Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc. Natl Acad. Sci. USA 100, 14 052–14 056 10.1073/pnas.2331023100 (doi:10.1073/pnas.2331023100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nunez-Iturri G., Olsson O., Howe H. F. 2008. Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biol. Conserv. 141, 1536–1546 10.1016/j.biocon.2008.03.020 (doi:10.1016/j.biocon.2008.03.020) [DOI] [Google Scholar]
- 63.Poulsen J. R., Osenberg C. W., Clark C. J., Levey D. J., Bolker B. M. 2007. Plants as reef fish: fitting the functional form of seedling recruitment. Am. Nat. 170, 167–183 10.1086/518945 (doi:10.1086/518945) [DOI] [PubMed] [Google Scholar]
- 64.Svenning J.-C., Wright S. J. 2005. Seed limitation in a Panamanian forest. J. Ecol. 93, 853–862 10.1111/j.1365-2745.2005.01016.x (doi:10.1111/j.1365-2745.2005.01016.x) [DOI] [Google Scholar]
- 65.Bond W., Slingsby P. 1984. Collapse of an ant-plant mutualism: the Argentine ant (Iridomyrmex humilis) and myrmecochorous Proteaceae. Ecology 65, 1031–1037 10.2307/1938311 (doi:10.2307/1938311) [DOI] [Google Scholar]
- 66.Chapman C. A., Chapman L. J. 1995. Survival without dispersers: seedling recruitment under parents. Conserv. Biol. 9, 675–678 10.1046/j.1523-1739.1995.09030675.x (doi:10.1046/j.1523-1739.1995.09030675.x) [DOI] [Google Scholar]
- 67.Janzen D. H. 1974. Deflowering of Central America. Nat. Hist. 83, 49–53 [Google Scholar]