Abstract
The environment is rarely constant and organisms are exposed to temporal and spatial variations that impact their life histories and inter-species interactions. It is important to understand how such variations affect epidemiological dynamics in host–parasite systems. We explored effects of temporal variation in temperature on experimental microcosm populations of the ciliate Paramecium caudatum and its bacterial parasite Holospora undulata. Infected and uninfected populations of two P. caudatum genotypes were created and four constant temperature treatments (26°C, 28°C, 30°C and 32°C) compared with four variable treatments with the same mean temperatures. Variable temperature treatments were achieved by alternating populations between permissive (23°C) and restrictive (35°C) conditions daily over 30 days. Variable conditions and high temperatures caused greater declines in Paramecium populations, greater fluctuations in population size and higher incidence of extinction. The additional effect of parasite infection was additive and enhanced the negative effects of the variable environment and higher temperatures by up to 50 per cent. The variable environment and high temperatures also caused a decrease in parasite prevalence (up to 40%) and an increase in extinction (absence of detection) (up to 30%). The host genotypes responded similarly to the different environmental stresses and their effect on parasite traits were generally in the same direction. This work provides, to our knowledge, the first experimental demonstration that epidemiological dynamics are influenced by environmental variation. We also emphasize the need to consider environmental variance, as well as means, when trying to understand, or predict population dynamics or range.
Keywords: epidemiology, host–parasite, variation, temperature stress, virulence, extinction
1. Introduction
Environmental variation influences natural patterns of parasite epidemiology and host demography [1,2]. However, it is difficult to ascertain the precise underlying processes driving dynamics as changes may be owing to hidden variables that also correlate with environmental variation. Hence, it is unresolved exactly how parasite incidence and distribution are affected by changes in the environment [3–5]. The effect of different environments on host–parasite dynamics may be attributable to direct effects on host or parasite life history, or effects on host populations may indirectly affect parasite transmission opportunities. The mechanism by which the environment influences parasite dynamics may lead to opposing predictions regarding the direction of the parasite response to stress [6]. Short-term correlative studies have linked mean temperatures in the field to differences in parasite incidence and disease across years [7,8]. Similarly, experimental studies have considered how differences in environmental means affect outcomes for host–parasite dynamics [4,9,10]. These studies demonstrate the sensitivity of the host and parasite to mean values of several environmental parameters. However, it is important to remember that environmental differences comprise not only means, but also variance.
Theoretical work demonstrates that changes in climate may be associated with a greater frequency of extreme events; in other words, the degree of variability is modified as well as the means [11,12]. Seasonal temperature extremes can have a strong influence on species distribution [11], e.g. owing to tolerance of certain organisms to frost. The inclusion of temporal environmental variation in epidemiological models has proved critical for accurate retrospective prediction of epidemics in the field [13] and for describing factors that influence spatial and temporal differences in parasite prevalence [14–16]. Even limited exposure to a stressful environment can modify host resistance traits [17–19] and experimental studies on non-parasite systems show that population dynamics are strongly influenced by fluctuation between permissive and restrictive environmental states [20–22]. This work highlights that both differences in mean and variance are important to consider when trying to understand how variation in environment influences the behaviour of natural systems. Recent theoretical analyses of Plasmodium dynamics [13] and a single generation study of its development in mosquitoes [23] advocate strongly that environmental variation is a key determinant for parasite epidemiology. However, experimental demonstration of the influence of a variable versus constant environment for long-term host–parasite dynamics is not yet established.
Here we explore how epidemiological dynamics are influenced over multiple generations across a gradient of mean temperatures ranging from permissive to restrictive conditions using a Paramecium–bacteria system in experimental microcosms. We compare these constant conditions to variable environments with the same mean temperature. Variable conditions are composed of random daily changes in temperature between permissive (23°C) conditions, where populations thrive, and restrictive (35°C) conditions, where growth is inhibited. Microcosm experiments are powerful in that they allow estimates of parasite epidemiology and host demography to be determined. Paramecium species [24] and this bacterial parasite are both sensitive to heat stress [25]. There is evidence that infected Paramecium can sometimes benefit from a transient protection against heat and osmotic stress owing to a general stress response induced by infection ([26–28] but see [29]). Parasite viability declines rapidly at higher temperature thus hindering transmission [25]. We therefore expected both host and parasite populations to be negatively affected by higher mean environmental temperatures in our experimental treatments. We also expected variable environments to be worse than constant environments, especially for the parasite as even very short periods at high temperatures can lead to declines in transmission [25]. Specifically, we identify: (i) how host and parasite are impacted by different constant mean temperatures; and (ii) how variable temperature environments with the same mean temperature impact host and parasite dynamics.
2. Material and methods
(a). Study organisms
Paramecium caudatum is a freshwater ciliate found in still water bodies in the Northern Hemisphere that feeds on bacteria and detritus within the water column [24]. Reproduction is predominantly asexual through mitotic division. Under exponential growth conditions, P. caudatum divides up to three times every 24 h and optimal growth temperatures generally range between 24°C and 28°C [24]. There is also evidence of within-species genetic variation in growth and survival at different temperatures [25]. Paramecium were maintained in a culture medium of dried organic lettuce supplemented with the bacterium Serratia marcescens as food [30]. Paramecium occupy shallow water habitats at the edge of still water bodies which can vary dramatically in temperature [31]. Thus, they are most probably exposed to rapid changes in temperature in their natural environment. Paramecium are found across a geographical region spanning a large temperature gradient [24].
Holospora undulata, a gram-negative α-proteobacterium, is a natural parasite of P. caudatum. Infection occurs through ingestion of infectious forms (15–20 µm) while feeding [32]. They are transferred to the micronucleus upon its fusion with food vesicles containing the parasite. After approximately 24 h, infectious forms differentiate into reproductive forms (5 µm), which multiply and begin to fill the micronucleus. After 7–10 days, an accumulation of reproductive forms is followed by the production of infectious forms [33]. Hosts can simultaneously harbour both spore types and a heavily infected host can be infected with up to several hundred infectious spores. Vertical transmission is achieved through segregation of reproductive forms into the two daughter micronuclei during asexual reproduction. Horizontal transmission occurs when infectious forms are released into the environment during host division or upon host death. Holospora undulata reduces host division rate and carrying capacity of P. caudatum and increases host mortality [29,34,35].
(b). Host and parasite origins
Two Paramecium clones were used in this experiment—clones K8 and VEN (see [29] for details). Prior to the experiment, we seeded mass cultures of each clone from single individuals. Five days before the start of the experiment, we established infected and uninfected mass cultures of each clone with identical densities. Infected cultures of each clone were initiated by adding Paramecium from infected populations to achieve parasite prevalence of approximately 30 per cent. Infected Paramecium populations originated from a portion of the uninfected cultures that were infected with inocula comprising bacteria collected from six different Paramecium clones (see [36]).
(c). Protocol
On day 1 of the experiment, the density of each mass culture and levels of infection were measured. Experimental replicate populations were set up by placing 17 ml of each of the four mass cultures (2 genotypes × 2 infection status) in fifty 50 ml Falcon tubes, with 3 ml of fresh culture medium. Tubes were then randomly allocated to one of four temperature levels (26°C, 28°C, 30°C and 32°C), which were replicated with constant and variable means. Means with variable temperatures were achieved by alternating tubes daily between 23°C and 35°C over a 15-day period. Electronic supplementary material, table S1 shows the temperatures that populations in the constant and variable temperature treatments were exposed to and the proportion of time populations in each of the variable treatments spent at 35°C. Each tube was randomly allocated an individual sequence corresponding to its mean temperature. Each sequence was created by placing the number of days required at each temperature in a random order using randomly generated number sequences. Fisher's Kappa spectral density tests were used to ensure no serial autocorrelation in the ordering of days. Each population followed two 15-day sequences consecutively (electronic supplementary material, figure S1 shows an example sequence a replicate population assigned to a variable treatment might experience). In addition, there were two constant control treatments at 23°C and 35°C.
The experiment contained a total of 200 replicate populations comprising 40 treatments, each with five replicates (2 genotypes × 2 infection status × 10 temperature treatments × 5 replicate populations). The experiment was divided in two blocks that were treated on consecutive days. Each block contained at least two replicates from each treatment with the third being randomly allocated to one of the blocks.
On days 6, 13, 20 and 27 of the experiment, 3 ml of each population was removed and replaced with fresh culture medium containing 50 P. caudatum from source populations of the same clone and infection status, kept at 23°C. Thus, weekly immigration permitted re-colonization of extinct populations for both Paramecium and the parasite. We used the 3 ml removed from each population prior to the immigration events to measure population density and infection prevalence. In addition, we also measured densities and infection prevalences on days 2, 4, 9 and 30 using 3 × 50 µl samples. When population numbers were too low to estimate density but we could still visually detect hosts in the microcosm, we assumed the presence of one paramecium in our 3 ml sample (i.e. 0.0167 hosts ml−1). Infection prevalence was estimated by making lacto-aceto-orcein fixations [33] of more than 20 Paramecium where possible, and never less than five. The proportion of infected individuals was determined at 1000× magnification (phase contrast). Infection prevalence was taken as the total (established plus new) proportion of infected individuals.
(d). Statistical analysis
(i). Host populations
We used analysis of variance (ANOVA) to assess how Paramecium population size, constancy and extinction probability were affected by infection status, mean temperature, a constant versus variable environment, and host clone. Host clone was included as a fixed factor as they were not randomly chosen representatives of the greater population. Each was chosen owing to their varied response to temperature stress and parasite infection. Temperature was included as a categorical variable as discrete levels were used and we discerned, using post hoc t-tests, where differences in our population measures between temperatures arose. However, treating temperature as a continuous variable does not influence the results (see the electronic supplementary material, table S3). We only consider populations at mean temperatures with corresponding variable and constant environments. These analyses exclude populations at constant temperatures of 23°C and 35°C. Inclusion of these temperatures in analyses including constant treatments only does not change the results (not shown). Incidence of host extinction was measured as the proportion of times the host population, arcsine square-root transformed, was found to be extinct across the four main sampling days (6, 13, 20, 27) of the experiment. Analyses investigating host extinction excluded populations in constant environments as extinctions were only observed in constant environments of 35°C. Host population size was measured as the log total area under the curve (AUC) during the experiment. This was approximated by calculating the sum of the total number of Paramecium in the population between sampling points (weighted by the number of days between sampling points). An additional, more complicated analysis is presented in the electronic supplementary material producing similar results where time is included in a model investigating the effect of our temperature treatments on the log mean population size (electronic supplementary material, table S2). Fluctuation in host population density was measured using the coefficient of variation calculated using the log-transformed Paramecium density per millilitre.
(ii). Parasite populations
We used ANOVA to investigate how host clone, temperature and a constant versus a variable environment affect mean parasite prevalence and parasite extinction. Temperature and Paramecium clone were treated as fixed factors. Analyses with temperature treated as a continuous variable does not change the results (electronic supplementary material, table S4). Parasite extinction was assumed when it could not be detected among Paramecium from infected populations on the fixation slide. Consequently, parasite extinction was sometimes estimated from very few Paramecium on a slide, which biased estimates of extinction for certain treatments. As observing fewer hosts naturally leads to a lower probability of finding parasites, extinction estimates were corrected for the number of Paramecium observed on each slide. This was achieved by taking the residuals over the entire dataset from a generalized linear model with a binomial error structure, with extinction as the response variable and number of Paramecium from which extinction was estimated (number on slide) as the explanatory variable. Mean residual extinction was calculated across the four main sampling days (6, 13, 20, 27) of the experiment. We then took the mean of the residuals across sampling days 6, 13, 20 and 27 as a measure of extinction. Parasite prevalence was calculated as the arcsine-transformed mean proportion of infected individuals across all sampling days. Owing to low host numbers and many extinctions on day 27, we combined parasite observations over sampling days 27 and 30. An additional analysis is presented in the electronic supplementary material, table S2, where time is included in a model investigating the effect of our temperature treatments on the parasite prevalence.
In all analyses, block was included in all models as a random factor and non-significant terms (p > 0.10) were removed in a stepwise fashion. The presented analyses do not include populations at constant temperatures of 23°C and 35°C, but they are sometimes included in figures to illustrate that they correspond to permissive and restrictive conditions, respectively. Inclusion of these populations in analyses across constant treatments only does not change the results. All analysis was done in JMP 8 [37].
3. Results
(a). Paramecium population dynamics
Population densities remained high throughout the experiment, 200–300 individuals ml−1, when in constant permissive conditions at 23°C. Conversely, population densities declined by 80 per cent during the first week of the experiment when in constant restrictive conditions of 35°C, reaching final levels of less than 20 individuals ml−1 (electronic supplementary material, figure S2). This confirms that our high-temperature treatment imposes a high level of stress on our Paramecium populations. The results of the statistical analyses of host population traits are summarized in table 1.
Table 1.
Effect of Paramecium host clone, parasite infection, temperature and constant versus variable environment on host population size, population constancy and incidence of Paramecium population extinction. (Statistics shown represent values taken from minimal models only. Italicized numbers indicate where different results are obtained if temperature is defined as a continuous variable.)
| numerator d.f. |
Paramecium population size |
coefficient of variation |
Paramecium extinction |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| denominator d.f. | F-ratio | p-value | denominator d.f. | F-ratio | p-value | denominator d.f. | F-ratio | p-value | ||
| clone | 1 | 142 | 15.01 | ** | 141 | 10.55 | ** | 71 | 4.35 | * |
| parasite | 1 | 142 | 617.18 | *** | 141 | 25.66 | *** | 71 | 6.67 | * |
| variable | 1 | 142 | 170.37 | *** | 141 | 95.99 | *** | |||
| temperature | 3 | 142 | 111.60 | *** | 141 | 31.59 | *** | 71 | 7.89 | * |
| clone × parasite | 1 | 142 | 75.05 | *** | 141 | 9.95 | ** | |||
| clone × temperature | 3 | 142 | 4.18 | ** | 141 | 8.89 | *** | |||
| parasite × temperature | 3 | 142 | 4.13 | ** | 141 | 2.76 | * | 71 | 2.65 | * |
| variable × clone | 1 | 142 | 4.18 | ** | 141 | 22.33 | *** | |||
| variable × parasite | 1 | |||||||||
| variable × temperature | 3 | 142 | 4.4440 | ** | ||||||
| clone × parasite × temperature | 1 | 141 | 4.69 | ** | ||||||
*p < 0.05.
**p < 0.01.
***p < 0.0001.
(i). Population density
Overall, infected populations had lower densities than uninfected populations (figure 1a), with reductions of up to 50 per cent. There were also significant main effects of the two environmental treatments (different constant temperatures and the constant versus variable environment). Densities were generally lower at higher temperatures (>30°C), and reductions were stronger for populations in variable temperature regimes. That is, for the same mean temperature, the variable environment, fluctuating between 23°C and 35°C, caused up to 30 per cent lower mean densities than the corresponding constant temperature. This effect was particularly pronounced at higher mean temperatures with more frequent exposure to 35°C in the variable environment (temperature × variable interaction; figure 1a).
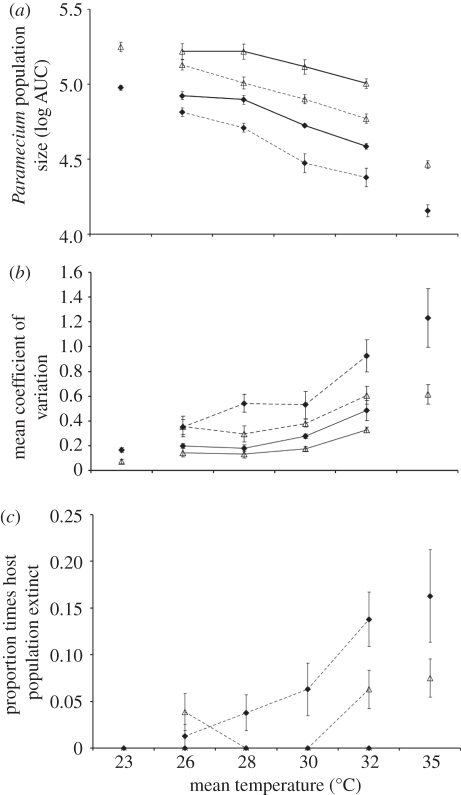
Figure 1.
Paramecium population parameter means for infected (closed triangle) and uninfected (open triangle) populations, in constant (solid lines) and variable (dashed lines) treatments (±s.e.) for (a) population size measured as the log10 area under the curve from d0 to d30, (b) coefficient of variation, and (c) proportion of times the population was observed extinct. The data shown are the combined means for host clones K8 and VEN. Figures also represent population parameters for infected and uninfected populations in constant environments at 23°C and 35°C to illustrate these temperatures that correspond to permissive and restrictive conditions, respectively.
The additional effect of infection on host population density depended on the experimental treatment. Infected populations experienced a disproportionate reduction in density compared with uninfected populations at higher temperatures (significant temperature × parasite interaction). By contrast, the non-significant variable × parasite interaction indicates that the combined negative effects of infection and the variable environment acted independently and thus additively (figure 1a).
Although effects were qualitatively identical for the two clones, all three treatments caused greater reductions in density for the VEN Paramecium clone than the K8 clone (significant clone × variable, clone × temperature and clone × parasite interactions, respectively; electronic supplementary material, figure S3). However, none of their three-way interactions were significant which indicates similar effects of infection under the different temperature treatments.
An analysis including the effect of time on Paramecium population size did not alter results obtained from analyses on the population means. However, this analysis revealed that all main effects and interactions between temperature treatments and Paramecium clones were variable through time. This is most likely owing to the marked decline in population size during the second half of the experiment for populations at higher temperatures (electronic supplementary material, table S2 and figure S4).
(ii). Density fluctuation
Populations with a lower mean density also showed stronger variation in density over the course of the experiment. Indeed, across all populations, there was a strong negative correlation between mean population density and the coefficient of variation (r = −0.69, n = 200, p < 0.0001). Consequently, while the presence of the parasite, higher temperature and the variable treatment decreased population density, these same factors also increased density fluctuations (table 1 and figure 1b). Further, the additional effect of infection caused greater fluctuations in density at higher temperatures (parasite × temperature; figure 1b).
(iii). Population extinction
Lower mean density was also associated with a higher probability of population extinction events (r = −0.38, n = 200, p < 0.0001). Paramecium population extinction events were restricted to variable treatments and to populations at constant 35°C, and were mostly observed after day 13 of the experiment when densities had fallen. Statistical analysis was therefore restricted to populations in variable environments. Again, extinction probability was significantly higher for infected populations and particularly pronounced at higher temperatures (table 1 and figure 1c).
(b). Parasite populations
(i). Prevalence
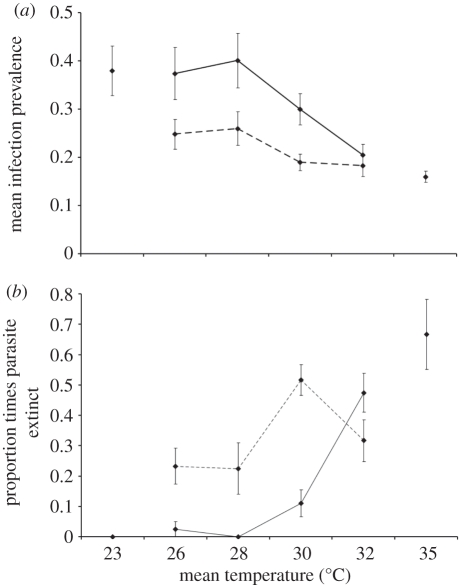
We found significant effects of both temperature treatments on mean parasite prevalence (results summarized in table 2). Initially adjusted to 30 per cent in all populations, prevalence declined markedly at temperatures above 28°C during the second half of the experiment (electronic supplementary material, figure S5). This resulted in lower overall mean prevalence at higher temperatures (figure 2). Furthermore, mean prevalence in the variable temperature environments was significantly lower than those in constant temperatures (figure 2a). This difference in prevalence between constant and variable environments was much greater at lower temperatures, with no difference being found at 32°C (variable × temperature interaction; figure 2). Mean parasite prevalence was low (approx. 25%) for all populations in variable environments and declined only moderately as mean temperature increased. Thus, more frequent exposure to 35°C in variable environments with higher mean temperatures did not have a strong additional negative influence on the parasite (figure 2). Indeed, reduced prevalence across variable environments may be owing to a threshold amount of time at 35°C, above which prevalence always declines.
Table 2.
Effect of Paramecium host clone, temperature and constant versus variable environment on parasite prevalence and incidence of parasite extinction. (Statistics shown represent values taken from minimal models only. Block described 14.06% of the variance for the model describing parasite prevalence.)
| numerator d.f. | parasite prevalence |
parasite extinction |
|||||
|---|---|---|---|---|---|---|---|
| denominator d.f. | F-ratio | p-value | denominator d.f. | F-ratio | p-value | ||
| clone | 1 | 65.03 | 231.68 | *** | 71 | 4.62 | * |
| variable | 1 | 65.16 | 79.23 | *** | 71 | 15.31 | ** |
| temperature | 3 | 65.01 | 29.96 | *** | 71 | 5.38 | ** |
| clone × temperature | 3 | 65.11 | 11.97 | *** | |||
| variable × clone | 1 | 65 | 20.85 | *** | |||
| variable × temperature | 3 | 65.09 | 4.55 | ** | 71 | 9.68 | *** |
*p < 0.05.
**p < 0.01.
***p < 0.0001.
Figure 2.
Parasite parameter means for infected populations in constant (solid lines) and variable (dashed lines) treatments (±s.e.) for (a) parasite prevalence and (b) proportion of times the parasite was observed extinct. The data shown are the combined means for host clones K8 and VEN. Figures also represent population parameters for infected and uninfected populations in constant environments at 23°C and 35°C to illustrate that these temperatures correspond to permissive and restrictive conditions, respectively.
Finally, mean parasite prevalence declined in populations of the more resistant VEN clone, and increased in the more susceptible K8 clone as might be expected. However, increasing temperature had a stronger negative effect on prevalence when infecting clone K8 (clone × temperature interaction; electronic supplementary material, figure S6).
An additional analysis including time in a model investigating the effects of the temperature treatments did not change the results obtained when looking at population means. However, all main effects and interactions were observed to be variable through time which is most probably attributable to the declines in prevalence observed during the second half of the experiment for populations at higher temperatures (electronic supplementary material, table S2 and figure S5).
(ii). Parasite extinction
The parasite was declared extinct, or could not be detected in just over half of all infected populations (51/100) on at least one occasion during the experiment. We observed a significant increase in extinction at higher temperatures and in variable environments (table 2). As with parasite prevalence, the difference in the incidence of extinction between populations in constant and variable environments was greater for populations at lower mean temperatures (temperature × variable interaction; figure 2b). Across all populations, there was a significant negative correlation between mean prevalence and the frequency of extinction (r = −0.31, n = 100, p = 0.0015). Thus, populations with a lower prevalence were more likely to go extinct.
4. Discussion
This work is, to our knowledge, the first to experimentally demonstrate how parasite epidemiology can be affected by temporal environmental variation between permissive and restrictive conditions. A key feature of environmental heterogeneity is that conditions can change both in terms of mean and variance. We tested how parasite epidemiology was affected by temporally variable temperature environments (effect of variance), compared with fixed temperatures (effect of mean), across a temperature gradient from permissive to restrictive conditions. Parasite spread and maintenance were highly sensitive to changes in both means and variances. Parasite infection reinforced the negative effects of a variable environment and higher mean temperatures on Paramecium population density.
We observed lower prevalence and an increased incidence of extinction of the parasite in variable treatments, alternating between 23°C and 35°C, and at higher temperatures. This is most probably attributable to an interplay between physiological and epidemiological factors. Paramecium become cured at higher temperatures thus reducing parasite prevalence [30]. Also, despite accelerated within-host development at higher temperatures (30°C) horizontal transmission is hampered owing to rapid subsequent deterioration of infectious spores in the environment [25]. This prevents an accumulation of infectious spores available to establish new infections, once environmental conditions become more favourable. This is likely to be aggravated by the reduction in Paramecium population density, which both further reduces the concentration of infectious forms in the environment, as well as contact rate with uninfected hosts. Indeed, this is consistent with the observed decline in the proportion of new infections, a proxy of the force of infection, at higher temperature and in the variable environment (electronic supplementary material, figure S7). Thus, despite recurrent immigration of infected Paramecium, the net effect was a decrease in prevalence. Lower prevalence was also associated with a higher frequency of parasite extinction events. This indicates that parasite persistence would be severely reduced at higher temperatures. Further, it is probable that the weekly culling coupled with low prevalence and Paramecium population sizes lead to the stochastic loss of the parasite. This is consistent with epidemiological models predicting minimum threshold densities for parasite maintenance [38,39]. It is possible that higher, or more frequent, introductions of infected Paramecium may have permitted parasite persistence. However, conditions imposed in this experiment show that parasite induced reduction in Paramecium density, coupled with a variable environment and increasing temperature, was not conducive for parasite maintenance.
Paramecium populations showed lower densities in variable environments and at higher temperatures. As in other organisms [40], tolerance of Paramecium to heat stress is partly mediated by the upregulation of heat-shock proteins, and infection with Holospora can further boost this response, thereby conferring heat stress protection [28]. We found no evidence for this, as infected populations were smaller and had higher extinction than uninfected populations, even in variable environments with low mean temperatures. This corroborates a recent study where we do not find evidence for parasite-mediated heat shock protection for the two clones used in this study [29]. Our results highlight the negative impact of even occasional, brief exposure to heat stress. Increased extinction for infected populations in these conditions is further detrimental for the parasite, hindering its persistence and spread.
The effect of fluctuating environments on the behaviour of biological systems can be considered within a source-sink framework [21], whereby short exposure to permissive conditions can allow population recovery, and enhance persistence, in otherwise restrictive conditions (sinks). In this experiment, we investigated how different variable environments, with mean temperatures ranging across a gradient presenting increasingly sink-like conditions, affected Paramecium and parasite populations. We found no evidence for recovery by either population in a variable environment following exposure to permissive conditions. On the contrary, both Paramecium and parasite populations across all variable environments suffered more than populations in equivalent constant environments with the same mean temperature. This was even true for populations in the variable environment with the lowest mean temperature. The absence of population recovery following periods in favourable conditions may be owing to an Allee effect, whereby populations are unable to grow below a certain density [41].
Environmental variation can vary in structure, time-scale and frequency. Here the sequences of environmental states were random (white noise), but they could have been structured in a negatively or positively autocorrelated (blue and red noise, respectively) fashion. Temporal fluctuations in natural populations are often positively auto-correlated with successive periods of time in either restrictive or permissive conditions [42]. Consequently populations that overall experience conditions purporting population decline are more likely to recover if exposed to enough days successively in permissive conditions assuming that there is a lag in recovery [43]. We are currently investigating how different types of environmental noise between restrictive and permissive conditions influence host–parasite dynamics.
Environmental variation also occurs across different timescales, and frequency of fluctuation will overlap differently with life-history stage and generation time, for different organisms. In our experiment, environmental fluctuations most probably occurred within one or two generations of both Paramecium and the parasite. Thus, changes in environment were long compared with the time required to mount a stress response. Indeed, parasite-mediated stress protection for Paramecium is transitory implying that perhaps shorter pulses of stress may have facilitated stress acclimatization in both organisms.
Our results complement a small body of work investigating the effects of environmental heterogeneity on community interactions. It has been shown that temporal environmental variation enhances coexistence between competing species [20,22,44], can destabilize predator communities [20] and promote population diversity [45] when compared with equivalent constant environments. These studies focus mainly on sequences of change that follow a random, white noise structure or fixed changes that occur at different day intervals. Although studies do not distinguish whether different species are stressed by the environments, this is probably species-specific. Only Jiang & Morin [22] explored how community demography was influenced by either positively correlated (red noise) or random (white noise) fluctuations between permissive and restrictive conditions. They found that Paramecium benefited from the impairment of the competitor species in variable environments, and observed greater population size when noise was positively correlated. Although we also observe stronger effects of environmental variation on the parasite, the antagonist species, we do not observe a beneficial feedback effect on Paramecium populations.
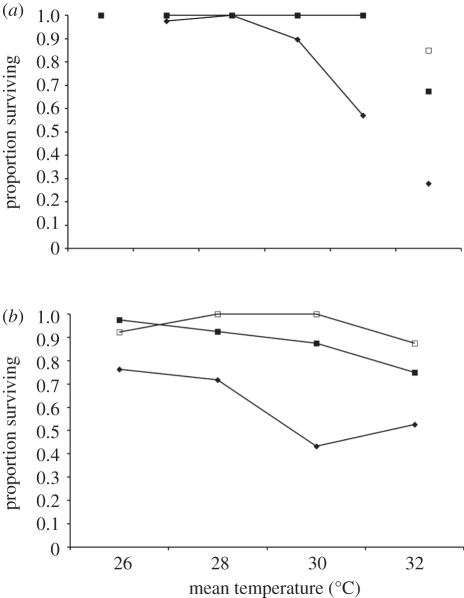
The duration of our experiment (30 days) spanned not more than 10–15 generations even in the most permissive conditions. Consequently, we do not expect evolution of either host or parasite to have occurred and emphasize that results obtained in this experiment essentially reflect variation in demographic and epidemiological dynamics. Theoretical [46,47] and empirical [48–50] studies show that host–parasite coevolution proceeds differently in spatially heterogeneous environments. Further, theory suggests that heterogeneous environments can select for reduced virulence when the frequency of marginal habitats which the host, but not the parasite can occupy increases [51]. Environmental variation is characterized by differences in means and variances. Here, we disentangled how both aspects of the environment affect host–parasite dynamics across a temperature gradient over which both populations may persist and interact. Understanding factors that limit, or permit, persistence and adaptation across environmental gradients is an important question for ecology and evolution [52]. It is often environmental variation or extremes, and not means, that constrains a species within a particular range [11]. Our results illustrate how environmental variation and difference in species tolerance ranges together may shape the geographical distribution of interacting species. Our results indicate that the range of stress tolerance for the parasite is less than that of uninfected and infected Paramecium populations (figure 3). Indeed, parasite persistence is restricted both by short exposure to stressful environments, across all variable environments, and at higher temperatures in constant environments. Thus, temporal environmental fluctuation, or populations spatially structured across a temperature gradient such as this one, would experience partially inhibited parasite persistence. Infected Paramecium populations tolerated a reduced range of environmental stress than uninfected populations, confirming the costs associated with infection. Populations exposed to this type of temperature variation, either spatially or temporally, may therefore create parasite-free refuges for populations where the parasite cannot persist. It may even be beneficial for the host population to be temporally exposed to these environments as a parasite cure. Further, if parasites are particularly harmful, there may be selection for infected hosts to actively seek these environments [53]. Indeed, the natural distribution of Holospora species are apparently restricted to temperate regions [32]. We are currently investigating how Paramecium and their parasite populations respond to constant and variable temperature regimes lower than optimal.
Figure 3.
Reductions in range for the parasite (closed diamonds) and infected (filled squares) and uninfected Paramecium (open squares) populations across the temperature gradient when in (a) constant and (b) variable conditions. The data shown are the mean proportion of times during the experiment each population was recorded extinct. Parasite extinctions in this figure include extinctions arising from host extinction. The data shown are the combined means for host clones K8 and VEN.
Recent theoretical work highlights how consideration of diurnal variation in temperature is crucial for accurate retrospective prediction of malaria epidemics [13]. This arises owing to its different effects on host and parasite life history when contrasting with constant environments. A follow-up empirical study based on individual observations, within one malaria-infected mosquito generation, corroborates the predictions and assumptions of this model [23]. However, direct extrapolation from individual observations in the laboratory to population level dynamics, is often not straightforward owing to long host generation times. Thus, our study directly complements the work of Paaijmans et al. by validating with experimental microcosm populations that environmental variation directly affects epidemiology.
5. Conclusions
This study is, to our knowledge, the first to experimentally show that temporal changes in environment, between permissive and restrictive conditions, influences parasite epidemiology, compared with equivalent constant conditions. Our work highlights the need to consider environmental variance, as well as means, when trying to understand, or predict, population dynamics and range. The discrepancy in results between populations in constant and variable environments, especially at lower mean temperatures, cautions against prediction of species range and dynamics based only on environmental means.
Acknowledgements
We are very grateful to Troy Day, Krijn Paaijmans, Owen Petchey and two anonymous reviewers for very helpful comments on this manuscript. We thank Claire Barbera for help in the laboratory. This work was supported by grants from Université Montpellier 2 (A.B.D.), Agence National de la Recherche (‘ANR-09-BLAN-0099’, S.F. and O.K.) and Institut National des Sciences de l'Univers (‘PIR EC2CO’, O.K.). This is ISEM contribution 2011-028.
References
- 1.Altizer S., Dobson A., Hosseini P., Hudson P., Pascual M., Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484 10.1111/j.1461-0248.2005.00879.x (doi:10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 2.Hosseini P. R., Dhondt A. A., Dobson A. 2004. Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. R. Soc. Lond. B 271, 2569–2577 10.1098/rspb.2004.2938 (doi:10.1098/rspb.2004.2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvell C. D., Mitchell C. E., Ward J. R., Altizer S., Dobson A. P., Ostfeld R. S., Samuel M. D. 2002. Ecology: climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 10.1126/science.1063699 (doi:10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 4.Lafferty K. D. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900 10.1890/08-0079.1 (doi:10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 5.McMichael A. J., Woodruff R. E., Hales S. 2006. Climate change and human health: present and future risks. Lancet 367, 859–869 10.1016/S0140-6736(06)68079-3 (doi:10.1016/S0140-6736(06)68079-3) [DOI] [PubMed] [Google Scholar]
- 6.Lafferty K. D., Holt R. D. 2003. How should environmental stress affect the population dynamics of disease? Ecol. Lett. 6, 654–664 10.1046/j.1461-0248.2003.00480.x (doi:10.1046/j.1461-0248.2003.00480.x) [DOI] [Google Scholar]
- 7.Hovmoller M. S. 2001. Disease severity and pathotype dynamics of Puccinia striiformis f.sp tritici in Denmark. Plant Pathol. 50, 181–189 10.1046/j.1365-3059.2001.00525.x (doi:10.1046/j.1365-3059.2001.00525.x) [DOI] [Google Scholar]
- 8.Powell E. N., Klinck J. M., Hofmann E. E. 1996. Modelling diseased oyster populations. Triggering mechanisms for Perkinsus marinus epizootics. J. Shellfish Res. 15, 141–165 [Google Scholar]
- 9.Lazzaro B. P., Little T. J. 2009. Immunity in a variable world. Phil. Trans. R. Soc. B 364, 15–26 10.1098/rstb.2008.0141 (doi:10.1098/rstb.2008.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolinska J., King K. C. 2009. Environment can alter selection in host–parasite interactions. Trends Parasitol. 25, 236–244 10.1016/j.pt.2009.02.004 (doi:10.1016/j.pt.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 11.Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 12.Schar C., Vidale P. L., Luthi D., Frei C., Haberli C., Liniger M. A., Appenzeller C. 2004. The role of increasing temperature variability in European summer heatwaves. Nature 427, 332–336 10.1038/nature02300 (doi:10.1038/nature02300) [DOI] [PubMed] [Google Scholar]
- 13.Paaijmans K. P., Read A. F., Thomas M. B. 2009. Understanding the link between malaria risk and climate. Proc. Natl Acad. Sci. USA 106, 13 844–13 849 10.1073/pnas.0903423106 (doi:10.1073/pnas.0903423106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Childs D. Z., Boots M. 2009. The interaction of seasonal forcing and immunity and the resonance dynamics of malaria. J. R. Soc. Interface 7, 309–319 10.1098/rsif.2009.0178 (doi:10.1098/rsif.2009.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari M. J., Djibo A., Grais R. F., Bharti N., Grenfell B. T., Bjornstad O. N. 2010. Rural-urban gradient in seasonal forcing of measles transmission in Niger. Proc. R. Soc. B 277, 2775–2782 10.1098/rspb.2010.0536 (doi:10.1098/rspb.2010.0536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkenstadt B. F., Grenfell B. T. 2000. Time series modelling of childhood diseases: a dynamical systems approach. J. R. Stat. Soc. 49, 187–205 10.1111/1467-9876.00187 (doi:10.1111/1467-9876.00187) [DOI] [Google Scholar]
- 17.Allen D. E., Little T. J. 2011. Identifying energy constraints to parasite resistance. J. Evol. Biol. 23, 224–229 10.1111/j.1420-9101.2010.02152.x (doi:10.1111/j.1420-9101.2010.02152.x) [DOI] [PubMed] [Google Scholar]
- 18.Allen D. E., Little T. J. 2010. Dissecting the effect of a heterogeneous environment on the interaction between host and parasite fitness traits. Evol. Ecol. 25, 499–508 10.1007/310682-010-9413-2 (doi:10.1007/310682-010-9413-2) [DOI] [Google Scholar]
- 19.Roth O., Kurtz J., Reusch T. B. H. 2010. A summer heat wave decreases the immunocompetence of the mesograzer, Idotea baltica. Mar. Biol. 157, 1605–1611 10.1007/s00227-010-1433-5 (doi:10.1007/s00227-010-1433-5) [DOI] [Google Scholar]
- 20.Gonzalez A., Descamps-Julien B. 2004. Population and community variability in randomly fluctuating environments. Oikos 106, 105–116 10.1111/j.0030-1299.2004.12925.x (doi:10.1111/j.0030-1299.2004.12925.x) [DOI] [Google Scholar]
- 21.Gonzalez A., Holt R. D. 2002. The inflationary effects of environmental fluctuations in source-sink systems. Proc. Natl Acad. Sci. USA 99, 14 872–14 877 10.1073/pnas.232589299 (doi:10.1073/pnas.232589299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L., Morin P. J. 2007. Temperature fluctuation facilitates coexistence of competing species in experimental microbial communities. J. Anim. Ecol. 76, 660–668 10.1111/j.1365-2656.2007.01252.x (doi:10.1111/j.1365-2656.2007.01252.x) [DOI] [PubMed] [Google Scholar]
- 23.Paaijmans K. P., Blanford S., Bell A. S., Blanford J. I., Read A. F., Thomas M. B. 2010. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl Acad. Sci. USA 107, 15 135–15 139 10.1073/pnas.1006422107 (doi:10.1073/pnas.1006422107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wichterman R. 1986. The biology of Paramecium. New York, NY: Plenum Press [Google Scholar]
- 25.Fels D., Kaltz O. 2006. Temperature-dependent transmission and latency of Holospora undulata, a micronucleus-specific parasite of the ciliate Paramecium caudatum. Proc. R. Soc. B 273, 1031–1038 10.1098/rspb.2005.3404 (doi:10.1098/rspb.2005.3404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujishima M., Kawai M., Yamamoto R. 2005. Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol. Lett. 243, 101–105 10.1016/j.femsle.2004.11.053 (doi:10.1016/j.femsle.2004.11.053) [DOI] [PubMed] [Google Scholar]
- 27.Hori M., Fujii K., Fujishima M. 2008. Micronucleus-specific bacterium Holospora elegans irreversibly enhances stress gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 55, 515–521 10.1111/j.1550-7408.2008.00352.x (doi:10.1111/j.1550-7408.2008.00352.x) [DOI] [PubMed] [Google Scholar]
- 28.Hori M., Fujishima M. 2003. The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 50, 293–298 10.1111/j.1550-7408.2003.tb00137.x (doi:10.1111/j.1550-7408.2003.tb00137.x) [DOI] [PubMed] [Google Scholar]
- 29.Duncan A. B., Fellous S., Accot R., Alart M., Chantung Sobandi A., Cosiaux A., Kaltz O. 2010. Parasite-mediated osmotic stress protection of Paramecium caudatum infected by Holospora undulata is host genotype specific. FEMS Microbiol. Ecol. 74, 353–360 10.1111/j.1574-6941.2010.00952.x (doi:10.1111/j.1574-6941.2010.00952.x) [DOI] [PubMed] [Google Scholar]
- 30.Nidelet T. 2007. L'effet de la structuration spatiale et de l'hétérogénéité environnementale sur les interactions hôte-parasite: une approche d'évolution expérimentale et d'épidémiologie. PhD thesis, UPMC Universite Paris, France [Google Scholar]
- 31.Lampert W. S. U. 1999. Limnoecology. Stuttgart, Germany: Thieme [Google Scholar]
- 32.Fokin S. I. 2004. Bacterial endocytobionts of ciliophora and their interactions with the host cell. Int. Rev. Cytol. 236, 181–249 10.1016/S0074-7696(04)36005-5 (doi:10.1016/S0074-7696(04)36005-5) [DOI] [PubMed] [Google Scholar]
- 33.Nidelet T., Koella J. C., Kaltz O. 2009. Effects of shortened host life span on the evolution of parasite life history and virulence in a microbial host-parasite system. BMC Evol. Biol. 9, 65. 10.1186/1471-2148-9-65 (doi:10.1186/1471-2148-9-65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magalon H., Nidelet T., Martin G., Kaltz O. 2010. Host growth conditions influence experimental evolution of life history and virulence of a parasite with vertical and horizontal transmission. Evolution 64, 2126–2138 [DOI] [PubMed] [Google Scholar]
- 35.Restif O., Kaltz O. 2006. Condition-dependent virulence in a horizontally and vertically transmitted bacterial parasite. Oikos 114, 148–158 10.1111/j.2006.0030-1299.14611.x (doi:10.1111/j.2006.0030-1299.14611.x) [DOI] [Google Scholar]
- 36.Adiba S., Huet M., Kaltz O. 2010. Experimental evolution of local parasite maladaptation. J. Evol. Biol. 23, 1195–1205 10.1111/j.1420-9101.2010.01985.x (doi:10.1111/j.1420-9101.2010.01985.x) [DOI] [PubMed] [Google Scholar]
- 37.SAS 2008. JMP statistics and graphics guide (v. 8.0.1.). Cary, NC: SAS Institute [Google Scholar]
- 38.Anderson R. M., May R. M. 1991. Infectious diseases of humans. Oxford, UK: Oxford University Press [Google Scholar]
- 39.Barlow N. D. 1996. The ecology of wildlife disease control: simple models revisited. J. Appl. Ecol. 33, 303–314 10.2307/2404752 (doi:10.2307/2404752) [DOI] [Google Scholar]
- 40.Feder M. E., Hofmann G. E. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann. Rev. Physiol. 61, 243–282 10.1146/annurev.physiol.61.1.243 (doi:10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 41.Courchamp F., Clutton-Brock T., Grenfell B. 1999. Inverse density dependence and the Allee effect. Trends Ecol. Evol. 14, 405–410 10.1016/S0169-5347(99)01683-3 (doi:10.1016/S0169-5347(99)01683-3) [DOI] [PubMed] [Google Scholar]
- 42.Ruokolainen L., Linden A., Kaitala V., Fowler M. S. 2009. Ecological and evolutionary dynamics under coloured environmental variation. Trends Ecol. Evol. 24, 555–563 10.1016/j.tree.2009.04.009 (doi:10.1016/j.tree.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 43.Petchey O. L., Gonzalez A., Wilson H. B. 1997. Effects on population persistence: the interaction between environmental noise colour, intraspecific competition and space. Proc. R. Soc. Lond. B 264, 1841–1847 10.1098/rspb.1997.0254 (doi:10.1098/rspb.1997.0254) [DOI] [Google Scholar]
- 44.Kassen R. 2002. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 15, 173–190 10.1046/j.1420-9101.2002.00377.x (doi:10.1046/j.1420-9101.2002.00377.x) [DOI] [Google Scholar]
- 45.Gallet R., Alizon S., Comte P. A., Gutierrez A., Depaulis F., Van Baalen M., Michel E., Muller-Graf C. D. M. 2007. Predation and disturbance interact to shape prey species diversity. Am. Nat. 170, 143–154 10.1086/518567 (doi:10.1086/518567) [DOI] [PubMed] [Google Scholar]
- 46.Gavrilets S., Michalakis Y. 2008. Effects of environmental heterogeneity on victim-exploiter coevolution. Evolution 62, 3100–3116 10.1111/j.1558-5646.2008.00513.x (doi:10.1111/j.1558-5646.2008.00513.x) [DOI] [PubMed] [Google Scholar]
- 47.Gomulkiewicz R., Drown D. M., Dybdahl M. F., Godsoe W., Nuismer S. L., Pepin K. M., Ridenhour B. J., Smith C. I., Yoder J. B. 2007. Dos and don'ts of testing the geographic mosaic theory of coevolution. Heredity 98, 249–258 10.1038/sj.hdy.6800949 (doi:10.1038/sj.hdy.6800949) [DOI] [PubMed] [Google Scholar]
- 48.Forde S. E., Thompson J. N., Bohannan B. J. M. 2004. Adaptation varies through space and time in a coevolving host–parasitoid interaction. Nature 431, 841–844 10.1038/nature02906 (doi:10.1038/nature02906) [DOI] [PubMed] [Google Scholar]
- 49.Vogwill T., Fenton A., Brockhurst M. A. 2010. How does spatial dispersal network affect the evolution of parasite local adaptation? Evolution 64, 1795–1801 10.1111/j.1558-5646.2009.00937.x (doi:10.1111/j.1558-5646.2009.00937.x) [DOI] [PubMed] [Google Scholar]
- 50.Thompson J. N. 2005. The geographic mosaic of coevolution. Chicago, IL: The University of Chicago Press [Google Scholar]
- 51.Hochberg M. E., Van Baalen M. 1998. Antagonistic coevolution over productivity gradients. Am. Nat. 152, 620–634 10.1086/286194 (doi:10.1086/286194) [DOI] [PubMed] [Google Scholar]
- 52.Kirkpatrick M., Barton N. H. 1997. Evolution of a species' range. Am. Nat. 150, 1–23 10.1086/286054 (doi:10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 53.Thomas M. B., Blanford S. 2003. Thermal biology in insect–parasite interactions. Trends Ecol. Evol. 18, 344–350 10.1016/S0169-5347(03)00069-7 (doi:10.1016/S0169-5347(03)00069-7) [DOI] [Google Scholar]